Abstract
Shallow lakes often alternate between two possible states: one clear with submerged macrophytes, and another one turbid, dominated by phytoplankton. A third type of shallow lakes, the inorganic turbid, result from high contents of suspended inorganic material, and is characterized by low phytoplankton biomass and macrophytes absence. In our survey, the structure and photosynthetic properties (based on 14C method) of phytoplankton were related to environmental conditions in these three types of lakes in the Pampa Plain. The underwater light climate was characterized. Clear-vegetated lakes were more transparent (K d 4.5–7.7 m−1), had high DOC concentrations (>45 mg l−1), low phytoplankton Chl a (1.6–2.7 μg l−1) dominated by nanoflagellates. Phytoplankton productivity and photosynthetic efficiency (α ~ 0.03 mgC mgChla −1 h−1 W−1 m2) were relatively low. Inorganic-turbid lakes showed highest K d values (59.8–61.4 m−1), lowest phytoplankton densities (dominated by Bacillariophyta), and Chl a ranged from 14.6 to 18.3 μg l−1. Phytoplankton-turbid lakes showed, in general, high K d (4.9–58.5 m−1) due to their high phytoplankton abundances. These lakes exhibited the highest Chl a values (14.2–125.7 μg l−1), and the highest productivities and efficiencies (maximum 0.56 mgC mgChla −1 h−1 W−1 m2). Autotrophic picoplankton abundance, dominated by ficocianine-rich picocyanobacteria, differed among the shallow lakes independently of their type (0.086 × 105–41.7 × 105 cells ml−1). This article provides a complete characterization of phytoplankton structure (all size fractions), and primary production of the three types of lakes from the Pampa Plain, one of the richest areas in shallow lakes from South America.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Shallow lakes often alternate between two possible states. One is characterized by more transparent waters, in which primary production is dominated by submersed rooted macrophytes, and the other is turbid, dominated by phytoplankton (Scheffer et al., 1993). Different mechanisms have been proposed to explain the low phytoplankton biomass in profusely vegetated lakes: reduction of nutrient availability, reduced resuspension, release of allelopathic substances by plants, shading, enhanced substrate for periphytic algae, and greater shelter availability for grazers (Scheffer, 1998).
On the other hand, another type of turbid shallow lakes, which are relatively common in tropical regions, are water bodies where the turbidity is the result of suspended inorganic material, deriving both from sediment resuspension and external loading (Roland & de Assis Esteves, 1998, and cites therein).
In the Pampa Plain some shallow lakes are dominated by submersed macrophytes, have a relatively low biomass of phytoplankton and occur in a clear state, but in general, water bodies exhibit high phytoplankton biomass and scarce macrophyte development, and are typically turbid. The third type of lake that can be encountered in this region corresponds to shallow lakes in which turbidity is mostly due to inorganic material, and in which both phytoplankton and macrophyte development is low. This type of lake does not fit in the categories proposed in the alternative equilibrium model described by Scheffer et al. (1993) and rather, prevails turbid during a long period of time.
According to Quirós et al. (2002) the three types of lakes that can be recognized in the Pampa Plain respond to the following factors: historically most of the shallow lakes in this region were clear-vegetated, but the increase in human activities (agriculture, fish introduction, livestock, and urbanization) lead to important changes in these lakes. Nowadays most of the shallow lakes located in the more impacted areas have evolved to phytoplankton-turbid mostly due to an increase in nutrients (Quirós et al., 2006). The inorganic turbid type encompasses lakes clearly limited in their productivity by light availability, and would be the result of the direct human impact on their drainage basin (Quirós et al., 2002). During the 20th century the shallow lakes of this region became more eutrophic due to human activities, mainly an increase in agricultural production. The eutrophication took place particularly in water bodies highly exposed to intense land use in their drainage basins (Quirós & Drago, 1999; Quirós et al., 2006; Renella & Quirós, 2006).
The first typology of shallow lakes of the Salado River Basin based on phytoplankton showed that the composition and biomass of this community could be strongly influenced by the degree of development of rooted macrophytes present in the lake (Izaguirre & Vinocur, 1994a). According to this former regional study, lakes densely colonized by submersed plants have the lowest phytoplankton densities and highest species diversity. Conversely, phytoplankton abundances are higher and algal blooms occur more frequently in lakes without rooted plants. A subsequent study (Izaguirre & Vinocur, 1994b) also showed that phytoplankton assemblages strongly differ among these lakes. In another survey, MacDonagh et al. (2000) reported the temporal fluctuations of the phytoplankton in one of the lakes of this basin, and suggested that the type of rooted plant that dominates the lake also influences the phytoplankton structure and dynamics.
Recently, Torremorell et al. (2007) analyzed the role of light limitation on the seasonal dynamics in three turbid Pampean shallow lakes. The results of this study showed that the low transparency of the lakes reduces the amount of photosynthetically available radiation (PAR) to levels that would limit primary production.
However, not only the development of macrophytes may influence the plankton structure in the lakes of the Pampa Plain, but also the water discharge. Due to their shallowness, these water bodies show a great variability in their residence time. For example, Gabellone et al. (2001) found that the degree of hydrological connection with the Salado River has a great effect on the limnological features and planktonic communities of an associated shallow lake. In a more recent study, Renella & Quirós (2006) have also demonstrated the role of hydrology, as one of the major factors that can regulate the plankton biomass of the lakes in this region.
In this study, we analyze the phytoplankton community structure of 10 shallow lakes from the Pampa Plain that correspond to the three above-mentioned types: clear-vegetated (with abundant submersed macrophytes), phytoplankton-turbid, and inorganic-turbid. We describe the biodiversity of the communities for each water body, as well as the contribution of each phytoplankton size fraction to the total phytoplankton density and biovolume. The pigment composition and primary production of phytoplankton are also assessed for each type of lake, and their relationships with the phytoplankton composition are discussed.
