Abstract
Floods are major determinants of ecological patterns and processes in river-floodplain systems. Although some general predictions of the effects of water level changes on ecological attributes have been identified, specific tests using the flood pulse concept are scarce, mainly in tropical areas, where large river-floodplain systems abound. We tested the hypothesis that floods decrease environmental and biological variability using data from a near-pristine floodplain in Central Amazon (Brazil). We recorded nine limnological variables and the zooplankton community structure at eleven sites during one low and one high water period. During the low water period, when the levels of hydrological connectivity were low, asynchronous processes (e.g., sediment disturbance by biota, decomposition, and predation) likely determined the large environmental and biological heterogeneity in the floodplain. On the other hand, environmental variability and zooplankton beta diversity were significantly decreased by the flood. We postulate that floods act as “rubber erasers”, reducing the environmental and ecological idiosyncrasies created during low water periods. Also, we suggest that dilution effects and enhanced connectivity during the high water period, along with species sorting during the low water period, may determine zooplankton beta diversity patterns in river-floodplain systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydrology plays a key role in modulating a number of ecological patterns and processes in aquatic ecosystems (Bunn & Arthington, 2002). Depending on the type of aquatic ecosystem, different paradigms are proposed to account for how hydrology affects ecological patterns (e.g., Vannotte et al., 1980, for streams, and Thornton et al., 1990, for reservoirs). Variations in water level and flow, along with variations in temperature and sediment load, for example, are the most important factors driving the structure and functioning of river-floodplain systems (RFS) (Junk et al., 1989; Neiff, 1990; Tockner et al., 2000). The broader paradigm surrounding the importance of floods in RFS became to be known as the flood pulse concept (FPC; Junk et al., 1989). For example, nutrients and organisms exchange between habitats, aquatic vegetation phenology, fish migrations, and feeding activity are mainly determined by the flood regime (Junk et al., 1989; Ward et al., 2002; Luz-Agostinho et al., 2009; Roach et al., 2014).
However, the consequences of the flood pulses on local habitats are still a matter of debate (Mayora et al., 2013). Some general predictions about the effects of water level changes on floodplain communities have been identified. One example of prediction concerns how richness of different aquatic assemblages, including macrophytes, amphibians, mollusks, fish, zooplankton, and insects, responds to a gradient of connectivity (Tockner et al., 1998; Ward & Tockner, 2001; Padial et al., 2009; Simões et al., 2013). Other trends include responses of fish species richness, abundance, and migration to floods duration and connectivity (Fernandes et al., 2009). Despite these general predictions, specific and measurable expectations of ecological attributes using the FPC are scarce, mainly in tropical areas, where large RFS abound.
Floods also tend to reduce the spatial variability of biological and environmental factors—the so-called “homogenization hypothesis” (Bozelli, 1992; Tockner et al., 2000; Thomaz et al., 2007). According to this hypothesis, during low water periods, when the floodplain aquatic habitats are more isolated from each other and disconnected from the main river channel, local forces (e.g., predation, competition, turbidity changes, and nutrient increments due to increased coupling between water and sediment) act with different intensities in each environment in the floodplain, thus creating habitats with different characteristics. On the other hand, spatial variability of ecological attributes decreases with water level rise, given that floods deliver a large amount of water from the main river channel, with particular environmental conditions and organisms.
Because of the temporal variability in the level of connectivity between aquatic habitats, which can be seen as a coarse proxy for dispersal possibilities (Ward et al., 1999), RFS are useful systems to investigate the role of niche-based and spatial processes on community structure (e.g., Algarte et al., 2014; Fernandes et al., 2014). Within the metacommunity theory, Gonzalez (2009) postulates that “between community diversity” (i.e., beta diversity) decreases as dispersal increases. This scenario is more likely during high water periods in RFS, when the habitats are more hydrologically connected. Thus, taking the above rationale proposed by the flood homogenization hypothesis into account, beta diversity and environmental heterogeneity are expected to decrease during flood events.
