Abstract
In order to understand the environmental variables which promote the proliferation of cyanobacteria and variation in microcystin concentrations in the Nui Coc reservoir, Vietnam, physicochemical parameters, the occurrence, and abundance of phytoplankton, cyanobacteria, and microcystin concentration were monitored monthly through the year 2009–2010. The relationships between these parameters were explored using principal component analysis (PCA) and Pearson correlation analysis. The phytoplankton community was mainly dominated by the cyanobacterium Microcystis with higher cyanobacteria abundance during summer and autumn season. PCA and Pearson correlation results showed that water temperature and phosphate concentration were the most important variables accounting for cyanobacteria, Microcystis, and microcystin occurrence. Analysis of the toxins by high-performance liquid chromatography demonstrated the presence of two microcystin variants: microcystin-LR (MC-RR) and microcystin-ddRR (MC-ddRR) with total concentrations of the toxins in filtered samples from surface water ranging from 0.11 to 1.52 μg MC-LR equiv L−1. The high concentrations of microcystin in the Nui Coc reservoir highlighted the potential risk for human health in the basin. Our study underlined the need for regular monitoring of cyanobacteria and toxins in lakes and reservoirs, which are used for drinking water supplies, not only in Vietnam but also in tropical countries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The cyanobacteria (commonly known as blue-green algae) are an ancient group of prokaryotic organisms found in many different aquatic environments all over the world (Whitton and Potts 2000). In aquatic environments, most cyanobacteria species occur in low concentrations; however, when environmental and hydrological conditions are favorable, cyanobacteria proliferate and form blooms. Mass occurrences of cyanobacteria are typically related to nutrient inputs resulting from fertilizer runoff and industrial, livestock, and human wastewater (Moreno et al. 2004). Cyanobacteria blooms cause a variety of water quality problems including unpleasant odors, dissolved oxygen depletion, and increased pH. They also release large amounts of dissolved organic carbon which fuels bacterial growth in drinking water supplies. Cyanobacteria blooms also reduce water transparency, can lead to diminished biodiversity, and can be responsible for fish kills as well as the death of other animals such as dogs and deer. Indeed, cyanobacteria can produce several potent toxins, and the presence of these compounds in drinking and bathing water supplies poses a serious hazard to human health (Falconer 1996). The World Health Organization recognize toxic cyanobacteria as a pathogenic agent that requires monitoring in water and have established provisional guideline value for microcystin-LR in drinking water (1 μg L−1) (WHO 1998). Surveys conducted in a wide range of countries have revealed that 25–95 % of the bloom samples expressed some degree of toxicity (Sivonen 1996). Cyanotoxins can toxicologically be divided into five major groups: hepatotoxins, neurotoxins, cytotoxins, dermatotoxins, and endotoxins. Among cyanobacterial toxins, microcystins (MCs) are probably seen to occur most frequently. Over 100 species of cyanobacteria belonging 40 genera such as Microcystis, Anabaena, Aphanizomenon, Oscillatoria, Nostoc, Cylindrospermopsis, Anabaenanopsis, Cylindrospermum, Haphalosiphon, Lyngbya, Nodularia, Phormidium, Planktothrix, Merismopedia, Leptolyngbya, Umezakia, and Pseudoanabaena are recognized as the most common genera having toxigenic species (Gkelis et al. 2005; Jayatissa et al. 2006; van Apeldoorn et al. 2007).
During the last decades, toxic cyanobacterial blooms have been reported with an increasing frequency worldwide and are often associated with the production of microcystins (Chorus 2001; Gkelis et al. 2005; Jayatissa et al. 2006; Quiblier et al. 2008; Joung et al. 2011). However, little information on toxic cyanobacteria and their toxins has been reported from freshwater bodies in Vietnam (Hummert et al. 2001; Nguyen et al. 2007; Dao et al. 2010; Nguyen et al. 2012). Preliminary studies on phytoplankton showed that potentially toxic cyanobacteria species occurred widely in Vietnamese natural waters (Hummert et al. 2001; Dang et al. 2003). Additionally, phytoplankton studies in several water bodies in northern Vietnam have shown that prolonged Microcystis species-dominated cyanobacterial blooms can occur (Dang et al. 2003). Moreover, different variants of microcystin in isolated Microcystis strains from the Thanh Cong lake have been identified (Hummert et al. 2001). More recently, several toxin-producing cyanobacteria species and their toxins have been reported in the central region of Vietnam, e.g., Thua Thien-Hue province, and in the Southern Vietnam, e.g., Tri An reservoir (Nguyen 2007; Nguyen et al. 2007; Dao et al. 2010).
