Abstract
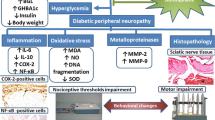
Diabetic peripheral neuropathy (DPN) is a common nerve disorder of diabetes. The aim of this study was to explore the protective effects of tropisetron in DPN. Type 1 diabetes was created by a single injection of streptozotocin (50 mg/kg, ip). Tropisetron (3 mg/kg, ip) was administered daily for 2 weeks. Our analysis showed that nerve fibers and their myelin sheaths were thinned with decreased myelinated fiber number in diabetic animals. The intensity of Bcl-2 staining decreased and the intensity of Bax staining increased in the sciatic nerves of diabetic rats by using immunohistochemical staining. Furthermore, diabetes significantly increased tumor necrosis factor-alpha, interleukin 1-β (TNFα and IL-1β) and Bax/Bcl-2 ratio in sciatic nerves of rats. However, intraperitoneal injection of tropisetron significantly reversed these alterations induced by diabetes. These findings suggest that tropisetron attenuates diabetes-induced peripheral nerve injury through its anti-inflammatory and anti-apoptotic effects, and may provide a novel therapeutic strategy to ameliorate the process of peripheral neuropathy in diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic peripheral neuropathy (DPN) is a serious chronic complication of diabetes (Shi et al. 2013). As a hallmark of diabetes, hyperglycemia can activate the polyol pathway which induces the accumulation of toxic metabolites in nervous tissues contributing to metabolic stress to the axon (Lós et al. 2019). Therefore, structural and functional impairment to peripheral nerves appears and causes neuronal disability through axonal atrophy, myelin sheath damage, and progressive nerve fiber loss (Ma et al. 2016).
Oxidative stress and inflammation are the most common pathological processes mediating diabetic complications, including DPN (Mirzakhani et al. 2018). Glucose neurotoxicity increases the expression of central inflammatory cytokines, such as TNFα and IL-1β, and leads to activation of the NF-kB pathway and, subsequently, apoptosis signaling and axonal degeneration (Dhaliwal et al. 2020,Lós et al. 2019,Ni et al. 2017).
Although insulin therapy is the standard method for type-1 diabetes mellitus (T1DM) treatment, it does not stop the several complications of DPN. Thus, there is a dire need to find an effective therapy for nerve damage in DPN.
Tropisetron, an antagonist of serotonin type 3 receptors, is extensively used as a safe antiemetic drug in chemotherapy (Barzegar-Fallah et al. 2014). Previous studies indicate that tropisetron exerts neuroprotective and anti-nociceptive effects in peripheral neuropathy models (Akada et al. 2006,Barzegar-Fallah et al. 2014).
The significant anti-diabetic activity of tropisetron via increasing GLUT2 gene expression and beta-cell mass in pancreatic tissue has recently been reported (Naderi et al. 2020b). Furthermore, tropisetron has been shown to suppress oxidative stress, pro-inflammatory cytokines, and apoptotic index in the pancreatic beta cells of diabetic rats (Naderi et al. 2020a, b).
Tropisetron also exerts its therapeutic effects against some diabetic tissues such as the kidney (Barzegar-Fallah et al. 2015), liver (Amini et al. 2020), and myocardial cells (Asadi et al. 2016). Swartz et al. reported that tropisetron has neuroprotective effects on glutamate-induced excitotoxicity in the central nervous system (Swartz et al. 2013). Tropisetron also managed to suppress vincristine-induced neurotoxicity by an anti-inflammatory mechanism (Barzegar-Fallah et al. 2014). However, there is no information on diabetic peripheral neuropathy after tropisetron treatment. Thus, herein, we decided to explore the effects of intraperitoneal injection of tropisetron on sciatic nerve damage in diabetic conditions.
