Abstract
The clinical application of the anticancer drug cisplatin is limited by its deleterious side effects, including male reproductive toxicity. In this context, the potential protective effect of carvedilol on testicular and spermatological damage induced by cisplatin in male Sprague–Dawley rats was investigated. Carvedilol was orally administered at a dose of 10 mg/kg for 2 weeks, and cisplatin was given as a single intraperitoneal injection of 10 mg/kg on the 12th day to induce toxicity. Cisplatin significantly reduced reproductive organ weight, sperm count and sperm motility, and increased sperm abnormalities and histopathological damage of testicular tissue. In addition, it resulted in a significant decline in serum testosterone as well as levels of testicular enzymatic and non-enzymatic antioxidants (superoxide dismutase, catalase, glutathione peroxides, and reduced glutathione). Moreover, cisplatin remarkably augmented malondialdehyde, nitric oxide, tumor necrosis factor-α, and nuclear factor-kappa B contents in testicular tissue. Conversely, carvedilol administration markedly mitigated cisplatin-induced testicular and spermatological injury as demonstrated by suppression of oxidative/nitrosative and inflammatory burden, amendment of antioxidant defenses, enhancement of steroidogenesis and spermatogenesis, and mitigation of testicular histopathological damage. The current study reveals a promising protective action of carvedilol against cisplatin-induced reproductive toxicity by virtue of its anti-inflammatory and antioxidant properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cisplatin, a crucial and widely prescribed anticancer drug, is used for the management of various types of malignant tumors including sarcomas, testicular, ovarian, breast, lung, bladder, and lymphomas (Kelland 2007; Todd and Lippard 2009). However, its therapeutic effectiveness is often limited owing to its undesirable toxic side effects, including male reproductive dysfunction (Stahl et al. 2006; Nakayama et al. 2008; Fung and Vaughn 2011; Beytur et al. 2012; Rezvanfar et al. 2013; Kaya et al. 2015). In fact, almost all treated patients experience temporary or permanent azoospermia and oligospermia, accompanied by testicular atrophy (Tomao et al. 2006).
Cisplatin adversely damages untargeted cells, in particular the highly proliferating testicular cell types Leydig, Sertoli and germ cells (Boekelheide 2005). Its toxicity is generally attributed to oxidative stress mediated by increased free radical production and decreased antioxidant defenses (Amin et al. 2008; Chirino and Pedraza-Chaverri 2009). The accumulation of reactive oxygen species (ROS) has been shown to induce lipid peroxidation and DNA fragmentation in sperm and testes (Boekelheide 2005). Furthermore, oxidative mediators are known to trigger inflammatory cascades by upregulating transcription factors, including nuclear factor-kappa B (NF-κB) (Savoia and Schiffrin 2007), which sequentially enhances the expression of pro-inflammatory cytokines, chemokines, adhesion molecules, and inducible nitric oxide synthase (iNOS) in a vicious cycle (Tak and Firestein 2001; Kleinert et al. 2004).
In this context, there remains a need for an adjuvant to improve cancer treatment by tackling the limiting side effects of chemotherapy. Carvedilol is a non-selective beta-blocker, which is used in the treatment of hypertension, ischemic heart disease, and congestive heart failure (Hayashi et al. 2010). It possesses a unique antioxidant property of scavenging and suppressing ROS, which has been attributed to the presence of a carbazole moiety in the molecule (Yue et al. 1992; Dandona et al. 2007). Carvedilol also exerts anti-inflammatory effects through balance modulation of pro- and anti-inflammatory mediators (Alfieri et al. 2008; Sari et al. 2011). Interestingly, previous studies revealed that carvedilol amended doxorubicin-induced cardiotoxicity (Oliveira et al. 2004), daunorubicin-induced cardiotoxicity and nephrotoxicity (Arozal et al. 2010), and cisplatin-induced nephrotoxicity (Rodrigues et al. 2011; Carvalho Rodrigues et al. 2013). Moreover, recent research revealed that the nephroprotection afforded by carvedilol was not associated with impairment of the antitumor activity of cisplatin (Carvalho Rodrigues et al. 2013). Rodrigues et al. (2011) suggested that, as cisplatin yields potent electrophilic intermediates that might react with carvedilol, carvedilol may compromise the antitumor activity of cisplatin. However, evidence was provided later by Ahmed et al. (2014) that carvedilol did not influence the cellular and sub-cellular distribution of cisplatin; instead, they suggested that its protective mechanism does not involve the formation of adducts with cisplatin. To the best of our knowledge, the effectiveness of carvedilol against cisplatin-induced testicular and spermatological damage has not yet been explored. Accordingly, the present study aimed to investigate the potential protective effect of carvedilol on reproductive organ weight, hormonal and spermatological changes, oxidative stress, inflammation, and the histopathological changes in testicular tissue following exposure to cisplatin in male rats.