The Nui Coc reservoir (surface area, 25.2 km2; effective storage capacity, 168.106 m3) receives water from the Cong River and is a well-known tourist site in North Vietnam as well being important for the local economy. The reservoir was constructed to provide drinking water supplies, agricultural irrigation, for aquaculture, flood control, recreation, hydroelectric power, and for flow management for the Thai Nguyen province and surrounding areas. In recent years, parallel with the rapid development of the local economy and the intensive use of water resources, the water quality of this reservoir has been considerably degraded due to untreated agricultural, industrial, and domestic wastewater inputs from the watershed. The reservoir requires an effective biomonitoring program, but at present, little is known about phytoplankton and cyanobacteria diversity in this system. Although the presence of the toxic Microcystis was first reported when massive bloom appeared, no data on cyanotoxins are available (Dang et al. 2003). The objective of this study was to document, through the monthly monitoring of the phytoplankton community, the concentration of toxic cyanobacteria and their toxins of the Nui Coc reservoir from May 2009 to May 2010. In addition, the relationship between the presence of cyanobacteria, toxin production, and environmental factors in the Nui Coc reservoir was also examined. In these systems that are integral the to economic development of these rural regions, a better understanding of toxic cyanobacteria occurrence and their toxins is essential for the protection of the water quality of this reservoir.
Material and methods
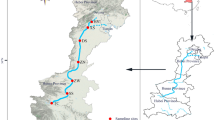
The Nui Coc reservoir is located in Thai Nguyen province (latitude 105°46′ E, Longitude 21°34′ N) and drains an area of 567 km2 (Fig. 1). This basin is located in the mountainous region, with the average elevation of 312 m and the mean slope of 41.3 % (Vu and Nguyen 2009). The climate in the Nui Coc reservoir basin is subtropical East Asian monsoon characterized by two distinct seasons: rainy and hot and dry and cooler. Yearly average rainfall in the basin is approximately 1,850 mm y−1 with an annual average temperature of 22 °C. The Nui Coc reservoir was constructed in 1970s; has a surface area of 25.2 km2 and an effective storage capacity of 168 × 106 m3 (Thai Nguyen center TDI 2002; Le et al. 2010). The mean depth of this reservoir is 23 m (Vu and Nguyen 2009).
Sample collection and analysis
Water samples were monthly collected from surface layer (0–35 cm) from May 2009 to May 2010 at six sites (NC1, NC2, NC3, NC4, NC5, and NC6) in the reservoir as shown in the Fig. 1. For the present study, average values of six sampling stations are presented. Physical factors (temperature, pH, dissolved oxygen (DO), and conductivity) were measured in situ using a multi-parameter probe (Model WQC-22 A, TOA). Water samples for nutrient determinations (PO4 3−-P, NH4 −-N, NO3 −-N, and NO2-N) were collected near the surface, filtered through Whatman GF/C filters (0.45 μm), and kept in polypropylene sampling at 4 °C in darkness analyzed as soon as possible. All analyses were conducted according to American Public Health Association (APHA 1998). Water samples for chlorophyll a (Chl a) determination were filtered through GF/C membrane filters, extracted in acetone, and measured spectrophotometrically (Lorenzen 1967). Phytoplankton samples were collected using plankton net (40 μm mesh size) and immediately fixed with formaldehyde solution at a 5 % (v/v) final concentration for species identification. Phytoplankton species were identified according to its morphology using light microscopic observation (Olympus BX51). The following taxonomic literature was used for the identification of cyanobacteria: Duong (1996) and Komárek and Anagnostidis (1989, 1999, 2005). Krammer and Lange-Bertalot (1986–1991) was used for diatom identification. The general phytoplankton identification was carried out using the techniques of Huber-Pestalozzi (1955, 1961, 1968, 1982 and 1983), van Den Hoek et al (1995) and Duong and Vo (1997). For phytoplankton enumeration, one-liter of water was preserved, fixed with 10 mL Lugol’s iodine solution, and then was sedimented for 48 h prior to counting. Number of cells was counted by using a Sedgewick-Rafter cell method (Hötzel and Croome 1998; Karlson et al. 2010).
Bloom samples were collected using plankton net. Samples were kept cool and delivered to the Department of Hydrobiology, Institute of Environmental Technology within 5 h after sampling. Samples were then concentrated by centrifugation and/or filtering through nylon mesh. Subsamples of cyanobacterial bloom which comprised of approximately 80–90 % Microcystis species were used for the isolation of Microcystis aeruginosa. This species was isolated using a sterilized Pasteur pipette to collect single cells or small colonies. Single cell or small colonies of Microcystis were rinsed in several drops of sterilized CB medium to remove the contaminating cells or suspended particles. The isolation was performed in CB liquid medium (Shirai et al. 1989). The cultures were maintained at 25 °C under 12:12 h light/dark regime, 14 μmol photons m−2 s−1 intensity. Biomass of isolated strains was harvested at the end of exponential growth phase and concentrated by centrifugation for 10 min. at 6,000 rpm at room temperature.
Sample preparation for microcystin determination
Surface water, colonies from the cyanobacterial bloom, and cultures from the Nui Coc reservoir were used for MC determination. Three litters of surface water from the reservoir was filtered onto GF/C filters (Whatman). Bloom, cultured, and the filters containing cyanobacteria cell samples were lyophilized under vacuum at −55 °C for 24 h and stored at −20 °C prior to toxin analysis.