Materials and methods
Experimental protocol
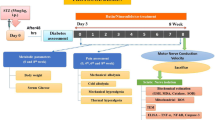
Twenty one male Wistar rats 250 ± 20 g weight, 3–4 months old) were kept in cages with a 12-h light/12-h dark cycle at room temperature (21 ± 2 °C) and had ad libitum access to food and water. All the experimental protocols were approved by the Ethics Committee of Urmia University of Medical Sciences (Ethical Code: IR.UMSU.REC.1398.305). Due to the mortality rate of diabetes, at first we considered 10 animals in diabetic groups, but finally 7 animals data were analyzed in the study. The rats were divided into three groups (n = 7): control (normal saline, daily), diabetes (STZ, 50 mg/kg as a single dose, intraperitoneally, freshly dissolved in normal saline) (Naderi et al. 2020b) , tropisetron + diabetes (STZ + 3 mg/kg tropisetron, daily for 2 weeks, intraperitoneally dissolved in normal saline) (Barzegar-Fallah et al. 2015,Naderi et al. 2020b).
The diabetes induced in rats after an overnight fast. After 72 h of STZ injection, FBS were measured by a standard digital glucometer (Elegance, CT-X10, Frankenberg, Germany), then diabetes is diagnosed at fasting blood glucose (FBS) ≥ 300mg/dl in each rat. After diabetes established, rats treated with tropiseton for 2 weeks according to the animal’s weight every day. Then, the animals euthanized with pentobarbital sodium (35 mg/kg, i.p.), (Sigma-Aldrich, Steinheim, Germany) and sciatic nerve dissected for later measurements.
FBS measurement
At the end of 2 weeks, blood samples were obtained from the tip of the tail, and glucose levels were measured by using a digital glucometer (Elegance, CT-X10, Frankenberg, Germany).
Preparation of sciatic nerve tissue for Luxol fast blue and immunohistochemical staining
After isolating sciatic nerves, apiece of this tissue was fixed with 10% formaldehyde. Then the samples were dehydrated and subsequently embedded in paraffin. Paraffin sections at 5-μm thick were prepared to evaluate the neuronal damage and myelination status by using Luxol fast blue staining. Myelin is stained blue, while axons remain white with Luxol fast blue staining. Myelin appeared as regular shapes, with a uniform thickness and clear boundary surrounding the myelin in healthy control group. By contrast, the myelin exhibited the worst fiber status and degeneration as evidenced by reduced myelin sheath thickness or loss of some myelinated nerve fibers leads to some disconnections after damage (Emir et al. 2016,Liu et al. 2016,Mustapha et al. 2019,Shi et al. 2013). The myelinated fiber number per 1 mm2, axone diameter, and myelin thickness of the nervous were analyzed using a histomorphometric lens (Olympus, HHA) under 400 × magnification and software (ImageJ software, National Institutes of Health, USA).
The expression of BAX and BCL2 were examined by immunohistochemistry. For this purpose, the cross-sections were prepared; three sections from each sciatic/rat (in total 18 cross-sections/group) were stained. Mean distributions of Bcl-2+ and Bax+ cells were examined. In brief, the histological sections were heated at 56 °C for 25 min, de-paraffinized in xylene (2×), and subsequently rehydrated through a series of graded alcohol concentrations (each for 5 min). The retrieval process for antigens was conducted by using 10 mM sodium citrate buffer (pH: 7.2), followed by blocking endogenous peroxidases using 1.5% hydrogen peroxide in 1× phosphate buffer (PBS, 20 min at room temperature), and incubated in a superblock solution (SCYTEK Co., AA025, Utah, USA, Lot: 43961) for 10 min. The control (with no primary antibody) and the experimental slides were incubated overnight (4 °C), respectively, with the blocking solution alone or primary antibodies: Bcl-2 (1:100, Cat N: E-AB-60788) and Bax (1:100, Cat N: EAB-13814, Elabsciences, USA. Subsequently, incubated with peroxidase/HRP conjugated goat anti-rabbit IgG secondary antibody (1:500, Elabscienece, USA, Cat N: E-AB1003) for 60 min at laboratory temperature. Finally, the slides were incubated with a standard 3,3′-diaminobenzidine chromogen solution (DAB; Sigma, St. Louis MO) for 5 min to visualize the labeled proteins and then counterstained with hematoxylin for 10 s. Moreover, the pixel-based intensity of brown reactions, defining the target proteins, was assessed by software (ImageJ software, National Institutes of Health, USA) at 2540 µm × 2540 µm of a cross-section from each animal. For this aim, images (20 megapixels) were captured by the onboard camera (Zeiss, Cyber-Shot, Japan). Next, the mean of pixel-based intensities of 3 images from one cross-section (in total 18 sections/each group) were examined, and the results were compared between groups. The normal IgG instead of primary antibody was used for negative control and positive control is healthy control rats (Shi et al. 2013).
