Abstract
Knowing the interactions between exotic and native species is essential to establish possible threats to the local fauna. In this study, we assessed the use of food resources and diet overlap between a recently introduced snail, Sinotaia quadrata, and a native species, Pomacea canaliculata. We analyzed the gut content and stable isotope of snails and resources in a lowland stream where both species coexist. Both Schoener’s and isotope dietary overlap indexes supported dietary overlap. Conversely, gut content analysis showed differences in consumption: S. quadrata consumed more detritus and diatoms than P. canaliculata, whose diet was characterized by detritus and macrophyte remains. Macrophytes were the resource that most contributed to the diet of both species, as shown by stable isotope mixing models. The combination of both techniques, gut content and stable isotope analysis, indicated that S. quadrata consumed macrophyte detritus while P. canaliculata ate fresh macrophytes. This difference indicates differential use of food resources between the studied species coexisting in a lowland stream. Although no negative trophic interaction was found, we highlight the importance of continuing to monitor interactions for other resources and studying possible risks to the local fauna.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The introduction of exotic species has increased between continents as a consequence of human activities (Schreiber et al. 2003; Strayer 2010). As a result, native species of the same taxonomic groups and surrounding biotic communities can be impacted negatively via species replacement, food web reorganization, and community composition simplification (Sor et al. 2017). Knowing the ecological aspects of an exotic species allows one to establish the degree of functional similarity with local species and determine possible risks to them (Solomon et al. 2010). These risks could even lead to competitive exclusion scenarios (Hardin 1960).
Several aquatic and terrestrial gastropod species have become pests around the world, causing severe environmental and socioeconomic damage (Kesner and Kumschick 2018). Exotic snails, such as the New Zealand mud snail Potamopyrgus antipodarum in the USA, can compete directly with native gastropods for resources, including space and food (Lysne et al. 2008; Riley et al. 2008). When competition for food resources exists, the introduced snails could either induce dietary shifts in the native species (Larson and Ross Black 2016) or promote the decline of native populations. An example of the latter is Batillaria attramentaria, which caused a decrease in native populations of Cerithidea californica in California (Byers 2000). However, the negative impacts of interspecific competition could be reduced if resource partitioning existed between species, allowing their coexistence (Schoener 1974; Fedosov et al. 2014).
Sinotaia quadrata (Benson 1842) is an Asiatic freshwater Viviparidae snail recently recorded for Argentina by Ovando and Cuezzo (2012) and Ferreira et al. (2017). This species has already gone through the introduction and establishment stages in its new area of distribution, two preconditions to be considered an invasive species (Ferreira et al. 2017). It has been warned that snails of this family can impact the benthic communities by altering algal biomass, algal species composition, and nutrient cycling (Johnson et al. 2009). However, like many other species introduced in this area, the impact of S. quadrata on native species remains poorly studied (Darrigran et al. 2020). Hence, the study of a potential threat of this species to the native fauna is relevant.
Pomacea canaliculata (Lamarck 1822) (Gastropoda: Ampullariidae), on the other hand, is a native species widely distributed lowland streams of Argentina (Seuffert and Martín 2013), where it plays a key ecosystem role in trophic webs (López van Oosterom et al. 2013, 2016; Cadierno et al. 2017) and represents one of the most relevant macrophyte consumer (Manara et al. 2018; Maldonado and Martín 2019). Consequently, considering the similar densities and sizes of both species that can be found where they coexist (Ferreira et al. 2017), the presence of S. quadrata might represent a threat, not only for native P. canaliculata populations, but also for the whole ecosystem.
Studying the use of resources by exotic and native species is essential to establish a potential diet overlap (Zahn Seegert et al. 2014). Studies of feeding habits have traditionally used gut content analysis as a tool for reconstructing the resource use of a particular species (Nielsen et al. 2018). However, not all the incorporated food is assimilated, and its analysis may lead to overestimating the importance of some items and in other cases to underestimating it because the identification of the digested food is not always possible, especially for detritivores (McCutchan and Lewis 2002). Therefore, this method is frequently complemented with stable isotope analysis, which provides information only on the assimilated material (Cabana and Rasmussen 1994). Thus, the simultaneous use of both methods provides more complete and detailed information over time than if they were used separately (Nielsen et al. 2018). In this sense, we used gut content and stable isotope analyses to assess the use of food resources and diet overlap of the exotic snail S. quadrata and the native snail P. canaliculata in a stream where they coexist. This would be the first step in establishing a possible risk for the local fauna by the introduced species and would provide relevant information about the ecology of this exotic snail.