Study area
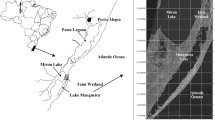
The studied lakes are located in the Pampa Plain (35°32′–36°48′S; 57°47′–58°07′W), in the Buenos Aires Province (Argentina), which is situated in the Warm Temperate Region (Fig. 1). Most of the selected shallow lakes (except Kakel Huincul) belong to the Salado River floodplain. According to Tricart (1973), the Pampa Plain lakes were formed by a combination of river and wind action. They have high levels of nutrients (Quirós & Drago, 1999), their ionic composition is dominated by sodium-bicarbonate (Fernández Cirelli & Miretzky, 2002), and are typically polymictic.
Location of the 10 studied lakes in the Pampa Plain (Buenos Aires—Argentina). The lakes are numbered in a North–South direction. The lake-types are clear-vegetated: Kakel Huincul (1), El Triunfo (2); inorganic-turbid: Yalca (3), La Limpia (4); phytoplankton-turbid: La Salada (5), El Burro (6), Chascomús (7), Vitel (8), Lacombe (9), San Jorge (10)
The climate of the region is temperate, with mean annual precipitation of about 935 mm, but with marked annual variability between wet and dry periods (Sierra et al., 1994). Mean annual temperature is about 15.3°C and winds have a mean annual speed of 10.1 km h−1 (Torremorell et al., 2007).
For this study, we selected 10 shallow lakes in this region. Two of them (Lake 1: Kakel Huincul; Lake 2: El Triunfo) are in clear-vegetated state, and are profusely colonized by submersed plants (mainly Myriophyllum sp. and Ceratophyllum demersum) and emergent macrophytes (Schoenoplectus californicus). Two other lakes (Lake 3: Yalca; Lake 4: La Limpia) are turbid and their turbidity is mainly due to suspended inorganic matter; nevertheless, it is important to point out that Yalca has two basins, one of them is located upstream and is vegetated. Six lakes are characterized by low Secchi depth values and high phytoplankton abundances (Lake 5: La Salada; Lake 6: El Burro; Lake 7: Chascomús; Lake 8: Vitel; Lake 9: Lacombe; Lake 10: San Jorge). All these lakes have a mean depth lower than 2 m. Most of the shallow lakes in this region are turbid with high algal biomass, and only a few are clear-vegetated or inorganic-turbid. Thus, we have selected more turbid organic systems in order to cover the variety of lakes, which can be dominated by different algal groups.
Among the studied lakes, three (from north to south: Vitel, Chascomús, El Burro) belong to a chain-system of seven lakes termed “lagunas encadenadas,” which are interconnected by streams and drain into the Salado River, thus their hydrological regime is influenced by the river discharge. As it was previously indicated, these three shallow lakes are phytoplankton-turbid.
At the time of sampling, the region was entering a drought period and therefore the horizontal water flow was minimal.
Materials and methods
The shallow lakes were sampled during the same week in November 2005. On the base of previous information obtained in lakes of this region (Izaguirre & Vinocur, 1994a; Torremorell et al., 2007) for this comparative study we took samples in late spring time, during the period of active algal and macrophyte growth, at three sampling sites in the pelagial zone of each water body.
Different physical and chemical parameters were measured in situ: temperature, pH, conductivity, and salinity were measured with Hanna HI 8314 and HI 8033 portable electronic sensors (HANNA, USA). In addition, vertical profiles of downward irradiance (from 350 to 750 nm) were obtained with a spectroradiometer (USB2000, Ocean Optics) attached to a fiber optic probe. Profiles were performed at noon, inside a large, black container filled with freshly collected lake water. This procedure was adopted to increase the accuracy of the measurements by eliminating the noise due to wave action observed in in situ profiles. Diffuse vertical attenuation coefficients for PAR, K d PAR, were calculated by regressing log-transformed irradiance measurements versus depth. Additionally, a measure of relative water transparency was recorded using a Secchi disk (Sd).
Water samples were collected directly from about 30 cm below the surface for chemical determination of nutrients. Nutrients were assayed as follows: total phosphorous (TP) concentrations were determined, after an acid digestion, by the molybdate-ascorbic method; total nitrogen (TN) was estimated as the sum of nitrates, nitrites, ammonia, and organic nitrogen; nitrate and nitrite were determined using a copperized cadmium reduction column followed by diazotation; ammonia was determined by the indophenol blue method; organic nitrogen was measured by means of the Kjeldahl method. In addition, the following parameters were also estimated: dissolved oxygen (Winkler method); total suspended solids (seston) were determined by weighing the residue resulting from the filtration of sample through pre-burned Whatman™ GF/F filters, and the percentage of organic matter in seston was estimated as the difference between dry weight and ash-free dry weight (550°C for 2 h). All the determinations were performed as follows (APHA, 1998). Aliquots of filtered water were stored at 4°C until analysis of dissolved organic carbon (DOC). DOC was measured by a high temperature Pt catalyst oxidation method (Shimadzu TOC-5000) following the recommendations of Sharp et al. (1993).
In order to assess which of these physical and chemical variables explain the variability among lakes a Principal Component Analyses was performed based on the correlation matrix of these variables.
Chlorophyll a concentration was estimated from triplicate samples (110–250 ml) collected onto glass-fiber filters (GF/F, Whatman). Filters were wrapped immediately in aluminum foil and stored at −80°C until processing (within 2 months of sampling). Chlorophyll was extracted using 90% aqueous acetone (V:V), at 10°C, in darkness and in a nitrogen saturated atmosphere. The extracts obtained were cleared by centrifugation at 3,000 rpm for 10 min. Pigment extracts were measured by ion pairing reverse-phase HPLC (modified from Mantoura & Llewellyn, 1983; Hurley, 1988) using a Äktabasic chromatograph (Amersham ®) controlled by the program Unicorn ® (C18 phenomenex ®; 5 μm particle size; 250 × 4.6 mm i.d.). The method applied has been described in detail by Laurion et al. (2002). The HPLC system was calibrated with a commercial primary standard (Sigma, Switzerland).