Here, we evaluated whether patterns predicted by the homogenization hypothesis hold for abiotic factors and zooplankton communities by assessing the effects of a large flood event in an Amazonian floodplain. First, we predict that floods decrease the spatial variability of environmental variables. Second, because floods homogenize floodplain habitats, we predict that beta diversity of zooplankton decreases during high water periods.
Methods
Study Area
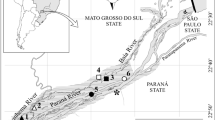
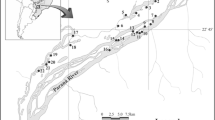
We sampled eleven sites in the Trombetas River floodplain (nine lakes and two sites in the main river channel; Fig. 1) in July 2002 (high water period; mean water depth in lakes = 8.5 m) and December 2002 (low water period, mean water depth in lakes = 3.0 m). This river is an affluent of the left bank of the Amazon River. It begins in the plateau of Guyana and, according to the classification of the Amazonian waters proposed by Sioli (1984), it is considered a clear water river. Initially it runs in a well-defined bed, with stable banks. This feature modifies when it reaches the Tertiary sediments of the formation Barreiras, where it forms, through erosion and sediment deposition process, many shallow and dendritic lakes (Radambrasil, 1976).
In the studied stretch, lakes are connected to the river and originated from erosion and deposition processes. These lakes can be grouped in two broad categories concerning their position in relation to the Trombetas River and also the connection type with the river. The first category refers to lakes originated from old and abandoned channels parallel to the river that usually have more than one point of connection with it and the number of connections tends to increase as the water level rises (lakes Flexal, Ajudante, Moura, Palhau and Curuçá-Mirim). The second category concerns lakes positioned in a more perpendicular position to the river that usually have a single connection through a continuous opening to the river main channel during most of the high water period (lakes Juquiri, Erepecu, Curuçá-Grande, and Mussurá). All these lakes present clear water as the Trombetas River. They have variable sizes and they are distributed in both banks of the river. The stretch of the river where lakes were located comprised a total extension of nearly 60 km.
Data
At each sampling site, the following variables were measured in the field: Secchi depth (cm), turbidity (NTU), water temperature (°C), electrical conductivity (μS cm−1), and pH (Digimed digital potentiometers). Concentrations of dissolved reactive phosphorus (µM), dissolved nitrogen (µM), dissolved organic carbon (mg l−1), and suspended matter (mg l−1) were determined in the laboratory according to APHA (2005).
Zooplankton samples were collected by one vertical haul through the water column with a plankton net of 50 µm mesh size in each sampling site, and were fixed in a solution of 4% formaldehyde buffered with calcium carbonate. The water volume sampled was estimated by multiplying the plankton net opening area by the water depth column reached by the net, and it varied from 0.12 to 0.31 m3 in the low water period; and from 0.55 to 0.74 m3 in the high water period. Species densities (individuals/m3) were estimated by counting triplicate aliquots in either a Sedgewick–Rafter cell under a microscope for rotifers and nauplii; or in open chambers under a stereomicroscope for cladocerans and copepods. At least 100 individuals per aliquot were counted. Young forms (nauplii and copepodites of Cyclopoida, Calanoida, and Harpacticoida) were also counted.
Data analysis
We used the standardized Euclidean coefficient and the modified Gower dissimilarity measure (see Anderson et al., 2006) to calculate the distance matrices between sampling sites according to the environmental and biological datasets, respectively. Using these matrices, two distance-based Redundancy Analyses (db-RDA; Legendre & Anderson, 1999) were run to test for differences in environmental characteristics and zooplankton community structure between water level periods. We used 999 permutations to test the significance of the F-statistic evaluating the hypothesis of no differences in limnological characteristics and zooplankton community structure between the water level periods. Then, analyses of multivariate homogeneity of group dispersions (PERMDISP; Anderson, 2006) were conducted to test for differences in multivariate dispersions between water level periods. The test for homogeneity of multivariate dispersions is a multivariate extension similar to Levene’s test (which was also used for each environmental variable). The F-statistic used in PERMDISP is estimated by an ANOVA “on the Euclidean distances from individual points within a group to their group centroid” (Anderson, 2006). In our study, the higher the average distance from sampling sites to their group centroid (as defined by the water level periods), the higher the environmental variability or the variation in community structure. Following Anderson (2006), the P values were obtained by permuting the least squares residuals 999 times. Each distance matrix was then submitted to a principal coordinate analysis (PCoA; Legendre & Legendre, 1998) to visualize the main patterns of similarity among the lakes according to each dataset. A clear difference between the scores of the water level periods produced by PCoA would reinforce the patterns detected, as this difference would be obtained independently of the explanatory variable (i.e., without the use of a constrained ordination technique).
