Abstract
Calcium (Ca) concentrations of many softwater lakes on the Canadian Shield have been in decline for decades as a response primarily to regional acid deposition and repeated cycles of forest harvesting. These Ca declines are of ecological interest, as many lakes have fallen to Ca levels detrimental to the fitness of ecologically important Ca-rich cladoceran taxa such as Daphnia pulex. However, distinguishing the impacts of reduced Ca from acidification (i.e., decreasing pH) on Ca-demanding fauna has proven difficult due to strong correlations between pH and Ca in softwater lakes. Here, we examine cladoceran sedimentary assemblages from low Ca lakes (mean present-day Ca < 2.0 mg l−1) in the Experimental Lakes Area (ELA) of Ontario, Canada, where Ca concentrations have declined since the 1980s, despite being closed to forestry and remote from (i.e., not downwind of) major sources of acid deposition. Differences between present-day and pre-industrial cladoceran assemblages were greatest among planktonic taxa, including Bosmina spp. and Holopedium glacialis. However, daphniid remains were present in only three of the ten study lakes with minimal directional trends. Overall, in these ELA lakes, daphniid abundance was low historically, and impacts possibly attributable to Ca declines have been obscured by the effects of regional climate warming.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Declines in Ca concentration have been observed in large numbers of softwater lakes on the Canadian Shield (Stoddard et al., 1999; Jeziorski et al., 2008), and are generally attributed to depletion of the base cation reservoirs in watershed soils at rates faster than their replenishment via geochemical weathering of the underlying bedrock, as well as reduced leaching rates from these reservoirs (Kirchner & Lydersen, 1995; Houle et al., 2006). Two principal mechanisms are typically responsible for base cation depletion in the catchments of softwater boreal lakes: biomass removal via repeated timber harvesting cycles (Watmough & Dillon, 2003), and the mobilization of base cations by acid deposition (Likens et al., 1996). The impact of aqueous Ca decline on surface waters is a topic of concern, as reduced lakewater Ca availability may have profound ecological consequences should it fall below physiological thresholds of Ca-demanding organisms, including cladoceran members of the crustacean zooplankton (e.g., daphniids; Cairns & Yan, 2009). Due to their intermediate trophic position, changes within cladoceran communities can have far-reaching impacts, as they are often the dominant algal grazers and prey item of both fish and invertebrate predators (Brooks & Dodson, 1965; Wissel et al., 2003; Korosi et al., 2012).
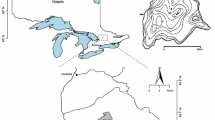
The Experimental Lakes Area (ELA), located in a sparsely populated region of northwestern Ontario ~50 km from Kenora (Fig. 1), is a location well-suited to the study of the impacts Ca decline in the absence of its two principal causes. The site was established in 1968 by the Government of Canada for the study of boreal lakes (including whole-lake manipulation studies) in a region that was relatively undisturbed by human and industrial activity and has since remained distant (and due to the prevailing westerlies, is not downwind) from sources of acid deposition while experiencing minimal forest harvesting due to its protected status (Johnson & Vallentyne, 1971; Jeffries et al., 2003; Blanchfield et al., 2009). The extensive monitoring history of many small lakes within the ELA and the relatively pristine conditions of these systems have proven ideal over the past four decades for studying a wide variety of environmental stressors, including cultural eutrophication, experimental lake acidification, climate warming, and aquaculture (Blanchfield et al., 2009). Reconstructions of diatom-inferred lakewater pH in northwestern Ontario show little change throughout the mid-twentieth century (e.g., Dickman et al., 1988; Paterson et al., 2002) despite the high levels of industrial sulfur emissions in eastern North America (e.g., Keller, 2009). This relative stability in lakewater pH is of particular importance for our study, as it is often difficult to separate the relative impacts of lake acidification (i.e., declines in pH) and Ca decline upon cladoceran communities (Jeziorski et al., 2012a), and although pH and Ca are typically positively correlated, the acidification of some softwater lakes may have been accompanied by an increase in Ca due to the accelerated leaching of base cations (e.g., Watmough & Aherne, 2008). In the absence of either of the two principal mechanisms of Ca decline (i.e., acidification and tree-harvesting), declining lakewater Ca concentrations have been documented in northwestern Ontario lakes (a mean decrease since the early 1980s of ~15% from 2.0 to 1.7 mg l−1 in 92 lakes; Jeziorski et al., 2008). Although decreases in pH have accompanied decreases in Ca in some cases, the lakes have remained circumneutral (mean pH = 6.6; Table 1), with pH values above thresholds of zooplankton community change (i.e., pH > 6: Holt et al., 2003; Jeziorski et al., 2008). Mechanisms that may be responsible for the small Ca declines in northwestern Ontario include: reduced atmospheric particulate deposition of base cations (including Ca; Hedin et al., 1994) in response to increased soil conservation practices and adoption of erosion control in the prairies (Agriculture and Agri-Food Canada, 1997), and variation in drought and fire frequency altering the acid neutralizing capacity of runoff (Bayley et al., 1992; Schindler et al., 1996). However, although increases in drought and fire frequency will increase the export of acid anions relative to base cations in stream runoff, base cation concentrations in lakes will likely rise as total runoff decreases, due to an increased release of base cations from lake sediments associated with increased water renewal time (Schindler et al., 1996).
