Abstract
Diatom assemblages in recent versus pre-industrial sediments were examined in 40 relatively undisturbed lakes from the Experimental Lakes Area (ELA). The ELA region of northwestern Ontario receives low amounts of acidic deposition and the lakes have been minimally disturbed by watershed development or other human activities. Consequently, this region represents an important location to detect possible changes in lakes due to climate change. In over half of the lakes, planktonic taxa (especially Discostella stelligera) increased between 10 and 40% since pre-industrial times. Changes in diatom assemblages are consistent with taxa that would benefit from enhanced stratification and a longer ice-free season. We hypothesized that there should be a relationship between stratification and measured chemical and physical characteristics of the study lakes. Multiple correlation analysis was undertaken to see the relationship between planktonic taxa and D. stelligera since pre-industrial times and the physical and chemical characteristics of the study lakes. Lake depth was consistently identified as an important variable. The timing of the increase in planktonic taxa within cores from these lakes will be needed to rule out other possible regional changes that may also be occurring in the ELA region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Changes in climatic conditions can have important and complex effects on lacustrine ecosystems (Smol and Cumming 2000). Climate is an important factor driving changes in physico-chemical properties of lake water such as water temperature and transparency (Blenkner et al. 2007), pH, nutrient cycling, which in turn may affect lake biota (Schindler et al. 1990; Snucins and Gunn 2000; Winder and Schindler 2004). For example, shifts in diatom species have been attributed to recent climatic warming in arctic (Smol and Douglas 2007), subarctic (Sorvari et al. 2002; Rühland et al. 2003), alpine (Karst-Riddoch et al. 2005), and temperate regions (Harris et al. 2006; Rühland et al. 2008). The lake-specific response of ecosystems to climatic forcing, including the degree of ecological change that occurs, is complex and may be modulated by differences in seasonality and/or related changes in thermal structure and mixing regimes (Gerten and Adrian 2001). Furthermore, changes related to human disturbance, such as alterations to lake-water pH and nutrient concentrations complicate our ability to isolate the relative importance of individual stressors on lake ecology.
In part, the complication of having to disentangle multiple environmental stressors may be overcome by studying comparatively undisturbed lakes in relatively remote regions (Rühland et al. 2008). For example, reference lakes in the Experimental Lakes Area (ELA) of northwestern Ontario, Canada have received minimal impact from human activities (Findlay and Shearer 1992). Most of these lakes are oligotrophic and circumneutral, and their physical, chemical, and biological processes, including nutrient cycling and primary production are very sensitive to climatic change (Xenopoulos and Schindler 2001). For example, the ELA reference lakes experienced an increase in lake temperature during the warmer and drier decades of the late-1970s and 1980s (Schindler et al. 1996; Fee et al. 1996; Xenopoulos and Schindler 2001). Warmer and drier conditions can result in limnological changes that include: enhanced water clarity, deeper thermoclines (Schindler et al. 1990, 1996), increased lake stability (Findlay et al. 2001; Winder and Schindler 2004), and changes in nutrient levels (Magnuson et al. 1997) and hypolimnetic dissolved oxygen concentrations (Blumberg and Di Toro 1990). Moreover, measurable changes occurred in lake biota at ELA, including an increase in algal biomass and number of phytoplankton species, with greater abundances of dinoflagellates and large chrysophytes (Findlay et al. 2001).
Sedimentary diatom assemblages from climate-sensitive lakes can provide an extended temporal record of environmental conditions, including changes related to variations in climate (Smol and Cumming 2000). Variations in temperature and precipitation may result in changes in lake depth, mixing regimes, salinity and nutrients, which can influence the specific composition and abundance of diatom assemblages (Bradbury et al. 2002; Fritz et al. 2010). Indeed, changes in diatom assemblages have been attributed to recent climatic warming in subarctic and alpine lakes (Sorvari et al. 2002; Rühland et al. 2003; Karst-Riddoch et al. 2005), and temperate regions (Forrest et al. 2002; Harris et al. 2006). In relatively undisturbed lakes, increases of at least 5% in planktonic Cyclotella taxa were present in 84 of 105 lakes, concurrent with declines in thickly silicified Aulacoseira and/or benthic Fragilaria taxa (Rühland et al. 2008).