We also compared the variability of species densities between periods by plotting the standard deviation (log-transformed) of individual taxa against their mean densities. Thus, for a given zooplankton density, we could assess whether density variability differed between water level periods. All statistical analyses were performed using the package vegan (Oksanen et al., 2013) for the R language and environment for statistical computing (v. 2.15.1; R Core Team, 2012).
Results
Limnological variability
Water level periods were clearly different regarding limnological characteristics (db-RDA; F 1;20 = 15.6; R 2 = 0.44; P < 0.001). Specifically, the Pearson’s correlation coefficients between the first canonical axis derived from the db-RDA and the original variables indicate an increase in the concentrations of dissolved organic carbon (r = 0.91), dissolved reactive phosphorus (r = 0.92), dissolved nitrogen (r = 0.92), and suspended matter (r = 0.79) during the low water period. Increases in water temperature (r = 0.76) and pH (r = 0.85), and a decrease in water transparency (r = −0.69) were also recorded in this period (Fig. 2 and results from PCoA in Fig. 3a; see also original data in Table S1).
In addition to a difference in mean limnological characteristics (as indicated by the db-RDA), the permutation test for homogeneity of multivariate dispersions indicated that lakes were significantly more dissimilar among themselves during the low water period (average distance to group centroid ± SD = 2.64 ± 1.12) than during the high water period (1.42 ± 0.58; F 1;20 = 9.33; P = 0.006). The hypothesis of homogeneity of variances, according to the univariate Levene’s test, was not rejected only for turbidity and pH. Significant heteroscedasticity was detected for all other variables and in all cases the variances (i.e., among-sampling sites variability) were higher during the low water period than during the high water level period (except for water temperature; Table 1). Figure 2 is also useful to show both the differences in central tendencies and variances between water level periods (with higher dispersion during the low than high water period).
Community variability
A total of 65 taxa of zooplankton were recorded (see Table S2 in Supplementary Material). Similarly to what was observed with the environmental data, water level variation strongly affected the zooplankton community structure, which differed significantly between the two level water periods (F 1;20 = 8.14; R 2 = 0. 29; P < 0.001; Fig. 3b). We detected a significant increase in beta diversity during the low water period (F 1;20 = 44.39; P < 0.001), as indicated by a higher average distance to the centroid during this period (2.95 ± 0.20) than during the high water period (2.03 ± 0.41). Among sites variability in densities of individual taxa was much higher during the low water period (Fig. 4), indicating a higher level of population aggregation during this period than during the high water period. These results indicated a clear difference between low and high water periods, not only in terms of average environmental conditions and community structure, but also in terms of their variability.
Discussion
Our results clearly support our predictions, and hence the flood homogenization hypothesis. The increased similarity among sites regarding limnological variables, zooplankton community structure, and zooplankton population densities during high water periods can be attributed to two complementary mechanisms (Bozelli & Esteves, 1995; Lewis et al., 2000; Thomaz et al., 2007). First, the enhanced connectivity among sites caused by flooding leads to higher exchanges of solutes and organisms. Second, the relative isolation of lakes during the low water period, which occurs once a year and lasts approximately 3 months, makes each habitat to be mostly influenced by local idiosyncratic function forces. Spatial variability in local function forces, in turn, causes the communities to follow different trajectories. We are confident in the causal relationship between flooding and the decrease in environmental and biological variability because this general pattern was detected for most of the variables analyzed in our investigation.