Methods
Study area
The study was carried out in the Carnaval stream, belonging to the Rio de la Plata Basin, located in the Pampa Ecoregion, close to La Plata city (Buenos Aires province, Argentina). It is a peri-urban stream (Hurtado et al. 2006) and, like other lowland Pampean streams, it is characterized by a low slope (< 1%), slow flow, high turbidity, and high content of suspended solids. In addition, the lack of riparian forest vegetation, the development of a dense macrophyte assemblage, and elevated algal growth are also characteristics of all Pampean streams (Feijoó and Lombardo 2007). Thus, in these ecosystems, autochthonous detritus, algal and macrophyte assemblages provided the basal resources (Feijoó et al. 2014). For this study, a section of the stream (34°51′28.22″ S, 58°4′5.17″ W) was selected where both species, P. canaliculata and S. quadrata, were abundant (corresponding to S4 in Ferreira et al. 2017). This section showed a macrophyte coverage higher than 30% on all sampling dates and the typical characteristics of the lowland Pampean streams described above. The site’s sediment was mainly composed of gravel followed by silt and sand and low percentages of clay. In addition, this reach passes through an urbanized area and receives untreated domestic wastewater, like most of the streams in the area.
Gut content analysis
Four samplings were carried out: April 2016, October 2016, April 2017, and October 2017. On each sampling date, 10 adult individuals of S. quadrata (n = 40, shell length between 19.40 and 29.15 mm, Ferreira et al. 2017) and 10 late juvenile and adult individuals of P. canaliculata (n = 40, shell length between 15.01 and 39.36 mm, Estebenet and Martín 2003) were collected for gut content analysis. Both species were present at the bottom of the stream and on top of the macrophytes. They were collected manually or with 500 µm sieves along a longitudinal transect of 100 m covering the two microhabitats where they were present (sediment and macrophytes) and fixed with 5% formaldehyde. In the laboratory, the snails were dissected under a stereoscopic microscope and the anterior part of the alimentary tract: the esophagus, of which the middle section is a crop, and the stomach (Andrews 1965) were separated and placed in vials with Bengal’s rose colorant for 24 h to stain their contents. This colorant stains organic matter and helps to differentiate organism tissue from other ingested material (Muñoz et al. 2009). Later, the content was homogenized in distilled water and mounted on microscope slides for observation under an optical microscope at a magnification of 400×. Photographs were taken by choosing 15 random fields (López van Oosterom et al. 2016) and analyzed with the Image J software.
For quantification, the area covered by each food item was considered relative to the total covered area of the digestive content (Jaarsma et al. 1998) and expressed as the relative frequency of each food item (Fi). Values of Fi were used as proxies of probabilities (pi) in the following formulas and in the Schoener’s index (see below). Based on the results of gut content analysis, richness (\(S = {\text{number}}\;{\text{of}}\;{\text{total}}\;{\text{food}}\;{\text{items}}\)), Shannon diversity index (\(H^{\prime} = - \mathop \sum \nolimits_{i = 1}^{S} p_{i} \log_{2} p_{i}\)), Dominance (\(D=1-\sum \nolimits_{i=1}^{S}{p}_{i}^{2}\), 1 − Simpson diversity), and Pielou evenness \(\left( {J^{\prime} = \frac{{H^{\prime}}}{{\log_{2} S}}} \right)\) of food items were calculated for each of the forty specimens of each species.
Stable isotope analyses
For stable isotope analysis, during the October-2017 sampling, 20 adult individuals of both species were collected in the studied section of the stream, using the same sampling technique as described above. The snails were taken to the laboratory alive, and placed in different containers with filtered stream water (filters with a pore size of 0.6 μm). Then, they were fasted for 24 h to ensure gut emptying and to include only assimilated substances in the analysis. The feces were siphoned out and individuals kept in a freezer at -20 °C until their processing. The shells were then mechanically discarded for the isotopic analyses (Bosley and Wainright 1999). Depending on the body mass of snails, two or three individuals were pooled to provide 3–4 mg dry weight samples, and three replicates were prepared per species. The potential food resources for both species were also sampled: macrophyte species present (Stuckenia striata, Hydrocotyle bonariensis, Gymnocoronis spilanthoides, Hydrocleys nymphoides, Sagittaria montevidensis, Egeria densa, Schoenoplectus californicus, Nasturtium microphyllum), fine particulate organic matter (FPOM < 65 μm), coarse particulate organic matter (CPOM > 65 µm), and epipelic biofilm (epipelon), the last three also taken in triplicate. For epipelon analysis, ten subsamples were collected by pipetting a superficial sediment layer (5–10 mm) in different places, following the recommendations of Gómez and Licursi (2001). In the laboratory, the coarse and fine fractions were separated with a 65-µm sieve. Macrophytes were sonicated for three 2-min cycles in an ultrasonic bath (Cleanson, 40-W power, 40-kHz frequency) to remove the attached algae from the plant surfaces (Romaní and Sabater 2001).
