Abstract
Many arid and semiarid regions are likely to become warmer and drier by the end of this century, due to human-induced climate change. We hypothesize that a reduction in water level caused by droughts will aggravate eutrophication, leading to higher cyanobacteria biomass and dominance in tropical regions. To test this hypothesis, we analyzed physical and chemical variables and plankton communities of 40 man-made lakes in warm semiarid northeastern Brazil at the end of the wet and dry seasons. We also constructed a predictive model of cyanobacteria biovolume in these lakes. The lakes had significantly lower water volume, transparency, and CO2 concentrations but higher water temperature, water column stability, electrical conductivity, pH, suspended solids, ammonium, total nitrogen concentrations, bacteria biomass, phytoplankton biomass, and cyanobacteria biomass and dominance in the dry than in the wet season. Our regression model suggested that cyanobacteria biovolume was positively related to water column stability, pH, and total nitrogen and negatively related to water transparency and concentrations of inorganic suspended solids. These results suggest that the projected warmer and drier climate in the future will reduce water quantity and quality of man-made lakes in the region, increasing the risks of salinization, anoxia, eutrophication, and cyanobacteria blooms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cultural eutrophication, resulting from excessive nutrient loading from human activities, is a worldwide problem for both ecological status and drinking-water quality. Eutrophication has many undesirable symptoms often including high phytoplankton biomass, dominance of harmful cyanobacteria, oxygen depletion, decrease in water transparency, unpleasant taste and odor, and drinking-water treatment problems (Smith & Schindler, 2009). Generally, phosphorus is considered the most important limiting nutrient in lakes and the one responsible for eutrophication (Schindler, 1977, 2012), although other investigators have advocated for consideration of both nitrogen and phosphorus (Conley et al., 2009; Paerl et al., 2011; Moss et al., 2013). Although Lewis (2000) hypothesized that nitrogen limitation of phytoplankton is common in tropical lakes, studies based on large datasets have reported no relationships with latitude (Huszar et al., 2006; Elser et al., 2007; Kosten et al., 2009). The concept of nutrient limitation for algal growth is the keystone of eutrophication research because it implies that (i) a single nutrient should be the primary limiting factor for algal growth in a given lake; (ii) algal growth in a given lake should be positively related to the supply of this nutrient; and (iii) practical control of algal growth and of eutrophication in the lake should involve restricting the input of this nutrient to the system (Smith, 1998).
Recent studies have suggested that climate change may exacerbate the symptoms of eutrophication (Moss et al., 2011; Jeppesen et al., 2014; Rigosi et al., 2014). According to the report of the Intergovernmental Panel on Climate Change (IPCC, 2013), many arid and semiarid regions are likely to become warmer and drier by the end of this century due to human-induced climate change. For instance, climate models for northeastern Brazil predict a temperature increase of about 4°C and rainfall reduction of about 40%, and increases in the intensity or duration of drought are likely by the end of the 21st century (Marengo et al., 2009, 2010). Lower precipitation and more frequent droughts are expected to accentuate fluctuations in water level, an important element of hydrology driving man-made lakes in arid and semiarid regions. A recent review indicates that water-level reduction often results in higher nutrient concentrations, higher phytoplankton biomass, and lower water transparency in both shallow and deep lakes and reservoirs (Jeppesen et al., 2015). In addition, reduction in water level due to drought and water removal for multiple purposes might increase salinity and conductivity (Jeppesen et al., 2015). Lower precipitation will reduce runoff and potentially decrease nutrient loading (Jeppesen et al., 2009, 2011). This reduction, however, is not expected to compensate for the negative consequences of water deficits that will lead to nutrient concentration from point sources and reinforcement of eutrophication in lakes (Beklioglu et al., 2007; Özen et al., 2010). Moreover, drought may increase the water residence time in lakes, and some studies have shown that longer water residence times during dry years increase cyanobacteria biomass and dominance (Elliott, 2012; Romo et al., 2012).
In comparison to temperate regions, very little is known about how water-level fluctuation affects the ecology of reservoirs in the tropics. Man-made lakes in warm semiarid northeastern Brazil show seasonal variations in water volume and increasing concentrations of nutrients and phytoplankton chlorophyll, and decreasing water level in dry season. However, below a critical threshold of water volume, the high levels of abiotic turbidity, caused by sediment resuspension by wind and fish, may inhibit phytoplankton growth, leading to a reduction in nutrient concentrations, phytoplankton biomass, and cyanobacteria dominance (Jeppesen et al., 2015; Medeiros et al., 2015). In the same semiarid region, Bouvy et al. (2000) showed that the lack of water renewal linked to a drought event was the major determinant of both eutrophic conditions and cyanobacteria dominance. A more recent study showed that the effects of drought on water quality and ecology in Ethiopian reservoirs depend on their propensity to dry out (Teferi et al., 2014). Reservoirs that refilled after drying had significantly lower nutrient concentrations; lower biomass of phytoplankton, cyanobacteria, and fish; greater macrophyte cover; and clearer water than reservoirs that did not dry (Teferi et al., 2014).
In tropical regions, cyanobacteria may show persistent annual dominance with relatively small changes during the year (Figueredo & Giani, 2009; Soares et al., 2009) and often are toxic (Bouvy et al., 1999; Molica et al., 2005; Duong et al., 2013). Although cyanobacteria blooms are typically associated with nutrient enrichment, their appearance has been related to several factors, such as adaptation to low light conditions (Scheffer et al., 1997), high temperature, and water column stability (Paerl & Huisman, 2009), low CO2 concentrations and high pH levels (Shapiro, 1990; Caraco & Miller, 1998), nitrogen limitation (Smith, 1983), ability to store phosphorus (Isvánovics et al., 2000), production of allopathic substances (Leão et al., 2009), and resistance to herbivory (Wilson et al., 2006). Nevertheless, cyanobacteria are sensitive to flushing (Elliott, 2012; Romo et al., 2012) and suspended clay particles (Cuker et al., 1990; Allende et al., 2009). Cyanobacteria form a diverse group of organisms that differ widely in functional traits, and as a result, a wide variety of environmental variables may determine their population dynamics (Soares et al., 2013; Rigosi et al., 2014).
There is an urgent need for empirical studies to improve our understanding of the impacts of current droughts and future climate change on tropical freshwater ecosystems. We hypothesized that a reduction in water level caused by droughts will aggravate eutrophication symptoms, leading to higher cyanobacteria biomass and dominance. To test this hypothesis, we analyzed physical and chemical variables and plankton communities of 40 man-made lakes in semiarid northeastern Brazil at the end of the wet and dry seasons. We also constructed a predictive model of cyanobacteria biovolume in these lakes.
Materials and methods
Study sites
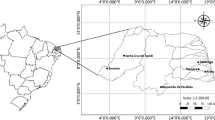
The 40 man-made lakes are located in a warm semiarid region (05°34′S to 06°44′S and 35°49′W to 38°25′W), encompassing six hydrographic basins in the state of Rio Grande do Norte in northeastern Brazil (Fig. 1). These lakes are impoundments of temporary streams constructed to store water for the long dry season typical of that region, and they are used for multiple purposes such as drinking water, irrigation, ranching, recreation, fishing, and aquaculture. The historical annual mean precipitation is 726 mm (1960 to 2006 period), with a well-defined rainy season between January and July, and an almost complete lack of precipitation during the remaining months. Precipitation levels exceeding the evapotranspiration rate usually occur only from February through April. Monthly mean air temperatures range from 24.2 to 26.5°C and are lowest from June through August (Data source: Agricultural Research Company of Rio Grande do Norte State—EMPARN). The local soils are shallow and highly susceptible to erosion; the predominant vegetation is a mosaic of thorn scrub and seasonally dry forest called Caatinga (Leal et al., 2005). In our study area, about 75% of the Caatinga has been devastated due to human activities (deforestation, fire, agriculture, pasture, and urbanization) (Santos, 2014). In addition, on average only about 12% of municipal sewage is treated (Data source: Water and Sewerage Company of Rio Grande do Norte State). Most of the lakes were constructed recently (mean age = 51 years) and are shallow (mean depth = 5 m), with surface areas ranging from 1 to 11 km2, and the maximum storage volume varies widely (Table 1).
