Abstract
Urban and peri-urban lakes experience a wider array of environmental stressors, and often at a higher intensity, than their rural counterparts, including road salt runoff. A paleolimnological approach was used to determine pre-disturbance limnological conditions and to evaluate the impact of environmental stressors (nutrient inputs, climate change, and winter de-icing salt) on the long-term (~ 150 years) water quality of a small urban kettle lake in the Oak Ridges Moraine near Toronto, Ontario, Canada. Specifically, we examined Cladocera and diatom subfossils in 210Pb-dated sediment cores from a lake with elevated measured chloride concentrations, Haynes Lake (Cl− = 201 mg/l), and a nearby reference lake located in a conservation area (Swan Lake, Cl− = 28 mg/l). In Haynes Lake, Cladocera compositional change is consistent with increasing Cl− concentrations, showing a shift from a Bosmina spp.-dominated cladoceran assemblage to a Daphnia spp.-dominated assemblage. Concurrently, we recorded increases in the relative abundances of the diatom taxon Achnanthidium minutissimum and benthic fragilarioid taxa. These biological changes coincided closely with the onset of road salting in the region (ca. 1940s). The reference site (Swan Lake), located ~ 1 km from our salt-impacted site, displayed only minimal changes in both Cladocera and diatom assemblages, suggesting road development and salting within the Haynes Lake watershed had a larger impact than regional stressors (i.e., climate).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Urban and peri-urban lakes are subjected to multiple local anthropogenic stressors, including residential development, recreation, and agriculture within their watersheds, as well as regional and global stressors, such as climate change (Reid et al., 2019). In north-temperate regions, the widespread use of road salt for de-icing purposes is an additional stressor that contributes to the gradual salinization of freshwater ecosystems (Dugan et al., 2017). Approximately, seven million tonnes of road salt are used annually in Canada, with the City of Toronto being the largest municipal user, applying an average of 135,000 tonnes of road salt per year (RSA, 2006; Environment Canada, 2012). Several de-icing chemicals are used for winter maintenance (CaCl2, MgCl2); however, rock salt (NaCl) is the most common because it is cost-effective, easy to apply, and suitable for most climates (i.e., NaCl is an effective de-icing compound for temperatures down to − 9 °C; Marsalek, 2003; Meriano et al., 2009). Rock salt has been used for winter road maintenance in the Greater Toronto Area (GTA) since the 1940s (Marsalek, 2003).
Because of their conservative nature, Cl− ions move into downstream waters, often in pulses of high Cl− in the spring, but also via gradual, long-term release over the summer from groundwater reservoirs (Lam et al., 2020; Yao et al., 2020). Consequently, many lakes in north-temperate regions of North America and Europe have experienced a gradual increase in Cl− concentrations (as well as conductivity) since the mid-twentieth century (Dugan et al., 2017; Hintz et al., 2022). These increases can impair biological, recreational, and drinking water quality (Corsi et al., 2010; Findlay and Kelly 2011) and can negatively impact aquatic organisms including algae, zooplankton, other invertebrates, and fish (Hintz et al., 2017; Arnott et al., 2020; Valleau et al., 2020, 2022b). For example, reductions in filamentous algal biomass have been observed in Cl− treatments > 500 mg/l (Hintz et al., 2017; Lind et al., 2018). With zooplankton, increases in Cl− can result in potentially fatal osmotic stress and behavioral changes (Tytler & Ireland, 1995). In laboratory experiments, cladocerans were shown to have reduced abundance, growth rates, fecundity, and increased egg mortality with increasing Cl− concentrations (Brown & Yan, 2015; Arnott et al., 2020).
Many governing agencies have set water quality criteria for Cl− for the protection of aquatic life. In Canada, the long-term exposure limit for Cl− is 120 mg/l, while the acute limit is 640 mg/L (CCME, 2011). Due to urbanization, many north-temperate lakes are now at risk of exceeding these guidelines (Dugan et al., 2020). In the province of Ontario, many recreational lakes record relatively low Cl− concentrations; for example, 76% of the lakes monitored as part of Ontario’s Broadscale Monitoring and Lake Partner programs have measured chloride concentrations below 5 mg/l (Arnott et al., 2020). However, 23% of the lakes have Cl− concentrations between 5 and 40 mg/l, and 1% of the monitored lakes have concentrations above 40 mg/l (Arnott et al., 2020). In southern Ontario, specifically, a greater proportion of lakes and streams record elevated Cl− concentrations, and data from the Provincial Stream Water Quality Monitoring Network (PSWQMN) show that summer Cl− concentrations in many streams in the Greater Toronto Area (GTA) are above the federal acute toxicity level (640 mg Cl−/l; Todd & Kaltenecker, 2012, Lawson & Jackson, 2021).
An understanding of pre-disturbance conditions is important when assessing lake health and for developing effective management strategies or recommendations. However, this information rarely exists due to a lack of long-term monitoring records (Smol, 2008, 2019). Fortunately, paleolimnology can be used to help fill this gap (Korhola et al., 2005; Sarmaja-Korjonen et al., 2006; Sweetman & Smol 2006; Smol, 2008). Paleolimnological methods use physical, chemical, and biological archives in sediments to understand long-term environmental change and to set realistic recovery targets for lake management (Ginn et al., 2015). Evaluating the effects of multiple drivers of change on urban lake ecosystems over centennial time scales helps provide a perspective for understanding contemporary ecological conditions.
Recent paleolimnological studies of urban lakes in north-temperate regions have shown marked changes in response to long-term eutrophication, often attributed to settlement and agricultural activities, and increases in brackish diatoms associated with road salt inputs (Pienitz et al., 2006; Rowell, 2009). Importantly, environmental sensitivity varies among species because of differences in their history of exposure to contaminants, size, habitat, and grazing efficiency (Hintz et al., 2019; Arnott et al., 2020). Moreover, different paleolimnological proxies (i.e., Cladocera and diatoms) may show differing responses to environmental stressors. Thus, multiple proxy studies can help tease apart the specific environmental drivers of ecosystem change.
Here, we evaluate the impacts of road salt additions on Haynes Lake, a small peri-urban lake with elevated chloride concentrations in comparison to a nearby reference system located in a conservation area (Swan Lake). Specifically, we applied paleolimnological techniques to assess changes in primary producers and primary consumers (diatoms and Cladocera, respectively) over the past ~ 150 years. Specifically, we sought to determine: (1) Have primary producers (diatoms) and primary consumers (Cladocera) changed over the past few decades in response to multiple stressors, and in particular the addition of road salt? If so, (2) how do the biological responses compare with previous paleolimnological studies in moderately impacted systems?