Two sets of quantitative phytoplankton samples were obtained at each of three stations in every lake. The samples for micro- and nanoplanktonic fractions were fixed with 1% acidified Lugol’s iodine solution, and those for the quantification of picophytoplankton were preserved with 2% ice-cold glutaraldehyde. The counts of micro- and nanoplanktonic fractions were performed using an inverted microscope following the Utermöhl (1958) technique, at 400× magnification. The picophytoplankton fraction was counted from the fluorescence given off by photosynthetic pigments. Samples were filtered through 0.2 μm black polycarbonate filters Isopore® GTPB 02500, which were then mounted on a microscope slide with a drop of immersion oil for fluorescence (Immersol Zeiss® 518 F). Each filter was examined for pigment autofluorescence with a Zeiss® Axioplan microscope equipped with an HBO 50 W lamp, a filter set for blue light excitation (BP 450–490 nm, FT 510 nm, LP 520 nm) for the identification of eukaryotic picoplankton, and green light excitation (BP 546 nm, FT 580 nm, LP 590 nm) for picocyanobacteria (Kemp et al., 1993). A minimum of 400 cells were counted (corresponding to at least 20 fields of view) at 1,000× magnification.
Photosynthetic rates were measured as a function of irradiance by the 14C-technique (Steeman Nielsen, 1952). Samples were incubated, in 45 ml quartz tubes placed at the surface of an outdoor water bath. The tubes were incubated for 3 h at nine irradiances. These nine irradiance levels were obtained by covering the tubes with different layers of neutral density filters. In addition, one tube was wrapped in aluminum foil and served as a dark control. One μCi of 14C labeled NaHCO3 was added to each tube. After incubation, samples were filtered through GF/F Whatman filters, placed in an HCl saturated atmosphere and dried overnight. The activity of filters was measured in a scintillation counter after adding 2.5 ml of OptiPhase ‘HiSafe’3 scintillation solution.
The P–I parameters were estimated with the equations of Platt et al. (1980)
where P, photosynthetic rate; P max, maximum photosynthesis in absence of photoinhibition under optimal light; α, the light-limited slope; β, the photoinhibition constant, i.e., the negative slope of the decline in photosynthesis which may occur at high light intensities; and I, irradiance (Platt et al., 1980). The projection of the intersection of the initial slope, α, with the plateau, P max, onto the abscissa defines the photoadaptation parameter, i.e., I k = P max/α. I k is useful as an index of photoadaptive state of phytoplankton communities.
The mean irradiance in the water column was calculated as follows (Ferrero et al., 2006)
where I 0 is the average irradiance at ground level with 1 h of local noon, i.e., between 12 and 14 Argentine official time, K d is the downdwelling diffuse attenuation coefficient for PAR, and z is the depth at the sampling site. I mean provides a reference for comparing I k values, i.e., phytoplankton primary production in lakes for which I mean < I k can be safely assumed to be light limited.
Results
Physical and chemical properties
Mean values of physical and chemical variables are shown in Table 1. The strong differences in the transparency of the lakes are evidenced in Secchi depth values, which ranged from 0.07 m to more than 1.02 m (down to upper limit of the submersed macrophyte bed) in Yalca and Kakel Huincul, respectively.
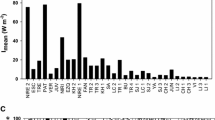
Mean diffuse vertical attenuation coefficients of the photosynthetically active radiation (PAR) varied between 3.4 and 47.0 m−1 for Kakel Huincul and Yalca, respectively. The contrasting K d values indicated strong differences in the euphotic zones of the shallow lakes, which varied between 0.10 and 0.15 m (Yalca, Chascomús, Vitel, and Limpia), and of about 0.7–1 m (Kakel Huincul, La Salada, and El Triunfo). In all surveyed Pampean lakes, maximum attenuation values were observed toward the blue and bluish green fractions (400–500 nm) of light spectrum.
Water temperature, pH, alkalinity, and conductivity varied in the range typical for the region. Values of DOC also differed among the lakes (Fig. 2). Highest concentrations were found in the shallow lakes with higher submersed macrophytes development (mean values between 45.9 and 82.4 mg l−1), and in those that experienced cyanobacteria blooms (mean values between 36.3 and 53.8 mg l−1). Yalca, which has two different basins, one of them vegetated, also had high DOC values.
Total phosphorous (TP) ranged from 0.14 to 0.81 mg l−1; most of the values were relatively high. Concentrations of dissolved inorganic nitrogen (DIN) were also relatively high (except in Lake El Triunfo), with mean values varying between 58 and 1,067 μg l−1. Total nitrogen (TN) ranged between 229 and 2,022 μg l−1, and the lowest concentrations occurred in lakes with high submersed macrophyte biomass (Table 1).
The first two factors of the PCA based on physical and chemical data account for 77.1% of the total variance. The ordination of the shallow lakes is shown in Fig. 3. The first component has a high positive correlation with seston and K d, and a high negative correlation with the percentage of organic matter in seston, conductivity, pH, and development of submerged macrophytes. The second axis is directly correlated with TP and inversely with DOC and TN. In this PCA, the more turbid lakes, both inorganic as well as some of the phytoplankton-turbid (Yalca, La Limpia, Vitel, Chascomús) are ordinated toward the right side of the figure, whereas the clear (El Triunfo and Kakel Huincul) and two organic water bodies (Lacombe and La Salada) are placed at the left side. The remaining two phytoplankton-turbid shallow lakes (El Burro and San Jorge) are ordinated at intermediate positions. The separation of the lakes in relation to the second axis reflects their differences in nutrient contents and concentration of DOC.
Phytoplankton (size fraction > 2 μm)
A total of 174 phytoplankton taxa were identified in 10 lakes. Marked differences in phytoplankton structure were found among lakes.
The relative contribution of the different algal classes to total phytoplankton density and biovolume is shown in Fig. 4a, b.