All the material was dried to constant weight for 48 h at 60 °C and then ground into powder to ensure homogeneity and analyzed in continuous flow isotope ratio mass spectrometry (CF-IRMS Thermo Scientific, Flash 2000-Delta V) coupled to an elemental analyzer (Centro de Isótopos Estáveis, UNESP, São Paulo). Isotopic ratios (13C/12C or 15N/14N) are typically expressed as the ratio between a heavier (higher atomic mass) and a lighter (lower atomic mass) isotope and converted into delta notation (δ values) through comparison of sample isotope ratios to ratios of internationally accepted standards. Standards for common systems include Vienna-Pee Dee Belemnite limestone (V-PDB) for carbon (δ13C) and atmospheric N2 for nitrogen (δ15N). The δ values are dimensionless ratios given in parts per thousand (‰) (Coplen 2011).
Data analysis
The differences in Fi, Shannon diversity, dominance, and evenness of Pielou of food items between species were analyzed with general linear mixed models (GLMMs). The Fi was first transformed by arcsin (√(X)) and Shannon diversity, dominance, and evenness of Pielou by log10(X + 1) to fit with a Gaussian error distribution, because model residuals without transformation were not normally distributed (Shapiro test: p < 0.01). For richness analysis, we used generalized linear mixed models with a Poisson error distribution, which is typically used for counting data. In all the models, the predictor variable was the mollusk species (levels: S. quadrata and P. canaliculata), and it was fitted with a random effect “year” (intercept) because of the lack of independence of the data. The notation of the models was as follows: \({y}_{i}=\beta 0+\beta 1i+b1j+\varepsilon ij\), where yi is the response variable (Fi, richness, Shannon diversity, dominance or evenness of Pielou), β0 is the intercept, β1i is the coefficient associated with the snail species, and b1j is the coefficient of the random effect “year” (2016, 2017). The null model that included no explanatory variables and the random effect was established for each model for assessing the relative explanatory power of models containing the predictors of interest. All the analyses were carried out with ‘R’ version 3.4.4 (R Core Team 2018) with lme4 (Bates et al. 2015) and lsmeans (Lenth 2016) packages.
We performed a Student’s t-test (α = 0.05) to compare δ13C and δ15N values between S. quadrata and P. canaliculata. Besides, the MixSiar Bayesian stable isotope mixing model (Semmens et al. 2013) was used to determine probability distributions for the proportional contribution of the food sources to the diet of each snail species. The trophic discrimination factor (TDF) used was 0.3 (± 0.14) ‰ for δ13C and 2.2 (± 0.3) ‰ for δ15N (McCutchan et al. 2003).
The diet overlap between species through gut content analysis was assessed using Schoener’s index (SI, Schoener 1970), following Reynaga and Rueda Martín (2014): \(I=1-0.5 \sum_{i=1}^{S}\left|{p}_{ij}-{p}_{ik}\right|\), where pij and pik represent the probability that item i is used by species j and k, respectively, being S the total number of food items used by the two species. On the other hand, the Isotopic dietary overlap index (IDO) was assessed following Miranda and Perissinotto (2012) with the same equation based also on Schoener’s index: \(\mathrm{IDO}=1-0.5 \sum_{i=1}^{S}\left|{p}_{ij}-{p}_{ik}\right|\), where p is the SIAR mean contribution of source i resulting from the final mixing models run for species j and k, respectively. Both indexes range from 0 to 1, as follows: 0 = no dietary overlap and 1 = complete overlap. A value equal to or greater than 0.6 is considered significant dietary overlap (Wallace 1981; Layman and Allgeier 2012).
Results
Gut content analysis
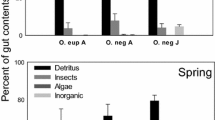
A total of nine food items were registered for P. canaliculata: detritus (unidentifiable organic matter), diatoms, macrophyte remains (fragments and seeds), invertebrate remains (legs, antennas, jaws, or chaetae), Chlorophyta, Charophyta, Cyanobacteria, Fungi, and Euglenophyta; and eight were found for S. quadrata (all the mentioned items except for Charophyta). The relative frequencies of food items are shown in Fig. 1. Although detritus was the dominant item for both species, its representation in the diet was significantly higher in the exotic species than in the native species (GLMM: p < 0.001, Table 1). In addition, the diet of S. quadrata was characterized by higher proportion of diatoms than that of P. canaliculata (GLMM: p = 0.043, Table 1), whereas P. canaliculata’s diet showed a higher frequency of macrophyte remains (GLMM: p < 0.001, Table 1). Macrophyte remains were present in the gut content of only one specimen of the exotic species. The diet of P. canaliculata contained significantly more items, was significantly more diverse, and had lower dominance than the diet of S. quadrata (Table 1). The result of Schoener’s Index was ecologically significant (SI = 0.73), indicating a diet overlap between S. quadrata and P. canaliculata.