Sampling
The lakes were sampled twice, in December 2007 (end of the dry season) and July 2008 (end of the wet season). Sampling was conducted in the deepest part of each lake, near the dam. Water samples for nutrients, suspended solids, chlorophyll a, and phytoplankton analysis were collected with a Van Dorn bottle at 1-m vertical depth intervals until 1 m above the bottom and integrated to obtain a representative sample from the water column of each lake. Filtration for dissolved nutrients and chlorophyll a analyses was conducted directly after collection, and the water and filters were frozen until analysis. Bacteria samples were fixed with formalin (2% final concentration). Phytoplankton samples were fixed in Lugol’s solution. Zooplankton samples were collected with a 50-µm-mesh plankton net towed vertically. The volume of filtered water was calculated as the product of the net mouth area and the tow depth, assuming a 100% filtering efficiency of the net. The zooplankton samples were preserved in a final concentration of 4% formaldehyde solution. Water temperature, dissolved oxygen, pH, electrical conductivity, and turbidity were measured in situ at 1-m intervals (Horiba model U-22 multiparameter probe, Kyoto, Japan). Water transparency was measured with a Secchi disk.
Climate variables (mean air temperature, precipitation and evapotranspiration) were estimated from isoline maps available from the web site of the Agricultural Research Company of Rio Grande do Norte State (www.emparn.rn.gov.br, July 30, 2010).
Sample analysis
Filtered samples were used for dissolved nutrient analysis. Nitrate was reduced to nitrite in alkaline-buffered solution by passing the sample through a column of copperized cadmium filings (Wetzel & Likens, 2000). Soluble reactive phosphorus (SRP) was determined through the reaction with a reagent composed of molybdate, ascorbic acid, and trivalent antimony (Wetzel & Likens, 2000). Ammonium nitrogen was analyzed by the phenol-hypochlorite method, using nitroprusside as a catalyst (Wetzel & Likens, 2000). Reactions with acidic ammonium molybdate and the reduction by sodium sulfite were used to analyze dissolved silica (Wetzel & Likens, 2000). Non-filtered samples were used to analyze total phosphorus, determined by oxidation using persulfate (Wetzel & Likens, 2000), and total organic nitrogen (Kjeldahl nitrogen) (Mackereth et al., 1978). Chlorophyll a (Chl-a) was extracted from filters (Whatman 934-AH) with 96% ethanol at room temperature for 20 h, and absorbance was measured at 665 and 750 nm (Jespersen & Christoffersen, 1988). To determine the concentrations of total suspended solids (TSS), the water samples were filtered with Whatman 934-AH filters and the residue retained on the filter was dried to constant weight at 105°C. To determine the concentration of inorganic suspended solids (ISS), the filters were incinerated in a muffle furnace at 550°C for 15 min and the ashes were weighed. Organic suspended solids (OSS) were determined by the difference between the TSS and ISS, according to APHA (1998).
Bacterioplankton samples were prepared according to Hobbie et al. (1977). Each sample was stained with acridine orange (final concentration: 0.01%) and then filtered through a polycarbonate membrane (Millipore, pore size 0.2 µm, diameter 25 mm, black). Bacteria abundance was estimated by direct counting at 1000x magnification under an epifluorescence microscope (Olympus BX-60), according to Daley & Hobbie (1975). In each sample, 10 random fields and at least 200 cells were counted.
Phytoplankton populations were enumerated in random fields (Uehlinger, 1964) using the settling technique (Utermöhl, 1958) under an inverted microscope (Zeiss Axiovert 10, Jena, Germany). The units (cells, colonies, and filaments) were enumerated for at least 100 specimens for the most frequent species (Lund et al., 1958) at ×400 magnification.
Zooplankton was counted under a microscope (Olympus, CX 41) in 1-ml Sedgwick-Rafter chambers. Between three and five subsamples were counted for each sample, until a minimum of 100 individuals of each taxonomic group (rotifers, copepods, cladocerans) were counted.
Calculations and data analysis
Relative water column stability (RWCS) was used as an index of water column stability at the time of sampling. RWCS was calculated according to the following equation (Padisák et al., 2003):
where D b is the density of the bottom water, D s is the density of the surface water, and D 4 and D 5 are the water densities at 4 and 5°C, respectively, according to Hutchinson (1957).
Dissolved inorganic nitrogen (DIN) was calculated as the sum of ammonium, nitrate, and nitrite. Total nitrogen was calculated as Kjeldahl nitrogen plus nitrate. CO2 concentrations were calculated indirectly, from measurements of alkalinity, pH, and water temperature, according to Stumm & Morgan (1996) and Weiss (1974). The trophic state of the lakes was defined based on Chl-a and nutrient concentrations, according to Nürnberg (1996). For that, Chl-a and nutrient concentrations were averaged over two seasons.
To evaluate the potential nutrient limitation on phytoplankton growth, we used the following indicators (Kosten et al., 2009): (1) if lake water TN/TP ratios were below 20 (molar based), a lake was considered N limited, and if they were above 38, a lake was P limited (Sakamoto, 1966); (2) if DIN/SRP ratios were below 13 (molar based), a lake was considered N limited, and if they were above 50, a lake was P limited (Morris & Lewis, 1988). If the ratios were in between those reference values, a lake was considered no N or P limited; and (3) SRP and DIN were compared to concentrations that have been considered to roughly limit phytoplankton growth; phosphorus was considered limiting below 10 µg P l−1 (Sas, 1989) and N below 100 µg P l−1 (Reynolds, 1999). Clearly, these are only approximations, as nutrient limitation depends on the attributes and storage capacities of the individual species (Reynolds, 1999).
Bacteria biomass was estimated by converting mean biovolume to biomass. To estimate the bacteria biovolume, we used an equation proposed by Massana et al. (1997): (4/π) × (W 2) × [L − (W/3)], where L is the length and W is the width. To convert biovolume to biomass, we used the equation of Norland (1993): m = CV a, where m is biomass, V is the estimated biovolume, C is the conversion constant (120 fg C cel−1), and a is a correction factor (0.76). The biovolume was calculated using the software Image-Pro Plus. At least 250 cells were measured to calculate the biovolume in each sample.
Phytoplankton biovolume (in cubic millimeters per liter) was estimated by multiplying the abundance of each species by the mean cell volume (Hillebrand et al., 1999), based on measurements of at least 30 individuals. To estimate zooplankton biomass, at least 30 randomly selected individuals of the most abundant species were measured. For rotifers, geometric formulas were used to estimate biovolume (Ruttner-Kolisko, 1977). Fresh weight was estimated from the biovolume of each individual, assuming that 106 µm3 corresponds to 1 µg of fresh weight (Ruttner-Kolisko, 1977). Dry weight was estimated as 10% of fresh weight (Pace & Orcutt, 1981). Microcrustacean biomass (copepods and cladocerans) was estimated using regression equations relating dry weight and body length, according to Bottrell et al. (1976). Zooplankton were classified according to their food preference: small filter-feeders (rotifers, nauplii, and copepodites), medium-sized filter-feeders (calanoid copepods and cladocerans), and omnivorous–carnivorous (cyclopoid copepods) (Loverde-Oliveira et al., 2009).
The ratio of zooplankton biomass (μg dry weight l−1)/algal biomass (Chl-a multiplied by 66, to convert μg l−1 to μg dry weight l−1) was used as a proxy for grazing pressure (Jeppensen et al., 2005), assuming that zooplankton use phytoplankton as their sole food source. The ratio gives a rough indication of the proportion of phytoplankton standing stock that is grazed per day, assuming that zooplankton consumes its biomass per day (Jeppesen et al., 1994).