Western blotting
IL-1β, TNFα, Bax, and Bcl-2 protein levels in the sciatic nerve were determined by Western immunoblotting according to the method as indicated in our previous study (Naderi et al. 2020b). In brief, another part of sciatic nerve was isolated and homogenized and then sonicated in the buffer containing 1% Triton X-100, 0.1% sodium dodecyl sulfate (SDS), 50 mM Tris hydrochloride (Tris-HCl), pH 7.5, 0.3 M sucrose, 5 mM ethylenediaminetetraacetic acid (EDTA), 2 mM sodium pyrophosphate, 1 mM sodium orthovanadate, and 1 mM phenylmethylsulfonyl fluoride, supplemented with a complete protease inhibitor cocktail. The samples were centrifuged (15 min at 1000 × g at 4 °C) to produce the supernatant, and then proteins were isolated through the SDS-PAGE and electrotransferred to the nitrocellulose membrane. After blocking, anti-IL-1β, anti-TNFα, anti-Bax, and anti-Bcl-2 antibodies were utilized. The concentration of the proteins was determined, and immunoreaction density was evaluated using the ImageJ software. Catalog numbers and companies of antibodies are presented in Table 1.
Statistical analysis
The data were expressed as mean ± SEM, and analyses were performed using SPSS 16.0. All the parameters were checked for normality using the one-sample Kolmogorov-Smirnov test. Data were statistically evaluated using one-way analysis of variance (ANOVA) followed by Tukey’s test. The significant level was expressed at P < 0.05.
Results
Tropisetron decreased blood glucose level (mg/dl)
As shown in Fig. 1, blood glucose level increased significantly (P < 0.001) at the end of the experiment in the diabetic group compared to the control group. However, after 2 weeks of treatment, tropisetron usage could attenuate blood glucose level markedly (P < 0.01) in diabetic rats.
Tropisetron ameliorated pathological changes of diabetic peripheral neuropathy
To examine the changes of axonal structure, Luxol fast blue staining of rat sciatic nerve was performed (Fig. 2). The axonal architecture in the sciatic nerves of the control rats was found normal. As shown in Table 2, some nerve fibers in the sciatic nerves of the diabetic group were thinned (7.849 ± 0.28) (P < 0.01) with decreased myelinated fiber number (73.13±3.03) (P < 0.05) compared to control group. In addition, myelin was degenerated leading to a reduced myelin sheath thickness (1.5 ± 0.3) in diabetic animals (P < 0.05). Tropisetron treatment mitigated these changes. Axon shrinkage (9.82 ± 0.44), myelinated fiber number (108 ± 5.01), and myelin thickness (3.8 ± 0.4) were improved partially by tropisetron treatment (P < 0.01). We further investigated the role of tropisetron in the intrinsic apoptotic signaling cascade which is called mitochondria apoptotic pathway. This pathway has been assumed to be critical in regulating apoptosis. The Bax and Bcl2 expression was evaluated by the immunohistochemistry in the sciatic nerve. Anti-apoptotic protein Bcl-2 as a key member in the Bcl-2 family, inhibits apoptosis; however, pro-apoptotic protein Bax could stimulate apoptosis. The diabetic rats showed strong positive staining for Bax (1.9 ± 0.4) and negative staining for Bcl2 (0.23 ± 0.11) in the sciatic nerve (P < 0.05), whereas the expression of Bax (0.95 ± 0.1) (P < 0.05) and Bcl2 (1.76 ± 0.35) (P < 0.001) in tropisetron-treated rats were lower and higher, respectively, as compared with the diabetic group (Fig. 3a-d), indicating a protective effect of tropisetron treatment in diabetic neuropathy.