Methods
Site description
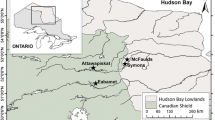
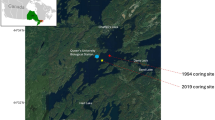
Haynes (43.958824, − 79.407891) and Swan (43.950867, − 79.414377) lakes are small (surface areas < 5 ha), shallow, oligo-mesotrophic (total phosphorus (TP) = 10.0 and 19.6 µg/l, respectively) kettle lakes, located on the Oak Ridges Moraine (ORM) in the GTA of Ontario, Canada, just north of the City of Richmond Hill (Fig. 1, Table 1). The ORM, which is composed of ~ 100-m thick, glaciofluvial-glaciolacustrine till sediments (Karrow, 1989; Sharpe et al., 2004), is ~ 160 km long, and extends from the Trent River in southeastern Ontario to the Niagara Escarpment. The ORM acts as a recharge area for ground water, is a drinking water source for local communities, and contains the largest concentration of headwater streams in the GTA (Gerber & Howard, 2002; Government of Ontariol, 2004).
Large-scale European settlement of the ORM began ca. 1800 and early activities were focused on agriculture and forestry, both of which involved land clearing (Sandberg et al., 2013). Settlement accelerated on the ORM from ca. 1800 to 1860 and with that came road building (including what is now known as Leslie Street). Due in part to the economic and cultural significance of the ORM, many studies have been completed on the kettle lakes in the region. Diatom and pollen analyses have been completed on Swan and Haynes lakes (Watchorn et al., 2008, 2012, respectively). Both lakes recorded shifts in diatom and pollen assemblages ca. 1850, associated with European settlement in the region (Watchorn et al., 2008, 2012). Although sediment records indicate that large changes in diatom assemblages have occurred over the past 300 years, the ecology has stabilized over the past ~ 50 years (Watchorn et al., 2008). More recently (ca. 1970s), kettle lakes in the region (Wilcox and Mussleman lakes) have been impacted by dense growths of aquatic plants, algal blooms, and poor water quality (Moos & Ginn, 2016); however, neither Haynes nor Swan lakes record elevated TP concentrations, and, to our knowledge, there have been no reports of algal blooms.
Haynes Lake is skirted by Leslie St. on its eastern shore, which curves around the lake. Road salt is routinely applied to this bend in the road and salt-laden runoff flows downslope into the lake. Just outside the Haynes Lake watershed is a golf course that was developed ca. 1993. In contrast, Swan Lake is located just 1 km to the southwest in a relatively undeveloped area operated by the Swan Lake Outdoor Education Center and the Toronto Regional Conservation Authority (est. 2002; Fig. 1).
Surface water chemistry collected in June 2019 from both lakes illustrates the difference in watershed development. Haynes Lake has elevated concentrations of many ions associated with road runoff (i.e., Cl−) relative to Swan Lake (Table 1), but with lower TP concentrations. Meteorological data from Toronto collected from 1981 to 2010 indicates the region reaches a maximum mean daily temperature of 26.8℃ in July and a minimum mean daily temperature of −10.2 °C in January (https://climate.weather.gc.ca/). Climate change has increased the mean annual air temperature across the GTA; in downtown Toronto, mean annual air temperature increased by ~ 0.9 °C from 1970 to 2000, while Richmond Hill records a greater increase of ~ 2.5 °C over the same period (Mohsin & Gough, 2010; ECCC, 2019).
Field sampling
Sediment cores were collected from Haynes and Swan lakes in June 2019. Cores were extracted from each lake using a Glew (1989) gravity corer and were sectioned at 0.5-cm intervals throughout using a Glew (1988) extruder. Secchi depth and lake depth (~ Zmax) were recorded at each site, and surface water samples were collected for water chemistry analysis. Water samples were stored in a cool location and analyzed at the Ontario Ministry of the Environment, Conservation and Parks’ (OMECP) Dorset Environmental Science Centre (DESC) within a week of collection.
Laboratory methods
Establishing core chronologies
To establish core chronologies, 210Pb dating with gamma spectroscopy was used, following the methodology outlined by Schelske et al. (1994). An Ortec high-purity Germanium crystal detector was used to measure gamma activity of radioisotopes 210Pb and 137Cs. The chronologies were based on unsupported 210Pb determined from activities of 210Pb and 214Pb, with the latter used to estimate background levels of 210Pb. Sediment ages were estimated using the constant rate of supply (CRS) model (Appleby, 2001). Ages between dated intervals were interpolated using a linear fit between consecutive intervals, and age estimates beyond background 210Pb concentrations were extrapolated using a second-order polynomial fit. We acknowledge that the extrapolated dates should be viewed with caution. Peaks in 137Cs were used as an independent chronological marker for the 1963 peak in atmospheric fallout prior to the global ban on atmospheric testing of nuclear weapons (signed August 5, 1963).
Cladocera analysis
Cladocera remains were prepared following the methods described by Frey (1986) and Korhola and Rautio (2001). In summary, between 0.1 and 0.5 g of freeze-dried sediment was used for each depth interval, and the sediment was deflocculated by mixing it with a 10% KOH solution and heating to ~ 80 °C for 60–90 min. The sediment/KOH mixture was then rinsed thoroughly through a 38-μm sieve with DI water, and ethanol and safranin were used to preserve and stain the samples. Between 1 and 4, ~ 50-μl aliquots of the concentrated remains were applied to each slide, followed by glycerin jelly and a coverslip.
The identification of cladoceran subfossils generally followed Korosi and Smol (2012a, 2012b) with additional identification references used as general guides (Smirnov 1996; Sweetman & Smol, 2006; Szeroczyńska & Sarmaja-Korjonen, 2007). Cladoceran remains were identified under × 200 or × 400 magnification using a Leica® compound microscope with brightfield illumination. All cladoceran remains (e.g., carapaces, headshields, and postabdominal claws) were tabulated separately, and the most abundant remain was used to calculate the number of individuals per taxon. Entire coverslips were scanned and a minimum of 70 individuals were enumerated for each interval and expressed as percent relative abundance (Kurek et al., 2010). Daphnia spp. were split into the Daphnia pulex complex and the Daphnia longispina complex based on the morphology of the postabdominal claw (Korosi & Smol 2012b). Bosmina spp. and Eubosmina spp. were differentiated using the placement of their headpores; however, due to the quality of some specimens, this could not be reliably done for every individual and therefore Bosmina and Eubosmina were combined into Bosmina spp. for analyses. As well, Alona affinis Leydig and Alona quadrangularis O. F. Müller, and Alona circumfimbriata Megard and Alona guttata Sars, were grouped for analysis. Dominant (> 5% in 2 intervals) Cladocera taxa were presented as percent relative abundance for plotting purposes.