Nanoplanktonic species dominated in Kakel Huincul and El Triunfo, and were mainly represented by the cryptophyceans Plagioselmis nanoplanctica (Skuja) Novar., Lucas & Morr. and Cryptomonas marssonii Skuja, and different species of chrysophyceans; the accompanying species in these two lakes were some chlorophyceans such as Monoraphidium contortum (Thur.) Kom.-Legn., M. circinale (Nyg.) Nyg., Chlamydomonas spp., Chlorella spp., and some diatoms like Fragilaria sp. and Cocconeis placentula Ehr.
Turbid inorganic lakes (Yalca and La Limpia) were dominated by diatoms; however, in Yalca the community was characterized by several diatom taxa with similar densities, while in La Limpia Navicula viridula (Kütz.) Ehr. dominated; in the latter lake Closterium aciculare West was subdominant in terms of density and co-dominant in terms of biomass.
The six shallow lakes with turbidity deriving from high abundances of phytoplankton, differed in their algal compositions. La Salada, El Burro, and Lacombe were clearly dominated by Chlorophyta (Oocystis lacustris Chod., Cosmarium leave Rabenh., and Oocystis nephrocystoides Fott & Cado, respectively), followed by colonial Cyanobacteria (Aphanocapsa delicatissima West & West). Chascomús and Vitel were very similar in phytoplankton composition, mainly represented by diatoms and chlorophycean species. Synedra berolinensis Lemm. was dominant in both lakes, and different species of Monoraphidium and Scenedesmus were also important. Aggregated forms, mainly species of Aphanocapsa and Merismopedia, represented cyanophyceans of Chascomús and Vitel. San Jorge was exclusively dominated by the filamentous cyanophycean Raphidiopsis mediterranea Skuja.
The densities and biovolumes of the >2 μm phytoplankton also strongly differed among lakes (Figs. 5a, 6a, respectively). Clear water bodies with macrophytes, and inorganic turbid shallow lakes presented low figures, with the lowest density (869 ind ml−1) and biovolume (1.97 × 106 μm3 ml−1) in Yalca. Among the phytoplankton-turbid lakes differences were also evident. The highest densities were found in lakes with Cyanobacteria blooms (1.13 × 105–2.23 × 105 ind ml−1). The biovolume registered in Vitel was the lowest one within the group of phytoplankton-turbid water bodies (4.62 × 107 μm3 ml−1), whereas Lacombe (1.62 × 109 μm3 ml−1) clearly exceeded the figures registered for all studied lakes.
Table 2 shows the pigment composition of the phytoplankton for each lake. Chl a concentrations displayed an among-lake variation of about 77-fold. The two clear-vegetated lakes presented the lowest Chl a concentrations ranging from 1.6 to 2.7 μg l−1, the inorganic-turbid lakes showed a range of 14.6 and 18.3 μg l−1, and phytoplankton-turbid ones exhibited the wider range, 14.2–125.7 μg l−1, and a mean value of 68.75 μg l−1. Accessory pigments showed among-lake differences in their concentrations. The pigment composition reflected the dominant algal classes in each shallow lake. The relative high proportion of fucoxanthin in El Triunfo is related with the high chrysophycean abundance in this lake, whereas in Chascomús and Vitel lakes it is associated with diatoms. The high abundance of chlorophyceans in all phytoplankton-turbid lakes (except San Jorge), accounts for the high concentrations of chlorophyll b and lutein + zeaxanthin. On the other hand, the very high values of carotenoid (16.54) in San Jorge lake are related to the filamentous Cyanobacteria bloom.
Phytoplankton (size fraction < 2 μm)
The two extremes of the density and biovolume range were registered within the group of phytoplankton-turbid lakes, with the lowest figures in those dominated by >20 μm Cyanobacteria (8.61 × 103 ind ml−1 and 1.36 × 104 ind ml−1, for Lacombe and San Jorge, respectively), and the highest abundances in Chascomús (2.31 × 106 ind ml−1), and Vitel (4.61 × 106 ind ml−1) (Figs. 5b, 6b).
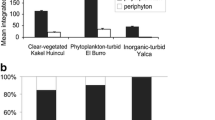
The small phytoplankton was almost exclusively represented by red-fluorescing phycocyanin-rich picocyanobacteria in all water bodies. The picoeukaryotic algae contribution to total <2 μm phytoplankton density was very low in all shallow lakes, and it only exceeded 10% in inorganic-turbid La Limpia and in phytoplankton-turbid La Salada and Lacombe (Fig. 7). Table 3 summarizes the main characteristics of the phytoplankton structure and composition for each type of shallow lakes.
Primary production and light limitation
The lowest P max values corresponded to the lakes where the phytoplankton production was less light limited, i.e., El Triunfo, Kakel Huincul, Lacombe, and La Salada. In addition, in these lakes we observed the lowest photoinhibition constants (β). The most turbid lakes were the most productive in terms of P max (Table 4). Figure 8 shows the values of I mean and I k. According to this figure, the phytoplankton production of lakes San Jorge, Burro, Chascomús, Yalca, La Limpia, and Vitel was light limited.
Discussion
Lakes from the Pampa Plain are naturally eutrophic or hypertrophic, are highly exposed to human uses of land and water resources, and are impacted by agricultural activities (Quirós & Drago, 1999). The low nitrogen values registered in the water column of the two most vegetated lakes (El Triunfo and Kakel Huincul) are probably related to a high uptake by macrophytes, as it was previously reported for Argentinean wetlands (Villar et al., 1998; Unrein, 2001).
Values of DOC observed in the shallow lakes from the Pampa Plain are within the range reported for eutrophic and dystrophic water bodies (Kalff, 2002). The highest concentrations were found both in clear water bodies with macrophytes beds, and in turbid shallow lakes with algal blooms, but the main source of DOC differs between these groups of lakes. In particular, the high DOC concentrations found in inorganic-turbid Yalca might derive from the upstream-vegetated basin.