Histological examination of Luxol fast blue stained sciatic nerve in control (C); diabetes (D); and diabetes + tropisetron (D + T) groups. Scale bars are as indicated. Magnification = 100× and 400×. Myelinated fiber (red arrow), axon (green arrow), myelin sheath (black arrow), myelin degeneration (orange arrow)
a Immunohistochemical staining for Bax and Bcl2 in control (C); diabetes (D); and diabetes + tropisetron (D + T) groups (brown arrows). (b, c) Quantitative Bax and Bcl2 intensity of the sciatic nerve; (d) software analysis for Bax and Bcl2 intensity in 2530 × 2530 µm of tissue. All the data are represented as mean ± SEM, *P < 0.05, compared with the control group. $P < 0.05, $$$P < 0.001, compared with the diabetes group. Scale bars are as indicated. Magnification = 300×
Tropisetron decreased TNFα, IL-1B, and Bax/Bcl-2 ratio in the sciatic nerve of STZ-induced diabetic rats
Cytokines can trigger inflammatory cascade contribute to severe diabetic neuropathy. For evaluation, the protein levels of IL-1β and TNFα as main cytokines in the sciatic nerve, Western blotting was used in different groups. The data analysis presented a significant increase in TNFα (3.25 ± 0.44) and IL-1β (3.66 ± 0.14) protein levels in the diabetic group in comparison with the control group (P < 0.001) (Fig. 4a-c). We analyzed the expression of Bax and Bcl-2 in the sciatic nerve tissue. Anti-apoptotic protein Bcl-2 as an important member in the Bcl-2 family mitigates apoptosis; however, pro-apoptotic protein Bax could stimulate apoptosis. As shown in Fig. 4a and d, Bax protein expression increased and Bcl-2 protein expression decreased in the sciatic nerves of the diabetic animals. So, the Bax/Bcl-2 ratio was increased (4.58 ± 0.86) in the diabetic rats compared to the control group (0.98 ± 0.03) (P < 0.001). However, tropisetron treatment decreased IL-1β (1.99 ± 0.32), TNFα (1.18 ± 0.11) (P < 0.001), and the Bax/Bcl-2 ratio (1.68 ± 0.23) (P < 0.01) in the sciatic nerve of the diabetic animals (Fig. 4a-d).
Evaluation of proinflammatory cytokines (TNFα and IL-1β) and Bax/Bcl-2 ratio in the sciatic nerves of different groups. (a) The blotting images of TNFα, IL-1β, Bax, and Bcl2; (b, c, d) The bar charts represent the quantitative analysis of TNFα, IL-1β, Bax/Bcl-2 ratio normalized against β-actin. **P < 0.01, ***P < 0.001, compared with control group. $$P < 0.01, $$$P < 0.001, compared with diabetic group. Control (C); diabetes (D); and diabetes + tropisetron (D + T)
Discussion
Currently, there is no effective treatment except aggressive glycemic control in preventing or halting the development of diabetic neuropathy (Dhaliwal et al. 2020). As previously stated, tropisetron is a 5-HT3R antagonist which has been reported to exhibit remarkable antioxidant, anti-inflammatory, and anti-hyperglycemic effects (Barzegar-Fallah et al. 2014). Recently, we demonstrated in our previous study that tropisetron-ameliorated hyperglycemia and body weight loss in the diabetic animals (Amini et al. 2020). Moreover, it has shown to be particularly effective against various diabetic complications (Asadi et al. 2016,Barzegar-Fallah et al. 2015,Barzegar-Fallah et al. 2014). Based on this, in the present study, we aimed to investigate the protective effects of tropisetron against experimentally induced peripheral neuropathy in diabetic rats.
This study reported for the first time that tropisetron treatment can protect peripheral nerve tissue against STZ-induced damages via anti-inflammatory and anti-apoptotic effects. Tropisetron-treated diabetic animals exhibited a low level of TNFα, IL-1β, Bax, and a high level of BCL2 expression.