An examination of diatom assemblages in recent versus pre-European settlement times from sediment cores (known as the ‘top–bottom paleolimnological approach’; Cumming et al. 1992) can provide a rapid assessment of regional environmental change in aquatic ecosystems (Smol 2008). Diatoms present in the uppermost lake sediments (top sample, 0–0.25–cm of sediment cores) represent present-day conditions, while diatoms preserved in deeper sediments (generally > 20 cm in Canadian Shield lakes) represent pre-industrial times, and can be considered to be a measure of natural lake conditions (Cumming et al. 1992; Rühland et al. 2003; Smol 2008).
The primary goal of this study is to determine if and how the relative abundance of diatom species have changed from pre-industrial to modern times in relatively undisturbed lakes from the ELA. In most temperate freshwater ecosystems, climate-related changes may be masked by direct anthropogenic stressors, such as eutrophication and acidic deposition. Lakes located in remote regions, such as the ELA from northern Ontario, have been minimally impacted by such anthropogenic stressors (Findlay and Shearer 1992) and can be considered “reference sites” (Schindler et al. 1996; Findlay et al. 2001). Such lakes should be useful in simplifying the number of anthropogenic stressors impacting lakes, and maximize the importance of climate and/or other potential regional stressors on diatom communities. We hypothesize that ‘reference’ lakes from the ELA should display changes in diatom assemblages that are similar to those observed in the meta-analysis of relatively undisturbed sites examined by Rühland et al. (2008) (i.e., an increase in planktonic diatoms, particularly small Cyclotella species). Although Rühland et al. (2008) examined dated cores from 105 ‘relatively’ undisturbed lakes, small to medium-sized lakes from north-western Ontario and the ELA were poorly represented. A secondary goal of this paper was to determine if the physio-chemical characteristics of ELA lakes can be used to predict change in diatom assemblages since pre-industrial times. If recent warming is an important causal mechanism for the observed changes in diatom assemblages, we hypothesize that deeper lakes (i.e., lakes that are more prone to exhibit thermal stratification), should display a stronger impact from recent increases in temperature (sensu Gerten and Adrian 2001), as these lakes should show larger changes in heat storage capacity, water column stability, and variation in the strength and period of mixing with climatic warming in comparison to shallower lakes.
Study area and sampling
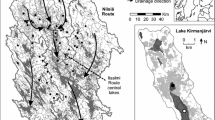
The 40 study lakes are located within the Experimental Lakes Area (ELA), northwestern Ontario (Fig. 1). The area is situated on the Canadian Shield with the bedrock constituted from Precambrian granites and gneisses (Findlay and Shearer 1992). The lakes ranged from 2 to 30 m in maximum depth and their watersheds consist of thin, poorly developed soils that are dominated by Picea mariana (black spruce) and Pinus banksiana (jack pine) forests. Duplicate sediment cores were sampled from all 40 lakes in June, 2006. Sediment cores were retrieved from the deepest basin of the lakes using a Glew corer (internal diameter 7.62 cm) ensuring that the sediment–water interface was preserved (Glew et al. 2001). Sediment samples from the top (0–0.25 cm, representing sediments recently accumulated or modern samples) and from the bottom of each core (20–20.25 cm, representing pre-industrial sediments) were analyzed to allow comparisons between modern and pre-industrial diatom species composition. The advantage of the so called ‘top–bottom’ approach is that it allows the investigator to quickly determine if consistent ecological changes have occurred across a region, which would be worthy of more detailed analyses. However, because the ‘top’ and ‘bottom’ sediment intervals vary slightly in the amount of time they represent (e.g., the ‘bottom’ sample will typically represent more time than a similarly sized interval from the top), it is possible that