Statistical analysis
Differences between the two study periods (dry and wet season) in relation to limnological variables were assessed by t test (α = 0.05). To explore the relationships among cyanobacteria biovolume and limnological variables, standard linear regressions were used. The potential multicollinearity among predictive variables was tested using the variance inflation factor (VIF > 10) (Legendre & Legendre, 2012). The 10 original variables were maximum depth, RWCS, Secchi depth, water temperature, pH, inorganic suspended solids, total nitrogen, total phosphorus, biomass of medium-sized zooplankton filter-feeders, and potential grazing pressure. The Akaike Information Criterion unbiased for small sample size (Akaike, 1974) was used to identify a single best model (Anderson, 2008). The Akaike weights were interpreted as the probability that i is the best model, given the data and set of candidate models. For t test and regression analyses, the biotic and abiotic data, except pH, were Log (x + 1) transformed to meet the assumptions of the analyses. The assumption of homogeneity of variance in the t test was assessed through Levene’s test. When this assumption was not satisfied, we used the t test with unequal variance to compare the differences between the seasons (Zar, 2010). The assumptions of linearity of relationships between the independent and dependent variables, and that the residuals are normally distributed in regression analyses were assessed through a scatterplot of predicted values versus residuals and a normal probability plot of residuals. All statistical analyses were performed using Statistica 7.0 (StatSoft, Inc., Tulsa, OK, U.S.A.).
Results
Abiotic environment
In 2007, the mean annual precipitation was lower (507 mm) and in 2008 was higher (980 mm) than the historical mean (726 mm) (Fig. 2).The total precipitation from January through December in 2007 was 36% lower than the cumulative precipitation from January through July in 2008. The mean air temperature in December 2007 (30.6°C) was four degrees higher than in July 2008 (26.5°C), when the water sampling was carried out.
Mean values of historical (1960–2006 period) monthly mean precipitation, air temperature, evapotranspiration, and precipitation in the two study years. Open circle indicates sampling month in 2007 and closed circle in 2008 (Data source: Empresa de Pesquisa Agropecuária do Rio Grande do Norte—EMPARN)
Large environmental differences were observed between the two contrasting climate conditions. As a consequence of the higher mean air temperature and evapotranspiration, and the lack of precipitation during the dry season (Fig. 2), significant reductions in water storage and maximum depth (z max) occurred in dry compared to wet conditions (Table 2; Fig. 3). Significantly higher water temperature and water column stability (RWCS) were also observed in dry than in wet conditions. The lakes showed low water transparency (Secchi depth < 1 m) in both climate conditions but significantly lower Secchi depth and higher turbidity, concentrations of total suspended solids (TSS), and organic suspended solids (OSS) were observed under the dry conditions. There was no significant difference, however, in the concentrations of inorganic suspended solids (ISS) between the seasons (Table 2; Fig. 3). Significantly higher values of electrical conductivity and pH, and lower CO2 concentrations occurred in the dry season (Table 2; Fig. 3). Although there was no significant difference in the mean oxygen concentrations from entire water column, oxygen concentrations near the bottom were significantly lower in dry than wet season (Table 2; Fig. 4). The water near the bottom seldom became anoxic (ODb, dissolved oxygen 1 m above the bottom <1 mg l−1), but this occurred somewhat more frequently in dry than in wet conditions (Fig. 3).
Box plots of a percentage of maximum storage volume; b z max maximum depth; c water temperature; d RWCS, relative water column stability; e Secchi disk depth; f turbidity; g TSS total suspended solids; h OSS organic suspended solids; i ISS, inorganic suspended solids, j Cond electrical conductivity; k pH; l CO 2 carbon dioxide concentrations; m DOb dissolved oxygen concentrations 1 m above the bottom found in 40 man-made lakes in Rio Grande do Norte, Brazil, in the dry and wet seasons (n = 80). The horizontal lines inside the box plots indicate the median, and the boundaries of the box plots indicate the 25th and 75th percentiles. Whiskers above and below indicate the 90th and 10th percentiles. Dots and stars are outliers and extreme points, respectively
Box plots of a SRSi, soluble reactive silica; b N NO − 3 nitrate; c N NH4 +, ammonium; d DIN dissolved inorganic nitrogen; e SRP soluble reactive phosphorus; f DIN:SRP molar ratio; g TN total nitrogen; h TP total phosphorus; i TN:TP molar ratio found in 40 man-made lakes in Rio Grande do Norte, Brazil, in the dry and wet seasons (n = 80). The horizontal lines inside the box plots indicate the median, and the boundaries of the box plots indicate the 25th and 75th percentiles. Whiskers above and below indicate the 90th and 10th percentiles. Dots and stars are outliers and extreme points, respectively. Light-gray areas represent potentially N-limited conditions; dark-gray areas represent potentially P-limited conditions; and white, no limitation
Higher N NH4 + and TN concentrations were observed in dry than in wet conditions, but no significant differences were found in SRSi, NO3 −, DIN, SRP, TP, DIN/SRP, and TN/TP ratios between the two seasons (Table 2; Fig. 4). Only 4% of the samples had TN/TP ratios >38 and 24% had SRP <10 µg l−1, suggesting no potential P limitation in most systems in both climate conditions (Fig. 4e, i). On the other hand, DIN/SRP ratios <13 and DIN concentrations <100 µg l−1 were observed in 85 and 84% of the samples, respectively (Fig. 4d, f). Therefore, analysis of the nutrient indicators suggested a potential N limitation to phytoplankton growth in the lakes in both seasons.
Plankton communities
Based on Chl-a and nutrient concentrations, most of the systems were hypereutrophic and eutrophic (Table 3). Bacteria and phytoplankton biomass (Chl-a concentrations and total phytoplankton biovolume) and cyanobacteria biovolume were significantly higher in dry than in wet conditions; the biovolume of diatoms, chlorophyceans, cryptomonads, zygnemaphyceans, and other minor phytoplankton groups (dinoflagellates, euglenoids, and xanthophyceans) did not change (Table 4; Fig. 5). The contribution of cyanobacteria to the total phytoplankton biovolume (cyanobacteria percentage) was significantly higher, while the percentages of diatoms and other minor phytoplankton groups were significantly lower under dry conditions; the percentages of chlorophyceans, cryptomonads, and zygnemaphyceans did not change with the season (Table 4; Fig. 6).
Box plots of a bacteria biomass; b Chl-a chlorophyll a; c Phyto total phytoplankton biovolume; and biovolume of d Cya cyanobacteria; e Dia diatoms; f Chl chlorophyceans; g Cry cryptomonads; h Zyg zygnemaphyceans; i Others other phytoplankton groups (dinoflagellates, euglenoids, and xanthophyceans), j Nost Nostocales cyanobacteria; k Osci Oscillatoriales cyanobacteria; l Chroo Chroococcales cyanobacteria found in 40 man-made lakes in Rio Grande do Norte, Brazil, in the dry and wet seasons (n = 80). The horizontal lines inside the box plots indicate the median, and the boundaries of the box plots indicate the 25th and 75th percentiles. Whiskers above and below indicate the 90th and 10th percentiles. Dots and stars are outliers and extreme points, respectively. Note the different scaling of the y-axis
Box plots of percentages of total phytoplankton biovolume of a cyanobacteria; b diatoms; c chlorophyceans; d cryptomonads; e zygnemaphyceans; f other phytoplankton groups (dinoflagellates, euglenoids, and xanthophyceans) found in 40 man-made lakes in Rio Grande do Norte, Brazil, in the dry and wet seasons (n = 80). The horizontal lines inside the box plots indicate the median, and the boundaries of the box plots indicate the 25th and 75th percentiles. Whiskers above and below indicate the 90th and 10th percentiles. Dots and stars are outliers and extreme points, respectively
Cyanobacteria contributed the most to total phytoplankton biovolume, in both dry (72.4%) and wet (53.8%) conditions (Table 4). The t test showed a significant increase in biovolume of Nostocales (filamentous potentially N2-fixing cyanobacteria) and Oscillatoriales (filamentous non-N2-fixing cyanobacteria) in dry conditions (Table 4; Fig. 5). Nostocales species were the dominant forms in both seasons, contributing 62 and 42% to cyanobacteria biovolume in dry and wet conditions, respectively. Oscillatoriales contributed 27 and 32% to cyanobacteria biovolume in dry and wet conditions, respectively. The most important phytoplankton species in the systems was the nostocalean Cylindrospermopsis raciborskii (Woloszynska) Seenaya & Subba Raju. On average, C. raciborskii contributed 22% to total phytoplankton biovolume; it was present in 70% of the samples and reached >30% of total phytoplankton biovolume in 30% of the lakes (Table 5). During the survey, heterocyted filaments, on average, comprised 31% of C. raciborskii abundance. No significant differences were observed in Chroococcales (unicellular or mucilaginous colonial cyanobacteria) between the two seasons (Table 4; Fig. 5).