Diatom analysis
Sediment preparation for diatom analysis followed the methods outlined by Rühland and Smol (2002). Briefly, ~ 0.02 g of sediment was digested with 15 ml of a 50:50 molar ratio of nitric acid and sulfuric acid. The resulting slurry was rinsed with deionized water until a circumneutral pH was reached. Slurries were left to resettle for 24 h between rinses. The neutral slurries where then plated onto cover slips, left to evaporate at room temperature, and then mounted onto microscope slides using Naphrax® as a mounting medium. For each interval, ~ 300 diatom valves were identified, with identifications made to the lowest taxonomic level possible. Diatom identification was made using an assortment of taxonomic references, including Krammer and Lange-Bertalot (1986, 1988, 1991a, b) and Camburn and Charles (2000). Identification was then verified using more recent publications and online databases (e.g., Diatoms of North America 2021). Dominant (> 5% in 2 intervals) diatom taxa were presented as percent relative abundance. For plotting purposes and to better identify trends, diatoms with like ecological criteria were group together; however, all statistical analyses were performed on ungrouped diatom data.
Statistical analyses
Stratigraphic zones were identified from the full assemblage data using constrained incremental sum of squares (CONISS) with Euclidean distance as the dissimilarity coefficient for both Cladocera and diatom assemblages (Grimm, 1987). A broken stick model (Bennett, 1996) was subsequently used to determine the number of important zones within each lake. To directly compare temporal changes in the diatom and cladoceran assemblages across the study lakes, species data from both cores were plotted together using detrended correspondence analysis (DCA). Separate DCAs were produced using diatoms and Cladocera relative abundance data, respectively, using Canoco version 4.5.
Results
Establishing core chronologies
The radiometric dating profiles in both lakes (Haynes and Swan) generally followed an exponential decline in 210Pb activity with depth (Figs. 2a, b). Background 210Pb levels were reached at 30.0–30.5 cm and 54.0–54.5 cm in Haynes and Swan lakes, respectively, corresponding to 210Pb-inferred dates of ca. 1938 and 1829 (Fig. 2a, b). Sediment cores from both lakes showed distinct peaks in 137Cs, corresponding to 210Pb-inferred dates of ca. 1961 and the early 1970s in Haynes and Swan lakes, providing independent verification that the chronologies developed from 210Pb were reliable.
Overall Cladocera assemblage trends
Before ca. 1950, both lakes (Haynes and Swan) were dominated by Bosmina spp. (67.0 ± 18.3% and 41.3 ± 6.6%, respectively), with notable relative abundances of Chydorus brevilabris Frey (22.2 ± 6.4%) in Swan Lake (Figs. 3, 4). The primary change in Cladocera assemblage in Haynes Lake occurred ca. 1954 (Fig. 3). This is marked by a pronounced decrease in Bosmina spp. and an associated increase in Daphnia spp. (first Daphnia longispina complex follow shortly by increases in Daphnia pulex complex). This shifted Haynes Lake from a Bosmina spp. dominated to a Daphnia spp.-dominated system. In contrast, the Cladocera assemblages of Swan Lake recorded only muted changes throughout the core. For example, Bosmina spp. relative abundance fluctuated through the core with a subtle yet notable increase post-1985, and C. brevilabris decreased slightly in recent sediment intervals (Fig. 3).
Stratigraphic profiles of dominant Cladocera and diatom taxa (relative abundance%) in Haynes Lake, plotted against core depth. Dashed horizontal lines represent the boundary between biostratigraphic zones identified using CONISS cluster analysis and deemed important by the broken stick model. The gray box delineates regional estimates for the approximate period of salting within the GTA. 210Pb dates are shown to the left
CONISS, followed by broken stick analysis, revealed two important shifts in Haynes Lake at 25 cm and 8 cm in the cladoceran record (ca. 1954 and ca. 2002, respectively; Fig. 3). CONISS and broken stick analysis did not identify any important zones in Swan Lake.
Overall diatom assemblage trends
Diatoms were well preserved in the sedimentary records from both lakes. Early in its sediment record (pre-1960), Haynes Lake was dominated by the planktonic taxa Discostella (Cyclotella) stelligera (Cleve and Grunow) Houk and Clee, Cyclotella bodanica Eulenstein ex Grunow, Cyclotella michiganiana Skvortsov, and Synedra tenera W. Smith (Fig. 3). Post-1960, there was a shift toward assemblages dominated by generalist benthic taxa (e.g., Achnanthidium minutissimum (Kützing) Czarnecki) and benthic fragilarioid taxa, particularly Staurosirella pinnata (Ehrenberg) D. M. Williams and Round and Staurosira construens (Ehrenberg). Although the temporal resolution of the cores is different, diatom assemblages in the reference lake (Swan Lake) showed little change throughout the sediment record and were dominated throughout by A. minutissimum and taxa, such as Fragilaria virescens Cleve-Euler, F. capucina Desmazières, S. pinnata, S. construens, and F. tenera W. Smith Lange-Bertalot (Fig. 4).
CONISS analysis of the diatom record, followed by broken stick analysis, identified two important groups in Haynes Lake, with the zone delineated at 22 cm (ca. 1960; Fig. 3). Broken stick analysis did not identify any important splits in Swan Lake.
Comparing cores using DCA
The first axes of the diatom and cladoceran DCAs explained 69 and 51% of the variation, respectively. In both plots, the sample scores from the reference lake (i.e., Swan Lake) showed little movement in ordination space (Fig. 5). In contrast, sample scores from Haynes Lake showed more variation over time in both the diatom and Cladocera plots. In the diatom DCA, sample scores in Haynes Lake moved closer to those of Swan Lake over time, driven by an increase in benthic taxa. In contrast, samples scores from Haynes Lake in the Cladocera DCA become more dissimilar from those of Swan Lake over time.