In the studied shallow lakes, K d (PAR) showed strong among-lake variations (14-fold), which translated into estimated euphotic depths from a few centimeters to the entire water column. Although spectral differences were also observed, the photons within the blue and bluish green wavelengths were always the first to be depleted from the water column (Pérez et al., in press).
The results of this study showed marked differences in the phytoplankton structure among clear-vegetated, inorganic-turbid and phytoplankton-turbid water bodies. Whereas nanoplanktonic algae, mainly flagellates were very abundant in the clearest vegetated shallow lakes, diatoms, chlorococcaleans, and some bloom-forming Cyanobacteria were dominant in the most turbid ones. The prevalence of small and often flagellate cells and colonial Cyanobacteria in clear shallow lakes with submersed macrophytes can be partially explained by their lower sedimentation rates as compared to large nonbuoyant cells (Søndergaard & Moss, 1998). One of the factors that may negatively affect phytoplankton community in these lakes is the increase of sedimentation and reduced resuspension due to the less turbulent and more quiescent waters resulting from the presence of macrophytes. Among the 10 surveyed shallow lakes Cryptophyceae showed the highest relative importance in terms of density in the two clear-vegetated water bodies. Cryptophyceae have many adaptations that allow them to succeed in humic lakes (Lepistö & Holopainen, 2003). Cryptophyceae tolerate occasional nutrient limitation, especially nitrogen. These flagellates have the ability to consume bacteria (Tranvik et al., 1989; Urabe et al., 2000; Sinistro et al., 2007; Unrein et al., 2007), and thus they are able to supplement their nutrient requirements by mixotrophy (Jones, 2000). Moreover, these algae can migrate vertically and stay at optimal light and nutrient conditions in the water column.
Another group of nanoflagellates that highly contributed to the total phytoplankton abundance in one of the clear vegetated shallow lakes (El Triunfo) was Chrysophyceae. The presence and abundance of this algal group in mesohumic waters was documented by Eloranta (1995). Regarding their ecological requirements, different factors may regulate the occurrence of chrysophytes in lakes (pH, phosphate, and dissolved organic matter), which were measured for many ecosystems (Kristiansen, 2005). Many chrysophytes have also the capability to be mixotrophic, and in particular, this behavior was well documented for Ochromonas spp. (Jones, 2000; Katechakis & Stibor, 2006).
The dominance of Cyanobacteria (like Aphanocapsa delicatissima, Microcystis spp., and the bloom-forming Raphidiopsis mediterranea), in phytoplankton-turbid lakes is related to the high nutrient availability deriving from the intense human activities in their catchments. On the other hand, the prevalence of diatoms in both types of turbid shallow lakes (e.g., Synedra berolinensis, Aulacoseira granulata, and Navicula viridula) is favored by their relatively high turbulence. It is important to point out that some of the encountered species are included in the functional groups well adapted to turbid waters and that can tolerate light deficiency according to Reynolds et al. (2002). Some diatom species registered in the plankton samples (such as several species of the genus Cocconeis, Cymbella, Epithemia, Gomphonema, Surirella, etc.) are meroplanktonic taxa, which frequently resuspend from the benthic community in these shallow-turbulent lakes.
Turbidity due to inorganic suspended matter or due to high phytoplankton biomass, particularly Cyanobacteria, shade the water column and this factor might negatively affect the growth of nonmotile green-algae (Happey-Wood, 1988). Thus, the relative low numbers of chlorococcalean species in the inorganic-turbid lakes, Yalca and La Limpia, and in cyanobacteria-dominated San Jorge can be, in part, explained by the fact that the light requirement of these algae are higher.
In a multivariate study of the clear and turbid water bodies of the Pampa Plain Quirós et al. (2002) found that the shallow lakes subjected to an intensive land use in their catchments were very turbid and were dominated by Cyanobacteria. Other studies on phytoplankton structure from shallow lakes of the Salado River Basin also revealed that this algal group was favored in water bodies with anthropogenic impact (Izaguirre & Vinocur, 1994a; Gabellone et al., 2001). Eutrophic and hypertrophic lakes are susceptible to bloom formation. In particular, enriched agricultural environments are among the most notorious sites for the growth and proliferation of nuisance cyanobacteria (Paerl, 1988).
In addition, to eutrophication derived from agriculture, many Pampean lakes were subject to human manipulations, such as fish introduction (Baigún & Quirós, 1985). Planktivorous fish have a remarkable cascade effect on the phytoplankton biomass by the reduction of zooplankton (Carpenter et al., 1985). Sosnovsky (2007) has experimentally demonstrated that the planktivorous fish introduction in Pampean shallow lakes increased algal biomass.
The lack of macrophytes in much enriched shallow lakes favors the development of Cyanobacteria blooms. These algae, in turn, are less prone to grazing which results in a positive feedback that favors their establishment and dominance. Particularly, filamentous and colonial forms of Cyanobacteria have evolved as structural adaptations that minimize zooplankton grazing (Paerl, 1988).
On the other hand, Cyanobacteria are able to grow at low light intensities and to harvest certain specific light wavelengths; as a result of this, they possess a competitive advantage in lakes which are turbid due to dense growth of other phytoplankton and present higher growth rates than other species (Chorus & Bartram, 1999).
Diatoms dominated in the two inorganic-turbid shallow lakes (Yalca and La Limpia), and their contribution was also important in Chascomús and Vitel. The absence of rooted macrophytes guarantees that these shallow lakes are continuously mixed. The success of diatoms in inorganic-turbid shallow lakes was also reported by other authors (Padisák & Dokulil, 1994; Cardoso et al., 2003). Diatoms depend on turbulent vertical mixing to counteract sedimentation losses. The ecological adaptations of diatoms, which according to Reynolds (1988) are included in the category of ruderal species (R-strategy), allow them to succeed in constantly turbid mixed lakes. In Chascomús and Vitel one of the dominant species is Synedra berolinensis, which can form star-like aggregates. In such turbid environments a morphological pre-adaptation to provide a good light antenna is one which places the photosynthetic pigment in the maximal area of cross section of the light field (Reynolds, 1994, 2006). Cells which are flattened in one plane or in two (like those of Synedra) project a variable area in a given plane, being maximum when the two longest axes are perpendicular to a unidirectional photon source (Reynolds, 1997). On the other hand, Padisák et al. (2003) experimentally demonstrated that star-like aggregates have a considerable form resistance factor, sharply increasing from 1 to 6 cells, which enhance the buoyancy. Thus, a star-like morphology would be and advantage in turbid water columns, both by maximizing the light harvesting, and by diminishing the sinking velocity.