Materials and methods
Animals
Adult male Sprague–Dawley rats, weighing 240–280 g, were obtained from the breeding colony of the National Organization for Drug Control and Research (NODCAR), Giza, Egypt. Animals were kept under controlled temperature (23 ± 2 °C) and relative humidity (60 ± 10 %) conditions, with a 12/12 h light/dark cycle. They were acclimatized for 1 week prior to any experimental procedures and were allowed standard chow diet and water ad libitum. This study complied with the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85–23, revised 1996) and was conducted in accordance with the ethical procedures and policies approved by the Ethical Committee of the Faculty of Pharmacy, Cairo University, Cairo, Egypt (Permit Number: PT 922).
Drugs and chemicals
Cisplatin was purchased from Mylan, Saint-Priest, France. Carvedilol was obtained from Multipharma, Cairo, Egypt. All other chemicals were of the highest purity and analytical grade.
Experimental design
Fifty rats were randomly allocated into five groups of 10 animals each. Group I (Control group) contained normal rats which received water. Group II (CMC group) contained normal rats which received 0.5 % carboxymethyl cellulose (10 ml/kg), the vehicle for carvedilol. Group III (CARV group) contained normal rats which received carvedilol at a dose of 10 mg/kg (Arab and El-Sawalhi 2013). Group IV (CIS group) contained rats with testicular and spermatological damage that was induced by administration of cisplatin at a dose of 10 mg/kg (Longo et al. 2011). Group V (CIS + CARV group) contained rats with testicular and spermatological damage treated with carvedilol, as previously described in group III. Carvedilol, water and the vehicle were orally administered through a feeding tube daily for 2 weeks, while cisplatin was administered as a single intraperitoneal injection on the 12th day. One day after the last treatment, rats were weighed and anesthetized, and blood samples were collected from the retro-orbital plexus. These were then centrifuged at 3000×g for 10 min to obtain serum for the determination of testosterone levels. Thereafter, animals were euthanized and the reproductive organs were immediately removed, cleaned of adhering tissue, and weighed. The epididymal content for each rat was immediately collected to examine the spermatological parameters. The left testis was fixed in 10 % formalin for histological examination. The right testis was decapsulated and divided into two parts, one of which was homogenized in 0.05 M potassium phosphate buffer (pH 7.4) for the determination of oxidative and inflammatory biomarkers and one of which was used for Western blot analysis of antioxidant enzymes. All samples were stored at −80 °C until analysis.
Spermatological examination
After collection of epididymal content, sperm count, motility, and the presence of abnormalities were determined for each rat according to the technique adopted by Bearden and Fluquary (1980).
Determination of serum testosterone level
Serum testosterone levels were measured with a rat-specific ELISA kit (Enzo Life Sciences, San Diego, CA, USA) used according to the manufacturer’s instructions.
Determination of testicular oxidative/nitrosative and inflammatory biomarkers
Testicular homogenate was divided into three portions, of which the first was used for the determination of malondialdehyde (MDA) according to the method described by Erdinçler et al. (1997). The second portion was deproteinized with 5 % sulfosalicylic acid and centrifuged at 1000×g for 15 min; the obtained supernatant was used for the determination of reduced glutathione (GSH) as described by Beutler et al. (1963). The third portion was centrifuged at 4000 rpm for 15 min at 4 °C and the resultant supernatant was used for the assay of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and NO using the corresponding Biodiagnostic colorimetric kits (Cairo, Egypt) according to the manufacturer’s instructions, while TNF-α was assayed using a rat-specific ELISA kit (Enzo Life Sciences, San Diego, CA, USA).
Western blot analysis of antioxidant enzymes and iNOS
Part of the right testis was homogenized in lysis buffer and quantified for protein levels using the Bio-Rad protein assay kit (Hercules, CA, USA). Protein expression was determined as previously described by Ahmed et al. (2014) using primary antibodies against SOD, CAT, GPx and beta-actin (β-actin) from Thermo Fisher Scientific Inc. (Waltham, MA, USA) as well as primary antibodies against iNOS from Stressgen Biotechnologies (Victoria, British Columbia, Canada). Protein amounts were quantified by densitometric analysis of the autoradiograms using a laser scanning densitometer (Biomed Instrument Inc., Fullerton, CA, USA). Results were expressed as arbitrary units after normalization for β-actin protein expression.