Irrespective of the cause, Ca declines observed in ELA lakes provide a “natural laboratory” for the study of Ca decline in the absence of acid deposition and associated pH changes. The declines are of interest due to the importance of ambient lakewater Ca concentration upon the distribution of Ca-rich herbivorous zooplankton taxa, and particularly members of Daphnia spp. (0.8–8% of dry body weight; Wærvågen et al., 2002; Jeziorski & Yan, 2006; Cairns, 2010). Laboratory analyses have identified Ca concentrations <1.5 mg l−1 to be detrimental to daphniid growth, reproduction, and survival (Hessen et al., 2000; Ashforth & Yan, 2008; Tan & Wang, 2010) due in part to a poor ability to retain body Ca in low Ca environments (Tan & Wang, 2010), impairing processes such as the production of antipredator defenses (Riessen et al., 2012). Despite the accumulating evidence of the importance of Ca decline for Ca-rich cladoceran communities, interpretations of the indirect ecological effects of Ca decline have been complicated by the direct effects of acid deposition. The interdependence of lake pH and Ca concentration has had a confounding effect on determining the unique impacts of these environmental stressors (Jeziorski et al., 2012a, b), as Ca is a major contributor to the alkalinity of lakes on the Canadian Shield. Furthermore, difficulties in distinguishing the effects of low pH from Ca limitation have been exacerbated by similar tolerances among some cladoceran taxa to both stressors (e.g., the relative sensitivity of Daphnia pulex to both low Ca availability and low pH contrasts strongly with the relative resilience of Daphnia catawba to these conditions; Malley & Chang, 1986; Ashforth & Yan, 2008; Cairns, 2010).
Here we use paleolimnological methods to examine cladoceran remains from the sediments of ten ELA lakes that have present-day Ca concentrations near 1.5 mg l−1 (demonstrated in laboratory conditions to be detrimental to the growth and survival of Ca-rich daphniids; Hessen et al., 2000; Ashforth & Yan, 2008; Cairns, 2010; Tan & Wang, 2010). We test the hypothesis that the present-day and pre-industrial cladoceran sedimentary assemblages of these low Ca ELA lakes have changed significantly through time and investigate whether there is evidence of long-term changes among the most Ca-sensitive taxa (i.e., the daphniids) that are indicative of changes in Ca concentrations. Analysis of sediment intervals representing modern-day and pre-industrial times using the so-called “top/bottom” paleolimnological approach (Smol, 2008) identified daphniid remains in three of the ten study lakes (lakes 164, 378, and 383). Sediment cores from these lakes were then radiometrically dated and analyzed in greater temporal detail to examine long-term changes in both daphniid populations and the broader cladoceran assemblage.
Methods
The ten ELA study lakes (Fig. 1) chosen for this paleolimnological analysis are a subset of 40 reference lakes used previously to examine changes in diatom assemblages in the ELA since pre-industrial times (Enache et al., 2011). Sediment cores were collected from each lake during the summer of 2006 using a Glew (1989) gravity corer, and sectioned on shore at a resolution of 0.5 cm using a Glew (1988) vertical extruder. The sediment cores were stored in a cold room at the Paleoecological Environmental Assessment and Research Laboratory (Kingston, Canada) prior to analysis. The subset of lakes selected for cladoceran sedimentary analysis have modern-day Ca concentrations nearing levels identified as detrimental to daphniid fitness (1.0 mg l−1 < Ca < 2.75 mg l−1; Hessen et al., 2000; Ashforth & Yan, 2008; Tan & Wang, 2010). To ensure that pH impacts would not confound the potential influence of Ca limitation, the subset was further restricted to lakes with measured pH above thresholds known to produce changes in crustacean zooplankton communities (6.0 < pH < 7.2; Holt et al., 2003). The lakes were also limited by maximum depth (<20 m), area (<25 ha), color (6.25 < TCU < 50), and total phosphorus (TP) concentrations (<10 μg l−1), to allow comparisons with prior investigations of the effects of Ca decline on cladocerans within south-central Ontario lakes (Jeziorski et al., 2012a, b). This screening procedure reduced the initial set of 40 lakes to ten (i.e., lakes 93, 99, 110, 129, 132, 163, 164, 378, 383, and 384; Table 1).
In our “top/bottom” paleolimnological analysis (the strengths and limitations of this approach are discussed in Smol, 2008) of the ten study lakes, we compared cladoceran remains present in recently deposited sediments (i.e., the “top” sample, sediment depth 1.5–2.0 cm) with deeper sediments (i.e., the “bottom” sample, sediment depth 20.0–20.5 cm) representing pre-1900 (Enache et al., 2011), and deposited prior to the onset of acid deposition in eastern North America (Cogbill & Likens, 1974; Charles et al., 1990). For the three lakes in which daphniid remains were identified in both the “top” and “bottom” sediment samples (lakes 164, 378, and 383), sediments were analyzed in greater detail to determine how cladoceran assemblages changed through time. Age–depth relationships were determined using 210Pb radioisotopic techniques (Appleby, 2001) and gamma emission analysis (Schelske et al., 1994) by an Ortec® germanium-crystal well detector at the Paleoecological Environmental Assessment and Research Lab (Queen’s University, Kingston, ON, Canada). Dates were calculated using the constant rate of supply (CRS) model (Appleby & Oldfield, 1978; Binford, 1990), revealing similar activity profiles for each of the three lakes (Fig. 2). Background 210Pb activities at a sediment depth of ~15 cm (corresponding to a calendar date of ~1875), validate that the deeper sediment depth considered in our “top–bottom” analysis (i.e., 20.0–20.5 cm) is representative of a period prior to major industrial development in eastern North America. Cladoceran analyses were performed on ~20 additional sediment intervals from each of these three sediment cores, providing a decadal-scale resolution over the past ~150–200 years.