Microcystin determination
Cyanobacterial crude extract was prepared according to Fastner et al. (1998) with minor modification. Briefly, the field samples on GF/C and lyophilized samples (50–100 mg DW) of cyanobacterial bloom and isolates were homogenized and first extracted overnight in 70 % methanol containing 5 % acetic acid and 0.1 % trifluoracetic acid followed by 3 × 60 min in MeOH 90 % containing 5 % acetic acid and 0.1 % TFA with 30-sec sonication during the last extraction. Each extraction step was followed by centrifugation (4,500 rpm, 10 min, 4 °C). The supernatants of all extractions from each sample were pooled, dried at 35 °C, redissolved in 1.5 mL MeOH (100 %), and centrifuged at 14,000 rpm, 1 °C for 10 min. The supernatant was kept at −20 °C prior to high-performance liquid chromatography (HPLC/MS).
Microcystin was analyzed by a HPLC/MS (Waters Alliance, Germany) on a reverse phase column (RP18; 5 μM LIChroSpher 100) by photodiode array detection between 200 and 300 nm according to Pflugmacher et al. (2001). Separation of 10-μL injection volume was achieved at 40 °C by a gradient of Milli-Q water and acetonitrile, both enriched with 0.1 % (v/v) TFA at a flow rate of 200 μL min−1, starting at 35 % acetonitrile (ACN), increasing to 55 % ACN within 15 min, cleaning at 100 % ACN and 10 min equilibration to start conditions. Measurements were made in the positive MRM Mode. The calibration is for MC-LR linear from 10 to 25 ng mL−1. The microcystin standard, MC-LR, was purchased from Axxora (Germany).
Data treatment
A principal component analysis (PCA) and Pearson correlation matrix using SPAD Software (version 5.6, Decisia, Paris, France) (StatSoft, Inc 2004) were performed to elucidate the relationships between the cyanobacteria, Microcystis and microcystin concentrations, and environmental factors.
Results
Physical and chemical characteristics
The results of physical and chemical characteristics during the study period from May 2009 to May 2010 in the Nui Coc reservoir are summarized in Table 1. The water temperature followed a seasonal pattern in this region. Temperature was lowest in January (18.8 °C), began to increase in April and May, and reached a maximum of 32.5 °C in September; after that, it decreased to 22 °C in December. The pH varied between a minimum of 6.6 and the maximum of 8.8 with little pattern. DO did not differ significantly over the year. The only exception was during April 2010 when DO was significantly lower than all other months (3.5 mg L−1). During the study period, conductivity ranged from 5.5 to 11.7 with the lowest value recorded in May 2009 and the highest value was in April 2010. Higher nitrite concentrations (0.02 mg N-NO2 L−1) were found during the rainy season (May–September), and nitrite were generally low in dry season (from 0.003 to 0.009 mg N-NO2 L−1; October–Jannuary). Nitrate concentration ranged from 0.24 to 0.41 mg N-NO3 L−1. The only exceptions occurred during early rainy season (May 2009, April 2010, and May 2010, when concentrations 0.55, 0.61, and 0.86 mg N-NO3 L−1 were observed; Table 1). Ammonium concentrations ranged between 0.03 and 0.23 mg N-NH4 L−1. Dissolved orthophosphate-P concentration varied by a factor 9 over the sampling period, with the lowest values generally observed during the cooler, dryer months. Total phosphorus concentrations varied between 0.09 and 0.53 mg P L−1. The maximum value was detected in February 2010 and the minimum value was in April 2010. Silicate-Si concentration remained relatively stable and varied between 3.5 and 4.4 mg Si-SiO2 L−1. No significant differences were found in silicate concentration among months (p > 0.05).
Phytoplankton abundance and composition
Changes in phytoplankton abundance and chlorophyll a concentration from May 2009 and May 2010 in the Nui Coc reservoir are shown in Fig. 2. Phytoplankton abundance varied with season with high values in summer and autumn (April to October) and low values in winter season (November to February). The maximum phytoplankton cell was observed in July 2009 (28 × 105 cells L−1), the lowest density occurred in December 2009 (2 × 104 cells L−1). Changes of phytoplankton density throughout the investigation coincided with closely Cyanobacteria abundance. Chl a concentration followed the same pattern as for phytoplankton abundance with highest Chl a concentration in July 2009 (16 μg L−1) when phytoplankton abundance was found to be the highest. During winter, both phytoplankton and Chl a concentrations were low (Fig. 2).
Phytoplankton from the Nui Coc reservoir was comprised of six groups: Chlorophyceae, Bacillariophyceae, Cryptophyceae, Euglenophyceae, Dinophyceae, and Cyanobacteria. The relative abundance of phytoplankton composition was dominated by the Cyanobacteria, Chlorophyceae, and Bacillariophyceae groups (Fig. 3, Table 2). During the study period, cyanobacteria dominated the phytoplankton community with a relative numerical abundance that ranged from 27 to 84 %. The most abundant cyanobacteria in this reservoir were colony-forming genera (Microcystis, Aphanocapsa, Snowella, and Woronichinia) as well as the solitary filamentous genera (Pseudanabaena, Cylindrospermopsis, Anabaena, and Oscillatoria). Among these cyanobacteria genera, only the Microcystis genus was present in all samples with M. aeruginosa, Microcystis wesenbergii, and Microcystis botrys being predominant in all samples. During the study period, three distinct blooms of Microcystis occurred (July, August, and September 2009). Eight morphospecies of Microcystis in bloom samples were observed, including Microcystis aeruginosa, Microcystis wesenbergii, Microcystis botrys, Microcystis flos-aquae, Microcystis panniformis, Microcystis protocystis, Microcystis novacekii, and Microcystis smithii.