Stable isotope analysis
Stable isotope signature value of δ15N was significantly higher in S. quadrata (18.18 ± 1.36; t = 5.171, df = 4, p = 0.007) than in P. canaliculata (14.11 ± 0.04, Fig. 2). The values of δ13C (S. quadrata = − 28.08 ± 0.76, P. canaliculata = − 27.46 ± 1.28, t = − 1.014, df = 4, p = 0.368), on the other hand, showed a similar use of resources, with high affinity for macrophytes, epipelon, and CPOM (Fig. 2).
The mean and standard deviations obtained from the MixSiar model analysis predicted the following contributions to the diet of P. canaliculata for each resource: macrophytes: 43.7% (± 16.9), epipelon: 20.6% (± 16.5), CPOM: 18.4% (± 14.6), FPOM: 17.2% (± 12.7); and for S. quadrata: macrophytes: 57.7% (± 21.4), epipelon: 14.8% (± 16.5); CPOM: 14.2% (± 15), FPOM: 13.3% (± 12.6). All the resources analyzed showed a considerable contribution (higher than 10%) to the diet of both species. Macrophytes were the resource with the highest contribution to the diet of both species. There was a significant overlap in the diet of S. quadrata and P. canaliculata (IDO = 0.86).
Discussion
Our study assessed the diet of an exotic species in its new distribution to estimate a possible diet overlap with a coexisting native species, by combining the gut content and stable isotopes analyses. The combination of these analyses allowed us to describe in detail the resource use by both species since, while gut content analysis provided a snapshot of the individual's consumption, stable isotope analysis provided information about the assimilated materials (Nielsen et al. 2018). Therefore, although their separate interpretation indicated a diet overlap, a comprehensive examination of the results of both analyses allowed us to infer a differential use of resources by the species.
The stable isotope analysis evinced a high contribution of macrophytes to the diet of both species, suggesting diet overlap; however, we did not find vegetal remains in the gut content of S. quadrata. Conversely, we found a substantial amount of detritus. This could be explained by considering that the detritus consumed by this species probably originated from the decomposition of the macrophytes. This is in agreement with Wolters et al. (2018), who stated that a possible route for the consumption of macrophyte-derived material might be the direct or incidental consumption of senescing macrophyte parts. As highlighted by other authors, the consumption of detritus and decaying macrophyte tissue is common in freshwater snails (Brönmark 1990; Madsen 1992; Qiu et al. 2011).
When aquatic ecosystems receive untreated wastewater discharges, like in the studied section of the stream, the environmental concentrations of δ15N can increase, leading to a higher concentration of this isotope in the detritus (Hamilton et al. 2001; Morrissey et al. 2013; Smucker et al. 2018). Consequently, primary consumers that feed upon this resource tend to show higher values of δ15N (Vander Zanden and Rasmussen 1999), as recorded for S. quadrata. Therefore, the difference in δ15N signal between both species supports the results of the gut content analysis, evidencing that detritus represents the most important food source for this exotic species. This is in accordance with Dudgeon and Yipp (1985), who classified S. quadrata as detritivore in its native range. Ovando and Cuezzo (2012), in contrast, mentioned this species as an herbivorous snail with a potential impact on the plant community in Argentina. However, their statement was not supported by a direct analysis of the diet of this species or a reference supporting that. On the other hand, the diet of P. canaliculata consisted mainly of detritus and macrophyte remains, in agreement with Kwong et al. (2010), Ocon et al. (2013), Hayes et al. (2015) and López van Oosterom et al. (2016). Kwong et al. (2010) found that this species was able to maintain a great proportion of macrophytes in its diet irrespective of seasonal changes in temperature, indicating that it forages on fresh macrophytes. Also, the higher food item richness and diversity show greater plasticity in the diet of P. canaliculata (López van Oosterom et al. 2016) than in that of S. quadrata.
The aforementioned differences between the diet of S. quadrata and P. canaliculata and the comprehensive examination of the results of both analyses allowed us to infer food partitioning between these sympatric species in a Pampean lowland stream. Such resource partitioning would allow both species to reduce interspecific competition and coexist. Several studies carried out in aquatic systems have demonstrated that species with similar ecological functions can coexist through the partitioning of food resources (Dubois et al. 2007; Novakowski et al. 2008; Borza et al. 2009). However, the possibility of overlapping with the native species due to other resources cannot be neglected. Miranda and Perissinotto (2012), for example, found a spatial overlap between Tarebia granifera and native gastropods. Other studies also reported that the increment in density of exotic snails, such as P. antipodarum, Pomacea maculata, and P. canaliculata, limited the growth of native species (Riley et al. 2008; Lysne and Koetsier 2008; Kwong et al. 2010; Posch et al. 2013). Effects on the reproduction of native species have also been reported. Anto et al. (2005), for example, showed that the ampullaridae snail Lanistes varicus, limited populations of Biomphalaria pfeifferi by reducing the number of egg masses produced. In this context, and considering that bigger snail species can affect the smaller ones (Maldonado and Martin 2019), further studies are needed to assess overlap of other resources or other negative interactions that could affect, for example, growth or reproduction of native species.