Histological examination
Light microscopic evaluation
The fixed testis was processed for paraffin embedding and 4 μm sections were prepared. Testicular sections were stained with hematoxylin and eosin (H&E) and examined using a light microscope. Qualitative histopathological damage in the seminiferous tubules was graded according to the severity of degenerative findings: (−) no obvious damage, (+) fewer than 25 % of seminiferous tubules affected (mild), (++) 25–50 % of seminiferous tubules affected (moderate), and (+++) over 50 % of seminiferous tubules affected (severe) (Rezvanfar et al. 2013). Histopathological examination of testes was interpreted by an experienced observer who was blind to the sample identity to avoid any bias.
Immunohistochemical analysis of testicular NF-κB
Paraffinized testicular sections were rehydrated in xylene and graded ethanol solutions. Samples were heated in citrate buffer (pH 6) for 20 min, cooled, and thereafter incubated with primary polyclonal rabbit anti-NF-κB antibody (1:200 dilution; Invitrogen, Carlsbad, CA, USA) overnight at 4 °C. Sections were washed with phosphate buffered saline, and incubated for 30 min at 37 °C with biotinylated secondary antibody (1:200 dilution, Dako, Glostrup, Denmark), and then with Avidin DH and biotinylated horseradish peroxidase H complex according to Elite ABC kit instructions (Vector Laboratories Inc., Burlingame, CA, USA). After another wash with phosphate buffered saline, the reaction was revealed by diaminobenzidine tetrahydrochloride (DAB Substrate Kit, Vector Laboratories Inc., Burlingame, CA, USA) and the sections were counterstained with hematoxylin, dehydrated, and cleared in xylene then cover slipped for light microscopic examination.
Statistical analysis
Data were expressed as mean ± SEM. Results were analyzed using one way analysis of variance test (one-way ANOVA) followed by Tukey–Kramer multiple comparison test. Statistical analysis was performed using GraphPad Prism software version 5 (San Diego, CA, USA); a probability level of less than 0.05 was accepted as statistically significant.
Results
Carvedilol attenuated cisplatin-induced alterations in relative reproductive organs weight in male rats
Cisplatin injection significantly decreased body weight, as well as the relative weight of reproductive organs (right and left testes, cauda epididymis, prostate, and seminal vesicles) to 87.7, 82.9, 85.2, 76.6, 73.7, and 80.7 % of the control group, respectively. Carvedilol administration reverted these alterations, except for body weight, back to their normal values (Table 1).
Carvedilol protects against cisplatin-induced spermatological damage and hormonal deficiency in male rats
Compared with the corresponding values of the control group, the CIS group exhibited a significant decline in sperm count and motility by almost half, along with a 2.7-fold increase in sperm abnormalities percentage (Fig. 1). In parallel, serum testosterone level was markedly reduced by 26.7 % compared to the control group (Fig. 2). Treatment with carvedilol amended the alterations in motility and sperm abnormalities by 30.3 and 46.0 %, respectively, versus the CIS group. In addition, carvedilol restored normal sperm count and serum testosterone to near-normal levels.
The effect of carvedilol on cisplatin-induced spermatological damage in male rats. Carvedilol (10 mg/kg, p.o) was administered daily for 2 weeks, during this period rats received a single intraperitoneal injection of cisplatin (10 mg/kg) on the 12th day to induce testicular toxicity. Each bar with vertical line represents the mean ± SEM of 10 animals in each group; *versus control, @versus CIS (one-way ANOVA followed by Tukey–Kramer multiple comparisons test; p < 0.05). CMC carboxymethyl cellulose, CARV carvedilol, CIS cisplatin
The effect of carvedilol on cisplatin-induced hormonal deficiency in male rats. Carvedilol (10 mg/kg, p.o) was administered daily for 2 weeks, during this period rats received a single intraperitoneal injection of cisplatin (10 mg/kg) on the 12th day to induce testicular toxicity. Each bar with vertical line represents the mean ± SEM of 10 animals in each group; *versus control, @versus CIS (one-way ANOVA followed by Tukey–Kramer multiple comparisons test; p < 0.05). CMC carboxymethyl cellulose, CARV carvedilol, CIS cisplatin
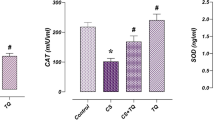
Carvedilol impeded cisplatin-induced testicular oxidative/nitrosative stress and inflammation in male rats
As shown in Table 2, cisplatin administration resulted in a marked augmentation in testicular MDA, NO, and TNF-α contents by 3.6-, 1.6- and 5.2-fold, respectively; it also resulted in a noticeable decline in testicular GSH content along with SOD, CAT and GPx activities by 60.5, 56.4, 47.1 and 65.9 %, respectively, as compared to the control group. In addition, the expression of these antioxidant enzyme genes was significantly reduced after cisplatin administration by 87.6, 53.9 and 68.9 %, respectively, as compared to the control group (Fig. 3a). Furthermore, cisplatin upregulated the expression of iNOS by 8.6-fold when compared to the control group (Fig. 3b). Carvedilol treatment prominently reversed cisplatin-induced alterations in the aforementioned oxidative/nitrosative and inflammatory genes and mediators.