Dominant species belonging to the Chlorophyceae group were represented by Scenedesmus acuminatus var. biceriatus, Scenedesmus quadricauda, Ankistrodesmus falcatus, Pediastrum simplex, Pediastrum duplex, Staurastrum sp., Cosmarium sp. Bacillariophyceae were represented by species Aulacoseira granulata, Melosira varians, Navicula placentula, Navicula gracilis, Nitzschia palea, and Nitzschia filiformis which accounted for the high relative abundance from late November 2009 to February 2010. Cryptophyceae, Euglenophyceae, and Dinophyceae were also observed in much lower numbers. These groups were represented mostly by Cryptomonas sp., Euglena acus, and Ceratium sp., respectively.
Toxin concentration
The monthly changes in MC concentrations in the water column of the Nui Coc reservoir are shown in Fig. 4. Measurable microcystin levels were detected for all of the investigated months with concentrations ranging from 0.12 to 1.52 μg MC-LR equiv L−1. Generally, the highest levels of MC were observed during the warmer months and the lowest levels during the cooler months. The only exception was during November when a concentration of 1.1 μg MC-LR equiv L−1was observed (Fig. 4).
Regarding the Microcystis bloom and cultured samples, detectable concentrations of microcystins were found in five samples (Table 3). The highest concentration of the total microcystin was found in Microcystis bloom sample from January 2010 (1,699 μg MC-LR equivalent/g DW), followed by sample from October 2009 (1,613 μg MC-LR equivalent g−1 DW). The lowest amount of microcystin (45 μgMC-LR equivalent g−1 DW) was detected in the samples collected in August 2009. The toxicity of M. aeruginosa strain isolated from the Nui Coc reservoir was confirmed with toxin concentration around 184 μg MC-LR equivalent g−1 DW.
Cyanobacteria, microcystis, and environmental factors
In order to identify the environmental factors associated with cyanobacteria appearance in the Nui Coc reservoir, we conducted a PCA analysis on the data. On the first plane, as defined by axes 1 and 2, representing around 50 % of the total variance, two seasonal conditions are clearly individualized. The rainy period is localized in the left part of PCA and characterized by high relative abundance of phytoplankton, Cyanobacteria, Microcystis, and microcystin concentrations, and dry season is positioned in the right part of PCA (Fig. 5). The relationships between relative abundance of Cyanobacteria, Microcystis, and environmental variables are presented in Table 4. Phytoplankton biomass, relative abundance of cyanobacteria, Microcystis, and microcystin concentration were positively correlated to the temperature and phosphate concentrations.
Discussion
The results presented in this study showed that the Nui Coc reservoir was eutrophic, and phytoplankton communities exhibited a seasonal pattern and dominated by the Cyanobacteria group. According to the Organization for Economic Cooperation and Development criteria (OECD 1982), the water quality of the Nui Coc reservoir can be classified as eutrophic as the annual average total phosphorous (TP) concentration is 0.19 mg L−1; and as meso-eutrophic based on the phytoplankton biomass and transparency values with the annual average of Chl a concentration of 7.1 μg L−1 and 2.5 m, respectively (Table 1). Two surveys conducted in 2001 and 2003 revealed some common features of the water quality from the Nui Coc reservoir including the high concentration of nutrients (TP and TN) and shifts of phytoplankton composition from diatoms to cyanobacteria (Dang et al. 2008). Dang et al. (2008) reported that the average phosphate and TP concentrations were 0.02 and 0.07, respectively. In the current study, the phosphate and TP concentration were relatively higher, suggesting that the trophic status in the Nui Coc reservoir were likely to get worse. Indeed, Le et al. (2010) have demonstrated that recently, human activities in the Nui Coc catchment have significantly impacted the aquatic system in this reservoir. The authors underline the fact that nutrient leaching from agricultural soils and from point sources such as domestic and industrial outlets are the main source. Due to the large fraction of agricultural land and fertilizer use in the watershed, the nitrogen and phosphorus fluxes leaching from agricultural soils accounted for 32.7 and 36.7 % of total nitrogen and phosphorus inputs to the reservoir hydrosystem, respectively (Le et al. 2010).