The results of the multivariate analyses (PCA) confirmed the similarities and differences among lakes previously described. The two clear-vegetated lakes, even when they belong to different basins, share similar physical and chemical features. The two inorganic-turbid shallow lakes ordinate similarly in the PCA in relation to K d and seston (first component). Nevertheless, their separation in relation to the second axis can be explained by their differences in total phosphorus and DOC; whereas La Limpia shows the highest TP concentration due to its location within an area with intense land-use, Yalca has higher values of DOC deriving from its vegetated basin located upstream. On the other hand, most of the phytoplankton-turbid shallow lakes ordinated similarly in relation to the first axis, due to their high K d and seston values resulting from the high algal biomass characteristic of this stable state.
This survey also showed that pico-algae were present in all 10 lakes at all sampling sites. However, clear differences in their relative abundances were found among the water bodies, regardless of the presence or absence of macrophytes. Even though all the lakes are eutrophic or hypertrophic, the picophytoplankton abundances did not always fit within the range reported for enriched water bodies (Sorokin, 1999). It is accepted that the relative contribution of picophytoplankton to total phytoplankton decreases as a function of increasing productivity (Søndergaard, 1991). However, picophytoplankton abundance and biomass typically increase with trophic state, but this trend does not continue in hypertrophic lakes that can present either too high or too low values (Sommaruga & Robarts, 1997). Vörös et al. (1998) surveyed 32 lakes covering a range of chlorophyll a concentrations from 0.2 to 390 μg l−1 and found that picophytoplankton abundance in hypertrophic lakes was either very high or as low as the ones measured in oligotrophic lakes. The same trend was observed in the 10 shallow lakes of our survey.
Additional abiotic factors, other than nutrients, were suggested as having a potential effect on the abundance of picoalgae. Among them, water temperature and light availability have been repeatedly reported (Agawin et al., 2000; Malinsky-Rushansky et al., 2002; Jasser & Arvola, 2003). Nevertheless, very different picoalgal cell numbers were observed in lakes which share a similar nutrient content, water temperature, and light climate (Chascomús and San Jorge). Biotic interactions might be responsible for these differences, which should be further explored in these shallow lakes. The lowest values occurred in systems with the highest abundances and biovolumes of >2 μm phytoplankton (San Jorge and Lacombe); these two shallow lakes were dominated by either filamentous Cyanobacteria or a shared dominance of chlorococcaleans and colonial Cyanobacteria, respectively. Callieri & Stockner (2002) suggested that the abundance of filamentous Cyanobacteria might be a main factor influencing picocyanobacterial numbers.
The influence of underwater light quality on the selection of picocyanobacteria types, yellow-autofluorescing phycoerithrin (PE cells) and red-autofluorescing phycocyanin (PC cells), has been studied in many lakes along a broad trophic gradient (Stockner et al., 2000, and cites therein). A general pattern is that PE cells more efficiently use blue and green light, whereas PC cells have and advantage where red light is prevalent (Sommaruga & Robarts, 1997; Stockner et al., 2000). In eutrophic lakes with high chlorophyll concentrations, red light prevails over blue and bluish green light. Our results agree with other studies showing that in productive or in humic waters, red-fluorescing cells are the dominant picophytoplankters (Søndergaard, 1991; Jasser & Arvola, 2003).
In this article, we have described the similarities and differences among clear-vegetated, inorganic-turbid and phytoplankton-turbid shallow lakes of the Pampa Plain. These three types of shallow lakes markedly differ in their optical features, which in turns affect their phytoplankton assemblages. Clear-vegetated and inorganic-turbid lakes both show characteristic physico-chemical conditions and phytoplankton structures. Phytoplankton-turbid lakes share some of their physical and chemical properties but they are more heterogeneous in phytoplankton composition; interestingly, the turbidity in these eutrophic shallow lakes is caused by different algal classes.
References
Agawin, N. S. R., C. M. Duarte & S. Agusti, 2000. Nutrient and temperature control of the contribution of picoplankton to phytoplankton biomass and production. Limnology and Oceanography 45: 591–600.
APHA (American Public Health Association), 1998. Standard Methods for the Examination of Water and Wastewater, 20th ed. APHA, Washington DC: 1268 pp.
Baigún, C. & R. Quirós, 1985. Introducción de peces exóticos en la República Argentina. Informe Técnico No 2. Departamento de Aguas Continentales (INIDEP), Mar del Plata, Argentina: 1–90.
Callieri, C. & J. G. Stockner, 2002. Freshwater autotrophic picoplankton: A review. Journal of Limnology 61: 1–14.
Cardoso, L. & D. da Motta Marques, 2003. Rate of change of the phytoplankton community in Itapeva Lake (North Coast of Rio Grande do Sul, Brazil), based on the wind driven hydrodynamic regime. Hydrobiologia 497: 1–12.
Carpenter, S. R., J. F. Kitchell & J. R. Hodgson, 1985. Cascading trophic interactions and lake productivity: Fish predation and herbivory can regulate lake ecosystems. Bioscience 35: 634–639.
Chorus, I. & J. Bartram, 1999. Toxic Cyanobacteria in Water. E & F.N. Spon, London.
Eloranta, P., 1995. Phytoplankton of the national park lakes in central and southern Finland. Annales Botanici Fennici 32: 193–203.