The effect of carvedilol on cisplatin-induced changes in testicular antioxidant enzymes and iNOS protein expression in male rats. a Antioxidant enzymes protein expression. b iNOS protein expression. Carvedilol (10 mg/kg, p.o) was administered daily for 2 weeks, during this period rats received a single intraperitoneal injection of cisplatin (10 mg/kg) on the 12th day to induce testicular toxicity. Each bar with vertical line represents the mean ± SEM of 10 animals in each group; *versus control, @versus CIS (one-way ANOVA followed by Tukey–Kramer multiple comparisons test; p < 0.05). CMC carboxymethyl cellulose, CARV carvedilol, CIS cisplatin
Carvedilol downregulated cisplatin-induced testicular NF-κB protein expression in male rats
The immunohistochemical analysis (Fig. 4; Table 3) revealed an enhancement in NF-κB protein expression (confined within the interstitial stroma adjacent to the basement membrane of degenerated tubules) in testes of cisplatin treated rats (Fig. 4d), an effect that was attenuated by carvedilol treatment (Fig. 4e).
The effect of carvedilol on cisplatin-induced testicular NF-κB protein expression in male rats. Representative photomicrographs illustrating the immunohistochemical staining of NF-κB in testicular sections from a control, b CMC, and c CARV groups showing no expression, d CIS group showing very extensive expression (arrows), as well as e CIS + CARV group displaying low expression (arrow) (Magnification: ×400). CMC carboxymethyl cellulose, CARV carvedilol, CIS cisplatin
Carvedilol mitigated cisplatin-induced histopathological damage in testes of male rats
As shown in Fig. 5 and Table 4, testicular sections of the CIS group revealed atypical morphological features manifested as massive degeneration in the seminiferous tubules, shrinkage in germ cell layers, disruption of spermatogenesis, interstitial edema, congestion in blood vessels, and replacement of the interstitial stroma with homogeneous eosinophilic material (Fig. 5d). Carvedilol mitigated these histopathological alterations and preserved the testicular structure, along with reinstating spermatogenesis in the majority of seminiferous tubules (Fig. 5e).
The effect of carvedilol on cisplatin-induced histopathological damage in testes of male rats. Representative photomicrographs of testicular sections from a control, b CMC, and c CARV groups showing normal intact testicular tissues with mature seminiferous tubules (s) and complete spermatogenic series, as well as from d CIS group displaying marked degeneration in seminiferous tubules (ds) and sever congestion in blood vessels (v) of tunica albuginea (a) with eosinophilic infiltration in the interstitial stroma (t), e CIS + CARV group showing normal testicular structure with mature active seminiferous tubules (s) (H&E ×40). CMC carboxymethyl cellulose, CARV carvedilol, CIS cisplatin
Discussion
The main finding of the present study was that carvedilol protected the male reproductive system against the toxic effects of cisplatin, as shown by restoration of reproductive organ weight, reinstatement of testicular architecture, enhancement of steroidogenesis, and preservation of spermatogenesis. Modulation of the inflammatory response and suppression of oxidative stress could serve as important mechanisms underlying the protective effects induced by carvedilol against cisplatin-induced testicular toxicity. These findings extend the conclusion of Ramzy et al. (2014) that carvedilol afforded protection against testicular damage in streptozotocin-induced diabetic rats via antioxidant and antiapoptotic mechanisms.
In the present study, cisplatin induced significant testicular injury evident by atrophy of seminiferous tubules, loss of germinal and stromal cells, and suppression of spermatogenesis. This in turn is in agreement with previous studies (Ilbey et al. 2009; Amin et al. 2012; Kaya et al. 2015; Hamza et al. 2016), which declared that such impairment in testicular architecture is mainly attributed to severe oxidative damage exerted by cisplatin on rat testis. These histopathological alterations were also associated with a decline in relative testicular weights along with serum testosterone level as previously documented by Ilbey et al. (2009) and Adejuwon et al. (2015). Moreover, we observed significant decreases in epididymal sperm concentration and sperm motility and increased sperm abnormalities in accordance with several recent studies (Rezvanfar et al. 2013; Kaya et al. 2015; Hamza et al. 2016).