It is well known that increasing blood glucose level in diabetes promotes glucose influx to neurons and leads to the accumulation of glycolysis products (Thornalley 2002). It can cause multiple organ dysfunction and structural changes, e.g., in the peripheral nervous system. Small fiber neuropathy, myelin fibers’ reduction, and axon loss in peripheral nerves may occur in high-glucose conditions (Sharma et al. 1981,Yagihashi et al. 1990). Therefore, the direct toxic effect of hyperglycemia, axon sheath abnormalities, and low blood flow are the major causes of pathophysiological alterations in diabetic neuropathy (Boulton and Malik 1998).
In agreement with previous studies (Erbaş et al. 2016, Liu et al. 2016), our histological analysis showed that the experimentally induced diabetes significantly reduced the density of myelinated fibers and myelin sheath thickness, as well as axonal thinning. Peng et al. declared that the morphology of the sciatic nerves showed signs of abnormality in the early stage of streptozotocin-induced diabetes in rats (Peng et al. 2015). Furthermore, some studies reported that the diabetic group showed a moderate irregular arrangement of nerve fibers after 2 weeks of diabetes induction (Moustafa et al. 2018a, b). However, in the present study, administrating tropisetron diminished the experimental diabetes-induced nerve damage and ameliorated the morphological characteristic of the peripheral nervous systems.
The mechanisms underlying the pathogenesis of DPN have not yet been fully established. Various reports have shown that inflammatory cytokines may play a key role in the development of DPN (Satoh et al. 2003). Long-lasting hyperglycemia can activate immune cells such as macrophages, neutrophils, and glial cells (Abcouwer 2011), thereby producing TNFα, IL-1β, and IL-6 levels (Shi et al. 2013). Pro-inflammatory cytokines can increase nerve excitability and myelin disturbances that contribute to nerve demyelination and inhibition cell survival (Alexandraki et al. 2008,Stettner et al. 2011). The anti-inflammation and neuroprotection effects of tropisetron were reviewed in ameliorating neurodegenerative diseases, including Alzheimer’s, multiple sclerosis, and stroke (Fakhfouri et al. 2015). In the present study, we demonstrated a marked reduction in pro-inflammatory cytokines, including IL-1β and TNFα, in the sciatic nerve of neuropathic rats following tropisetron treatment.
Similarly, in a model of vincristine-induced neuropathy, rats treated with tropisetron presented improvement in nerve injury in a receptor-independent manner (Barzegar-Fallah et al. 2014). The anti-inflammation effect of tropisetron was confirmed in colitis (Utsumi et al. 2016). Yu et al. (Yu et al. 2018) showed that tropisetron attenuated lipopolysaccharide-induced neuroinflammation in the mouse cerebral cortex. Several in vivo and in vitro studies have described the anti-apoptotic effect of tropisetron in various conditions. Aminzadeh reported that tropisetron improved the viability of cells and protected them from hyperglycemia-induced apoptosis by modulating Bax and BCL2 protein expressions in rat pheochromocytoma (PC12) cells (Aminzadeh 2017). In addition, tropisetron protects against amyloid-beta-induced neurotoxicity in vivo by reducing apoptotic and inflammatory markers (Rahimian et al. 2013). Strong evidence confirmed that HG induces apoptosis through the oxidative stress insult and, subsequently activation of the cytokine network (Safhi et al. 2019). Accordingly, TNFα as a major proinflammatory cytokine in DPN can promote the nuclear factor-kappa B (NF-κB) and apoptotic signaling pathways (Evangelista et al. 2018,Urabe et al. 2015). Bax, as a proapoptotic factor, and BCL2, as an antiapoptotic agent, play pivotal roles in the apoptosis process in T1DM (Edlich 2018). Exposure to long-lasting high glucose disturbs pro- and anti-apoptotic balance, which can result in apoptosis insult (Lee et al. 2009,Shi et al. 2018). Activation of pro-apoptotic protein Bax causes the release of cytochrome c from mitochondria that contribute to apoptosis. An elevated ratio of Bax/Bcl-2 may trigger caspase-3 release as a key mediator of apoptosis, and thus plays a critical role in programmed cell death or apoptosis (Busch et al. 2012).