Zooplankton biomass and the proxy for grazing pressure did not differ significantly between dry and wet conditions (Table 4). On average, medium-sized zooplankton filter-feeders dominated in both seasons, contributing 57 and 48% to the total zooplankton biomass under dry and wet conditions, respectively (Fig. 7).
Box plots of biomass of a SFZ small zooplankton filter-feeders; b MFZ medium-sized zooplankton filter-feeders; c OCZ omnivorous–carnivorous zooplankton; d Zoo total zooplankton; e Grazing, grazing pressure found in 40 man-made lakes in Rio Grande do Norte, Brazil, in the dry and wet seasons (n = 80). The horizontal lines inside the box plots indicate the median, and the boundaries of the box plots indicate the 25th and 75th percentiles. Whiskers above and below indicate the 90th and 10th percentiles. Dots and stars are outliers and extreme points, respectively. Note the different scaling of the y-axis
Regression analyses
The model probabilities (w i) indicated that a model including Secchi depth, RWCS, pH, inorganic suspended solids (ISS), and TN was likely to best explain the biovolume of cyanobacteria, given the data and set of candidate models (model 1, Table 6). Cyanobacteria biovolume was positively related to RWCS, pH, and TN and negatively related to Secchi depth and ISS. This model accounted for 40% of the variation in cyanobacteria biovolume and was (0.17/0.09) = 1.9 times more likely to be the best explanation than the extended model including grazing pressure (model 3, Table 6). All variance inflation factors were lower than the critical heuristic value of 10, suggesting that collinearity among the explanatory variables did not strongly affect the results.
Discussion
Our hypothesis that drought conditions may aggravate the symptoms of eutrophication was supported by the data and model in this study. Our results suggest that drought impact the hydrological, chemical, and physical characteristics of man-made lakes in tropical semiarid regions, favoring cyanobacteria blooms.
Studies conducted in other man-made lakes in semiarid northeastern Brazil have also shown that the annual rainfall deficit and lack of water renewal linked to an El Niño event seem to be the major factors responsible for both eutrophic conditions and cyanobacteria blooms (Bouvy et al., 1999, 2000). Studies elsewhere have also suggested that droughts will decrease the flushing rate and increase the water residence time, contributing to cyanobacteria blooms (Elliott, 2012; Romo et al., 2012). Indeed, at the end of the wet season, when the large majority of studied lakes reached their maximum capacity or overflowed, there were reductions in the total phytoplankton biovolume and the absolute and relative cyanobacteria biovolume, indicating that flushing was an important density-independent loss factor to control large cyanobacteria, because of their relatively slow growth rates (Scheffer, 1998; Reynolds, 2006). Thus, our study is in agreement, at least in part, with the general prediction of de Senerpont Domis et al. (2013a, b); that in tropical systems, temporal variability in precipitation can be an important driver of the seasonal development of plankton, and that intense precipitation events may disrupt cyanobacteria blooms. However, these authors assumed that precipitation intensity will increase in the tropics, even in semiarid regions, which is not the case (Sarmento et al., 2013). For semiarid northeastern Brazil, an increase in dry periods and reduction in intense precipitation events are projected (Marengo et al., 2009). Consequently, with the projected reduction in mean precipitation and intense precipitation events, and longer dry periods, we can expect more-prolonged cyanobacteria blooms in tropical semiarid regions.
Reduced precipitation also led to changes in the chemical conditions in these lakes. Lower precipitation and higher evaporation resulted in a reduction in water storage and a 3-fold increase in mean electrical conductivity, increasing the risk of salinization and threatening the water supply. Similarly, in Lake Doirani, Greece (warm semiarid Mediterranean climate), electrical conductivity increased from 0.49 to 1.24 mS cm−1 and showed a significant correlation with the water-level reduction (Jeppesen et al., 2015). Excessive phytoplankton growth sharply increases the CO2 demand to support photosynthesis, decreasing free CO2 availability and consequently raising pH levels (Paerl & Huisman, 2009). This may explain the lower CO2 concentrations and higher pH levels during the dry season in our study. The higher pH resulting from CO2 consumption by high phytoplankton biomass (chlorophyll a and total biovolume) might also have given a competitive advantage to cyanobacteria, at least in the dry conditions, when the mean pH was 8.5. Experimental studies have shown that at alkaline pH (8–9) the relative contribution from cyanobacteria is high, independently of CO2 concentrations, while at pH around 7, the cyanobacteria contribution is negatively related to CO2 concentrations, suggesting that high pH per se may favor cyanobacteria dominance (Caraco & Miller, 1998). In addition, the alkaline pH found in the warmer and drier climate conditions may have favored cyanobacteria dominance, because these phytoplankton can use bicarbonate as a carbon source (Paerl & Huisman, 2009; Holland et al., 2012).
There is a consensus among the scientific community that eutrophication is a major cause of cyanobacteria blooms. Our results showed that most of our systems are eutrophic or hypereutrophic, thus contributing to excessive growth of cyanobacteria. A study to evaluate the effect of land use on water quality in the same semiarid region as our study found a positive relationship between land use (bare soil and urban area) and lake trophic status (Santos, 2014). The higher the proportion of bare soil surrounding the lakes, the higher was the concentration of nutrients (nitrogen and phosphorus), and the higher the proportion of bare soil and urban areas, the higher was the concentration of chlorophyll (Santos, 2014). Furthermore, man-made lakes in semiarid regions tend to have small surface areas relative to their basins (Thornton & Rast, 1993), which together with their long water residence time (months to years) makes these systems highly vulnerable to eutrophication and siltation, due to high input and retention of nutrients and sediments from the basin (Jeppesen et al., 2015). Despite the lack of precipitation and external nutrient supply in the dry season, ammonium and total nitrogen concentrations increased in the lakes studied here, potentially related to internal processes (evaporation, decomposition and internal loading). This was evidenced by the higher phytoplankton and bacteria biomass, and lower oxygen concentration in the water near the bottom during the dry season. Decomposition of organic material normally leads to the release of nitrogen as ammonium, a process called ammonification (Scheffer, 1998). Similarly, a detailed mass-balance study in two Mediterranean lakes found that nutrient concentrations (total phosphorus and dissolved inorganic nitrogen) increased during dry years despite reduced external nutrient loading (Özen et al., 2010).