Discussion
Early development (ca. 1850) until the onset of road salting (ca. 1940)
European settlement in southern Ontario led to a tripling of the population between 1825 and 1842 and by 1851 it had doubled again (1850 population ~ 952,000; Hillmer & Bothwell 2021). Land clearing and farming also increased throughout the region at this time. Due to this rapid population growth, deforestation, and farming, changes in water quality occurred in both Haynes and Swan lakes. Specifically, Regional Road 12 (now known as Leslie St.) was constructed. This improved transportation corridor, along with land clearing around Haynes Lake, significantly increased the sedimentation rate ca. 1870 (Watchorn et al., 2012). Past work on Swan Lake showed changes in diatom-inferred-TP (DI-TP) corresponding with changing land-use practices (deforestation, reforestation, fertilizer application; Watchorn et al., 2008). Swan Lake was ultra- to oligotrophic prior to ca. 1800. However, from 1835 to 1850, increased land clearance and settlement doubled the DI-TP, and, by ca. 1850, DI-TP concentrations reached ~ 19 µg/l, remaining relatively stable thereafter (Watchorn et al., 2008). Despite historical evidence of these early cultural disturbances, the changes in Cladocera and diatom assemblages were subtle in the bottom sections of the sediment cores. Based on 210Pb-inferred dates, it is possible that the sediment cores were too short in length to capture this early development period (i.e., early 1800s).
Post-road salting ca. 1940 to ca. 1990 changes
Despite a multiple stressor environment (i.e., climate change and local development), road salt additions appear to have elicited the major response in our proxies and particularly in the cladoceran record in Haynes Lake. The species-specific changes beginning in the 1950s are indicative of changes in Cl− and conductivity (Valleau et al., 2020, 2022b). Overall, the Haynes Lake records show a decrease in the relative abundance of Cl−-sensitive Cladocera species and increases in diatoms that have been linked to increases in conductivity (i.e., small benthic fragilarioid taxa and A. minutissumum). Specifically, Haynes Lake tracked a dramatic reduction in the relative abundance of Bosmina spp. beginning ca. 1950, a trend that continued to the present. In recent laboratory experiments, Bosmina spp. have been shown to be more sensitive than larger bodied Cladocera (i.e., daphniids) to Cl− additions (Valleau et al., 2022a), in part because of their smaller size. Body size in Cladocera is inversely related to the surface area of salt-transporting organs and thus smaller taxa have higher relative salt influxes than larger taxa. Moreover, Bosmina spp. are less efficient grazers than Daphnia spp., and toxicity is also inversely related to food availability and nutrition, with increased mortality associated with decreasing nutrient availability (Heugens et al., 2001). Similar reductions in Bosmina spp. with road salt additions have been noted in recent paleolimnological research in other freshwater lakes, with daphniids and chydorids generally showing reduced sensitivity (Bos et al., 1999; Valleau et al., 2020).
In Haynes Lake, the abrupt increase in the relative abundance of the D. longispina complex and subsequent gradual increase in the D. pulex complex following the onset of road salt application may also be linked to increases in Cl−. Multi-specific groups often have wider tolerances and, in addition, species within the Daphnia pulex complex (in particular D. pulex Leydig and D. pulicaria Forbes) have relatively wide ranges of salinity tolerances and optima (Bos et al., 1999; Derry et al., 2003). Furthermore, Coldsnow et al. (2017) showed that D. pulex was able to rapidly acquire (over 2.5 months or within 10–15 generations) an increased tolerance to road salt, and many studies have shown that daphniids living at higher salinity have higher tolerances to salt than populations living at lower concentrations (Weider & Hebert, 1987; Teschner, 1995; Latta et al., 2012; Liao & Faulks, 2015). In Haynes Lake, as Cl− concentrations from road salt gradually increased over time, Daphnia spp. may have been better equipped to adapt to these changes.
Concurrently with the observed decreases in Bosmina spp., the relative abundances of the diatom species A. minutissimum and benthic fragilarioid taxa increased in Haynes Lake. Notably, there was a marked shift from planktonic to benthic taxa in the 1950s that corresponded closely to large increases in daphniid relative abundance. These diatom taxa are often described as generalists and increases in their relative abundances have been attributed to the elimination of more sensitive taxa (Medley & Clements, 1998; Porter-Goff et al., 2013). A. minutissimum and benthic fragilarioid taxa are commonly regarded as having a wide tolerance to a variety of environmental conditions, including conductivity, but have been specifically linked to increasing Cl− concentrations in freshwater lakes (MacDougall et al., 2017; Sivarajah et al., 2019; Valleau et al., 2022b). It is also possible that the observed shift in the diatom assemblage was influenced by top-down control. The switch to Daphnia spp., which are much more efficient grazers of phytoplankton than Bosmina spp., may have resulted in the decline in planktonic diatoms in favor of benthic forms. Thus, coincident changes in Cladocera and diatoms assemblages may indicate a paired response to increased road salt runoff, with the change in diatoms occurring in response to a dramatic change in grazing pressure.
In contrast to the Cladocera, which clearly show species assemblages in Haynes Lake becoming less similar to those in Swan Lake over time, the switch from planktonic to benthic diatom taxa resulted in diatom species assemblages in the impact lake that are now more similar to those of Swan Lake. Swan Lake is a shallow (Zmax = 6 m), weedy lake and so the dominance of benthic taxa was not unexpected. Haynes Lake is slightly deeper (Zmax = 16 m), and sedimentary assemblages were historically dominated by planktonic diatoms. However, the lake is still relatively clear (Secchi depth = 5.9 m) and so capable of supporting the benthic community that now dominates diatom assemblages, following the marked floristic changes that occurred in the mid-1950s.
The species-specific changes in both Cladocera and diatoms recorded in Haynes Lake are similar to those seen in lakes moderately impacted by road salt, such as in soft-water lakes in south-central Ontario (Valleau et al., 2020, 2022b). These moderately impacted lakes (Cl− = 33–90 mg/l) also recorded a decrease in Bosmina spp. and increases in A. minutissimum and benthic fragilarioid diatom taxa. Although Haynes Lake has measured Cl− concentrations more than double those recorded in the moderately impacted lakes, it still maintains a Cladocera population that includes some of the most sensitive species (i.e., Bosmina spp.; Valleau et al., 2020, 2022a). Salting practices began earlier in the GTA (ca. 1940) compared to south-central Ontario (ca. 1950); however, the biological changes are delayed in Haynes Lake in comparison to the moderately impacted lakes. This may illustrate the combined buffering capacity of hard water and food availability when considering toxicity (Elphick et al., 2011; Brown & Yan, 2015). While the moderately impacted lakes are oligotrophic, soft-water lakes, Haynes Lake is a mesotrophic, hard-water lake and both these factors have been shown to buffer the negative toxicological effects of increasing Cl− concentrations (Heugens et al., 2001; Elphick et al., 2011).