The preparation of microscope slides for the analysis of sedimentary cladoceran remains followed standard methods, as described in Korhola & Rautio (2001). Cladoceran remains (i.e., carapaces, headshields, postabdominal claws, etc.) were tabulated separately and the total number of individuals present was calculated using the most abundant remain for each taxon (Frey, 1986). A minimum of 90 individual cladocerans (a sub-sample large enough for a representative estimation of the sedimentary cladoceran assemblage; Kurek et al., 2010) were identified from each sediment interval using a Leica DMRB light microscope with bright field optics (10–40× objective, 10× ocular lens). To remove the potential bias of a non-random distribution of remains, each slide was counted in its entirety.
The primary taxonomic resources used for identification of the cladoceran remains included Smirnov (1996), Sweetman & Smol (2006), Szeroczyńska & Sarmaja-Korjonen (2007), and Korosi & Smol (2012a, b). The postabdominal claws from Daphnia spp. were attributed to a species complex according to the presence/absence of stout middle pecten (Korosi et al., 2011): the D. pulex complex (stout pecten present) in this region may include the species D. pulex, D. pulicaria, D. catawba, and D. minnehaha; whereas the D. longispina complex (stout pecten absent) may include D. mendotae, D. longiremis, D. dentifera, D. dubia, and D. retrocurva (Keller & Pitblado, 1989; Hebert, 1995).
To first examine broad differences in the cladoceran assemblages between the two time periods, an analysis of similarity (ANOSIM) was performed using the vegan package (Oksanen et al., 2010) for the R software environment (R Development Core Team, 2011). ANOSIM is a non-parametric multivariate test used to examine a similarity matrix (of square-root transformed cladoceran relative abundances) comparing the samples (lakes) from the two a priori defined groups (i.e., the “tops” vs. the “bottoms”) by ranking them according to Bray–Curtis similarity, with minimal statistical assumptions (Clarke, 1993). The ANOSIM test determines whether average pair-wise similarity within groups is greater than average pair-wise similarity between groups. To then test whether the relative abundances of any individual taxa significantly differed between the modern and pre-industrial samples, we used a non-parametric Wilcoxon Signed-Rank test, again using the vegan package (Oksanen et al., 2010).
Ordination techniques were used to summarize the patterns of change in the modern and pre-industrial cladoceran assemblages of the ten study lakes. The percent relative abundance species data were square-root transformed prior to the analyses to equalize the variance among taxa. An initial detrended correspondence analysis (DCA) of the modern-day and pre-industrial species assemblages revealed relatively short gradient lengths (1.82 and 1.40 SD units for the first and second axes in the modern samples, and 1.58 and 1.52 SD units for the pre-industrial assemblages). Consequently the relationships between species assemblages were explored with principal component analysis (PCA) using the vegan package (Oksanen et al., 2010) for the R software environment (R Development Core Team, 2011). Both modern and pre-industrial samples were run in a combined ordination to allow modern and pre-industrial niche space to be observed in the PCA biplot, thus providing an assessment of change in the lakes over time.
The sediment cores from the three lakes with daphniid remains present in the “top/bottom” samples were examined in greater detail both for general trends over time in the crustacean zooplankton assemblages, and more rigorously using cluster analyses (constrained incremental sum of squares (CONISS); Grimm, 1987), with the number of zones determined via the broken stick model (Bennett, 1996). CONISS was performed using the vegan package (Oksanen et al., 2010) for the R software environment (R Development Core Team, 2011).
Results
The sedimentary cladoceran assemblages identified in the initial “top/bottom” analysis of the ten study lakes were broadly typical of softwater lakes on the Canadian Shield, with relatively simple assemblages dominated by the pelagic taxa Bosmina spp. and a diverse collection of littoral chydorid taxa (principally Alona spp. and Chydorus spp.; Fig. 3). The ANOSIM did not identify significant differences between the two time periods across the full dataset (R = −0.01, P = 0.53), nor did any individual taxa show significant directional changes through time across all lakes in the Wilcoxon Signed-Rank tests. Differences between the two time periods were characterized by an increase in the relative abundances of Bosmina spp. of 10% or more in 5 of the 10 lakes, as well as smaller increases in Alona intermedia, and a coincident decrease in the other chydorid taxa (notably Alona affinis/quadrangularis; Fig. 4). As the ten study lakes were screened a priori to ensure similar physical and chemical environments, the modern-day crustacean zooplankton sedimentary assemblages were broadly similar, demonstrated by their similar site scores in the PCA biplot (47% of the variation present in the relative abundance data (square-root transformed) was captured by the first two ordination axes; Fig. 5).
Principal component analysis (PCA) biplot showing the relationship between species and site scores for the ten lakes of the “top/bottom” analysis (modern-day site scores, black triangles; pre-industrial site scores, gray triangles). Species names and associated symbols are Acroperus harpae (Aharp), Alonella excisa (Aexci), Alonella nana (Anana), Alona affinis (Aaffi), Alona circumfimbriata (Acirc), Alona guttata (Agutt), Alona intermedia (Aint), Alona quadrangularis (Aquad), Bosmina spp. (Bosm), Chydorus brevilabris (Cbrev), Chydorus gibbus (Cgibb), Daphnia longispina complex (Dlong)
Daphniid remains were recorded in only three of the ten lakes (164, 378, and 383; Fig. 3), albeit at small relative abundances: Lake 164 had daphniids present in low (~1%) relative abundances in the “bottom” interval; Lake 378 had daphniids present in relatively high (~10%) abundances in the “top” and “bottom” intervals; and in Lake 383, daphniids were present in low (~2%) relative abundance in both the “top” and “bottom” intervals.