Contrarily to recent studies (Huszar et al., 2006; Elser et al., 2007; Kosten et al., 2009; Rangel et al. 2012), our study supports the hypothesis that nitrogen limitation is common in tropical lakes (Lewis, 2000), as evidenced by our indicators of nutrient limitation. In general, those studies argue that local factors (land use, catchment characteristics, and hydrology) have a stronger influence on which nutrient is limiting than climate. There are two main potential explanations to the trend toward N limitation in these ecosystems. Firstly, the studied lakes have received urban wastewater effluents, which are proportionally richer in phosphorus than nitrogen (Moss et al., 2013), for decades and have been phosphorus overfertilized, as evidenced by high TP and SRP concentrations. Secondly, the high temperature in the sediment, intense bacterial activity, and sediment deoxygenation increase the release of phosphorus from sediments to water column, while nitrogen may decrease owing to denitrification (Lewis, 2000; Veraart et al., 2011; Moss et al., 2013).
Commonly, a major consequence of the N limitation is the excessive growth of N2-fixing cyanobacteria (Schindler et al., 2008; Moss et al., 2013), as observed in our study. We also found a positive relationship between cyanobacteria and TN concentrations. Interpretation of causality in such regression models is problematic, because fixation of atmospheric nitrogen by N2-fixing cyanobacteria may increase the input of nitrogen into the lake. The high incidence of filaments of C. raciborskii with heterocytes (31%) compared to other systems (<10%, Branco & Senna, 1994; Bouvy et al., 1999; Huszar et al., 2000; Soares et al., 2009) indicates that N2-fixation is a potentially important process in these systems. However, N2-fixation by cyanobacteria is not sufficient to compensate for N deficiency in many lakes (Lewis & Wurtsbaugh, 2008; Scott & McCarthy, 2010; Paerl et al., 2011).
In addition to nutrients, light is also a bottom-up control for phytoplankton growth (Reynolds 2006). Water transparency remained low in the lakes during the entire study period. Turbidity increased in the dry season, and these highly turbid conditions seem to be a decisive factor in favoring filamentous cyanobacteria. These cyanobacteria show a high product of the maximum linear dimension and surface/volume ratio, becoming good light antennae (Reynolds, 2006). The higher proportion of organic to inorganic suspended solids, particularly at the end of the dry season, also suggests that high algal biomass in the lakes is responsible for much of the underwater light attenuation. The patterns observed in the field as well as the physiologically based competition model indicate that dominance by shade-tolerant cyanobacteria, as originally proposed for Oscillatoriales, can be an alternative stable state of the algal community in shallow lakes, because these cyanobacteria are able to cause an increase in turbidity that augments their competitive advantage (Scheffer et al., 1997). The turbid conditions in these systems may have conferred a competitive advantage on C. raciborskii, and because of its superior shade tolerance, it is considered to be a ‘heterocystous Oscillatoria’ (Padisák & Reynolds, 1998).
In addition to the reduction in water storage, and its physical and chemical consequences, the higher temperatures in the dry season may also have favored the cyanobacteria. Recent studies suggest that warming will probably increase the magnitude, frequency and persistence until autumn of cyanobacteria blooms (Kosten et al., 2012; O’Neil et al., 2012; Jeppesen et al., 2014; Izaguirre et al. 2015). High temperatures may give a competitive advantage to cyanobacteria through direct and indirect processes (Paerl & Huisman, 2009). However, Lürling et al. (2013) recently found that cyanobacteria did not show higher growth rates than green algae, for example, with increased temperature. These authors suggested that the competitive advantage of cyanobacteria with global warming is more likely related to the indirect effect of stronger stratification, enhanced by their ability to migrate vertically and avoid settling. In our study, stratification was more stable in the dry season, and our regression model showed a positive relationship between the cyanobacteria biovolume and water column stability. Stratification should favor organisms with functional traits that allow them to regulate their position in the water column. When water column mixing is weak, cyanobacteria may form dense layers at the surface, shading their competitors (Huisman et al., 2004; Jöhnk et al., 2008). Nevertheless, although thermal stability may contribute to cyanobacteria dominance, destratification per se does not necessarily lead to cyanobacteria decline. Water column mixing may be sufficient to restrict the dominance of cyanobacteria such as Microcystis that are dependent on floating to compete for light (Huisman et al., 2004; Jöhnk et al., 2008) but may be less effective for shade-tolerant species such as C. raciborskii (Antenucci et al., 2005; Soares et al., 2013). This is a possible explanation for the high relative contribution of filamentous cyanobacteria at the end of the wet season, although with considerably lower biovolume.
Our best regression model also showed a negative relationship between cyanobacteria biovolume and inorganic suspended solids. Observational and experimental studies have shown that suspended clay may negatively affect cyanobacteria. For instance, a recent study conducted in a man-made lake in the same semiarid region as our study showed that sediment resuspension during a severe drought led to the collapse of the cyanobacteria biomass and dominance (Medeiros et al., 2015). Allende et al. (2009) showed that in inorganic-turbid lakes of the Pampa Plain (Argentina), phytoplankton communities were dominated by diatoms. An experimental study with mesocosms found that additions of suspended clay changed a phytoplankton community dominated by flagellated algae and Chroococcales cyanobacteria to a sparse, flagellate-dominated community (Cuker et al., 1990). Recently, modified clay has been used in combination with flocculents to control cyanobacteria blooms in lakes (Lürling & van Oosterhout, 2013).
In summary, the present study showed that drought increases the risk of salinization and deep anoxia, aggravates the symptoms of eutrophication, and increases the intensity of cyanobacteria blooms, most notably the potentially N2-fixing filamentous forms. Our regression model suggested that cyanobacteria biovolume was positively related to water column stability, pH, and TN, and negatively related to water transparency and concentrations of suspended inorganic solids. Our results imply that the future warmer and drier climate predicted for the semiarid region of northeastern Brazil will reduce the water quantity and quality of man-made lakes. Therefore, more effective reduction of external nutrient loading (both nitrogen and phosphorus) will be needed to manage the water quality of these ecosystems.
References
Akaike, H., 1974. A new look at the statistical model identification. IEEE Transactions on Automatic Control 19: 716–723.
Anderson, D. R., 2008. Model Based Inference in the Life Sciences: A Primer on Evidence. Springer, New York.
Allende, L., G. Tell, H. Zagarese, A. Torremorell, G. Pérez, J. Bustingorry, R. Escaray & I. Izaguirre, 2009. Phytoplankton and primary production in clear-vegetated, inorganic-turbid, and algal-turbid shallow lakes from the pampa plain (Argentina). Hydrobiologia 624: 45–60.
Antenucci, J. P., A. Ghadouani, M. A. Burford & J. R. Romero, 2005. The long-term effect of artificial destratification on phytoplankton species composition in a subtropical reservoir. Freshw Biology 50: 1081–1093.
APHA, 1998. Standard Methods for the Examination of Water and Wastewater. American Public Health Association, Washington DC.
Beklioglu, M., S. Romo, I. Kagalou, X. Quintana & E. Bécares, 2007. State of the art in the functioning of shallow Mediterranean lakes: workshop conclusions. Hydrobiologia 584: 317–326.
Bottrell, H. H., A. Duncan, Z. M. Gliwicz, E. Grygierek, A. Herzing, H. A. Ilkowska, P. Larsson & T. Weglenska, 1976. A review of some problems in zooplankton production studies. Norwegian Journal of Zoology 24: 419–456.
Bouvy, M., R. Molica, S. De Oliveira, M. Marinho & B. Beker, 1999. Dynamics of a toxic cyanobacterial bloom (Cylindrospermopsis raciborskii) in a shallow reservoir in the semi-arid region of northeast Brazil. Aquatic Microbial Ecology 20: 285–297.
Bouvy, M., D. Falcão, M. Marinho, M. Pagano & A. Moura, 2000. Occurrence of Cylindrospermopsis (Cyanobacteria) in 39 Brazilian tropical reservoirs during 1998 drought. Aquatic Microbial Ecology 23: 13–27.
Branco, C. W. C. & P. A. C. Senna, 1994. Factors influencing the development of Cylindrospermopsis raciborskii and Microcystis aeruginosa in Paranoá Reservoir, Brasília, Brazil. Algological Studies 75: 85–96.