In the current study, treatment with carvedilol pronouncedly alleviated the aforementioned architectural and functional testicular disruptions induced by cisplatin together with restoring the weight of reproductive organs. This is supported by findings of Ramzy et al. (2014), who concluded that carvedilol attenuated diabetes-induced testicular tissue damage. Interestingly, it was suggested that the increase in testicular weight might be ascribed to the prevention of oxidative cellular damage which cisplatin exerted on Sertoli, Leydig and germ cell populations (Ilbey et al. 2009; Rezvanfar et al. 2013). This improvement was further confirmed in the current study by the enhancement in testosterone levels and sperm quality following carvedilol treatment. Importantly, Rezvanfar et al. (2013) showed that diminished testosterone secretion might be attributed to cisplatin-related deficiency in the enzymatic antioxidants within Leydig cells. Sperm were also extremely susceptible to cisplatin-induced peroxidative damage due to their high content of polyunsaturated fatty acids and low antioxidant capacity, resulting in reduced sperm function and infertility accordingly as reported by Aitken and Curry (2011). It is worth mentioning that alterations in the oxidant/antioxidant milieu are known to play a significant role in cisplatin-induced side effects.
In the present investigation, cisplatin induced a significant reduction in the expression and activities of SOD, CAT, and GPx along with a prominent decline in GSH content in testicular tissues, which is in accordance with previous studies (Longo et al. 2011; Khan et al. 2013; Rezvanfar et al. 2013; Ciftci et al. 2014; Almaghrabi 2015). Such decline in antioxidant status is partly attributable to the overproduction of toxic free radicals by damaged mitochondria as a result of cisplatin exposure (Kawai et al. 2006; Rezvanfar et al. 2013). This may also have occurred because of direct binding of cisplatin to GSH and essential sulfhydryl groups at the active sites of SOD, CAT and GPx (Rezvanfar et al. 2013) as well as the reported downregulation of these antioxidant enzymes’ protein expression in cisplatin-treated rats (Almaghrabi 2015). Cisplatin-induced disruption of both enzymatic and nonenzymatic antioxidants and overproduction of toxic free radicals were associated with an increased oxidative stress burden (Atessahin et al. 2006), evident in the current study by a surge in testicular MDA content, in agreement with the results of preceding studies (Salem et al. 2012; Rezvanfar et al. 2013; Hamza et al. 2016). Indeed, ROS generation stimulates transcription factors, particularly NF-κB (Bubici et al. 2006). Several studies reported that cisplatin-induced oxidative stress upregulated the expression of NF-κB, which subsequently increased the expression of numerous inflammatory genes (including iNOS), resulting in apoptotic death of testicular tissue through excessive NO production (Ilbey et al. 2009; Amin et al. 2012; Sherif et al. 2014; Hamza et al. 2016). Furthermore, and as concluded by Yao et al. (2007), the overproduction of TNF-α triggers apoptosis, generates ROS, and modulates the activation of a network of cytokines that all contribute to cellular injury. This is in accord with results the current study, which revealed that cisplatin promoted the expression of NF-κB and iNOS and their downstream inflammatory molecules TNF-α and NO, leading to an escalation in the inflammatory burden within the testicular tissue.
We showed that carvedilol administration boosted endogenous antioxidant defenses in testicular tissue, evident by augmented GSH content and SOD, CAT and GPx activity and expression. Carvedilol also mitigated cisplatin-induced lipid peroxidation, as demonstrated by decreased MDA content. These results are in agreement with recent reports, which reported that carvedilol attenuated lipid peroxidation and enhanced the antioxidant defenses in ischemic reperfusion- and diabetes-induced testicular tissue damage (Parlaktas et al. 2014; Ramzy et al. 2014). We further found that carvedilol attenuated NF-κB and iNOS expression and decreased TNF-α and NO release. Consistent with this, Sari et al. (2011) and Sahu et al. (2014) reported that carvedilol suppressed the activation of NF-κB and the expression of iNOS and TNF-α in daunorubicin-induced cardiotoxicity and potassium dichromate-induced nephrotoxicity in rats. Given these findings, we suggest that the antioxidant and anti-inflammatory properties of carvedilol are implicated in its protective actions against cisplatin-induced reproductive toxicity.
The powerful antioxidant activity of carvedilol is mainly accredited to its antioxidant and/or free radical scavenging activity (Yue et al. 1992; Dandona et al. 2007). By virtue of its iron-chelating properties, carvedilol suppresses the formation of hydroxyl radicals from both hydrogen peroxide via the Fenton reaction and superoxide anions via the Haber–Weiss reaction (Wilop et al. 2009). Carvedilol has also been shown to protect the intrinsic antioxidant defenses (Kumar and Dogra 2009), which is attributable to radical scavenging via the nitrogen and aromatic ring stabilization of carbazole moiety (Yue et al. 1992; Migliavacca et al. 1998). In addition, some carvedilol metabolites appear to have even more potent antioxidant properties than the parent compound (Naidu et al. 2002). Of note, carvedilol is a strong NF-κB inhibitor through the inhibition of IKKα activity and IκBα degradation, as well as the suppression of NF-κB binding to κB sites (Yang et al. 2003; Lin et al. 2010). Thus, the observation in the current work of carvedilol lowering NF-κB expression may be implicated in the observed decrease of TNF-α, iNOS and consequently NO. Moreover, it has been documented that carvedilol inhibited the protein expression of NF-κB during the inflammatory process both in vivo (Sari et al. 2011; Hamdy and El-Demerdash 2012) and in vitro (Yang et al. 2003; Lin et al. 2010), which accounts for its anti-inflammatory mechanism of action together with the prevention of ROS from activating NF-κB and its downstream genes.