The down-core sedimentary cladoceran assemblages from Lake 164 remained stable throughout the core (Fig. 6). Bosminid remains dominated the assemblages (~90% relative abundance) for the entire record, whereas daphniids (solely represented by the Daphnia longispina complex) were present only sporadically in low abundances. The most notable changes were the appearance and gradual increase of Holopedium glacialis beginning in the 1960s, coinciding with the disappearance of Alonella nana from the littoral community. After performing the cluster analysis (CONISS), no “significant” zones were identified by the broken stick model (Bennett, 1996).
Lake 378 contained the most diverse assemblages of the three lakes (Fig. 7), with 31 species or groups identified. Although bosminid remains dominated the assemblages, daphniids (particularly members of the D. longispina complex) also comprised a sizable proportion (~10%) of the assemblage. Despite variability among the littoral species, the assemblages have remained relatively stable throughout the last two centuries. After performing the cluster analysis (CONISS), no “significant” zones were identified by the broken stick model (Bennett, 1996).
Of the three lakes analyzed in detail, Lake 383 had the most pronounced changes in its crustacean zooplankton assemblages (Fig. 8). Bosminid remains were present in high abundances throughout the core; however, their dominance increased from ~65% in the late 1800s to ~80% at the start of the twentieth century. Remains from the D. longispina complex were present in low, but consistent abundances from the 1880s until the 1940s, at which point they were virtually eliminated from the sediments and subsequently only present in a few intervals at trace levels. Concurrent with the disappearance of the daphniids in the 1940s, H. glacialis appeared and has gradually increased in relative abundance in recent sediment intervals. The littoral community was dominated throughout the record by the combination of A. nana and Chydorus spp.; however, both have declined in relative abundance since the beginning of the record, coincident with general increases in Bosmina spp. The only zonation identified as “significant” by the broken stick model (Bennett, 1996) occurs at ~1900.
Relative frequency diagram of the most common crustacean zooplankton remains found in the sediments of Lake 383. Sediments were radiometrically dated using 210Pb. Results of the constrained incremental sum of squares (CONISS) are also shown (the horizontal dashed line marks the zonation identified by the broken stick model)
Discussion
The water chemistry of the ELA study lakes is typical of softwater lakes located on the Canadian Shield; however, over the past several decades, there has been a regional decline in lakewater Ca concentration of ~15% while pH has remained circumneutral (a 2002 survey of 92 ELA lakes revealed 45% were <1.5 mg Ca l−1, relative to only 21% 20 years earlier; Findlay & Shearer, 1992; Jeziorski et al., 2008). Eight of the ten study lakes (the exceptions were Lake 163 and Lake 378) examined in the “top/bottom” sediment analysis were included in the Canadian Wildlife Service helicopter surveys described by Jeziorski et al. (2008) and seven of these eight lakes have experienced declines of ~15–20% in aqueous Ca since the 1980s (presently all of the study lakes are below 2.8 mg l−1 Ca; Table 1). However, despite declines in lakewater Ca, the sedimentary cladoceran assemblages have remained relatively stable (Figs. 3, 4), and radiometric dating of the three sediment cores (lakes 164, 378, and 383) revealed that background 210Pb activity (i.e., ~1875; Binford, 1990) corresponded with a sediment depth of ~15 cm (Fig. 2), a shallower depth than was used for the “bottoms” of the “top/bottom” analysis (~15 vs. 20.0–20.5 cm). Despite this disparity, the assemblages from the “bottom” interval and the bottom of the dated sediments were very similar for each lake, further demonstrating the relative stability of these assemblages.
Small differences between the “top” and “bottom” cladoceran sedimentary assemblages were identified via PCA analysis, with the principal changes being an increase in the pelagic cladocerans, especially Bosmina spp. (Fig. 5). The lakes exhibiting the greatest changes over time (notably 132, 378, and 384) were those that had the greatest changes in Bosmina spp. since the pre-industrial period (Fig. 3). However, the accompanying changes in the relative abundances of the littoral taxa, Alona spp. and Chydorus spp., as well as the D. longispina complex, differ in both composition and direction among lakes, suggesting multiple factors may be responsible for the species shifts.
The general trend among cladoceran assemblages within both the “top/bottom” samples and the detailed cores were declines in littoral species (notably Alona spp. and Chydorus spp.) since the pre-industrial period and, in some cases, their complete disappearance from the assemblage. The dwindling abundances of littoral species resulted in the only zonation identified in the cluster analyses (at ~1900 in Lake 383; Fig. 8). The reduction in the relative abundances of littoral species is accompanied by a concurrent increase in Bosmina spp., pre-dating the changes in Daphnia spp. and H. glacialis relative abundance during the mid-twentieth century. The ELA’s location has kept it relatively undisturbed with respect to local anthropogenic stressors (the watershed of Lake 383 did experience a fire during 1974, but there is little evidence of its impact on the cladoceran sedimentary assemblage; Fig. 8), yet there has been some regional environmental changes over recent decades, including a prolonged warm period during the 1970s and 1980s which resulted in lakes becoming warmer and clearer (Schindler et al., 1996). The decrease in stream export associated with this period of drought reduced chemical inputs to the lakes decreasing concentrations of dissolved organic carbon (DOC; Schindler et al., 1997), a change that may have increased the vulnerability of daphniids to visual predators relative to Holopedium (Wissel et al., 2003). However, the changes among the cladoceran sedimentary assemblages (i.e., in the early and mid-twentieth century; Figs. 6, 7, 8) pre-date this recent warm period and are more likely associated with an earlier climate response detected in paleolimnological analyses of ELA phytoplankton (Enache et al., 2011). Broad ecological shifts within the algal diatom communities of temperate lakes (i.e., increases in the relative abundances of pelagic taxa concurrent with decreases in littoral taxa) have been attributed to increases in the intensity of lake stratification and longer ice-free periods associated with regional climate warming (Rühland et al., 2008), and the substantive modern-day increases in planktonic diatom abundances in lakes 99, 132, and 378 (Enache et al., 2011) coincide with some of the largest bosminid increases in the ten study lakes.