Caraco, N. & R. Miller, 1998. Direct and indirect effects of CO2 on competition between a cyanobacteria and eukaryotic phytoplankton. Canadian Journal of Fisheries and Aquatic Sciences 55: 54–62.
Conley, D. J., H. W. Paerl, R. W. Howarth, D. F. Boesch, S. P. Seitzinger, K. E. Havens, C. Lancelot & G. E. Likens, 2009. Ecology controlling eutrophication: nitrogen and phosphorus. Science 323: 1014–1015.
Cuker, B. E., P. T. Gama & J. M. Burkholder, 1990. Type of suspended clay influences lake productivity and phytoplankton community and response to phosphorus loading. Limnology and Oceanography 35: 830–839.
Daley, R. J. & J. E. Hobbie, 1975. Direct counts of aquatic bacteria by a modified epifluorescence technique. Limnology and Oceanography 20: 875–882.
de Senerpont Domis, L. N., J. J. Elser, A. S. Gsell, V. L. M. Huszar, B. W. Ibelings, E. Jeppesen, S. Kosten, W. M. Mooij, F. Roland, U. Sommer, E. Van Donk, M. Winder & M. Lürling, 2013a. Plankton dynamics under different climatic conditions in space and time. Freshwater Biology 58: 463–482.
de Senerpont Domis, L. N., J. J. Elser, A. S. Gsell, V. L. M. Huszar, B. W. Ibelings, E. Jeppesen, S. Kosten, W. M. Mooij, F. Roland, U. Sommer, E. Van Donk, M. Winder & M. Lürling, 2013b. Plankton dynamics under different climate conditions in tropical freshwater systems (a reply to the comment by Sarmento, Amado and Descy, 2013). Freshwater Biology 58: 2211–2213.
Duong, T. T., T. P. Q. Le, T.-S. Dao, S. Pflugmacher, E. Rochelle-Newall, T. K. Hoand, T. N. Vu, C. T. Ho & D. K. Dang, 2013. Seasonal variation of cyanobacteria and microcystins in the Nui Coc Reservoir, Northern Vietnam. Journal of Applied Phycology 25: 1065–1075.
Elliott, J. A., 2012. The seasonal sensitivity of cyanobacteria and other phytoplankton to changes in flushing rate and water temperature. Global Change Biology 16: 864–876.
Elser, J. J., M. E. S. Bracken, E. E. Cleland, D. S. Gruner, W. S. Harpole, H. Hillebrand, J. T. Ngai, E. W. Seabloom, J. B. Shurin & J. E. Smith, 2007. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecology Letters 10: 1135–1142.
Figueredo, C. C. & A. Giani, 2009. Phytoplankton community in the tropical lake of Lagoa Santa (Brazil): conditions favoring a persistent bloom of Cylindrospermopsis raciborskii. Limnologica 39: 264–272.
Hillebrand, H., C. D. Dürselen, D. Kirschtel, U. Pollingher & T. Zohary, 1999. Biovolume calculation for pelagic and benthic microalgae. Journal of Phycology 35: 403–424.
Hobbie, J. E., R. J. Daley & S. Jasper, 1977. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Applied and Environmental Microbiology 33: 1225–1228.
Holland, D. P., A. Pantorno, P. T. Orr, S. Stojkovic & J. Beardall, 2012. The impacts of a high CO2 environment on a bicarbonate user: the cyanobacterium Cylindrospermopsis raciborskii. Water Research 46: 1430–1437.
Huisman, J., J. Sharples, J. M. Stroom, P. M. Visser, W. E. A. Kardinaal, J. M. H. Verspagen & B. Sommeijer, 2004. Changes in turbulent mixing shift competition for light between phytoplankton species. Ecology 85: 2960–2970.
Huszar, V. L. M., L. H. S. Silva, M. Marinho, P. Domingos & C. L. S. Anna, 2000. Cyanoprokaryote assemblages in eight productive tropical Brazilian waters. Hydrobiologia 424: 67–77.
Huszar, V. L. M., N. F. Caraco, F. Roland & J. Cole, 2006. Nutrient–chlorophyll relationships in tropical–subtropical lakes: do temperate models fit? Biogeochemistry 79: 239–250.
Hutchinson, G. E., 1957. A Treatise on Limnology: Vol. I. Geography, Physics and Chemistry. Wiley, New York.
IPCC, 2013. Summary for policymakers. In Stocker, T. F., D. Qin, G. K. Plattner, M. Tignor, S. K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex & P. M. Midgley (eds), Climate Change 2013: The physical science basis. Contribution of working group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge: 3–29.
Isvánovics, V., H. M. Shafik, M. Présing & S. Juhos, 2000. Growth and phosphate uptake kinetics of the cyanobacterium, Cylindrospermopsis raciborskii (Cyanophyceae) in throughflow cultures. Freshwater Biology 43: 257–275.
Izaguirre, I., M. L. Sánchez, M. R. Schiaffino, I. O’Farrell, P. Huber, N. Ferrer, J. Zunino, L. Lagomarsino & M. Mancini, 2015. Which environmental factors trigger the dominance of phytoplankton species across a moisture gradient of shallow lakes? Hydrobiologia 752: 47–64.
Jeppesen, E., M. Søndergaard, E. Kanstrup & B. Petersen, 1994. Does the impact of nutrients on the biological structure and function of brackish and freshwater lakes differ? Hydrobiologia 276: 15–30.
Jeppensen, E., M. Søndergaard, J. P. Jensen, K. E. Havens, O. Anneville, L. Carvalho, M. F. Coveney, R. Deneker, M. T. Dokulil, B. Foy, D. Gerdeaux, S. E. Hampton, S. Hilt, K. Kangur, J. Köhler, E. H. H. R. Lammens, T. L. Lauridsen, M. Manca, M. R. Miracle, B. Moss, P. Nõges, G. Persson, G. Phillips, R. Portielje, S. Romo, C. L. Schelske, D. Straile, I. Tatrai, E. Willén & M. Winder, 2005. Lake response to reduced nutrient loading – an analysis of contemporary long-term data from 35 case studies. Freshwater Biology 50: 1747–1771.
Jeppesen, E., B. Kronvang, M. Meerhoff, M. Søndergaard, K. M. Hansen, H. E. Andersen, T. L. Lauridsen, M. Beklioglu, A. Özen & J. E. Olesen, 2009. Climate change effects on runoff, catchment phosphorus loading and lake ecological state, and potential adaptations. Journal of Environmental Quality 38: 1930–1941.
Jeppesen, E., B. Kronvang, J. E. Olesen, J. Audet, M. Søndergaard, C. C. Hoffmann, H. E. Andersen, T. L. Lauridsen, L. Liboriussen, S. E. Larsen, M. Beklioglu, M. Meerhoff, A. Özen & K. Özkan, 2011. Climate change effects on nitrogen loading from cultivated catchments in Europe: implications for nitrogen retention, ecological state of lakes and adaptation. Hydrobiologia 663: 1–21.
Jeppesen, E., M. Meerhoff, T. A. Davidson, D. Trolle, M. Søndergaard, T. L. Lauridsen, M. Beklioglu, S. Brucet, P. Volta, I. González-Bergonzoni & A. Nielsen, 2014. Climate change impacts on lakes: an integrated ecological perspective based on a multi-faceted approach, with special focus on shallow lakes. Journal of Limnology 73: 88–111.
Jeppesen, E., S. Brucet, L. Naselli-Flores, E. Papastergiadou, K. Stefanidis, T. Nõges, P. Nõges, J. L. Attayde, T. Zohary, J. Coppens, T. Bucak, R. F. Menezes, F. R. S. Freitas, M. Kernan, M. Søndergaard & M. Beklioglu, 2015. Ecological impacts of global warming and water abstraction on lakes and reservoirs due to changes in water level and related changes in salinity. Hydrobiologia 750: 201–227.