Several reports indicate the anti-apoptotic effect of tropisetron in vivo and in vitro. The neuroprotective effect of tropisetron has been shown in high glucose-induced apoptosis in PC12 cells by reducing the ratio of Bax/Bcl-2 as an in vitro model of diabetic neuropathy (Aminzadeh 2017). Furthermore, the anti-aging effect of tropisetron in the mouse brain was reported by attenuating apoptosis as indicated by Bax and BCL2 changes (Mirshafa et al. 2020). We have recently observed that tropisetron diminished pancreas apoptosis by altering the mitochondrial apoptotic pathway in STZ-induced diabetic rats (Naderi et al. 2020a). Most notably, our findings indicate that diabetes causes a marked increase in Bax and a marked decrease in BCL2 expression in the sciatic nerve tissue, this effect being amenable to reversal by tropisetron. These data may have etiological and therapeutic implications for the management of diabetic neuropathy.
Conclusion
These findings suggest that tropisetron plays a protective role in sciatic nerve injury induced by diabetes. Moreover, the inflammatory cytokines and apoptosis indices in DPN were inhibited after treatment with tropisetron. This result implied that tropisetron might protect the sciatic nerve by inhibiting the inflammatory response and apoptosis. Thus, we can propose that tropisetron may have healing effects on nerve damage in a neuropathy model induced by STZ. Further studies are essential to detect the probable molecular mechanisms by which tropisetron exerts its protective effects against diabetic neuropathy.
References
Abcouwer SF (2011) Neural inflammation and the microglial response in diabetic retinopathy. J Ocul Biol Dis Infor 4(1–2):25–33
Akada Y, Mori R, Matsuura K, Suzuki K, Kato K, Kamiya M, Naba H, Kurokawa M, Ogihara T, Kato Y, Yamasaki F, Yamamoto I (2006) Pharmacological profiles of the novel analgesic M58996 in rat models of persistent and neuropathic pain. J Pharmacol Sci 102(2):205–212
Alexandraki KI, Piperi C, Ziakas PD, Apostolopoulos NV, Makrilakis K, Syriou V, Diamanti-Kandarakis E, Kaltsas G, Kalofoutis A (2008) Cytokine secretion in long-standing diabetes mellitus type 1 and 2: associations with low-grade systemic inflammation. J Clin Immunol 28(4):314–321
Amini M, Saboory E, Pourheydar B, Bagheri M, Naderi R (2020) Involvement of endocannabinoid system, inflammation and apoptosis in diabetes induced liver injury: role of 5-HT3 receptor antagonist. Int Immunopharmacol 79:106158
Aminzadeh A (2017) Protective effect of tropisetron on high glucose induced apoptosis and oxidative stress in PC12 cells: roles of JNK, P38 MAPKs, and mitochondria pathway. Metab Brain Dis 32(3):819–826
Asadi F, Razmi A, Dehpour AR, Shafiei M (2016) Tropisetron inhibits high glucose-induced calcineurin/NFAT hypertrophic pathway in H9c2 myocardial cells. J Pharm Pharmacol 68(4):485–493
Barzegar-Fallah A, Alimoradi H, Asadi F, Dehpour AR, Asgari M, Shafiei M (2015) Tropisetron ameliorates early diabetic nephropathy in streptozotocin-induced diabetic rats. Clin Exp Pharmacol Physiol 42(4):361–368
Barzegar-Fallah A, Alimoradi H, Mehrzadi S, Barzegar-Fallah N, Zendedel A, Abbasi A, Dehpour AR (2014) The neuroprotective effect of tropisetron on vincristine-induced neurotoxicity. Neurotoxicology 41:1–8
Boulton AJ, Malik RA (1998) Diabetic neuropathy. Med Clin North Am 82(4):909–929
Busch F, Mobasheri A, Shayan P, Stahlmann R, Shakibaei M (2012) Sirt-1 is required for the inhibition of apoptosis and inflammatory responses in human tenocytes. J Biol Chem 287(31):25770–25781