Recent changes (ca. 1990)—present
In the topmost intervals of Haynes Lake, there is a second stepwise decrease in Bosmina spp. and an increase in Daphnia spp., which may be linked to an intensification of salting or increased residential development in the region. In contrast to the decreasing trend in Bosmina spp. recorded in Haynes Lake, Swan Lake showed a modest increase in the relative abundance of Bosmina spp. ca. 1990. Increases in the relative abundance of Bosmina spp. in many freshwater lakes have been attributed to the effects of climate warming, as warmer water temperatures have been shown to favor smaller body size (Hargan et al., 2016; Armstrong and Kurek 2019).
Management implications
A community-level shift occurred in Haynes Lake in both primary producers (diatoms) and primary consumers (Cladocera; Fig. 3). The paired response in both indicators in Haynes Lake may be a warning of broader ecosystem effects. Changes in Cladocera grazing pressure can be a contributing factor in algal blooms, which negatively impact water quality and aquatic habitats (Korosi et al., 2012; Hintz et al., 2017, 2019). Although paleolimnology cannot provide information on the interactive effects between Cladocera and diatoms, our highly coupled response in Haynes Lake suggests that both the primary producer and primary consumer community structure are changing with Cl− additions. Importantly, our work shows that the building blocks of lake ecosystems (primary producers and consumers) are being affected by Cl− at much lower concentrations than shown in some experimental work (Goncalves et al., 2007; Van Meter et al., 2011; Petranka & Francis 2013; Van Meter & Swan 2014; Hinz & Relyea, 2017) and well below water quality guidelines for the protection of aquatic ecosystems (Arnott et al., 2020; Hintz et al., 2022).
The ORM is the headwater source for numerous streams and lakes in southern Ontario. The high Cl− concentrations measured in Haynes Lake, a small, highly connected, headwater lake, suggest that Cl− seepage into ground water is a possibility. Contaminated ground water is discharged into downstream waterbodies via streams through baseflow and this can lead to elevated concentrations of Cl− year-round. This has implications for toxicity from prolonged exposure to aquatic species. Extended exposure, even at low concentrations, has been shown to negatively impact population viability through reductions in reproduction (decrease in total neonates and egg viability in Cladocera; Brown & Yan, 2015; Arnott et al., 2020; Valleau et al., 2022a). For freshwater lakes, chloride retention within ground water may prolong biological recovery with implications for conservation and management strategies.
Conclusion
With urbanization, a wide range of ecosystem services offered by urban lakes are likely to be compromised. Consequently, it is important to understand the complex interactions of human and natural drivers influencing ecological changes in these ecosystems. Despite multiple regional stressors, the primary driver of change in both Cladocera and diatom assemblage in Haynes Lake appears to be road salt application. The species-specific changes are indicative of increasing Cl− concentrations, tracking a highly coupled response in the biological assemblages in Haynes Lake. In contrast, minimal changes were recorded in the nearby reference lake (Swan Lake). This work illustrates the need for further study of the impacts of road salt on lake food webs, in the context of other environmental stressors (e.g., development, climate warming, and eutrophication).
Data availability
Data available by request.
Code availability
N/A.
Change history
12 September 2024
A Correction to this paper has been published: https://doi.org/10.1007/s10750-024-05682-4
References
Appleby, P. G., 2001. Chronostratigraphic techniques in recent sediments. In Last, W. M. & J. P. Smol (eds), Tracking Environmental Change Using Lake Sediments Volume 1: Basin Analysis, Coring, and Chronological Techniques. Kluwer Academic Publishers, Dordrecht.
Armstrong, Z. & J. Kurek, 2019. Sensitivity and response of low-nutrient lakes to post twentieth century environmental change in New Brunswick, Canada. Journal of Paleolimnology 61: 85–89. https://doi.org/10.1007/s10933-018-0046-8.
Arnott, S. E., M. P. Celis-Salgado, R. E. Valleau, A. M. DeSellas, A. M. Paterson, N. D. Yan, J. P. Smol & J. A. Rusak, 2020. Road salt impacts freshwater zooplankton at concentrations below current water quality guidelines. Environmental Science & Technology 43: 9398–9407. https://doi.org/10.1021/acs.est.0c02396.
Bennett, K. D., 1996. Determination of the number of zones in a biostratigraphical sequence. New Phytologist 132: 155–170.
Bos, D. G., B. F. Cumming & J. P. Smol, 1999. Cladocera and Anostraca from the Interior Plateau of British Columbia, Canada, as paleolimnological indicators of salinity and lake level. Hydrobiologia 392: 129–141. https://doi.org/10.1023/A:1003542709450.
Brown, A. H. & N. D. Yan, 2015. Food quantity affects the sensitivity of Daphnia to road salt. Environmental Science & Technology 49: 4673–4680. https://doi.org/10.1021/es5061534.
Camburn, K. E. & D. F. Charles, 2000. Diatoms of Low-Alkalinity Lakes in the Northeastern United States, Academy of Natural Sciences, Philadelphia:
Canadian Council of Ministers of the Environment (CCME), 2011. Canadian Environmental Quality Guidelines for the Protection of Aquatic Life: Chloride. http://ceqg-rcqe.ccme.ca/download/en/337?redir=1571165242.
Coldsnow, K. D., B. M. Mattes, W. D. Hintz & R. A. Relyea, 2017. Rapid evolution of tolerance to road salt in zooplankton. Environmental Pollution 222: 367–373. https://doi.org/10.1016/j.envpol.2016.12.024.
Corsi, S. R., D. J. Graczyk, S. W. Geis, N. L. Booth & K. D. Richards, 2010. A fresh look at road salt: aquatic toxicity and water-quality impacts on local, regional, and national scales. Environmental Science & Technology 44: 7376–7382.
Derry, A. M., E. E. Prepas & P. D. N. Hebert, 2003. A comparison of zooplankton communities in saline lakewater with variable anion composition. Hydrobiologia 505: 199–215.
Dugan, H. A., S. L. Bartlett, S. M. Burke, J. P. Doubek, F. E. Krivak-Tetley, N. K. Skaff, J. C. Summers, K. J. Farrell, I. M. McCullough, A. M. Morales-Williams, D. C. Roberts, Z. Ouyang, F. Scordo, P. C. Hanson & K. C. Weathers, 2017. Salting our freshwater lakes. PNAS 114: 4453–4458. https://doi.org/10.1073/pnas.1620211114.