In conclusion, this current study demonstrates, for the first time, the potential protective effects of carvedilol against cisplatin-induced testicular and spermatological toxicity in rats. These beneficial actions are attributable to carvedilol’s unique anti-inflammatory, antioxidant and free radical scavenging properties. Additionally, carvedilol has the advantage of being a safe and well-tolerated drug, which is already used clinically. Accordingly, carvedilol is considered a promising add-on therapy aiming at minimizing cisplatin-associated side effects, thereby improving the effectiveness of chemotherapy together with patients’ quality of life.
References
Adejuwon SA, Femi-Akinlosotu OM, Omirinde JO (2015) Cisplatin-induced testicular dysfunction and its amelioration by Launaea taraxacifolia leaf extract. Andrologia 47:553–559
Ahmed LA, Shehata NI, Abdelkader NF, Khattab MM (2014) Tempol, a superoxide dismutase mimetic agent, ameliorates cisplatin-induced nephrotoxicity through alleviation of mitochondrial dysfunction in mice. PLoS One 9:e108889
Aitken RJ, Curry BJ (2011) Redox regulation of human sperm function: from the physiological control of sperm capacitation to the etiology of infertility and DNA damage in the germ line. Antioxid Redox Signal 14:367–381
Alfieri AB, Briceno L, Fragasso G, Spoladore R, Palloshi A, Bassanelli G, Montano C, Arioli F, Cuko A, Ruotolo G, Margonato A (2008) Differential long-term effects of carvedilol on proinflammatory and antiinflammatory cytokines, asymmetric dimethylarginine, and left ventricular function in patients with heart failure. J Cardiovasc Pharmacol 52:49–54
Almaghrabi OA (2015) Molecular and biochemical investigations on the effect of quercetin on oxidative stress induced by cisplatin in rat kidney. Saudi J Biol Sci 22:227–231
Amin A, Hamza AA, Kambal A, Daoud S (2008) Herbal extracts counteract cisplatin-mediated cell death in rat testis. Asian J Androl 10:291–297
Amin A, Abraham C, Hamza AA, Abdalla ZA, Al-Shamsi SB, Harethi SS, Daoud S (2012) A standardized extract of Ginkgo biloba neutralizes cisplatin-mediated reproductive toxicity in rats. J Biomed Biotechnol. doi:10.1155/2012/362049
Arab HH, El-Sawalhi MM (2013) Carvedilol alleviates adjuvant-induced arthritis and subcutaneous air pouch edema: modulation of oxidative stress and inflammatory mediators. Toxicol Appl Pharmacol 268:241–248
Arozal W, Watanabe K, Veeraveedu PT, Ma M, Thandavarayan RA, Sukumaran V, Suzuki K, Kodama M, Aizawa Y (2010) Protective effect of carvedilol on daunorubicin-induced cardiotoxicity and nephrotoxicity in rats. Toxicol 274:18–26
Atessahin A, Sahna E, Turk G, Ceribasi AO, Yilmaz S, Yuce A, Bulmus O (2006) Chemoprotective effect of melatonin against cisplatin-induced testicular toxicity in rats. J Pineal Res 41:21–27
Bearden HJ, Fluquary J (1980) Applied animal reproduction. Restore Publishing Co. Inc., Reston
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Beytur A, Ciftci O, Oguz F, Oguzturk H, Yilmaz F (2012) Montelukast attenuates side effects of cisplatin including testicular, spermatological, and hormonal damage in male rats. Cancer Chemother Pharmacol 69:207–213
Boekelheide K (2005) Mechanisms of toxic damage to spermatogenesis. J Natl Cancer Inst Monogr 34:6–8
Bubici C, Papa S, Dean K, Franzoso G (2006) Mutual cross-talk between reactive oxygen species and nuclear factor-kappa B: molecular basis and biological significance. Oncog 25:6731–6748
Carvalho Rodrigues MA, Silva Faria MC, Santos NA, Gobe GC, Dos Santos AC (2013) Carvedilol efficiently protects kidneys without affecting the antitumor efficacy of cisplatin in mice. Chem Biol Interact 206:90–99
Chirino YI, Pedraza-Chaverri J (2009) Role of oxidative and nitrosative stress in cisplatin-induced nephrotoxicity. Exp Toxicol Pathol 61:223–242