Although our intent was to examine lakewater Ca decline in circumneutral ELA lakes using changes in daphniid abundance, this objective was complicated by the absence or low relative abundance of daphniids in nine of the ten study lakes (Fig. 3). The relative scarcity of daphniids may be attributable to a focus on the extreme low end of daphniids’ Ca range, as laboratory estimates of a 1.5 mg l−1 Ca limitation threshold for D. pulex (Ashforth & Yan, 2008) are substantially lower than recent field estimates of optimal Ca concentrations for many daphniid taxa common to softwater Shield lakes (2.76–16.10 mg l−1; Cairns, 2010). The daphniid remains identified in both the “top/bottom” (Fig. 5) and the detailed core analyses (Figs. 6, 7, 8) were almost exclusively from the D. longispina species complex (the complex most sensitive to Ca limitation; Cairns, 2010; Jeziorski et al., 2012a), with only a minimal presence of the D. pulex complex in the sediments despite historical records indicating the existence of D. catawba (a species tolerant of both low Ca and low pH conditions; Malley & Chang, 1986; Cairns, 2010) in low abundances in this region (Patalas, 1971). Of the three lakes containing daphniid remains, the greatest changes in daphniid relative abundance occurred in Lake 383 with a decrease from low, but consistent abundances (~2%) to near absence during the 1940s. Coincident with this daphniid decline/disappearance was the arrival and persistence of the planktonic taxon H. glacialis. Although little is known regarding the Ca requirements of the littoral Cladocera, three littoral taxa encountered in the “top/bottom” analyses (Acroperus harpae, Alonella excisa, and Chydorus brevilabris; Fig. 3) have been identified as having relatively low Ca content (i.e., % of dry body weight; Shapiera et al., 2011). However, none of these three species exhibited much directional change between the two time periods (Figs. 3, 4).
Among pelagic cladocerans, there have been small (~0–5%) increases in H. glacialis since the mid-twentieth century in lakes 164 and 383, a decrease in the D. longispina complex since ~1940 in Lake 383, and an increase in Bosmina spp. in 383 since ~1900. The rise of the Ca-poor taxa H. glacialis and Bosmina spp. (0.2–0.4% of dry body weight; Wærvågen et al., 2002; Jeziorski & Yan, 2006) at the expense of Ca-rich daphniid species (0.8–8% of dry body weight; Wærvågen et al., 2002; Jeziorski & Yan, 2006) in lakes 164 and 383 are changes consistent with reduced Ca availability, whereby taxa with low Ca requirements realize a competitive advantage. In other locations, the subtle changes since the 1940s in lakes 164 and 383 would likely be attributed to the onset of acid deposition (i.e., an increase in acid-tolerant taxa at the expense of acid-sensitive taxa). However, the ELA is remote from sources of industrial emissions and the study lakes are presently circumneutral (Table 1), with no evidence to suggest that lake pH has been low enough to constitute a stressor for acid-sensitive crustacean zooplankton taxa either since the establishment of the ELA (Patalas, 1971; Enache et al., 2011) or in diatom-inferred pH reconstructions dating back to pre-industrial times (e.g., Dickman et al., 1988; Paterson et al., 2002).
Although changes in the relative abundances of cladoceran remains from pelagic species in the sediment records of lakes 164 and 383 provide limited evidence of Ca limitation impacts on aquatic communities in the absence of concurrent pH changes and timber harvesting, the changes in Ca-rich versus Ca-poor species are subtle, despite indications of a threshold response in laboratory conditions (Hessen et al., 2000; Ashforth & Yan, 2008; Tan & Wang, 2010). The relative stability of the cladoceran assemblages (with the principal changes suggesting regional warming) and low daphniid abundances in most of the study lakes suggest that perhaps Ca limitation presents a competitive disadvantage prior to reaching lethal levels in natural environments, or that the poor taxonomic resolution of the daphniid species complexes in the sediment record is obscuring a progressive pattern of species replacement within the complex due to differing Ca thresholds (Cairns, 2010). As declines in lakewater Ca availability are expected to continue (Watmough et al., 2003) and the endpoints remain uncertain due to the site-specific nature of the issue, the next logical step in the development of the study of Ca decline using paleolimnological techniques will be to determine the taxa and/or communities that are benefitting from decreasing Ca availability (e.g., H. glacialis). Increasing our understanding of the broader response of aquatic communities in regions experiencing Ca declines will avoid the principal difficulties of recent observational studies focused upon daphniid changes (e.g., Jeziorski et al., 2012a). To take full advantage of the abundant information present in the sediment record, it is necessary to increase our knowledge of the Ca demands of a wider variety of fauna with remains that preserve well in sediments, and integrate this information with both direct monitoring programs in softwater regions and targeted sediment core analyses.