The phytoplankton of the Nui Coc reservoir showed that the community structure was dominated quantitatively by the Cyanobacteria group during the warmer months. However, during autumn and winter, Chlorophyceae and Bacillariophyceae dominated although cyanobacteria were always present with relative abundances of over 4 and 1 %, respectively (Fig. 3). These results were consistent with those of other studies which found that phytoplankton succession in tropical aquatic systems were characterized by a distinct shift between dry and rainy seasons: Chlorophytes—Chroococcales/Cyanobacteria in Lake Tanganyika (Descy et al. 2005), Diatoms/Cyanobacteria in Lake Victoria (Kenya), the Lake Guiers (Senegal) and Lake Dongping (Lung’ayia et al. 2000; Bouvy et al. 2006; Tian et al. 2012), and Bacillariophyceae/Chlorophyceae in Lake Ogeube (West African lake) (Nweze 2006). Moreover, there was a sharp contrast between low cell density and biomass during the winter–spring period and the marked increase during summer and autumn (Fig. 2). In fact, the mean cell density and Chl a concentrations were three- and eightfold higher in summer–autumn than in winter–spring period, respectively. The highest phytoplankton densities and Chl a concentration observed during the rainy season are probably due to the increasing input of nutrients from terrestrial run-off, thus stimulating phytoplankton growth (Nweze 2006). The two peaks of phytoplankton biomass during the summer period were dominated by Microcystis. Cyanobacteria are known to be an abundant group in most tropical lakes (Lung’ayia et al. 2000; Descy et al. 2005; Jayatissa et al. 2006; Tian et al. 2012). In general, cyanobacteria have a high temperature optimum for growth compared with other phytoplankton groups (Dokulil and Teubner 2000; Paerl and Huisman 2008). This was also the case in the Nui Coc reservoir as both the cyanobacteria and Microcystis in particularly were positively correlated with temperature during the study period (Table 4). The combination of high temperature, increased light availability, and the increased nutrient load from terrestrial run-off entering the reservoirs during the rainy period probably gives the cyanobacteria a competitive advantage over the other phytoplankton in this system (Sekadende et al. 2005).
It has been well documented that environmental factors such as temperature, pH, dissolved oxygen, and nutrient availability play an important role in regulating the structure and distribution of phytoplankton communities in lakes or rivers (Charpin et al. 1998; Paerl and Huisman 2008; Lacerda et al. 2004). In the present study, the relationships between the environmental factors and the presence of cyanobacteria, particularly Microcystis population, were investigated by using principal correlation analysis (Fig. 5, Table 4). Our results indicated that the abundance and variation of cyanobacteria and particularly Microcystis were linked to water temperature and phosphate concentrations. These results seemed to be consistent with other work that found that the high water temperature, stratification, and high phosphorous concentrations were the main factors in affecting Microcystis abundance and persistence (Paerl and Huisman 2008; Imai et al. 2009; Davis et al. 2009; Xu et al. 2010; Liu et al. 2011; Joung et al. 2011).
It has been also been found that both high temperature and P concentration promoted the growth rates of both toxic and non-toxic populations of Microcystis (Davis et al. 2009; Joung et al. 2011). The cyanobacterial community from the Nui Coc reservoir was composed predominantly of M. aeruginosa which is known as the most common MC producer and bloom-forming species (Codd et al. 2005). M. botrys and M. wesenbergii were also found as codominant species. These morphospecies are common to different regions throughout the world (Chorus 2001) and are known to cause blooms in Vietnamese freshwaters (Dang et al. 2008; Nguyen et al. 2007; Dao et al. 2010).
In the present study, total MCs were found in phytoplankton samples and showed variation ranging from 0.11 to 1.52 μg MC-LR equiv L−1 (Fig. 4). These values are high and are above the guideline values of the World Health Organization for drinking water supply. High microcystin concentrations were recorded from samples collected in July and August when phytoplankton density and biomass were peaked. Surprisingly, MCs were correlated to water temperature but were poorly correlated with other parameters such as nutrients, cyanobacterial biomass, Chl a, Microcystis abundance. Our results correspond to the observations of Amé et al. (2003) and Dai et al. (2008) who suggested that among physicochemical factors, water temperature was an important factor related to MCs. Although clear correlations between MCs, cyanobacteria, Microcystis and the environment factors were evident and noted in many studies, such relationship was not always consistent (Graham et al. 2006; Jacoby et al. 2000; Vezie et al. 1998; Wicks and Thiel 1990; Wu et al. 2006). For examples, a survey of 30 subtropical shallow lakes in China was conducted by Wu et al. (2006) who underlined a significant correlation between MCs and Chl a, cyanobacteria biomass, Microcystis, and temperature. In contrast, Kotak et al. (2000), working on eutrophic lakes in Canada found no relationship between temperature, M. aeruginosa, and MC production. It has also been reported that MC in phytoplankton samples was strongly correlated with TN from water bodies in America (Graham et al. 2006), with TP in eutrophic lakes in Alberta, Canada (Kotak et al. 2000). In contrast, according to Jungmann et al. (1996), MC was not correlated with any measured variables in a hypereutrophic reservoir in Germany. This contradictory data may be due to different responses of microcystin producing species to various environmental factors.
The microcystin production of isolated strain of M. aeruginosa was confirmed and microcystin content in this strain was high compared with previous records in Vietnam. In fact, the toxin content in isolated strain was less toxic than most field blooms. This could be explained by the presence of various toxic cyanobacteria strains in the different blooms or the relative abundance of cyanobacteria species varying according to the environmental conditions. The total MC concentrations in the bloom samples dominated by Microcytis varied from 45.3 to 161 μg MC-LR in which MC-RR plus desmethyl (ddMC-RR) was dominant and recently reported for Vietnam (Nguyen et al. 2012). The variation of total microcystin concentration in these samples suggested that the potential toxicity of microcystin-producing species was variable. In general, both toxic and nontoxic species coexisted during the bloom period, and changes in the proportion of these species may partly explain the variation in MCs observed in our work.