Ciftci O, Cetin A, Aydin M, Kaya K, Oguz F (2014) Fish oil, contained in eicosapentaenoic acid and docosahexaenoic acid, attenuates testicular and spermatological damage induced by cisplatin in rats. Andrologia 46:1161–1168
Dandona P, Ghanim H, Brooks DP (2007) Antioxidant activity of carvedilol in cardiovascular disease. J Hypertens 25:731–741
Erdinçler DS, Seven A, Inci F, Beǧer T, Candan G (1997) Lipid peroxidation and oxidant status in experimental animals: effect of aging and hypercholestrolemic diet. Clin Chim Acta 265:77–84
Fung C, Vaughn DJ (2011) Complications associated with chemotherapy in testicular cancer management. Nat Rev Urol 8:213–222
Hamdy N, El-Demerdash E (2012) New therapeutic aspect for carvedilol: antifibrotic effects of carvedilol in chronic carbon tetrachloride-induced liver damage. Toxicol Appl Pharmacol 261:292–299
Hamza AA, Elwy HM, Badawi AM (2016) Fenugreek seed extract attenuates cisplatin-induced testicular damage in Wistar rats. Andrologia 48:211–221
Hayashi T, De Velasco MA, Saitou Y, Nose K, Nishioka T, Ishii T, Uemura H (2010) Carvedilol protects tubular epithelial cells from ischemia–reperfusion injury by inhibiting oxidative stress. Int J Urol 17:989–995
Ilbey YO, Ozbek E, Simsek A, Otunctemur A, Cekmen M, Somay A (2009) Potential chemoprotective effect of melatonin in cyclophosphamide- and cisplatin-induced testicular damage in rats. Fertil Steril 92:1124–1132
Kawai Y, Nakao T, Kunimura N, Kohda Y, Gemba M (2006) Relationship of intracellular calcium and oxygen radicals to Cisplatin-related renal cell injury. J Pharmacol Sci 100:65–72
Kaya K, Ciftci O, Cetin A, Dogan H, Basak N (2015) Hesperidin protects testicular and spermatological damages induced by cisplatin in rats. Andrologia 47:793–800
Kelland L (2007) The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer 7:573–584
Khan MA, Liu J, Kumar G, Skapek SX, Falck JR, Imig JD (2013) Novel orally active epoxyeicosatrienoic acid (EET) analogs attenuate cisplatin nephrotoxicity. FASEB J 27:2946–2956
Kleinert H, Pautz A, Linker K, Schwarz PM (2004) Regulation of the expression of inducible nitric oxide synthase. Eur J Pharmacol 500:255–266
Kumar A, Dogra S (2009) Neuroprotective effect of carvedilol, an adrenergic antagonist against colchicine induced cognitive impairment and oxidative damage in rat. Pharmacol Biochem Behav 92:25–31
Lin P-Y, Shen H-C, Chen C-J, Wu S-E, Kao H-L, Huang J-H, Wang D, Chen S-C (2010) The inhibition in tumor necrosis factor-α-induced attenuation in endothelial thrombomodulin expression by carvedilol is mediated by nuclear factor-κB and reactive oxygen species. J Thromb Thrombolysis 29:52–59
Longo V, Gervasi PG, Lubrano V (2011) Cisplatin induced toxicity in rat tissues: the protective effect of Lisosan G. Food Chem Toxicol 49:233–237
Migliavacca E, Ancerewicz J, Carrupt P-A, Testa B (1998) Theoretical parameters to characterize antioxidants. Part 2. The cases of melatonin and carvedilol. Helv Chim Acta 81:1337–1348
Naidu PS, Singh A, Kulkarni SK (2002) Carvedilol attenuates neuroleptic-induced orofacial dyskinesia: possible antioxidant mechanisms. Br J Pharmacol 136:193–200
Nakayama K, Milbourne A, Schover LR, Champlin RE, Ueno NT (2008) Gonadal failure after treatment of hematologic malignancies: from recognition to management for health-care providers. Nat Clin Pract Oncol 5:78–89
Oliveira PJ, Bjork JA, Santos MS, Leino RL, Froberg MK, Moreno AJ, Wallace KB (2004) Carvedilol-mediated antioxidant protection against doxorubicin-induced cardiac mitochondrial toxicity. Toxicol Appl Pharmacol 200:159–168
Parlaktas BS, Atilgan D, Gencten Y, Akbas A, Markoc F, Erdemir F, Ozyurt H, Uluocak N (2014) The effects of carvedilol on ischemia-reperfusion injury in the rat testis. Int Braz J Urol 40:109–117