Conclusions
As neither acid deposition nor timber harvesting is present to any significant degree in the ELA region (limited logging occurred in the watersheds of selected ELA lakes between 1970 and 1980, including lakes 164 and 378), recent declines in lakewater Ca are most likely due to an alternate mechanism such as the reduced atmospheric deposition of Ca or increased stream acidity related to changes in drought (i.e., reduced base cation export) and forest fire frequency. Regardless of the underlying mechanism, Ca concentrations in some ELA lakes have fallen to levels that are detrimental to Ca-rich daphniids and although significant assemblage-wide differences among the sedimentary cladoceran remains were not observed, there have been subtle decreases in daphniid relative abundance and increases in their Ca-poor competitors (Bosmina spp. and H. glacialis). However, although the shifts in daphniid fauna and Ca availability have occurred at circumneutral pH levels, the minor changes among the cladoceran sedimentary assemblages have likely been exacerbated by climate warming in recent decades. Due to the taxonomic limitations of daphniid sedimentary remains, future paleolimnological work examining the impact of Ca decline using cladocerans should focus on taxa such as H. glacialis that are likely to benefit from reduced Ca availability.
References
Agriculture and Agri-Food Canada, 1997. Profile of Production Trends and Environmental Issues in Canada’s Agriculture and Agri-Food Sector. Minister of Public Works and Government Services, Ottawa, Canada.
Appleby, P. G., 2001. Chronostratigraphic techniques in recent sediments. In Last, W. M. & J. P. Smol (eds), Tracking Environmental Changes Using Lake Sediments, Vol. 1: Basin Analysis, Coring and Chronological Techniques. Kluwer Academic Press, Dordrecht: 171–204.
Appleby, P. G. & F. Oldfield, 1978. The calculation of 210Pb dates assuming a constant rate of supply of unsupported 210Pb to the sediments. Catena 5: 1–8.
Ashforth, D. & N. D. Yan, 2008. The interactive effects of calcium concentration and temperature on the survival and reproduction of Daphnia pulex at high and low food concentrations. Limnology and Oceanography 53: 420–432.
Bayley, S. E., D. W. Schindler, B. R. Parker, M. P. Stainton & K. G. Beaty, 1992. Effects of forest fire and drought on acidity of a base-poor boreal forest stream: similarities between climatic warming and acidic precipitation. Biogeochemistry 17: 191–204.
Bennett, K. D., 1996. Determination of the number of zones in a biostratigraphical sequence. New Phytologist 132: 155–170.
Binford, M. W., 1990. Calculation and uncertainty analysis of 210Pb dates for PIRLA project lake sediment cores. Journal of Paleolimnology 3: 253–267.
Blanchfield, P. J., M. J. Paterson, J. A. Shearer & D. W. Schindler, 2009. Johnson and Vallentyne’s legacy: 40 years of aquatic research at the Experimental Lakes Area. Canadian Journal of Fisheries and Aquatic Sciences 66: 1831–1836.
Brooks, J. L. & S. I. Dodson, 1965. Predation, body size, and composition of plankton. Science 150: 28–35.
Cairns, A., 2010. Field assessments and evidence of impact of calcium decline on Daphnia (Crustacea, Anomopoda) in Canadian Shield lakes. M.Sc. Thesis, York University, Toronto, ON, Canada.
Cairns, A. & N. D. Yan, 2009. A review of laboratory and field evidence of the influence of low ambient calcium concentrations on daphniids, gammarids and crayfish. Environmental Reviews 17: 67–79.
Charles, D. F., M. W. Binford, E. T. Furlong, R. A. Hites, M. J. Mitchell, S. A. Norton, F. Oldfield, M. J. Paterson, J. P. Smol, A. J. Uutala, J. R. White, D. R. Whitehead & R. J. Wise, 1990. Paleoecological investigation of recent lake acidification in the Adirondack Mountains, NY. Journal of Paleolimnology 3: 195–241.
Clarke, K. R., 1993. Non-parametric multivariate analyses of changes in community structure. Australian Journal of Ecology 18: 117–143.
Cogbill, C. V. & G. E. Likens, 1974. Acid precipitation in the northeastern United States. Water Resources Research 10: 1133–1137.
Dickman M., H. G. Thode, S. Rao & R. Anderson, 1988. Downcore sulphur isotope ratios and diatom inferred pH in an artifically acidified Canadian Shield lake. Environmental Pollution 49: 265–288.
Enache, M. D., A. M. Paterson & B. F. Cumming, 2011. Changes in diatom assemblages since pre-industrial times in 40 reference lakes from the Experimental Lakes Area (northwestern Ontario, Canada). Journal of Paleolimnology 46: 1–15.
Findlay, D. L. & J. A. Shearer, 1992. Relationships between sedimentary diatom assemblages and lakewater pH values in the Experimental Lakes Area. Journal of Paleolimnology 7: 145–156.
Frey, D. G., 1986. Cladocera analysis. In Berglund, B. E. (ed.), Handbook of Holocene Palaeoecology and Palaeohydrology. Wiley, New York: 667–692.
Glew, J. R., 1988. A portable extruding device for close interval sectioning of unconsolidated core samples. Journal of Paleolimnology 1: 235–239.
Glew, J. R., 1989. A new trigger mechanism for sediment samplers. Journal of Paleolimnology 2: 241–243.
Grimm, E. C., 1987. CONISS: a Fortran 77 program for stratigraphically constrained cluster analysis by the method of incremental sum of squares. Computers & Geosciences 13: 13–35.