In accordance with Miranda and Perissinotto (2012) and Nielsen et al. (2018), our findings demonstrate the importance of combining the gut content and the isotope analysis in this type of study. Both analyses are complementary, and their results allowed us to infer the origin of the detritus that S. quadrata was consuming. To conclude, the exotic species consumed macrophyte detritus while the native one ate fresh macrophytes. Because Pampean streams are autotrophic, macrophytes provide the main source of food as detritus upon death (Minshall 1978; Feijoó and Lombardo 2007). These resources are, in general, abundant in the streams of the region (López van Oosterom et al. 2013; Ocon et al. 2013) and may contribute to the successful establishment of S. quadrata in streams with similar characteristics. Additionally, as mentioned above, this exotic snail could affect local species in different ways. For this reason, we highlight the importance of continuing to study possible negative interactions between S. quadrata and other detritivore native species coexisting in its new areas of distribution.
Data availability
We affirm that the data support the results. In turn, laboratory and field standards were met.
Code availability
Not applicable.
References
Andrews EB (1965) The functional anatomy of the gut of the prosobranch gastropod Pomacea canaliculata and of some other pilids. Proc Zool Soc Lond 145:19–36. https://doi.org/10.1111/j.1469-7998.1965.tb01998.x
Anto F, Bosompem K, Kpikpi J, Adjuik M, Edoh D (2005) Experimental control of Biomphalaria pfeifferi, the intermediate host of Schistosoma mansoni, by the ampullariid snail Lanistes varicus. Ann Trop Med Parasitol 99(2):203–209. https://doi.org/10.1179/136485905X17425
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed–effects models using lme4. J Stat Softw 67:1–48
Borza P, Erős T, Oertel N (2009) Food resource partitioning between two invasive gobiid species (Pisces, Gobiidae) in the littoral zone of the river Danube, Hungary. Int Rev Hydrobiol 94(5):609–621. https://doi.org/10.1002/iroh.200911134
Bosley K, Wainright LSC (1999) Effects of preservatives and acidification on the stable isotope ratios (15N:14N, 13C:12C) of two species of marine animals. Can J Fish Aquat Sci 56(11):2181–2185. https://doi.org/10.1139/f99-153
Brönmark C (1990) How do herbivorous freshwater snails affect macrophytes?–A comment. Ecology 71(3):1212–1215. https://doi.org/10.2307/1937391
Byers JE (2000) Competition between two estuarine snails: implications for invasions of exotic species. Ecology 81(5):1225–1239. https://doi.org/10.1890/0012-9658(2000)081[1225:CBTESI]2.0.CO;2
Cabana G, Rasmussen JB (1994) Modelling food chain structure and contaminant bioaccumulation using stable nitrogen isotopes. Nature 372(6503):255–257. https://doi.org/10.1038/372255a0
Cadierno MP, Burela S, Dreon MS, Martín PR, Heras H (2017) The influence of energy, nutritional value and noxiousness of prey in sex-and size-biased predation by Snail Kites in southern South America. Emu Austral Ornithol 117(4):382–387. https://doi.org/10.1080/01584197.2017.1338113
Coplen TB (2011) Guidelines and recommended terms for expression of stable isotope-ratio and gas-ratio measurement results. Rapid Commun Mass Spectrom 25(17):2538–2560. https://doi.org/10.1002/rcm.5129
Darrigran G, Agudo-Padrón I, Baez P, Belz C et al (2020) Non-native mollusks throughout South America: emergent patterns in an understudied continent. Biol Invasions 22(3):853–871. https://doi.org/10.1007/s10530-019-02178-4
Dubois S, Orvain F, Marin-Léal JC, Ropert M, Lefebvre S (2007) Small-scale spatial variability of food partitioning between cultivated oysters and associated suspension-feeding species, as revealed by stable isotopes. Mar Ecol Prog Ser 336:151–160. https://doi.org/10.3354/meps336151
Dudgeon D, Yipp MW (1985) The diets of Hong Kong freshwater gastropods. In: Morton B, Dudgeon D (eds) The Malacofauna of Hong Kong and Southern China, II: Proceedings of the second international workshop on the Malacofauna of Hong Kong and Southern China. Hong Kong University Press, Hong Kong, pp 491–509