Dhaliwal J, Dhaliwal N, Akhtar A, Kuhad A, Chopra K (2020) Beneficial effects of ferulic acid alone and in combination with insulin in streptozotocin induced diabetic neuropathy in Sprague Dawley rats. Life Sci 255:117856
Edlich F (2018) BCL-2 proteins and apoptosis: recent insights and unknowns. Biochem Biophys Res Commun 500(1):26–34
Emir A, Kalkan Y, Bostan H (2016) Histopathological effects of intramuscular metamizole sodium on rat sciatic nerve. Iran J Basic Med Sci 19(8):829–836
Erbaş O, Oltulu F, Yılmaz M, Yavaşoğlu A, Taşkıran D (2016) Neuroprotective effects of chronic administration of levetiracetam in a rat model of diabetic neuropathy. Diabetes Res Clin Pract 114:106–116
Evangelista AF, Vannier-Santos MA, de Assis Silva G S, Silva DN, Juiz PJL, Nonaka CKV, Dos Santos RR, Soares MBP, Villarreal CF (2018) Bone marrow-derived mesenchymal stem/stromal cells reverse the sensorial diabetic neuropathy via modulation of spinal neuroinflammatory cascades. J Neuroinflammation 15(1):189
Fakhfouri G, Mousavizadeh K, Mehr SE, Dehpour AR, Zirak MR, Ghia JE, Rahimian R (2015) From chemotherapy-induced emesis to neuroprotection: therapeutic opportunities for 5-HT3 receptor antagonists. Mol Neurobiol 52(3):1670–1679
Lee JH, Song MY, Song EK, Kim EK, Moon WS, Han MK, Park JW, Kwon KB, Park BH (2009) Overexpression of SIRT1 protects pancreatic beta-cells against cytokine toxicity by suppressing the nuclear factor-kappaB signaling pathway. Diabetes 58(2):344–351
Liu GM, Xu K, Li J, Luo YG (2016) Curcumin upregulates S100 expression and improves regeneration of the sciatic nerve following its complete amputation in mice. Neural Regen Res 11(8):1304–1311
Lós DB, Oliveira WH, Duarte-Silva E, Sougey WWD, Freitas E, de Oliveira AGV, Braga CF, França MER, Araújo S, Rodrigues GB, Rocha SWS, Peixoto CA, Moraes SRA (2019) Preventive role of metformin on peripheral neuropathy induced by diabetes. Int Immunopharmacol 74:105672
Ma J, Liu J, Yu H, Chen Y, Wang Q, Xiang L (2016) Beneficial effect of metformin on nerve regeneration and functional recovery after sciatic nerve crush injury in diabetic rats. Neurochem Res 41(5):1130–1137
Mirshafa A, Mohammadi H, Shokrzadeh M, Mohammadi E, Talebpour Amiri F, Shaki F (2020) Tropisetron protects against brain aging via attenuating oxidative stress, apoptosis and inflammation: the role of SIRT1 signaling. Life Sci 248:117452
Mirzakhani N, Farshid AA, Tamaddonfard E, Imani M, Erfanparast A, Noroozinia F (2018) Carnosine improves functional recovery and structural regeneration after sciatic nerve crush injury in rats. Life Sci 215:22–30
Moustafa PE, Abdelkader NF, El Awdan SA, El-Shabrawy OA, Zaki HF (2018) Extracellular matrix remodeling and modulation of inflammation and oxidative stress by sulforaphane in experimental diabetic peripheral neuropathy. Inflammation 41(4):1460–1476
Moustafa PE, Abdelkader NF, El Awdan SA, El-Shabrawy OA, Zaki HF (2018) Liraglutide ameliorated peripheral neuropathy in diabetic rats: involvement of oxidative stress, inflammation and extracellular matrix remodeling. J Neurochem 146(2):173–185
Mustapha OA, Olude MA, Bello ST, Taiwo A, Jagun A, Olopade JO (2019) Peripheral axonopathy in sciatic nerve of adult Wistar rats following exposure to vanadium. J Peripher Nerv Syst 24(1):94–99
Naderi R, Shirpoor A, Samadi M, Pourheydar B, Moslehi A (2020) Tropisetron attenuates pancreas apoptosis in the STZ-induced diabetic rats: involvement of SIRT1/NF-κB signaling. Pharmacol Rep 72(6):1657–1665