Hebert, P. D. N., 1995. The Daphnia of North America: An Illustrated Fauna (CD-ROM). University of Guelph, Guelph.
Hedin, L. O., L. Granat, G. E. Likens, T. A. Buishand, J. N. Galloway, T. J. Butler & H. Rodhe, 1994. Steep declines in atmospheric base cations in regions of Europe and North America. Nature 367: 351–354.
Hessen, D. O., N. E. W. Alstad & L. Skardal, 2000. Calcium limitation in Daphnia magna. Journal of Plankton Research 22: 553–568.
Holt, C. A., N. D. Yan & K. M. Somers, 2003. pH 6 as the threshold to use in critical load modeling for zooplankton community change with acidification in lakes of south-central Ontario: accounting for morphometry and geography. Canadian Journal of Fisheries and Aquatic Sciences 60: 151–158.
Houle, D., R. Ouimet, S. Couture & C. Gagnon, 2006. Base cation reservoirs in soil control the buffering capacity of lakes in forested catchments. Canadian Journal of Fisheries and Aquatic Sciences 63: 471–474.
Jeffries, D. S., T. A. Clair, S. Couture, P. J. Dillon, J. Dupont, W. Keller, D. K. McNicol, M. A. Turner, R. Vet & R. Weeber, 2003. Assessing the recovery of lakes in southeastern Canada from the effects of acidic deposition. Ambio 32: 176–182.
Jeziorski, A. & N. D. Yan, 2006. Species identity and aqueous calcium concentrations as determinants of calcium concentrations of freshwater crustacean zooplankton. Canadian Journal of Fisheries and Aquatic Sciences 63: 1007–1013.
Jeziorski, A., N. D. Yan, A. M. Paterson, A. M. DeSellas, M. A. Turner, D. S. Jeffries, B. Keller, R. C. Weeber, D. K. McNicol, M. E. Palmer, K. McIver, K. Arseneau, B. K. Ginn, B. F. Cumming & J. P. Smol, 2008. The widespread threat of calcium decline in fresh waters. Science 322: 1374–1377.
Jeziorski, A., A. M. Paterson & J. P. Smol, 2012a. Changes since the onset of acid deposition among calcium-sensitive cladoceran taxa within softwater lakes of Ontario, Canada. Journal of Paleolimnology 48: 323–337.
Jeziorski, A., A. M. Paterson & J. P. Smol, 2012b. Crustacean zooplankton sedimentary remains from calcium-poor lakes: complex responses to threshold concentrations. Aquatic Sciences 74: 121–131.
Johnson, W. E. & J. R. Vallentyne, 1971. Rationale, background and development of experimental lake studies in Northwestern Ontario. Journal of the Fisheries Research Board of Canada 28: 123–128.
Keller, W., 2009. Limnology in northeastern Ontario: from acidification to multiple stressors. Canadian Journal of Fisheries and Aquatic Sciences 66: 1189–1198.
Keller, W. & J. R. Pitblado, 1989. The distribution of crustacean zooplankton in Northern Ontario, Canada. Journal of Biogeography 16: 249–259.
Kirchner, J. W. & E. Lydersen, 1995. Base cation depletion and potential long-term acidification of Norwegian catchments. Environmental Science & Technology 29: 1953–1960.
Korhola, A. & M. Rautio, 2001. Cladocera and other branchiopod crustaceans. In Smol, J. P., H. J. B. Birks & W. M. Last (eds), Tracking Environmental Change Using Lake Sediments 4: Zoological Indicators. Kluwer Academic Publishers, Dordrecht: 5–41.
Korosi, J. B. & J. P. Smol, 2012a. An illustrated guide to the identification of cladoceran subfossils from lake sediments in northeastern North America: part 1—the Daphniidae, Leptodoridae, Bosminidae, Polyphemidae, Holopedidae, Sididae, and Macrothricidae. Journal of Paleolimnology 48: 571–586.
Korosi, J. B. & J. P. Smol, 2012b. An illustrated guide to the identification of cladoceran subfossils from lake sediments in northeastern North America: part 2—the Chydoridae. Journal of Paleolimnology 48: 587–622.
Korosi, J. B., A. Jeziorski & J. P. Smol, 2011. Using morphological characters of subfossil daphniid postabdominal claws to improve taxonomic resolution within species complexes. Hydrobiologia 676: 117–128.
Korosi, J. B., S. M. Burke, J. R. Thienpont & J. P. Smol, 2012. Anomalous rise in algal production linked to lakewater calcium decline through food web interactions. Proceedings of the Royal Society B 279: 1210–1217.
Kurek, J., J. B. Korosi, A. Jeziorski & J. P. Smol, 2010. Establishing reliable minimum count sizes for cladoceran subfossils sampled from lake sediments. Journal of Paleolimnology 44: 603–612.
Likens, G. E., C. T. Driscoll & D. C. Buso, 1996. Long-term effects of acid rain: response and recovery of a forest ecosystem. Science 272: 244–246.
Malley, D. F. & P. S. S. Chang, 1986. Increase in the abundance of Cladocera at pH 5.1 in experimentally-acidified lake 223, Experimental Lakes Area, Ontario. Water, Air and Soil Pollution 30: 629–638.
Oksanen, J., F. G. Blanchet, R. Kindt, P. Legendre, R. B. O’Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens & H. Wagner, 2010. Vegan: Community Ecology Package. R package version 1.17-4. http://CRAN.R-project.org/package=vegan.