Estebenet AL, Martín PR (2003) Shell interpopulation variation and its origin in Pomacea canaliculata (Gastropoda: Ampullariidae) from Southern Pampas, Argentina. J Molluscan Stud 69(4):301–310. https://doi.org/10.1093/mollus/69.4.301
Fedosov AE, Tiunov AV, Kiyashko SI, Kantor YI (2014) Trophic diversification in the evolution of predatory marine gastropods of the family Terebridae as inferred from stable isotope data. Mar Ecol Prog Ser 497:143–156. https://doi.org/10.3354/meps10585
Feijoó CS, Lombardo RJ (2007) Baseline water quality and macrophyte assemblages in Pampean streams: a regional approach. Water Res 41(7):1399–1410. https://doi.org/10.1016/j.watres.2006.08.026
Feijoó C, Leggieri L, Ocón C, Muñoz I, Rodrigues Capítulo A, Giorgi A, Colautti D, Ferreiro N, Licursi M, Gómez N, Sabater S (2014) Stoichiometric homeostasis in the food web of a chronically nutrient–rich stream. Freshw Sci 33(3):820–831. https://doi.org/10.1086/677056
Ferreira AC, Paz EL, Rumi A, Ocon C, Altieri P, Rodrigues Capítulo A (2017) Ecology of the non-native snail Sinotaia cf quadrata (Caenogastropoda: Viviparidae) A study in a lowland stream of South America with different water qualities. Anais Acad Brasil Ci 89(2):1059–1072. https://doi.org/10.1590/0001-3765201720160624
Gómez N, Licursi M (2001) The Pampean Diatom Index (IDP) for assessment of rivers and streams in Argentina. Aquat Ecol 35(2):173–181. https://doi.org/10.1023/A:1011415209445
Hamilton SK, Tank JL, Raikow DF, Wollheim WM, Peterson BJ, Webster JR (2001) Nitrogen uptake and transformation in a midwestern US stream: a stable isotope enrichment study. Biogeochemistry 54(3):297–340. https://doi.org/10.1023/A:1010635524108
Hardin G (1960) The competitive exclusion principle. Science 131(3409):1292–1297
Hayes KA, Burks RL, Castro-Vazquez A et al (2015) Insights from an integrated view of the biology of apple snails (Caenogastropoda: Ampullariidae). Malacologia 58(1–2):245–302. https://doi.org/10.4002/040.058.0209
Hurtado MA, Giménez JE, Cabral MG et al (2006) Análisis ambiental del Partido de La Plata, Aportes al Ordenamiento Territorial. Instituto de Geomorfología y Suelos, Buenos Aires, p 129
Jaarsma NG, De Boer SM, Townsend CR, Thompson RM, Edwards ED (1998) Characterising food–webs in two New Zealand streams. N Z J Mar Freshw Res 32(2):271–286. https://doi.org/10.1080/00288330.1998.9516825
Johnson PT, Olden JD, Solomon CT, Vander Zanden MJ (2009) Interactions among invaders: community and ecosystem effects of multiple invasive species in an experimental aquatic system. Oecologia 159(1):161–170. https://doi.org/10.1007/s00442-008-1176-x
Kesner D, Kumschick S (2018) Gastropods alien to South Africa cause severe environmental harm in their global alien ranges across habitats. Ecol Evol 8(16):8273–8285. https://doi.org/10.1002/ece3.4385
Kwong KL, Dudgeon D, Wong PK, Qiu J (2010) Secondary production and diet of an invasive snail in freshwater wetlands: implications for resource utilization and competition. Biol Invasions 12(5):1153–1164. https://doi.org/10.1007/s10530-009-9537-x
Larson M, Ross Black A (2016) Assessing interactions among native snails and the invasive New Zealand mud snail, Potamopyrgus antipodarum, using grazing experiments and stable isotope analysis. Hydrobiologia 763(1):147–159. https://doi.org/10.1007/s10750-015-2369-z
Layman CA, Allgeier JE (2012) Characterizing trophic ecology of generalist consumers: a case study of the invasive lionfish in The Bahamas. Mar Ecol Prog Ser 448:131–141. https://doi.org/10.3354/meps09511
Lenth RV (2016) Least-squares means: the R package lsmeans. J Stat Softw 69:1–33
López Van Oosterom MV, Ocon CS, Brancolini F, Maroñas ME, Sendra ED, Rodrigues Capítulo A (2013) Trophic relationships between macroinvertebrates and fish in a pampean lowland stream (Argentina). Iheringia Ser Zool 103(1):57–65. https://doi.org/10.1590/S0073-47212013000100009
López Van Oosterom MV, Ocon C, Ferreira AC, Rodrigues Capítulo A (2016) The diet of Pomacea canaliculata (Gastropoda: Ampullariidae) in its native habitat based on gut content and stable isotopes analysis. Intropica 11(1):73–83. https://doi.org/10.21676/23897864.1864