Jespersen, A. M. & K. Christoffersen, 1988. Measurements of chlorophyll-a from phytoplankton using ethanol as extraction solvent. Archiv für Hydrobiologie 109: 445–454.
Jöhnk, K. D., J. Huisman, J. Sharples, B. Sommeijer, P. M. Visser & J. M. Stroom, 2008. Summer heatwaves promote blooms of harmful cyanobacteria. Global Change Biology 14: 495–512.
Kosten, S., V. L. M. Huszar, N. Mazzeo, M. Scheffer, L. S. S. Sternberg & E. Jeppesen, 2009. Lake and watershed characteristics rather than climate influence nutrient limitation in shallow lakes. Ecological Applications 19: 1791–1804.
Kosten, S., V. L. M. Huszar, E. Bécares, L. S. Costa, E. van Donk, L. A. Hansson, E. Jeppesen, C. Kruk, G. Lacerot, N. Mazzeo, L. D. Meester, B. Moss, M. Lürling, T. Nõges, S. Romo & M. Scheffer, 2012. Warmer climates boost cyanobacterial dominance in shallow lakes. Global Change Biology 18: 118–126.
Leal, I. R., J. M. C. Silva, M. Tabarelli & T. E. Lacher Jr, 2005. Changing the course of biodiversity conservation in the Caatinga of northeastern Brazil. Conservation Biology 19: 701–706.
Leão, P. N., M. T. S. D. Vasconcelos & V. M. Vasconcelos, 2009. Allelopathy in freshwater cyanobacteria. Critical Reviews in Microbiology 35: 271–282.
Legendre, P. & L. Legendre, 2012. Numerical Ecology. Elsevier, Amsterdam.
Lewis Jr, W. M., 2000. Basis for the protection and management of tropical lakes. Lakes Reservoirs: Research and Management 5: 35–48.
Lewis Jr, W. M. & W. A. Wurtsbaugh, 2008. Control of lacustrine phytoplankton by nutrients: erosion of the phosphorus paradigm. International Review of Hydrobiology 93: 446–465.
Loverde-Oliveira, S. M., V. L. M. Huszar, N. Mazzeo & M. Scheffer, 2009. Hydrology-driven regime shifts in a shallow tropical lake. Ecosystems 12: 807–819.
Lund, J. W. G., C. Kipling & E. D. Lecren, 1958. The inverted microscope method of estimating algae number and the statistical basis of estimating by counting. Hydrobiologia 11: 143–170.
Lürling, M. & F. van Oosterhout, 2013. Controlling eutrophication by combined bloom precipitation and sediment phosphorus inactivation. Water Research 47: 6527–6537.
Lürling, M., F. Eshetu, E. J. Faassen, S. Kosten & V. L. M. Huszar, 2013. Comparison of cyanobacterial and green algal growth rates at different temperatures. Freshwater Biology 58: 552–559.
Mackereth, F. J. H., J. Heron & J. F. Talling, 1978. Water Analysis: Some Revised Methods for Limnologists. Freshwater Biological Association, Cumbria.
Marengo, J. A., R. Jones, L. M. Alves & M. C. Valverde, 2009. Future change of temperature and precipitation extremes in South America as derived from the PRECIS regional climate modeling system. International Journal of Climatology 29: 2241–2255.
Marengo, J. A., T. Ambrizzi, R. P. Rocha, L. M. Alves, S. V. Cuadra, M. C. Valverde, R. R. Torres, D. C. Santos & S. E. T. Ferraz, 2010. Future change of climate in South America in the late twenty-first century: intercomparison of scenarios from three regional climate models. Climate Dynamics 35: 1073–1097.
Massana, R., J. M. Gasol, P. K. Bjørnsen, N. Blackburn, A. Hagstrom, S. Hietanen, B. H. Hygum, J. Kuparinen & C. PedrosAlio, 1997. Measurement of bacterial size via image analysis of epifluorescence preparations: description of an inexpensive system and solutions to some of the most common problems. Scientia Marina 61: 397–407.
Medeiros, L. C. M., A. Mattos, M. Lürling & V. Becker, 2015. Is the future blue-green or brown? The effects of extreme events on phytoplankton dynamics in a semi-arid man-made lake. Aquatic Ecology 49: 293–307.
Molica, R. J. R., E. J. A. Oliveira, P. V. V. C. Carvalho, A. N. S. F. Costa, M. C. C. Cunha, G. L. Melo & S. M. F. O. Azevedo, 2005. Occurrence of saxitoxins and an anatoxin-a(s)-like anticholinesterase in a Brazilian drinking water supply. Harmful Algae 4: 743–753.
Morris, D. P. & W. M. Lewis Jr, 1988. Phytoplankton nutrient limitation in Colorado mountain lakes. Freshwater Biology 20: 315–327.
Moss, B., S. Kosten, M. Meerhoff, R. W. Battarbee, E. Jeppesen, N. Mazzeo, K. Havens, G. Lacerot, Z. Liu, L. De Meester, H. Paerl & M. Scheffer, 2011. Allied attack: climate change and eutrophication. Inland Waters 1: 101–105.
Moss, B., E. Jeppesen, M. Søndergaard, T. L. Lauridsen & Z. Liu, 2013. Nitrogen, macrophytes, shallow lakes and nutrient limitation: resolution of a current controversy? Hydrobiologia 710: 3–21.
Norland, S., 1993. The relationship between biomass and volume of bacteria. In Kemp, P. F., B. F. Sherr, E. B. Sherr & J. J. Cole (eds), Aquatic Microbial Ecology. Lewis Publishers, Boca Raton: 303–307.
Nürnberg, G. K., 1996. Trophic state of clear and colored, soft- and hardwater lakes with special consideration of nutrients, anoxia, phytoplankton and fish. Lake and Reservoir Management 12: 432–447.
O’Neil, J. M., T. W. Davis, M. A. Burford & C. J. Gobler, 2012. The rise of harmful cyanobacteria blooms: the potential roles of eutrophication and climate change. Harmful Algae 14: 313–334.
Özen, A., B. Karapınar, I. Kucuk, E. Jeppesen & M. Beklioglu, 2010. Drought-induced changes in nutrient concentrations and retention in two shallow Mediterranean lakes subjected to different degrees of management. Hydrobiologia 646: 61–72.
Pace, M. L. & J. D. Orcutt Jr, 1981. The relative importance of protozoans, rotifers and crustaceans in a freshwater zooplankton community. Limnology and Oceanography 26: 822–830.
Padisák, J. & C. S. Reynolds, 1998. Selection of phytoplankton associations in Lake Balaton, Hungary, in response to eutrophication and restoration measures, with special reference to cyanoprokaryotes. Hydrobiologia 384: 41–53.
Padisák, J., W. Scheffler, P. Kasprzak, R. Koschel & L. Krienitz, 2003. Interannual variability in the phytoplankton composition of Lake Stechlin (1994-2000). Archiv für Hydrobiologie Special Issues in Advanced Limnology 58: 101–133.
Paerl, H. W. & J. Huisman, 2009. Climate change: a catalyst for global expansion of harmful cyanobacterial blooms. Environmental Microbiology Reports 1: 27–37.
Paerl, H. W., H. Xu, M. J. McCarthy, G. Zhu, B. Qin, Y. Li & W. S. Gardner, 2011. Controlling harmful cyanobacterial blooms in a hyper-eutrophic lake (Lake Taihu, China): the need for a dual nutrient (N & P) management strategy. Water Research 45: 1973–1983.
Rangel, L. M., L. H. S. Silva, P. Rosa, F. Roland & V. L. M. Huszar, 2012. Phytoplankton biomass is mainly controlled by hydrology and phosphorus concentrations in tropical hydroelectric reservoirs. Hydrobiologia 693: 13–28.
Reynolds, C. S., 1999. Non-determinism to probability, or N: p in the community ecology of phytoplankton. Archiv für Hydrobiologie 146: 23–35.