Patalas, K., 1971. Crustacean plankton communities in forty-five lakes in the Experimental Lakes Area, Northwestern Ontario. Journal of the Fisheries Research Board of Canada 28: 231–244.
Paterson, A. M., D. S. Morimoto, B. F. Cumming, J. P. Smol & J. M. Szeicz, 2002. A paleolimnological investigation of the effects of forest fire on lake water quality in northwestern Ontario over the past ca. 150 years. Canadian Journal of Botany 80: 1329–1336.
R Development Core Team, 2011. R: A language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. ISBN 3-900051-07-0. http://www.R-project.org/.
Riessen, H. P., R. D. Linley, I. Altshuler, M. Rabus, T. Söllradi, H. Clausen-Schaumann, C. Laforsch & N. D. Yan, 2012. Changes in water chemistry can disable plankton prey defenses. Proceedings of the National Academy of Sciences of the United States of America 109: 15377–15382.
Rühland, K., A. M. Paterson & J. P. Smol, 2008. Hemispheric-scale patterns of climate-related shifts in planktonic diatoms from North American and European lakes. Global Change Biology 14: 1–15.
Schelske, C. L., A. Peplow, M. Brenner & C. N. Spencer, 1994. Low-background gamma counting: applications for 210Pb dating of sediments. Journal of Paleolimnology 10: 115–128.
Schindler, D. W., S. E. Bayley, B. R. Parker, K. G. Beaty, D. R. Cruikshank, E. J. Fee, E. U. Schindler & M. P. Stainton, 1996. The effects of climatic warming on the properties of boreal lakes and streams at the Experimental Lakes Area, Northwestern Ontario. Limnology and Oceanography 41: 1004–1017.
Schindler, D. W., P. J. Curtis, S. E. Bayley, B. R. Parker, K. G. Beaty & M. P. Stainton, 1997. Climate-induced changes in the dissolved organic carbon budgets of boreal lakes. Biogeochemistry 36: 9–28.
Shapiera, M., A. Jeziorski, N. D. Yan & J. P. Smol, 2011. Calcium content of littoral Cladocera in three softwater lakes of the Canadian Shield. Hydrobiologia 678: 77–83.
Smirnov, N. N., 1996. Cladocera: The Chydorinae and Sayciinae (Chydoridae) of the World. SPB Academic Publishing, Amsterdam.
Smol, J. P., 2008. Pollution of Lakes and Rivers: A paleoenvironmental Perspective, 2nd ed. Blackwell Publishing, Oxford.
Stoddard, J. L., D. S. Jeffries, A. Lukewille, T. A. Clair, P. J. Dillon, C. T. Driscoll, M. Forsius, M. Johnannessen, J. S. Kahl, J. H. Kellogg, A. Kemp, J. Mannio, D. T. Monteith, P. S. Murdoch, S. Patrick, A. Rebsdorf, B. L. Skjelkvale, M. P. Stainton, T. Traaen, H. van Dam, K. E. Webster, J. Wieting & A. Wilander, 1999. Regional trends in aquatic recovery from acidification in North America and Europe. Nature 401: 575–578.
Sweetman, J. N. & J. P. Smol, 2006. A guide to the identification of cladoceran remains (Crustacea, Branchiopoda) in Alaskan lake sediments. Archiv für Hydrobiologie (Supplementbände) Monograph Studies 151: 353–394.
Szeroczyńska, K. & K. Sarmaja-Korjonen, 2007. Atlas of Subfossil Cladocera from Central and Northern Europe. Friends of the Lower Vistula Society, Świecie.
Tan, Q. & W. Wang, 2010. Interspecies differences in calcium content and requirement in four freshwater cladocerans explained by biokinetic parameters. Limnology and Oceanography 55: 1426–1434.
Wærvågen, S. B., N. A. Rukke & D. O. Hessen, 2002. Calcium content of crustacean zooplankton and its potential role in species distribution. Freshwater Biology 47: 1866–1878.
Watmough, S. A. & J. Aherne, 2008. Estimating calcium weathering rates and future lake calcium concentrations in the Muskoka–Haliburton region of Ontario. Canadian Journal of Fisheries and Aquatic Sciences 65: 821–833.
Watmough, S. A. & P. J. Dillon, 2003. Base cation and nitrogen budgets for seven forested catchments in central Ontario, 1983–1999. Forest Ecology and Management 177: 155–177.
Watmough, S. A., J. Aherne & P. J. Dillon, 2003. Potential impact of forest harvesting on lake chemistry in south-central Ontario at current levels of acid deposition. Canadian Journal of Fisheries and Aquatic Sciences 60: 1095–1103.
Wissel, B., W. J. Boeing & C. W. Ramcharan, 2003. Effects of water color on predation regimes and zooplankton assemblages in freshwater lakes. Limnology and Oceanography 46: 1965–1976.
Acknowledgments
This project was funded through a Natural Sciences and Engineering Research Council (NSERC) grant to J. P. Smol. Field work was funded by a NSERC grant to Brian F. Cumming. We would also like to thank Dr. J. Kurek, as well as two journal reviewers, for their insightful comments on this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Karl E. Havens
Rights and permissions
About this article
Cite this article
Jeziorski, A., Paterson, A.M., Watson, I. et al. The influence of calcium decline and climate change on the cladocerans within low calcium, circumneutral lakes of the Experimental Lakes Area. Hydrobiologia 722, 129–142 (2014). https://doi.org/10.1007/s10750-013-1691-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-013-1691-6