Naderi R, Shirpoor A, Samadi M, Pourheydar B, Moslehi A (2020) Tropisetron improves pancreas function and increases insulin synthesis and secretion in the STZ-induced diabetic rats: involvement of UCP2/ZnT8 pathway. J Pharm Pharmacol 72(8):1082–1091
Ni GL, Cui R, Shao AM, Wu ZM (2017) Salidroside ameliorates diabetic neuropathic pain in rats by inhibiting neuroinflammation. J Mol Neurosci 63(1):9–16
Peng L, Liu W, Zhai F, He L, Wang H (2015) Microvessel permeability correlates with diabetic peripheral neuropathy in early stage of streptozotocin-induced diabetes rats. J Diabetes Complications 29(7):865–871
Rahimian R, Fakhfouri G, Ejtemaei Mehr S, Ghia JE, Genazzani AA, Payandemehr B, Dehpour AR, Mousavizadeh K, Lim D (2013) Tropisetron attenuates amyloid-beta-induced inflammatory and apoptotic responses in rats. Eur J Clin Invest 43(10):1039–1051
Safhi MM, Alam MF, Sivakumar SM, Anwer T (2019) Hepatoprotective potential of Sargassum muticum against STZ-induced diabetic liver damage in Wistar rats by inhibiting cytokines and the apoptosis pathway. Anal Cell Pathol (Amst) 2019:7958701
Satoh J, Yagihashi S, Toyota T (2003) The possible role of tumor necrosis factor-alpha in diabetic polyneuropathy. Exp Diabesity Res 4(2):65–71
Sharma AK, Bajada S, Thomas PK (1981) Influence of streptozotocin-induced diabetes on myelinated nerve fibre maturation and on body growth in the rat. Acta Neuropathol 53(4):257–265
Shi X, Chen Y, Nadeem L, Xu G (2013) Beneficial effect of TNF-α inhibition on diabetic peripheral neuropathy. J Neuroinflammation 10:69
Shi X, Pi L, Zhou S, Li X, Min F, Wang S, Liu Z, Wu J (2018) Activation of Sirtuin 1 attenuates high glucose-induced neuronal apoptosis by deacetylating p53. Front Endocrinol (Lausanne) 9:274
Stettner M, Dehmel T, Mausberg AK, Köhne A, Rose CR, Kieseier BC (2011) Levetiracetam exhibits protective properties on rat Schwann cells in vitro. J Peripher Nerv Syst 16(3):250–260
Swartz MM, Linn DM, Linn CL (2013) Tropisetron as a neuroprotective agent against glutamate-induced excitotoxicity and mechanisms of action. Neuropharmacology 73:111–121
Thornalley PJ (2002) Glycation in diabetic neuropathy: characteristics, consequences, causes, and therapeutic options. Int Rev Neurobiol 50:37–57
Urabe H, Terashima T, Lin F, Kojima H, Chan L (2015) Bone marrow-derived TNF-α causes diabetic neuropathy in mice. Diabetologia 58(2):402–410
Utsumi D, Matsumoto K, Amagase K, Horie S, Kato S (2016) 5-HT3 receptors promote colonic inflammation via activation of substance P/neurokinin-1 receptors in dextran sulphate sodium-induced murine colitis. Br J Pharmacol 173(11):1835–1849
Yagihashi S, Kamijo M, Watanabe K (1990) Reduced myelinated fiber size correlates with loss of axonal neurofilaments in peripheral nerve of chronically streptozotocin diabetic rats. Am J Pathol 136(6):1365–1373
Yu Y, Zhu W, Liang Q, Liu J, Yang X, Sun G (2018) Tropisetron attenuates lipopolysaccharide induced neuroinflammation by inhibiting NF-κB and SP/NK1R signaling pathway. J Neuroimmunol 320:80–86
Acknowledgements
We are thankful to the Urmia University of Medical Sciences for supporting this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghazipour, A.M., Pourheydar, B. & Naderi, R. The effect of tropisetron on peripheral diabetic neuropathy: possible protective actions against inflammation and apoptosis. Cell Stress and Chaperones 27, 513–521 (2022). https://doi.org/10.1007/s12192-022-01287-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-022-01287-9