The decrease in nutrient concentrations during the high water period was consistent with a dilution effect of flooding. The dilution is expected when main river waters are poor in nutrients (e.g., Carvalho et al., 2001), as it is the case of the Trombetas River. Zooplankton populations may also be diluted during high water periods in the Trombetas River floodplain (Bozelli, 1994) and in other river-floodplain systems (Brandorff & Andrade, 1978; Lansac-Tôha et al., 2009). Complementarily, during the low water period, different processes (especially wind action in shallow environments and sediment bioturbation) act, with different intensities and asynchronously (promoting heterogeneity), to increase the nutrient concentrations and phytoplankton primary productivity (Roland et al., 1998).
A decrease in beta diversity during high water periods, also observed for zooplankton in other floodplains (e.g., Bonecker et al., 2005), is consistent with metacommunity predictions as one can assume that connectivity is higher during high water periods than during low water periods. During high water periods, we postulate that flood flows increase passive dispersal rates, redistributing organisms among lakes (Gurnell et al., 2008), which may enhance homogenization at the floodplain scale (Gonzalez, 2009). Another important response to the predominance of water from a main source (the river) is that lakes become more similar in terms of physical and chemical characteristics during floods (which may last several months), also resulting in decreased beta diversity. Thus, zooplankton community homogenization (i.e., low beta diversity and low variability in population densities) during high waters seems to be a response to enhanced connectivity and similarity in physical and chemical conditions among the sites.
Our results also suggest a main role of deterministic assembly processes during the low water period. Besides an increase in beta diversity, we found that the variation in surrogates of productivity (e.g., nitrogen and phosphorus concentrations; see Mihaljević et al., 2009) among lakes was positively associated with the mean values of these surrogates (i.e., a simultaneous increase in average and variation; see Chase, 2010). In fact, dissolved phosphorus (0.51–2.02 µM) and dissolved nitrogen (29–106 µM) were extremely variable in the low water period. As a consequence, one can infer that different species of zooplankton were selected in each lake, according to their degree of trophy preferences. Thus, selection of zooplankton through habitat filters leads to convergence of different lake communities during high water periods but to divergences among lakes during low water periods, when the importance of niche-based mechanisms on structuring zooplankton assemblages seems to be enhanced. Langenheder et al. (2012), for example, also found a positive association between beta diversity and nutrient concentrations in a bacterial metacommunity and similarly interpreted this finding as a result of species sorting mechanisms (i.e., “selection of taxa by the prevailing environmental conditions”; see also Leibold et al., 2004). In addition to lake trophy, the resuspension processes that occur more often during the low water period (see Bozelli et al., 2009) and whose degree differs among lakes may also contribute to greater heterogeneity of zooplankton populations in this period. This is because the greater abundance of suspended particles with different size fractions can be used directly as food or serve as a substrate for bacterial growth after adsorption of dissolved organic carbon (Lind &Dávalos-Lind, 1991). We cannot rule out, however, the role of other processes as, for instance, priority effects (e.g., Louette & De Meester, 2007) and variation in zooplanktivorous fish composition, as small-sized fish uses zooplankton as feeding resources in floodplain habitats (Novakowski et al., 2008), especially during low water periods (Pereira et al., 2011).
We predict that the construction of reservoirs in relative pristine RFS, as projected for the Amazon basin (Ferreira et al., 2014), has the potential to change the patterns we identified here in at least two ways. First, because reservoirs release water with low nutrient and solid concentrations (e.g., Thornton et al., 1990), floodplains located downstream will tend to be more homogeneously oligotrophic during high water periods. Second, if the artificial control of water flow by reservoirs shortens the period of isolation of floodplain habitats, assemblages may not have enough time to follow different trajectories in each floodplain habitat during low water periods. The long-term consequences of these impacts are uncertain, but the former may reduce RFS productivity (with cascade effects on RFS communities), while the latter may have negative consequences on RFS beta (and probably gama) diversity.