Fernández Cirelli, A. & P. Miretzky, 2002. Lagos poco profundos de la Pampa Argentina. Relación con aguas subterráneas someras. In Fernández Cirelli, A. & G. Chalar Marquisá (eds), El agua en Iberoamérica. De la limnología a la gestión en Sudamérica. CYTED XVII, CETA—Centro de estudios Transdisciplinarios del Agua, Facultad de Ciencias Veterinarias, Buenos Aires: 43–52.
Ferrero, E., M. Eöry, G. Ferreyra, I. Schloss, H. Zagarese, M. Vernet & F. Momo, 2006. Vertical mixing and ecological effects of ultraviolet radiation in planktonic communities. Photochemistry and Photobiology 82: 898–902.
Gabellone, N., L. C. Solari & M. C. Claps, 2001. Planktonic and physico-chemical dynamics of markedly fluctuating backwater pond associated with a lowland river (Salado River, Buenos Aires, Argentina). Lakes and Reservoirs: Research and Management 6: 133–142.
Happey-Wood, C. M., 1988. Ecology of freshwater planktonic green algae. In Sandgren, C. D. (ed.), Growth and Reproductive Strategies of Freshwater Phytoplankton. Cambridge University Press, Cambridge: 175–226.
Hurley, J. P., 1988. Analysis of aquatic pigments by high performance liquid chromatography. Journal of Analytical Purification 3: 12–16.
Izaguirre, I. & A. Vinocur, 1994a. Typology of shallow lakes of the Salado River Basin (Argentina), based on phytoplankton communities. Hydrobiologia 277: 49–62.
Izaguirre, I. & A. Vinocur, 1994b. Algal assemblages from shallow lakes of the Salado River Basin (Argentina). Hydrobiologia 289: 57–64.
Jasser, I. & L. Arvola, 2003. Potential effects of abiotic factors on the abundance of autotrophic picoplankton in four boreal lakes. Journal of Plankton Research 25: 873–883.
Jones, R. I., 2000. Mixotrophy in planktonic protists: An overview. Freshwater Biology 45: 219–226.
Kalff, J., 2002. Limnology. Prentice Hall, New Jersey, USA.
Katechakis, A. & H. Stibor, 2006. The mixotroph Ochromonas tuberculata may invade and suppress specialist phago- and photoautotroph plankton communities depending on nutrient conditions. Oecologia 148: 692–701.
Kemp, P. F., B. F. Sherr, E. B. Sherr & J. J. Cole, 1993. Handbook of Methods in Aquatic Microbial Ecology. Lewis Publishers, Boca Raton, FL.
Kristiansen, J., 2005. Golden Algae. A Biology of Chrysophytes. A.R.G. Gantner Verlag Kommanditgesellschaft, Ruggell, Liechtenstein.
Laurion, I., A. Lami & R. Sommaruga, 2002. Distribution of mycosporine-like aminoacids and photoprotective caraotenoids among freshwater phytoplankton assemblages. Aquatic Microbial Ecology 26: 283–294.
Lepistö, L. & A.-L. Holopainen, 2003. Occurrence of Cryptophyceae and katablepharids in boreal lakes. Hydrobiologia 502: 307–314.
MacDonagh, M., G. Ruiz, L. C. Solari & N. Gabellone, 2000. Fitoplancton de una laguna de moderada eutrofia en la provincia de Buenos Aires. Diversidad y Ambiente 1: 37–43.
Malinsky-Rushansky, N., T. Berman, T. Berner, Y. Yacobi & Z. Dubinsky, 2002. Physiological characteristics of picophytoplankton, isolated from Lake Kinneret: Responses to light and temperature. Journal of Plankton Research 24: 1173–1183.
Mantoura, R. F. C. & C. A. Llewellyn, 1983. The rapid determination of algal chlorophyll and carotenoid pigments and their breakdown products in natural waters by reverse-phase high-performance liquid chromatography. Analytica Chimica Acta 151: 297–314.
Padisák, J. & M. Dokulil, 1994. Meroplankton dynamics in a saline, turbulent shallow lake (Neusiedlersee, Austria and Hungary). Hydrobiologia 289: 23–42.
Padisák, J., E. Soróczki-Pintér & Z. Rezner, 2003. Sinking properties of some phytoplankton shapes and the relation of form resistance to morphological diversity plankton—An experimental study. Hydrobiologia 500: 243–257.
Paerl, H. W., 1988. Growth and reproductive strategies of freshwater blue-green algae. In Sandgren, C. D. (ed.), Growth and Reproductive Strategies of Freshwater Phytoplankton. Cambridge University Press, Cambridge: 261–315.
Pérez, G. L., A. Torremorell, J. Bustingorry, R. Escaray, A. P. Pérez, M. Diéguez & H. E. Zagarese, in press. Optical characteristics of shallow lakes from the Pampa and Patagonia regions of Argentina. Limnologica.
Platt, T. C., L. Gallegos & W. G. Harrison, 1980. Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. Journal of Marine Research 38: 687–701.
Quirós, R. & E. Drago, 1999. The environmental state of Argentinean lakes: An overview. Lakes and reservoirs: Research and Management 4: 55–64.
Quirós, R., A. M. Renella, M. B. Boveri, J. J. Rosso & A. Sosnovsky, 2002. Factores que afectan la estructura y el funcionamiento de las lagunas pampeanas. Ecología Austral 12: 175–185.
Quirós, R., M. B. Boveri, C. A. Petrachi, A. M. Renella, J. J. Rosso, A. Sosnovsky & H. T. von Bernard, 2006. Los efectos de la agriculturización del humedal pampeano sobre la eutrofización de sus lagunas. In Tundizi, J. G., T. Matsumura-Tundisi & C. Sidagis Galli (eds), Eutrofização na América do Sul: Causas, conseqüèncias e tecnologias de gerenciamento e controle: 1–16.
Renella, A. M. & R. Quirós, 2006. The effects of hydrology on plankton biomass in shallow lakes of the Pampa Plain. Hydrobiologia 556: 181–191.
Reynolds, C. S., 1988. Functional Morphology and the Adaptive Strategies of Freshwater Phytoplankton. Cambridge University Press, Cambridge.