In conclusion, the current study contributed to understanding the seasonal variations in phytoplankton community and microcystin production in the Nui Coc reservoir. Our investigation indicated that cyanobacteria were a common component of phytoplankton communities in the Nui Coc reservoir. Moreover, such abiotic factors as water temperature and phosphate concentration played an important role in the occurrence and variation of cyanobacteria abundance and microcystin concentration in the Nui Coc reservoir. The high concentrations of microcystin in the Nui Coc reservoir highlighted the potential risk for human health in the basin. This underlines the need for regular monitoring of cyanobacteria and toxins in lakes and reservoirs used for drinking water supplies.
References
Amé MV, Díaz MP, Wunderlin DA (2003) Occurrence of toxic cyanobacterial blooms in San Roque Dam (Córdoba–Argentina): a field and chemometrics study. Environ Toxicol 18:192–201
APHA (1998) American Public Health Association. American Water Works Association (AWWA), Water Environment Federation (WEF). Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington
Bouvy M, Ba N, Ka S, Sane S, Pagano M, Arfi R (2006) Phytoplankton community structure and species assemblage succession in a shallow tropical lake (Lake Guiers, Senegal). Aquat Microb Ecol 45:147–161
Charpin MF, Maurin N, Amblard C, Devaux J (1998) Seasonal variations of phytoplankton photosynthate partitioning in two lakes of different trophic level. J Plankton Res 20:901–921
Chorus I (2001) Cyanotoxins: occurrence, causes, consequences. Springer, Berlin, 357 pp
Codd GA, Lindsay J, Young FM, Morrison LF, Metcalf JS (2005) Harmful cyanobacteria: from mass mortalities to management measures. In: Huisman J, Matthijs HCP, Visser PM (eds) Harmful cyanobacteria. Springer, Dordrecht, pp 1–23
Dai R, Liu H, Qu J, Ru J, Hou Y (2008) Cyanobacteria and their toxins in Guanting Reservoir of Beijing, China. J Hazard Mater 153:470–477
Dang HPH, Dang DK, Nguyen SN, Duong TT, Dang TT, Dahlmann J (2003) Toxicity and toxins of some cyanobacterial natural bloom samples and isolates from HaNoi and adjacent area waterbodies. 3rd International Toxic Algae Control Symposium–Strategies on Toxic Algae Control in Lakes and Reservoirs for Establishment of International Network. 21–25/10/2003. Wuxi City, China
Dang HPH, Dang DK, Nguyen SN, Dang TT, Duong TT (2008) Study of toxic microalgae in fresh inland waterbodies for the purpose of monitoring, management and defense of surface fresh water sources. Vietnam J Sci Technol 46:46–53
Dao TS, Cronberg G, Nimptsch J, Do HLC, Wiegand C (2010) Toxic cyanobacteria from Tri An Reservoir, Vietnam. Nova Hedwigia 90:433–448
Davis TW, Berry DL, Boyer GL, Gobler CJ (2009) The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae 8:715–725
Descy JP, Hardy MA, Sténuite S, Pirlot S, Leporcq B, Kimirei I, Sekadende B, Mwaitega SR, Sinyenza D (2005) Phytoplankton pigments and community composition in Lake Tanganyika. Freshw Biol 50:668–684
Dokulil MT, Teubner K (2000) Cyanobacterial dominance in lakes. Hydrobiologia 438:1–12
Duong DT (1996) Taxonomy of cyanobacteria of Vietnam. Agriculture publishing house, Hanoi, p 220
Duong DT, Vo H (1997) Fresh algae of Vietnam: taxonomy of chlorococcales. Agriculture publishing house, Hanoi, p 503
Falconer IR (1996) Potential impact on human health of toxic cyanobacteria. Phycologia 35:6–11
Fastner J, Flieger I, Neumann U (1998) Optimal extraction of microcystin from field samples – a comparison of different solvent and procedures. Wat Res 32:3177–3181
Gkelis S, Harjunpää V, Lanaras T, Sivonen K (2005) Diversity of hepatotoxic microcystins and bioactive anabaenopeptins in cyanobacterial blooms from Greek freshwaters. Environ Toxicol 20:249–256
Graham JL, Jones JR, Jones SB, Clevenger TE (2006) Spatial and temporal dynamics of microcystin in a Missouri reservoir. Lakes Reserv Manag 22:59–68
Hötzel G, Croome R (1998) A phytoplankton methods manual for Australian rivers. Occasional Paper No. 18/98. Land and Water Resources Research and Development Corporation, Canberra
Huber-Pestalozzi G (1955) Das Phytoplankton de Süβwasseres. Systematik und Biologie. 4. Teil. Euglenophyceen. E. Schweizerbart’sche, Stuttgart, p 606, CXIV plates
Huber-Pestalozzi G (1961) Das Phytoplankton de Süβwasseres. Systematik und Biologie. 5. Teil. Chlorophyceae (Grünalgen) Ordnung: volvocales. E. Schweizerbart’sche, Stuttgart, p 743, CLVIII plates