In conclusion, we showed that floods play a key and highly predictable role in determining, not only average conditions, but also environmental and biological variability (in terms of zooplankton community composition and population densities), at least in unregulated floodplain systems. Also, we suggest that beta diversity patterns in the Trombetas River floodplain were driven by at least two community assembly processes (dilution effects and faunal “redistribution” or enhanced dispersal rates, during the high water period and species sorting, during the low water period). Therefore, the search for a single community assembly process in high variable systems is a quixotic task and one should evaluate the relative roles of different mechanisms in explaining variation in community structure.
References
Algarte, V. M., L. Rodrigues, V. L. Landeiro, T. Siqueira & L. M. Bini, 2014. Variance partitioning of deconstructed periphyton communities: does the use of biological traits matter? Hydrobiologia 722: 279–290.
Anderson, M. J., 2006. Distance-based tests for homogeneity of multivariate dispersions. Biometrics 62: 245–253.
Anderson, M. J., K. E. Ellingsen & B. H. McArdle, 2006. Multivariate dispersion as a measure of beta diversity. Ecology Letters 9: 683–693.
APHA – American Public Health Association, 2005. Standard Methods for the Examination of Water and Wastewater, 21st edn, Washington, D.C.
Bonecker, C. C., C. L. da Costa, L. F. M. Velho & F. A. Lansac-Tôha, 2005. Diversity and abundance of the planktonic rotifers in different environments of the Upper Paraná River floodplain (Paraná State – Mato Grosso do Sul State, Brazil). Hydrobiologia 546: 405–414.
Bozelli, R. L., 1992. Composition of the zooplankton community of Batata and Mussurá lakes and of the Trombetas River, Brazil. Amazoniana 12: 239–261.
Bozelli, R. L., 1994. Zooplankton community density in relation to water fluctuation and inorganic turbidity in an Amazonian lake, Lago Batata, State of Pará, Brazil. Amazoniana 13: 17–32.
Bozelli, R. L. & F. A. Esteves, 1995. Species diversity, evenness and richness of the zooplankton community of Batata and Mussurá lakes and of the Trombetas River, Amazonia, Brazil. In International Conference on Tropical Limnology, 1995, Salatiga, Indonesia. Tropical Limnology, Satya Wacana Christian University Press, Salatiga 1995. 2: 87–93.
Bozelli, R. L., A. Caliman, R. D. Guariento, L. S. Carneiro, J. M. Santangelo, M. P. Figueiredo-Barros, J. J. F. Leal, A. Rocha, L. B. Quesado, P. M. Lopes, V. F. Farjalla, C. C. Marinho, F. Roland & F. A. Esteves, 2009. Interactive effects of environmental variability and human impacts on the long-term dynamics of an Amazonian floodplain lake and a South Atlantic coastal lagoon. Limnologica 39: 306–313.
Brandorff, G.-O. & E. R. Andrade, 1978. The relationship between the water level of the Amazon River and the fate of the zooplankton population in Lago Jacaretinga e varzea lake in Central Amazon. Studies on Neotropical Fauna and Environment 13: 63–70.
Bunn, S. E. & A. H. Arthington, 2002. Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity. Environmental Management 30: 492–507.
Carvalho, P., L. M. Bini, S. M. Thomaz, L. G. Oliveira, B. Robertson, W. L. G. Tavechio & A. J. Darwich, 2001. Comparative limnology of South-American lakes and lagoons. Acta Scientiarum 23: 265–273.
Chase, J. M., 2010. Stochastic community assembly causes higher biodiversity in more productive environments. Science 328: 1388–1391.
Fernandes, R., A. A. Agostinho, E. A. Ferreira, C. S. Pavanelli, H. I. Suzuki, D. P. Lima & L. C. Gomes, 2009. Effects of the hydrological regime on the ichthyofauna of riverine environments of the Upper Paraná River floodplain. Brazilian Journal of Biology 69: 669–680.
Fernandes, I. M., R. Henriques-Silva, J. Penha, J. Zuanon & P. R. Peres-Neto, 2014. Spatiotemporal dynamics in a seasonal metacommunity structure is predictable: the case of floodplain-fish communities. Ecography 37: 464–475.