Dugan, H. A., N. K. Skaff, J. P. Doubek, S. L. Bartlett, S. M. Burke, F. E. Krivak-Tetley, J. C. Summers, P. C. Hanson & K. C. Weathers, 2020. Lakes at risk of chloride contamination. Environmental Science & Technology 54: 6639–6650.
Elphick, J. R., K. D. Bergh & H. C. Bailey, 2011. Chronic toxicity of chloride to freshwater species: effects of hardness and implications for water quality guidelines. Environmental Toxicology and Chemistry 30: 239–246.
Environment and Climate Change Canada (ECCC), 2019. Temperature and Precipitation Graph for 1981 to 2010 Canadian Climate Normals: Richmond Hill. https://climate.weather.gc.ca/climate_normals/results_1981_2010_e.html?stnID=5016&autofwd=1.
Environment Canada, 2012. Five-year Review of Progress: Code of Practice for the Environmental Management of Road Salts. https://www.ec.gc.ca/sels-salts/45D464B1-96CC-4A27-8B96-42224F3C3CD5/COM1481_five_year_Code_E-_v3.pdf.
Findlay, S. E. & V. R. Kelly, 2011. Emerging indirect and long-term road salt effects on ecosystems. Annals of the New York Academy of Sciences 1223: 58–68.
Frey, D. G., 1986. Cladocera Analysis. In Berglund, B. E. (ed), Handbook of Holocene Palaeoecology and Palaeohydrology Wiley, New York: 667–692.
Gerber, R. E. & K. Howard, 2002. Hydrogeology of the Oak Ridges Moraine aquifer system: implications for protection and management from the Duffins Creek watershed. Canadian Journal of Earth Sciences 39: 1333–1348.
Ginn, B. K., T. Rajaratnam, B. F. Cumming & J. P. Smol, 2015. Establishing realistic management objectives for urban lakes using paleolimnological techniques: an example from Halifax Region (Nova Scotia, Canada). Lake and Reservoir Management 31: 92–108.
Glew, J. R., 1988. A portable extruding device for close interval sectioning of unconsolidated core samples. Journal of Paleolimnology 1: 235–239. https://doi.org/10.1007/BF00177769.
Glew, J. R., 1989. A new trigger mechanism for sediment samplers. Journal of Paleolimnology 2: 241–243.
Goncalves, A. M., B. B. Castro, M. A. Pardal & F. Goncalves, 2007. Salinity effects on survival and life history of two freshwater cladocerans (Daphnia magna and Daphnia longispina). International Journal of Limnology 43: 13–20. https://doi.org/10.1051/limn/2007022.
Government of Ontario. 2004. http://www.mah.gov.on.ca/.
Grimm, E. C., 1987. CONISS: a FORTRAN 77 program for stratigraphically constrained cluster analysis by the method of incremental sum of squares. Computers & Geosciences 13: 13–35.
Hargan, K. E., C. Nelligan, A. Jeziorski, K. M. Rühland, A. M. Paterson, W. Keller & J. P. Smol, 2016. Tracking the long-term responses of diatoms and cladocerans to climate warming and human influences across lakes of the Ring of Fire in the Far North of Ontario, Canada. Journal of Paleolimnology 56: 153–172. https://doi.org/10.1007/s10933-016-9901-7.
Heugens, E. H., A. J. Hendriks, T. Dekker, N. M. V. Straalen & W. Admiraal, 2001. A review of the effects of multiple stressors on aquatic organisms and analysis of uncertainty factors for use in risk assessment. Critical Reviews in Toxicology 31: 247–284.
Hillmer, N. and R. Bothwell. 2021. Ontario. In The Canadian Encyclopedia. https://www.thecanadianencyclopedia.ca/en/article/ontario
Hintz, W. D., B. M. Mattes, M. S. Schuler, D. K. Jones, A. B. Stoler, L. Lind & R. A. Relyea, 2017. Salinization triggers a trophic cascade in experimental freshwater communities with varying food-chain length. Ecological Applications 27: 833–844. https://doi.org/10.1002/eap.1487.
Hintz, W. D., D. K. Jones & R. A. Relyea, 2019. Evolved tolerance to freshwater salinization in zooplankton: life-history trade-offs, cross-tolerance and reducing cascading effects. Philosophical Transactions of the Royal Society B 374: 20180012.
Hintz, W. D., S. E. Arnott, C. C. Symons, D. A. Greco, A. McClymont, J. A. Brentrup, M. Cañedo-Argüelles, A. M. Derry, A. L. Downing, D. K. Gray, S. J. Melles, R. A. Relyea, J. A. Rusak, C. L. Searle, L. Astorg, H. K. Baker, B. B. Beisner, K. L. Cottingham, Z. Ersoy, C. Espinosa, J. Franceschini, A. T. Giorgio, N. Göbeler, E. Hassal, M.-P. Hébert, M. Huynh, S. Hylander, K. L. Jonasen, A. E. Kirkwood, S. Langenheder, O. Langvall, H. Laudon, L. Ling, M. Lundgren, L. Proia, M. S. Schuler, J. B. Shurin, C. F. Steiner, M. Striebel, S. Thibodeau, P. Urrutia-Cordero, L. Vendrell-Puigmitja & G. A. Weyhenmeyer, 2022. Current water quality guidelines across North America and Europe do not protect lakes from salinization. PNAS 119: e2115033119. https://doi.org/10.1073/pnas.2115033119.
Hintz, W. & R. Relyea, 2017. A salty landscape of fear: responses of fish and zooplankton to freshwater salinization and predatory stress. Oecologia 185: 147–156. https://doi.org/10.1007/s00442-017-3925-1.
Karrow, P. F., 1989. Quaternary geology of the Great Lakes subregion. In Fulton, R. J. (ed), Quaternary Geology of Canada and Greenland. Geological Society of America, Boulder.
Korhola, A. & M. Rautio, 2001. Cladocera and other branchiopod crustaceans. In Smol, J. P., H. J. B. Birks & W. M. Last (eds), Tracking Environmental Change Using Lake Sediments. Volume 4: Zoological Indicators Kluwer Academic Publishers, Dordrecht: 4–41.
Korhola, A., M. Tikkanen & J. Weckström, 2005. Quantification of Holocene lake-level changes in Finnish Lapland using a Cladocera–lake depth transfer model. Journal of Paleolimnology 34: 175–190.