Reynolds, C. S., 2006. Ecology of Phytoplankton. Cambridge University Press, Cambridge.
Rigosi, A., C. C. Carey, B. W. Ibelings & J. D. Brookes, 2014. The interaction between climate warming and eutrophication to promote cyanobacteria is dependent on trophic state and varies among taxa. Limnology and Oceanography 59: 99–114.
Romo, S., J. Soria, F. Fernández, Y. Ouahid & A. Barón-Solá, 2012. Water residence time and the dynamics of toxic cyanobacteria. Freshwater Biology 58: 513–522.
Ruttner-Kolisko, A., 1977. Suggestions for biomass calculation of plankton rotifers. Archiv für Hydrobiologie, Beihefte Ergebnisse der Limnologie 8: 71–76.
Sakamoto, M., 1966. Primary production by phytoplankton community in some Japanese lakes and its dependence on lake depth. Archiv für Hydrobiologie 62: 1–28.
Santos, A. C. 2014. Efeitos das características do uso do solo da bacia de drenagem sobre a qualidade da água e biodiversidade de ecossistemas aquáticos. Master thesis. Universidade Federal do Rio Grande do Norte, Natal.
Sarmento, H., A. M. Amado, J. P. Descy, et al., 2013. Climate change in tropical freshwaters (comment on the paper ‘Plankton dynamics under different climatic conditions in space and time’ by de Senerpont Domis. Freshwater Biology 58: 2208–2210.
Sas, H., 1989. Lake Restoration by Reduction of nutRient Loading: Expectations, Experiences, Extrapolations. Academia Verlag Richarz, St. Augustin.
Scheffer, M., 1998. Ecology of Shallow Lakes. Kluwer Academic Publishers, Dordrecht.
Scheffer, M., S. Rinaldi, A. Gragnani, L. R. Mur & E. H. van Nes, 1997. On the dominance of filamentous cyanobacteria in shallow, turbid lakes. Ecology 78: 272–282.
Schindler, D. W., 1977. Evolution of phosphorus limitation in lakes. Science 195: 260–262.
Schindler, D. W., 2012. The dilemma of controlling cultural eutrophication of lakes. Proceedings of The Royal Society B 279: 4322–4333.
Schindler, D. W., R. E. Hecky, D. L. Findlay, M. P. Stainton, B. R. Parker, M. J. Paterson, K. G. Beaty, M. Lyng & S. E. M. Kasian, 2008. Eutrophication of lakes cannot be controlled by reducing nitrogen input: results of a 37-year whole-ecosystem experiment. Proceedings of the National Academy of Sciences 105: 11254–11258.
Scott, J. T. & M. McCarthy, 2010. Nitrogen fixation may not balance the nitrogen pool in lakes over timescales relevant to eutrophication management. Limnology and Oceanography 55: 1265–1270.
Shapiro, J., 1990. Current beliefs regarding dominance by blue-greens: the case for the importance of CO2 and pH. Verhandlungen of Internationale Vereinigung für Theoretische und Angewandte Limnologie 24: 38–54.
Smith, V. H., 1983. Low nitrogen to phosphorus ratios favor dominance by blue-green algae in lake phytoplankton. Science 221: 669–671.
Smith, V. H., 1998. Cultural eutrophication of inland, estuarine, and coastal waters. In Pace, M. L. & P. M. Groffman (eds), Successes, Limitations and Frontiers in Ecosystem Science. Springer-Verlag, New York: 7–49.
Smith, V. H. & D. W. Schindler, 2009. Eutrophication science: where do we go from here? Trends in Ecology & Evolution 24: 201–207.
Soares, M. C. S., M. I. A. Rocha, M. M. Marinho, S. M. F. O. Azevedo, C. W. C. Branco & V. L. M. Huszar, 2009. Changes in species composition during annual cyanobacterial dominance in a tropical reservoir: physical factors, nutrients and grazing effects. Aquatic Microbial Ecology 57: 137–149.
Soares, M. C. S., V. L. M. Huszar, M. N. Miranda, M. M. Mello, F. Roland & M. Lürling, 2013. Cyanobacterial dominance in Brazil: distribution and environmental preferences. Hydrobiologia 717: 1–12.
Stumm, W. & J. J. Morgan, 1996. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters. Wiley Interscience, Hoboken.
Teferi, M., S. A. J. Declerck, T. De Bie, P. Lemmens, A. Gebrekidan, T. Asmelash, T. Dejenie, K. Gebrehiwot, H. Bauer, J. A. Deckers, J. Snoeks & L. D. Meester, 2014. Strong effects of occasional drying on subsequent water clarity and cyanobacterial blooms in cool tropical reservoirs. Freshwater Biology 59: 870–884.
Thornton, J. A. & W. Rast, 1993. A test of hypothesis relating to the comparative limnology and assessment of eutrophication in semi-arid man-made lakes. In Straškraba, M., J. G. Tundisi & A. Duncan (eds), Comparative Reservoir Limnology and Water Quality Management. Kluwer Academic Publishers, Dordrecht: 1–24.
Uehlinger, V., 1964. Étude statistique des méthodes de dénobrement planctonique. Archives des Sciences 77: 121–123.
Utermöhl, H., 1958. Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. Mitteillungen der Internationalen Vereinigung für Theoretische und Angewandte Limnologie 9: 1–38.
Veraart, A. J., J. J. M. de Klein & M. Scheffer, 2011. Warming can boost denitrification disproportionately due to altered oxygen dynamics. PloS One 6(3): e18508. doi:10.1371/journal.pone.0018508.
Weiss, R. F., 1974. Carbon dioxide in water and seawater: the solubility of a non-ideal gas. Marine Chemistry 2: 203–215.
Wetzel, R. G. & G. E. Likens, 2000. LIMNOLOGICAL Analyses, Book 3. Springer-Verlag, New York.
Wilson, A. E., O. Sarnelle & A. R. Tillmanns, 2006. Effects of cyanobacterial toxicity and morphology on the population growth of freshwater zooplankton: meta-analyses of laboratory experiments. Limnology and Oceanography 51: 1915–1924.
Zar, J. H., 2010. Biostatistical Analysis, 5th ed. Prentice Hall, New Jersey.
Acknowledgments
The authors thank the staff of the Secretaria Estadual do Meio Ambiente e dos Recursos Hídricos do Rio Grande do Norte (SEMARH), especially Celso Veiga, Gláucia Costa, Maria Pereira, Selma Silva, Antônio Paiva, Josenilton Silva, and Fernando Silva for their logistical assistance during the sampling work and for providing data from the lakes. We thank the staff of the Empresa de Pesquisa Agropecuária do Rio Grande do Norte (EMPARN) and the Companhia de Águas e Esgotos do Rio Grande do Norte for providing climatological and sewage treatment data, respectively. We are especially grateful to Fabiana Araújo and Jamila Pereira for laboratory assistance, and to Fábio Roland, Natália Noyma, Luciana Vidal, Anderson Freitas, Lúcia Lobão and Guilherme Dias for the nutrient and bacteria analyses. We thank Janet Reid for English correction. We also thank Mariana Meerhoff and two anonymous reviewers for their suggestions that helped improve the quality of the manuscript. JB was partially supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/CT-HIDRO, doctoral grant 141682/2007-8) and the Coordenadoria de Aperfeiçoamento de Pessoal Superior (CAPES/WUR, PhD sandwich scholarship grant BEX2977/09-5). VLMH was partially supported by CNPq (Grant 307727/2009-2).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Mariana Meerhoff
Rights and permissions
About this article
Cite this article
Brasil, J., Attayde, J.L., Vasconcelos, F.R. et al. Drought-induced water-level reduction favors cyanobacteria blooms in tropical shallow lakes. Hydrobiologia 770, 145–164 (2016). https://doi.org/10.1007/s10750-015-2578-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2578-5