Ferreira, J., L. E. O. C. Aragão, J. Barlow, P. Barreto, E. Berenguer, M. Bustamante, T. A. Gardner, A. C. Lees, A. Lima, J. Louzada, L. Parry, C. A. Peres, R. Pardini, P. S. Pompeu, M. Tabarelli & J. Zuanon, 2014. Brazil´s environmental leadership at risk: mining and dams threaten protected areas. Science 346: 706–707.
Gonzalez, A., 2009. Metacommunities: spatial community ecology. In Encyclopedia of Life Sciences (ELS). Wiley, Chichester.
Gurnell, A., K. Thompson, J. Goodson & H. Moggridge, 2008. Propagule deposition along river margins: linking hydrology and ecology. Journal of Ecology 96: 553–565.
Junk, W. J., P. B. Bayley & R. E. Sparks, 1989. The flood pulse concept in river-floodplain systems. Canadian Special Publication of Fisheries and Aquatic Sciences 106: 110–127.
Langenheder, S., M. Berga, Ö. Östman & A. J. Székely, 2012. Temporal variation of β-diversity and assembly mechanisms in a bacterial metacommunity. The ISME Journal 6: 1107–1114.
Lansac-Tôha, F. A., C. C. Bonecker, L. F. M. Velho, N. R. Simões, J. D. Dias, G. M. Alves & E. M. Takahashi, 2009. Biodiversity of zooplankton communities in the Upper Paraná River. Brazilian Journal of Biology 69: 539–549.
Legendre, P. & L. Legendre, 1998. Numerical Ecology. Elsevier, Amsterdam.
Legendre, P. & M. J. Anderson, 1999. Distance-based redundancy analysis: testing multispecies responses in multifactorial ecological experiments. Ecological Monographs 69: 1–24.
Leibold, M. A., M. Holyoak, N. Mouquet, P. Amarasekare, J. M. Chase, M. F. Hoopes, R. D. Holt, J. B. Shurin, R. Law, D. Tilman, M. Loreau & A. Gonzalez, 2004. The metacommunity concept: a framework for multi-scale community ecology. Ecology Letters 7: 601–613.
Lewis Jr, W. M., S. K. Hamilton, M. A. Lasi, M. Rodríguez & J. F. Saunders III, 2000. Ecological determinism on the Orinoco floodplain. BioScience 50: 681–692.
Lind, O. T. & L. Dávalos-Lind, 1991. Association of turbidity and organic carbon with bacterial abundance and cell size in a large, turbid and tropical lake. Limnology and Oceanography 36: 1200–1208.
Louette, G. & L. De Meester, 2007. Predation and priority effects in experimental zooplankton communities. Oikos 116: 419–426.
Luz-Agostinho, K. D. G., A. A. Agostinho, L. C. Gomes, H. F. Júlio-Jr & R. Fugi, 2009. Effects of flooding regime on the feeding activity and body condition of piscivorous fish in the Upper Paraná River floodplain. Brazilian Journal of Biology 69: 481–490.
Mayora, G., M. Devercelli & F. Giri, 2013. Spatial variability of chlorophyll-a and abiotic variables in a river-floodplain system during different hydrological phases. Hydrobiologia 717: 51–63.
Mihaljević, M., F. Stević, J. Horvatić & B. H. Kutuzović, 2009. Dual impact of the flood pulses on the phytoplankton assemblages in a Danubian floodplain lake (KopačkiRit Nature Park, Croatia). Hydrobiologia 618: 77–88.
Neiff, J. J., 1990. Ideas para la interpretacion ecológica del Paraná. Interciencia 15: 424–441.
Novakowski, G. C., N. S. Hahn & R. Fugi, 2008. Diet seasonality and food overlap of the fish assemblage in a pantanal pond. Neotropical Ichthyology 6: 567–576.
Oksanen, J., F. G. Blanchet, R. Kindt, P. Legendre, P. R. Minchin, R. B. O’Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens & H. Wagner, 2013. Vegan: Community Ecology Package [available on internet at http://www.CRAN.R-project.org/package=vegan].