Huber-Pestalozzi G (1968) Das Phytoplankton de Süβwasseres. Systematik und Biologie. 3. Teil. Cryptophyceae, Chloromonadophyceae, Dinophyceae. 2. Auflage E. Schweizerbart’sche, Stuttgart
Huber-Pestalozzi G (1982) Das Phytoplankton des Süßwassers Systematik und Biologie, 8. Teil, 1.Halfte Conjugatophyceae Zygnematales und Desmidiales (excl.Zygnemataceae), Von Kurt Föster, Pfronten/ Allgäu, E. Schweizerbarth’sche Verlagsbuchhandlung (Nagele u. Obermiller). Mit 785 Abbildungrn in zahlreichen Einzeldarstellungen auf 65 Tafelen und 11 Abbildungrn in Text. Stuttgart
Huber-Pestalozzi G (1983) Das Phytoplankton des Süßwassers Systematik und Biology, 7.Teil, 1. Hälfte, Chlorophyceae (Grunalgen) Ordnung: chlorococcales. In: Komarek J, Fott B, Schweizerbart'sche Verlagsbuchhandlung E (Nagele u. Obermiller). Mit 253 Tafeln, 14 Abbidungen und 43 Tabellen in Text, Stuttgart, pp 1043
Hummert C, Dahlmann J, Reinhardt K, Dang HPH, Dang DK, Luckas B (2001) Liquid chromatography-mass spectrometry identification of microcystins in Microcystis aeruginosa strain from lake Thanh Cong, Hanoi, Vietnam. Chromatographia 24:569–575
Imai H, Chang KH, Kusaba M, Nakano S (2009) Temperature-dependent dominance of Microcystis (Cyanophyceae) species: M. aeruginosa and M. wesenbergii. J Plankton Res 31:171–178
Jacoby JM, Collier DC, Welch EB, Hardy FJ, Crayton M (2000) Environmental factors associated with a toxic bloom of Microcystis aeruginosa. Can J Fish Aquat Sci 57:231–240
Jayatissa LP, Silva EIL, McElhiney J, Lawton LA (2006) Occurrence of toxigenic cyanobacterial blooms in freshwaters of Sri Lanka. Syst Appl Microbiol 29:156–164
Joung SH, Oh HM, Ko SR, Ahn CY (2011) Correlations between environmental factors and toxic and non-toxic Microcystis dynamics during bloom in Daechung Reservoir, Korea. Harmful Algae 10:188–193
Jungmann D, Ludwichowski KU, Faltin V, Benndorf J (1996) A field study to investigate environmental factors that could effect microcystin synthesis of a Microcystis population in the Bautzen reservoir. Int Rev Hydrobiol 81:493–501
Karlson B, Cusack C, Bresnan E (2010) Microscopic and molecular methods for quantitative phytoplankton. Intergovernmental Oceanographic Commission of UNESCO, p 109
Komárek J, Anagnostidis K (1989) Modern approach to the classification system of Cyanophytes 4-Nostocales. Arch Hydrobiol Suppl 82:247–345
Komárek J, Anagnostidis K (1999) Cyanoprokaryota, 1. Teil, Chroococcales. In: Ettl H, Gärtner G, Heynig H, Mollenhauer D (eds) Süsswasserflora von Mitteleuropa 19/1. Fischer Verlag, Jena, pp 1–548
Komárek J, Anagnostidis K (2005) Cyanoprokaryota, 2. Teil/ 2nd part: oscillatoriales. In: Büdel B, Krienitz L, Gärtner G, Schagerl M (eds) Süßwasserflora von Mitteleuropa 19/2. Elsevier/Spektrum, Heidelberg
Kotak BG, Lam AKY, Prepas EE, Hrudey SE (2000) Role of physical and chemical variables in regulating microcystin-LR concentration in phytoplankton of eutrophic lakes. Can J Fish Aquat Sci 57:1584–1593
Krammer K, Lange-Betarlot H (1986–1991) Bacillariophyceae. 1.Teil: Naviculaceae. 876 p; 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae, 596 p; 3. Teil: Centrales, Fragilariaceae, Eunotiaceae, 576 p; 4. Teil: Achnanthaceae. Kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema. 437 p. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds.) Süßwasserflora von Mitteleuropa. Gustav Fischer Verlag, Stuttgart, p 2485
Lacerda SR, Koening ML, Neumann-Leitão S, Flores Montes MJ (2004) Phytoplankton nyctemeral variation at a tropical river estuary (Itamaracá-Pernambuco-Brazil). Braz J Biol 64:81–94
Le TPQ, Nghiem XA, Vu HH, Duong TT, Tran VT, Dang DK (2010) Estimation of the nutrient (N, P) load from different wastewater sources in the Nui Coc reservoir basin (Thai Nguyen province). Vietnam J Sci Technol 48:369–375
Liu X, Lu X, Chen Y (2011) The effects of temperature and nutrient ratios on Microcystis blooms in Lake Taihu, China: an 11-year investigation. Harmful Algae 11:337–343
Lorenzen J (1967) Determination of chlorophyll and pheo-pigments: spectrophotometric equations. Limnol Oceanogr 12:343–346
Lung'ayia HBO, M'harzi A, Tackx M, Gichuki J, Symoens JJ (2000) Phytoplankton community structure and environment in the Kenyan waters of Lake Victoria. Freshw Biol 43:529–543
Moreno IM, Pereira P, Franca S, Cameán A (2004) Toxic cyanobacteria strains isolated from blooms in the Guadiana River (southwestern Spain). Biol Res 37:405–417
Nguyen LTT (2007) Planktic cyanobacteria from freshwater localities in ThuaThien-Hue province, Vietnam.–PhD Thesis, Copenhagen, p 158