Korosi, J. B. & J. P. Smol, 2012a. An illustrated guide to the identification of cladoceran subfossils from lake sediments in northeastern North America: part 1—the Daphniidae, Leptodoridae, Bosminidae, Polyphemidae, Holopedidae, Sididae, and Macrothricidae. Journal of Paleolimnology 48: 571–586. https://doi.org/10.1007/s10933-012-9632-3.
Korosi, J. B. & J. P. Smol, 2012b. An illustrated guide to the identification of cladoceran subfossils from lake sediments in northeastern North America: part 2—the Chydoridae. Journal of Paleolimnology 48: 587–622. https://doi.org/10.1007/s10933-012-9636-z.
Korosi, J. B., S. M. Burke, J. R. Thienpont & J. P. Smol, 2012. Anomalous rise in algal production linked to lakewater calcium decline through food web interactions. Proceedings of the Royal Society (London) Series B 279: 1210–1217.
Krammer, K. & H. Lange-Bertalot, 1986. Bacillariophyceae. 1. Teil: Naviculaceae. In Ettl, H., J. Gerloff, H. Heynig & D. Mollenhauer (eds), Süsswasserflora von Mitteleuropa, Band 2/1 Gustav Fischer Verlag, New York: 1–876.
Krammer, K. & H. Lange-Bertalot, 1988. Bacillariophyceae. 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae. In Ettl, H., J. Gerloff, H. Heynig & D. Mollenhauer (eds), Süsswasserflora von Mitteleuropa, Band 2/2 Gustav Fischer Verlag, Stuttgart: 1–596.
Krammer, K. & H. Lange-Bertalot, 1991a. Bacillariophyceae. 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. In Ettl, H., J. Gerloff, H. Heynig & D. Mollenhauer (eds), Süsswasserflora von Mitteleuropa, Band 2/3 Gustav Fischer Verlag, Stuttgart: 1–576.
Krammer, K. & H. Lange-Bertalot, 1991b. Bacillariophyceae. 4. Teil: Achnanthaceae, Kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema, Gesamtliteraturverzeichnis Teil 1–4. In Ettl, H., J. Gerloff, H. Heynig & D. Mollenhauer (eds), Süsswasserflora von Mitteleuropa, Band 2/4 GustavFischer Verlag, Stuttgart: 1–437.
Kurek, J., J. B. Korosi, A. Jeziorski & J. P. Smol, 2010. Establishing reliable minimum count sizes for cladoceran subfossils sampled from lake sediments. Journal of Paleolimnology 44: 603–612. https://doi.org/10.1007/s10933-010-9440-6.
Lam, W. Y., D. Lembcke & C. Oswald, 2020. Quantifying chloride retention and release in urban stormwater management ponds using a mass balance approach. Hydrological Processes 34: 4459–4472.
Latta, L. C., L. J. Weider, J. K. Colbourne & M. E. Pfrender, 2012. The evolution of salinity tolerance in Daphnia: a functional genomics approach. Ecology Letters 15: 794–802. https://doi.org/10.1111/j.1461-0248.2012.01799.x.
Lawson, L. & D. A. Jackson, 2021. Salty summertime streams—road salt contaminated watersheds and estimates of the proportion of impacted species. Facets 6: 317–333.
Liao, Y.-F. & L. K. Faulks, 2015. Stress tolerance and population stability of rock pool Daphnia in relation to local conditions and population isolation. Hydrobiologia 742: 267–278.
Lind, L., M. S. Schuler, W. D. Hintz, A. B. Stoler, D. K. Jones, B. M. Mattes & R. A. Relyea, 2018. Salty fertile lakes: how salinization and eutrophication alter the structure of freshwater communities. Ecosphere 9: e02383. https://doi.org/10.1002/ecs2.2383.
MacDougall, M. J., A. M. Paterson, J. G. Winter, F. C. Jones, L. A. Knopf & R. I. Hall, 2017. Response of periphytic diatom communities to multiple stressors influencing lakes in the Muskoka River watershed, Ontario, Canada. Freshwater Science 36: 77–89.
Marsalek, J., 2003. Road salts in urban stormwater: an emerging issue in stormwater management in cold climates. Water Science and Technology 48: 61–70.
Medley, C. N. & W. H. Clements, 1998. Responses of diatom communities to heavy metals in streams: the influence of longitudinal variation. Ecological Applications 8: 631–644.
Meriano, M., N. Eyles & K. W. F. Howard, 2009. Hydrogeological impacts of road salt from Canada’s busiest highway on a Lake Ontario watershed (Frenchman’s Bay) and lagoon, City of Pickering. Journal of Contaminant Hydrology 107: 66–81.
Mohsin, T. & W. A. Gough, 2010. Trend analysis of long-term temperature time series in the Greater Toronto area (GTA). Theoretical and Applied Climatology 101: 311–327.
Moos, M. T. & B. K. Ginn, 2016. Developing a lake management strategy by dovetailing lake monitoring with paleolimnological techniques: a case study from a kettle lake on the Oak Ridges Moraine (Ontario, Canada). Lake and Reservoir Management 32: 234–245.
Petranka, J. W. & R. A. Francis, 2013. Effects of road salts on seasonal wetlands: poor prey performance may compromise growth of predatory salamanders. Wetlands 33: 707–715. https://doi.org/10.1007/s13157-013-0428-7.
Pienitz, R., K. Roberge & W. F. Vincent, 2006. Three hundred years of human-induced change in an urban lake: paleolimnological analysis of Lac Saint-Augustin, Quebec City, Canada. Canadian Journal of Botany 84: 303–320.
Porter-Goff, E. R., P. C. Frost & M. A. Xenopoulos, 2013. Changes in riverine benthic diatom community structure along a chloride gradient. Ecological Indicators 32: 97–106.
Reid, A. J., A. K. Carlson, I. F. Creed, E. J. Eliason, P. A. Gell, P. T. Johnson, K. A. Kidd, T. J. MacCormack, J. D. Olden, S. J. Ormerod, J. P. Smol, W. W. Taylor, K. Tockner, J. C. Vermaire, D. Dudgeon & S. J. Cooke, 2019. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biological Reviews 94: 849–873.
RiverSides Stewardship Alliance and Sierra Legal Defence Fund (RSA), 2006. A Low-Salt Diet for Ontario’s Roads and Rivers: 40.
Rowell, H. C., 2009. Paleolimnology of Onondaga Lake: the history of anthropogenic impacts on water quality. Lake and Reservoir Management 12: 35–45.