Padial, A. A., P. Carvalho, S. M. Thomaz, S. M. Boschilia, R. B. Rodrigues & J. T. Kobayashi, 2009. The role of an extreme flood disturbance on macrophyte assemblages in a Neotropical floodplain. Aquatic Sciences 71: 389–398.
Pereira, J. O., M. T. da Silva, L. J. S. Vieira & R. Fugi, 2011. Effects of flood regime on the diet of Triportheuscurtus (Garman, 1890) in an Amazonian floodplain lake. Neotropical Ichthyology 9: 623–628.
Radambrasil Ministério das Minas e Energia, 1976. Levantamento de recursos naturais: Santarém, Rio de Janeiro, Folha SA 21, 10: 220.
R Core Team, 2012. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna.
Roach, K., K. O. Winemiller & D. S. Davis III, 2014. Autochthonous production in shallow littoral zones of five floodplain rivers: effects of flow, turbidity and nutrients. Freshwater Biology 59: 1278–1293.
Roland, F., F. A. Esteves & F. A. R. Barbosa, 1998. The influence of bauxite tailings on the light and its consequence on the phytoplankton primary production in an Amazonian floodplain lake. Verhandlung der InternationaleVereinigung Limnologie 26: 765–767.
Simões, N. R., J. D. Dias, C. M. Leal, L. S. M. Braghim, F. A. Lansac-Tôha & C. C. Bonecker, 2013. Floods control the influence of environmental gradients on the diversity of zooplankton communities in a neotropical floodplain. Aquatic Sciences 75: 607–617.
Sioli, H., 1984. The Amazon and its main affluents: hydrography, morphology of the river courses, and river types. In Sioli, H. (Ed.), The Amazon: Limnology and Landscape Ecology of a Mighty Tropical River and its Basin. Dr. W. Junk Publishers, Dordrecht: 127–165.
Thomaz, S. M., L. M. Bini & R. L. Bozelli, 2007. Floods increase similarity among aquatic habitats in river-floodplain systems. Hydrobiologia 579: 1–13.
Thornton, K. W., B. L. Kimmel & F. F. Payne, 1990. Reservoir Limnology: Ecological Perspectives. Wiley, New York.
Tockner, K., F. Schiemer & J. V. Ward, 1998. Conservation by restoration: the management concept for a river-floodplain system on the Danube River in Austria. Aquatic Conservation: Marine and Freshwater Ecosystems 8: 71–86.
Tockner, K., F. Malard & J. V. Ward, 2000. An extension of the flood pulse concept. Hydrological Processes 14: 2861–2883.
Vannote, R. L., G. W. Minshall, K. W. Cummins, J. R. Sedell & C. E. Cushing, 1980. The river continuum concept. Canadian Journal of Fisheries and Aquatic Sciences 24: 277–304.
Ward, J. V. & K. Tockner, 2001. Biodiversity: towards a unifying theme for river ecology. Freshwater Biology 46: 807–819.
Ward, J. V., K. Tockner, D. B. Arscott & C. Claret, 2002. Riverine landscape diversity. Freshwater Biology 47: 517–539.
Ward, J. V., K. Tockner & F. Schiemer, 1999. Biodiversity of floodplain river ecosystems: ecotones and connectivity. Regulated Rivers: Research & Management 15: 125–139.
Acknowledgments
All authors have been continuously supported by grants provided by the National Council for Scientific and Technological Development (CNPq) and extend sincere thanks to the company Mineração Rio do Norte S/A for supporting field activities. We acknowledge our colleagues of the Laboratório de Limnologia of the Universidade Federal do Rio de Janeiro for helping with samples and to Felipe Siqueira for preparing the map. We thank Dr. Tadeu Siqueira for helpful suggestions on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Piet Spaak
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bozelli, R.L., Thomaz, S.M., Padial, A.A. et al. Floods decrease zooplankton beta diversity and environmental heterogeneity in an Amazonian floodplain system. Hydrobiologia 753, 233–241 (2015). https://doi.org/10.1007/s10750-015-2209-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2209-1