Ramzy MM, El-Sheikh AA, Kamel MY, Abdelwahab SA, Morsy MA (2014) Mechanism of testicular protection of carvedilol in streptozotocin-induced diabetic rats. Indian J Pharmacol 46:161–165
Rezvanfar MA, Rezvanfar MA, Shahverdi AR, Ahmadi A, Baeeri M, Mohammadirad A, Abdollahi M (2013) Protection of cisplatin-induced spermatotoxicity, DNA damage and chromatin abnormality by selenium nano-particles. Toxicol Appl Pharmacol 266:356–365
Rodrigues MA, Rodrigues JL, Martins NM, Barbosa F, Curti C, Santos NA, Santos AC (2011) Carvedilol protects against cisplatin-induced oxidative stress, redox state unbalance and apoptosis in rat kidney mitochondria. Chem Biol Interact 189:45–51
Sahu BD, Koneru M, Bijargi SR, Kota A, Sistla R (2014) Chromium-induced nephrotoxicity and ameliorative effect of carvedilol in rats: involvement of oxidative stress, apoptosis and inflammation. Chem Biol Interact 223C:69–79
Salem EA, Salem NA, Maarouf AM, Serefoglu EC, Hellstrom WJ (2012) Selenium and lycopene attenuate cisplatin-induced testicular toxicity associated with oxidative stress in Wistar rats. Urology 79:1184.e1–1184.e6
Sari FR, Arozal W, Watanabe K, Harima M, Veeravedu PT, Thandavarayan RA, Suzuki K, Arumugam S, Soetikno V, Kodama M (2011) Carvedilol attenuates inflammatory-mediated cardiotoxicity in daunorubicin-induced rats. Pharmaceuticals 4:551–566
Savoia C, Schiffrin EL (2007) Vascular inflammation in hypertension and diabetes: molecular mechanisms and therapeutic interventions. Clin Sci 112:375–384
Sherif IO, Abdel-Aziz A, Sarhan OM (2014) Cisplatin-induced testicular toxicity in rats: the protective effect of arjunolic acid. J Biochem Mol Toxicol 28:515–521
Stahl O, Eberhard J, Jepson K, Spano M, Cwikiel M, Cavallin-Stahl E, Giwercman A (2006) Sperm DNA integrity in testicular cancer patients. Hum Reprod 21:3199–3205
Tak PP, Firestein GS (2001) NF-kappa B: a key role in inflammatory diseases. J Clin Invest 107:7–11
Todd RC, Lippard SJ (2009) Inhibition of transcription by platinum antitumor compounds. Metallomics 1:280–291
Tomao F, Miele E, Spinelli G, Tomao S (2006) Anticancer treatment and fertility effects. Literature review. J Exp Clin Cancer Res 25:475–481
Wilop S, Von Hobe S, Crysandt M, Esser A, Osieka R, Jost E (2009) Impact of angiotensin I converting enzyme inhibitors and angiotensin II type 1 receptor blockers on survival in patients with advanced non-small-cell lung cancer undergoing first-line platinum-based chemotherapy. J Cancer Res Clin Oncol 135:1429–1435
Yang SP, Ho LJ, Lin YL, Cheng SM, Tsao TP, Chang DM, Hsu YL, Shih CY, Juan TY, Lai JH (2003) Carvedilol, a new antioxidative beta-blocker, blocks in vitro human peripheral blood T cell activation by downregulating NF-kappaB activity. Cardiovasc Res 59:776–787
Yao X, Panichpisal K, Kurtzman N, Nugent K (2007) Cisplatin nephrotoxicity: a review. Am J Med Sci 334:115–124
Yue TL, Cheng HY, Lysko PG, Mckenna PJ, Feuerstein R, Gu JL, Lysko KA, Davis LL, Feuerstein G (1992) Carvedilol, a new vasodilator and beta adrenoceptor antagonist, is an antioxidant and free radical scavenger. J Pharmacol Exp Ther 263:92–98
Acknowledgments
The authors are grateful to Prof. Dr. Bakeir A and Prof. Dr. Ahmed KA (Department of Histology, Faculty of Veterinary Medicine, Cairo University, Cairo, Egypt) for performing the histopathological and immunohistochemical examinations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Eid, A.H., Abdelkader, N.F., Abd El-Raouf, O.M. et al. Carvedilol alleviates testicular and spermatological damage induced by cisplatin in rats via modulation of oxidative stress and inflammation. Arch. Pharm. Res. 39, 1693–1702 (2016). https://doi.org/10.1007/s12272-016-0833-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-016-0833-6