Lysne S, Koetsier P (2008) Comparison of desert valvata snail growth at three densities of the invasive New Zealand mudsnail. West N Am Nat 68(1):103–106. https://doi.org/10.3398/1527-0904(2008)68[103:CODVSG]2.0.CO;2
Lysne SJ, Perez KE, Brown KM, Minton RL, Sides JD (2008) A review of freshwater gastropod conservation: challenges and opportunities. J N Am Benthol Soc 27(2):463–470. https://doi.org/10.1899/07-061.1
Madsen H (1992) Food selection by freshwater snails in the Gezira irrigation canals, Sudan. Hydrobiologia 228(3):203–217. https://doi.org/10.1007/BF00006587
Maldonado MA, Martín PR (2019) Dealing with a hyper–successful neighbor: effects of the invasive apple snail Pomacea canaliculata on exotic and native snails in South America. Curr Zool 65(3):225–235. https://doi.org/10.1093/cz/zoy060
Manara E, Maldonado MA, Martín PR (2018) The role of an invader in its native range: could differential grazing by apple snails structure the submersed macrophytes assemblages in Southern Pampas (Argentina)? Hydrobiologia 828(1):229–242. https://doi.org/10.1007/s10750-018-3834-2
McCutchan JH Jr, Lewis WM Jr (2002) Relative importance of carbon sources for macroinvertebrates in a Rocky Mountain stream. Limnol Oceanogr 47(3):742–752. https://doi.org/10.4319/lo.2002.47.3.0742
McCutchan JH Jr, Lewis WM Jr, Kendall C, McGrath CC (2003) Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 102(2):378–390. https://doi.org/10.1034/j.1600-0706.2003.12098.x
Minshall GW (1978) Autotrophy in stream ecosystems. Bioscience 28(12):767–771. https://doi.org/10.2307/1307250
Miranda NA, Perissinotto R (2012) Stable isotope evidence for dietary overlap between alien and native gastropods in coastal lakes of northern KwaZulu-Natal, South Africa. PLoS ONE. https://doi.org/10.1371/journal.pone.0031897
Morrissey CA, Boldt A, Mapstone A, Newton J, Ormerod SJ (2013) Stable isotopes as indicators of wastewater effects on the macroinvertebrates of urban rivers. Hydrobiologia 700(1):231–244. https://doi.org/10.1007/s10750-012-1233-7
Muñoz I., Romaní AM., Rodrigues Capítulo A, Esteban, JG, Berthou EG (2009) Relaciones tróficas en el ecosistema fluvial. In: Elosegi A, Sabater S (eds) Conceptos y técnicas en ecología fluvial. Fundación BBVA, Bilbao, España, pp 347–366
Nielsen JM, Clare EL, Hayden B, Brett MT, Kratina P (2018) Diet tracing in ecology: method comparison and selection. Methods Ecol Evol 9(2):278–291. https://doi.org/10.1111/2041-210X.12869
Novakowski GC, Hahn NS, Fugi R (2008) Diet seasonality and food overlap of the fish assemblage in a pantanal pond. Neotrop Ichthyol 6(4):567–576. https://doi.org/10.1590/S1679-62252008000400004
Ocon C, López Van Oosterom MV, Muñoz MI, Rodrigues Capítulo A (2013) Macroinvertebrate trophic responses to nutrient addition in a temperate stream in South America. Fundam Appl Limnol 182(1):17–30. https://doi.org/10.1127/1863-9135/2013/0382
Ovando MXC, Cuezzo MG (2012) Discovery of an established population of a non–native species of Viviparidae (Caenogastropoda) in Argentina. Molluscan Res 32(3):121–131
Posch H, Garr AL, Reynolds E (2013) The presence of an exotic snail, Pomacea maculata, inhibits growth of juvenile Florida apple snails, Pomacea Paludosa. J Molluscan Stud 79(4):383–385. https://doi.org/10.1093/mollus/eyt034
Qiu JW, Chan MT, Kwong KL, Sun J (2011) Consumption, survival and growth in the invasive freshwater snail Pomacea canaliculata: does food freshness matter? J Molluscan Stud 77(2):189–195. https://doi.org/10.1093/mollus/eyr005
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Reynaga MC, Rueda Martín PA (2014) Trophic analysis of three species of Marilia (Trichoptera: Odontoceridae) from the neotropics. Rev Biol Trop 62(2):543–550
Riley LA, Dybdahl MF, Hall JRRO (2008) Invasive species impact: asymmetric interactions between invasive and endemic freshwater snails. J N Am Benthol Soc 27(3):509–520. https://doi.org/10.1899/07-119.1