Rühland, K. M. & J. P. Smol, 2002. Freshwater diatoms from the Canadian Arctic treeline and development of paleolimnological inference models. Journal of Phycology 38: 249–326.
Sandberg, L. A., G. R. Wekerle & L. Gilbert, 2013. The Oak Ridges Moraine Battles: Development, Sprawl, and Nature Conservation in the Toronto Region, University of Toronto Press:
Sarmaja-Korjonen, K., M. Nyman, S. Kultti & M. Väliranta, 2006. Palaeolimnological development of Lake Njargajavri, northern Finnish Lapland, in a changing Holocene climate and environment. Journal of Paleolimnology 35: 65–81.
Schelske, C., A. Peplow, M. Brenner & C. Spencer, 1994. Low-background gamma counting: applications for 210Pb dating of sediments. Journal of Paleolimnology 10: 115–128. https://doi.org/10.1007/BF0068250.
Sharpe, D., A. Pugin, S. Pullan & J. Shaw, 2004. Regional unconformities and the sedimentary architecture of the Oak Ridges Moraine area, southern Ontario. Canadian Journal of Earth Sciences 41: 183–198.
Sivarajah, B., J. B. Korosi, J. M. Blais & J. P. Smol, 2019. Multiple environmental variables influence diatom assemblages across an arsenic gradient in 33 subarctic lakes near abandoned gold mines. Hydrobiologia 841: 133–151.
Smirnov, N. N., 1996. Cladocera: The Chydorinae and Sayciinae (Chydoridae) of the World, SPB Academic Publishing, Amsterdam:
Smol, J. P., 2008. Pollution of Lakes and Rivers, 2nd ed. Blackwell Publishing, Hoboken:
Smol, J. P., 2019. Under the radar: long-term perspectives on ecological changes in lakes. Proceedings of the Royal Society B 286: 20190834. https://doi.org/10.1098/rspb.2019.0834.
Sweetman, J. N. & J. P. Smol, 2006. A guide to the identification of cladoceran remains (Crustacea, Branchipoda) in Alaskan lake sediments. Archiv für Hydrobiologie (Supplementbände) Monograph Studies 151: 353–394.
Szeroczyńska, K. & K. Sarmaja-Korjonen, 2007. Atlas of Subfossil Cladocera from Central and Northern Europe, Friends of the Lower Vistula Society, Świecie:
Teschner, M. 1995. Effects of salinity on the life history and fitness of Daphnia magna: variability within and between populations. In Cladocera as Model Organisms in Biology: Proceedings of the Third International Symposium on Cladocera, held in Bergen, Norway, 9–16 August 1993. Springer, Dordrecht: 33–41.
Todd, A. K. & M. G. Kaltenecker, 2012. Warm season chloride concentrations in stream habitats of freshwater mussel species at risk. Environmental Pollution 171: 199–206.
Tytler, P. & J. Ireland, 1995. The influence of temperature and salinity on the structure and function of mitochondria in chloride cells in the skin of the larvae of the turbot (Scophthalmus maximus). Journal of Thermal Biology 20: 1–14.
Valleau, R. E., A. M. Paterson & J. P. Smol, 2020. Effects of road-salt application on Cladocera assemblages in shallow Precambrian shield lakes in south-central Ontario, Canada. Freshwater Science 39: 824–836.
Valleau, R. E., M. P. Celis-Salgado, S. E. Arnott, A. M. Paterson & J. P. Smol, 2022a. Assessing the effect of salinization (NaCl) on the survival and reproduction of two ubiquitous Cladocera species (Bosmina longirostris and Chydorus brevilabris). Water Air and Soil Pollution 233: 135. https://doi.org/10.1007/s11270-021-05482-9.
Valleau, R. E., K. M. Rühland, A. M. Paterson & J. P. Smol, 2022b. Using diatoms to track road-salt seepage into small, shallow, softwater Ontario lakes. Canadian Journal of Fisheries and Aquatic Sciences 79: 1514–1528. https://doi.org/10.1139/cjfas-2021-0072.
Van Meter, R. J. & C. M. Swan, 2014. Road salts as environmental constraints in urban pond food webs. PLoS ONE 9: e90168.
Van Meter, R. J., C. M. Swan, J. Leips & J. W. Snodgrass, 2011. Road salt stress induces novel food web structure and interactions. Wetlands 31: 843–851. https://doi.org/10.1007/s13157-011-0199-y.
Watchorn, M., P. Hamilton, T. Anderson, H. Roe & R. T. Patterson, 2008. Diatoms and pollen as indicators of water quality and land-use change: a case study from the Oak Ridges Moraine, Southern Ontario, Canada. Journal of Paleolimnology 39: 491–509. https://doi.org/10.1007/s10933-007-9126-x.
Watchorn, M., P. Hamilton & R. T. Patterson, 2012. The paleolimnology of Haynes Lake, Oak Ridges Moraine, Ontario, Canada: documenting anthropogenic and climatic disturbances. Environmental Earth Sciences 68: 1823–1834. https://doi.org/10.1007/s12665-012-1870-1.
Weider, L. J. & P. D. Hebert, 1987. Microgeographic genetic heterogeneity of melanic Daphnia pulex at a low-arctic site. Heredity 58: 391–399.
Yao, H., A. M. Paterson, A. L. James, C. McConnell, T. Field, R. Ingram, D. Zhang, S. E. Arnott & S. N. Higgins, 2020. Contrasting long-term trends of chloride levels in remote and human-disturbed lakes in south-central Ontario, Canada. Lake and Reservoir Management 37: 19–33.
Acknowledgements
The authors would like to thank Michael Pope for his assistance in the field. This research was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) via Grants to JPS and AMP.
Funding
This research was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) via Grants to JPS and AMP.
Author information
Authors and Affiliations
Contributions
REV designed the project, participated in field work, analyzed diatoms remains, conducted statistical analysis, and was the lead author on the manuscript. KGM participated in field work, analyzed the Cladocera remains, and contributed to Cladocera interpretation. All authors contributed to editing the manuscript. All authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
N/A.
Consent to participate
N/A.
Consent for publication
N/A.
Additional information
Handling editor: Erik Jeppesen
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: a duplicate version of Figure 5 was also used as Figure 1.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Valleau, R.E., Murray, K.G., Paterson, A.M. et al. Comparing long-term changes in cladoceran and diatom assemblages from a lake impacted by road salt seepage to a nearby reference lake near Toronto (Ontario, Canada). Hydrobiologia 851, 3841–3854 (2024). https://doi.org/10.1007/s10750-024-05510-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-024-05510-9