Reynolds, C. S., 1994. The long, the short and the stalled: On the attributes of phytoplankton selected by physical mixing in lakes and rivers. Hydrobiologia 189: 9–21.
Reynolds, C. S., 1997. Vegetation Processes in the Pelagic: A Model for Ecosystem Theory. Ecology Institute, Oldendorf/Luhe.
Reynolds, C. S., 2006. Ecology of Phytoplankton. Cambridge University Press, Cambridge, UK.
Reynolds, C. S., V. L. M. Huszar, C. Kruk, L. Naselli-Flores & S. Melo, 2002. Towards a functional classification of the freshwater phytoplankton. Journal of Plankton Research 24: 417–428.
Roland, F. & F. de Assis Esteves, 1998. Effects of bauxite tailing on PAR attenuation in an Amazonian crystalline water lake. Hydrobiologia 377: 1–17.
Scheffer, M., 1998. Ecology of Shallow Lakes. Chapman & Hall, London.
Scheffer, M., S. H. Hosper, M. L. Meijer, B. Moss & E. Jeppesen, 1993. Alternative equilibria in shallow lakes. Trends in Ecology and Evolution 8: 275–279.
Sharp, J. H., E. T. Peltzer, M. J. Alperin, G. Cauwet, J. W. Farrington, B. Fry, D. M. Karl, J. H. Martin, A. Spitzy, S. Tugrul & C. A. Carlson, 1993. Procedures subgroup report. Marine Chemistry 41: 37–49.
Sierra, E. M., M. E. Fernández Long & C. Bustos, 1994. Cronología de inundaciones y sequías en el noreste de la provincia de Buenos Aires 1911-89. Revista de la Facultad de Agronomía 14: 241–249.
Sinistro, R., I. Izaguirre & V. Asikian, 2007. Experimental study on the microbial plankton community in a South American wetland (Lower Paraná River Basin), and the effect of the light deficiency due to floating macrophytes. Journal of Plankton Research 28: 753–768.
Sommaruga, M. & R. D. Robarts, 1997. The significance of autotrophic and heterotrophic picoplankton in hypereutrophic ecosystems. FEMS Microbiology Ecology 24: 187–200.
Søndergaard, M., 1991. Phototrophic picoplankton in temperate lakes: Seasonal abundance and importance along a trophic gradient. Internationale Revue der Gesamten Hydrobiologie 76: 505–522.
Søndergaard, M. & B. Moss, 1998. Impact of submerged macrophytes on phytoplankton in shallow freshwater lakes. Ecological Studies 131: 115–132.
Sorokin, Y. I., 1999. Aquatic Microbial Ecology. Backhuys Publishers, Leiden.
Sosnovsky, A., 2007. Factores que determinan la estructura del zooplancton en pequeños cuerpos de agua de la Región Pampeana. Ph.D. Dissertation, Universidad de Buenos Aires, Buenos Aires, Argentina.
Steeman Nielsen, E., 1952. The use of radiocarbon (14C) for measuring organic production in the sea. Journal du Conseil International pour l’Exploration de la Mer 18: 117–140.
Stockner, J. G., C. Callieri & G. Cronberg, 2000. Picoplankton and other non-bloom-forming Cyanobacteria in lakes. In Whitton, B. A. & M. Potts (eds), The Ecology of Cyanobacteria. Kluwer Academic Publishers, Dordrecht: 195–231.
Torremorell, A., J. Bustingorry, R. Escaray & H. Zagarese, 2007. Seasonal dynamics of a large, shallow lake, laguna Chascomús: The role of light limitation and other physical variables. Limnologica 37: 100–108.
Tranvik, L. J., K. G. Porter & J. M. Sieburth, 1989. Occurrence of bacterivory in Cryptomonas, a common freshwater phytoplankter. Oecologia 78: 473–476.
Tricart, J. L., 1973. Geomorfología de la Pampa Deprimida. INTA, Colección Científica no XII, Buenos Aires.
Unrein, F. 2001. Efecto de los nutrientes y el pH sobre la estructura del fitoplancton en ambientes de la llanura aluvial del Paraná Inferior. Ph.D. Dissertation, Universidad de Buenos Aires, Buenos Aires, Argentina.
Unrein, F., R. Massana, L. Alonso-Sáez & J. M. Gasol, 2007. Significant year-round effect of small mixotrophic flagellates on bacterioplankton in an oligotrophic coastal system. Limnolology and Oceanography 52: 456–469.
Urabe, J., T. B. Gurung, T. Yoshida, T. Sekino, M. Nakanishi, M. Maruo & E. Nakayama, 2000. Diel changes in phagotrophy by Cryptomonas in Lake Biwa. Limnology and Oceanography 45: 1558–1563.
Utermöhl, H., 1958. Zur Vervollkommnung der quantitativen Phytoplankton Methodik. Mitteilungen Internationale Vereinigung Limnologie 9: 1–38.
Villar, C., L. de Cabo, P. Vaithiyanathan & C. Bonetto, 1998. River–floodplain interactions: Nutrient concentrations in the Lower Paraná River. Archiv für Hydrobiologie 142: 433–450.
Vörös, L., C. Callieri, K. V. Balogh & R. Bertoni, 1998. Freshwater picocyanobacteria along a trophic gradient and light quality range. Hydrobiologia 369/370: 117–125.
Acknowledgments
This research was supported by a grant from Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET) PIP 5354, and a grant from the University of Buenos Aires UBACyT X838, and ANPCyT PICT 13550. We thank the reviewers for their valuable suggestions on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: J. Padisak
Rights and permissions
About this article
Cite this article
Allende, L., Tell, G., Zagarese, H. et al. Phytoplankton and primary production in clear-vegetated, inorganic-turbid, and algal-turbid shallow lakes from the pampa plain (Argentina). Hydrobiologia 624, 45–60 (2009). https://doi.org/10.1007/s10750-008-9665-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-008-9665-9