Nguyen LTT, Cronberg G, Annadotter H, Larsen J (2007) Planktic cyanobacteria from freshwater localities in Thuathien-Hue Province, Vietnam II. Algal biomass and microcystin production. Nova Hedwigia 85:35–49
Nguyen LVA, Tanabe Y, Matsuura H, Kaya K, Watanabe MM (2012) Morphological, biochemical and phylogenetic assessments of water-bloom-forming tropical morphospecies of Microcystis (Chroococcales, Cyanobacteria). Phycol Res 60:208–222
Nweze NO (2006) Seasonal variations in phytoplankton in Ogelube lake, a small natural West African lake. Lakes Reserv Res Manag 11:63–72
OECD (1982) Eutrophication of waters—monitoring, assessment and control. Organization for Economic Cooperation and Development, Paris
Paerl HW, Huisman J (2008) Blooms like it hot. Sci 320:57–58
Pflugmacher S, Wiegand C, Beattie KA, Krause E, Steinberg CEW, Codd GA (2001) Uptake, effects and metabolism of cyanobacterial toxins in the emergent reed plant Phragmites australis (Cav.) Trin. ex Steud. Environ Toxicol Chem 20:846–852
Quiblier C, Leboulanger C, Sané S, Dufour P (2008) Phytoplankton growth control and risk of cyanobacterial blooms in the lower Senegal River delta region. Water Res 42:1023–1034
Sekadende BC, Lyimo TJ, Kurmayer R (2005) Microcystin production by cyanobacteria in the Mwanza Gulf (Lake Victoria, Tanzania). Hydrobiologia 543:299–304
Shirai M, Matumaru K, Ohotake A, Takamura Y, Aida T, Nakano M (1989) Development of a solid medium for growth and isolation of axenic Microcystis strains (Cyanobacteria). Appl Environ Microbiol 55:2569–2571
Sivonen K (1996) Cyanobacterial toxins and toxin production. Phycologia 35:12–24
StatSoft, Inc. (2004) STATISTICA (data analysis software system), version 7. www.statsoft.com
Tian C, Lu X, Pei H, Hu W, Xie J (2012) Seasonal dynamics of phytoplankton and its relationship with the environmental factors in Dongping Lake, China. Environ Monit. doi:10.1007/s10661-012-2736-4
Thai Nguyen center TDI (Thai Nguyen center for technology development and investigation) (2002) Final report of the project “Investigation and assessment of water environment of the Nui Coc reservoir. Provincial project on protection environment and effective utilization of this reservoir”. 146 pp
Van Apeldoorn ME, Egmond HPV, Speijers GJA, Bakker GJI (2007) Toxins cyanobacteria. Mol Nutr Food Res 51:7–60
van den Hoek C, Mann DG, Jahns HM (1995) Algae. An introduction to phycology. Cambridge University Press, Cambridge
Vezie C, Brient L, Sivonen K, Bertru G, Lefeuvre JC, Salkinoja-Salonen M (1998) Variation of microcystin content of cyanobacterial blooms and isolated strains in Lake Grand-Lieu (France). Microb Ecol 35:126–135
Vu TL, Nguyen KH (2009) Assessment of water resources (rainfalls and surface water) in the Nui Coc reservoir basin. Branch report for the ĐTĐL.2009T/08 project. 30 pp
Whitton BA, Potts M (2000) Indroduction to the cyanobacteria. In: Whitton BA, Potts M (eds) The ecology of cyanobacteria: their diversity in time and space. Kluwer Academic, Dordrecht, pp 1–11
WHO (1998) Guidelines for drinking-water quality. Addendum to vol. 1. Recommendations, 2nd edn. World Health Organization, Geneva
Wicks RJ, Thiel PG (1990) Environmental factors affecting the production of peptide toxins in floating scums of the cyanobacterium Microcystis aeruginosa in a hypertrophic African reservoir. Environ Sci Technol 24:1413–1418
Wu SK, Xie P, Liang GD, Wang SB, Liang XM (2006) Relationships between microcystins and environmental parameters in 30 subtropical shallow lakes along the Yangtze Rives, China. Freshwat Biol 51:2309–2319
Xu Y, Wang G, Yang W, Li R (2010) Dynamics of the water bloom-forming Microcystis and its relationship with physicochemical factors in Lake Xuanwu (China). Environ Sci Pollut Res 17:1581–1590
Acknowledgments
This work was supported by the National Foundation for Science and Technology Development (NAFOSTED) 106.16-2010.71 project and the International Foundation for Science (IFS) W/4674-1 projects. The authors are grateful for the financial supports from Vietnam's NAFOSTED and the IFS. The authors thank many individuals for their help in collecting samples in the field.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Duong, T.T., Le, T.P.Q., Dao, TS. et al. Seasonal variation of cyanobacteria and microcystins in the Nui Coc Reservoir, Northern Vietnam. J Appl Phycol 25, 1065–1075 (2013). https://doi.org/10.1007/s10811-012-9919-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-012-9919-9