Romaní AM, Sabater S (2001) Structure and activity of rock and sand biofilms in a Mediterranean stream. Ecology 82:3232–3245. https://doi.org/10.1890/0012-9658(2001)082[3232:SAAORA]2.0.CO;2
Schoener TW (1970) Nonsynchronous spatial overlap of lizards in patchy habitats. Ecology 51(3):408–418. https://doi.org/10.2307/1935376
Schoener TW (1974) Resource partitioning in ecological communities. Science 185(4145):27–39. https://doi.org/10.1126/science.185.4145.27
Schreiber ESG, Quinn GP, Lake PS (2003) Distribution of an alien aquatic snail in relation to flow variability, human activities and water quality. Freshw Biol 48(6):951–961. https://doi.org/10.1046/j.1365-2427.2003.01070.x
Semmens BX, Stock BC, Ward E (2013) MixSIAR: a Bayesian stable isotope mixing model for characterizing intrapopulation niche variation. Ecological Society of America, Minneapolis, pp 04–09
Seuffert ME, Martín PR (2013) Distribution of the apple snail Pomacea canaliculata in Pampean streams (Argentina) at different spatial scales. Limnologica 43(2):91–99. https://doi.org/10.1016/j.limno.2012.06.002
Smucker NJ, Kuhn A, Cruz-Quinones CJ, Serbst JR, Lake JL (2018) Stable isotopes of algae and macroinvertebrates in streams respond to watershed urbanization, inform management goals, and indicate food web relationships. Ecol Indic 90:295–304. https://doi.org/10.1016/j.ecolind.2018.03.024
Solomon CT, Olden JD, Johnson PTJ, Dillon JRRT, Vander Zanden MJ (2010) Distribution and community-level effects of the Chinese mystery snail (Bellamya chinensis) in northern Wisconsin lakes. Biol Invasions 12(6):1591–1605. https://doi.org/10.1007/s10530-009-9572-7
Sor R, Boets P, Lek S, Goethals P (2017) Spatio-temporal co-occurrence of alien and native molluscs: a modelling approach using physical-chemical predictors. Aquat Invasions 12(2):147–158. https://doi.org/10.3391/ai.2017.12.2.03
Strayer D (2010) Alien species in fresh waters: ecological effects, interactions with other stressors, and prospects for the future. Freshw Biol 55:152–174. https://doi.org/10.1111/j.1365-2427.2009.02380.x
Vander Zanden MJ, Rasmussen JB (1999) Primary consumer δ13C and δ15N and the trophic position of aquatic consumers. Ecology 80(4):1395–1404
Wallace JRRK (1981) An assessment of diet-overlap indexes. Trans Am Fish Soc 110(1):72–76. https://doi.org/10.1577/1548-8659(1981)110%3c72:AAODI%3e2.0.CO;2
Wolters JW, Verdonschot RCM, Schoelynck J, Brion N, Verdonschot PFM, Meire P (2018) Stable isotope measurements confirm consumption of submerged macrophytes by macroinvertebrate and fish taxa. Aquat Ecol 52(4):269–280. https://doi.org/10.1007/s10452-018-9662-7
Zahn Seegert SE, Rosi-Marshall EJ, Baxter CV, Kennedy TA, Hall JRRO, Cross WF (2014) High diet overlap between native small-bodied fishes and nonnative fathead minnow in the Colorado River, Grand Canyon, Arizona. Trans Am Fish Soc 143(4):1072–1083. https://doi.org/10.1080/00028487.2014.901250
Acknowledgements
We are grateful for the valuable suggestions from three anonymous reviewers who helped us to improve the paper. This study was supported by the Consejo Nacional de Investigaciones Científicas y Técnicas PIP N° 0570 and Universidad Nacional de La Plata N° 738-869. This paper is Scientific Contribution N° 1170 of the Institute of Limnology “Dr. Raúl A. Ringuelet” (ILPLA, CONICET-La Plata, UNLP).
Funding
This study was supported by the Consejo Nacional de Investigaciones Científicas y Técnicas PIP N° 0570 and the Universidad Nacional de La Plata N° 738-869.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This research does not present conflict of interest and has not being considered for publication elsewhere.
Ethical approval
Not applicable.
Consent to participate
All the authors have fully participated in this manuscript and accept responsibility for it.
Additional information
Handling Editor: Eric Larson.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Altieri, P., Paz, L.E., Ferreira, A.C. et al. Differential use of trophic resources between an exotic and a coexisting native snail. Limnology 23, 103–110 (2022). https://doi.org/10.1007/s10201-021-00671-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10201-021-00671-1