a change could emerge that is due to temporal differences between ‘top’ and ‘bottom’ samples. However, in practice, results from the ‘top–bottom’ approach have been verified by complete core analyses (Cumming et al. 1992, 1994; Ginn et al. 2007a, b). Although the specific dates of individual bottom sediment samples from the 40 lakes were not dated, full-core 210Pb profiles from 17 lakes from ELA show that supported 210Pb activity is reached at core depths of ~20 cm (mean and median = 20 cm; SD = 6; n = 17) (Cumming et al. Department of Biology, Queen’s University, Kingston, ON, unpublished data). Based on these results and the relatively high unsupported 210Pb in these cores, we are confident that a depth of 20 cm represents pre-industrial conditions in the majority of the study lakes. As mentioned above, the length of time represented by a sediment interval can also be important. Based on cores collected to date from northwestern Ontario, typically the 0.25-cm interval would represent between 1 and 2 years, whereas a sample between 20 and 20.25 cm would represent between 1 and 5 years. These results are similar to 210Pb dating results from many published studies of cores taken from lakes on the southern Canadian Shield (Mills et al. 2009) and in northwestern Ontario (Paterson et al. 2002), including Lake 239 from the Experimental Lakes Area (Laird and Cumming 2008).
Methods
Water chemistry
Water samples were collected from 35 of the 40 study lakes and analyzed at the Ontario Ministry of the Environment’s Dorset Environmental Science Centre using standard MOE protocols (Ontario Ministry of the Environment 1983). The five lakes not sampled for water chemistry are sampled regularly by ELA staff as reference lakes, and consequently spot samples from these systems were deemed unnecessary at the time. Chemical analyses included concentrations of chloride, sulphate, sodium, potassium, magnesium, calcium, NH4/NH3, NO2/NO3, total Kjeldahl nitrogen (TKN), total phosphorus (TP), dissolved organic carbon (DOC), reactive silicate (SiO3), specific conductivity, and true colour. Lake-water pH was measured in the field using a Fisher-Accumet pH meter (model 925). Of the study lakes, ELA lakes 110, 114, and 226 have been experimentally manipulated. In 1993, northern pike were introduced to Lake 110. Lake 114 was manipulated for epilimnetic acidification (1979–1986), including the addition of aluminum in 1984. Experiments conducted in Lake 226 include epilimnetic fertilization from 1973 to 1980, epilimnetic addition of radioisotope tracers (1977–1978; 1989) and metals (1987), and the lowering of water levels from 1995 to 1997. The watersheds of the study lakes have been minimally impacted by recent human disturbances, but in the past (between 1970 and 1980), some of the watersheds were partially logged (ELA lakes: 114, 115, 149, 164, 165, 373, 377, 378, 626, 627, 629, and 938), and some have experienced fires. Low-impact bait fishing and angling has likely occurred in many of the study lakes, as these activities are restricted but not monitored closely.
Diatom preparation and enumeration
Slides for diatom analysis were prepared using standard techniques (Cumming et al. 1992) for the top (0–0.25 cm) and the 20–20.25-cm sediment intervals. A small amount of wet sediment was suspended in a 50:50 (molar) mixture of sulfuric and nitric acid in a 20-ml glass vial for 24 h prior to being submersed in a 70°C water bath for approximately 5 h. The remaining sedimentary material was settled for a period of 24 h, at which time the acid above the sample was removed. The sample was rinsed with distilled water and allowed to settle once again for 24 h. The rinsing with distilled water was repeated approximately 8 times until the sample was neutral (litmus test). The samples were settled onto coverslips in a series of four 100% dilutions, which when dry, were mounted onto glass slides using Naphrax®, a high-resolution mounting medium. For each sample, at least 400 diatom valves were enumerated with a Leica DMRB microscope equipped with DIC optics at 1000× magnification (Numerical Aperature of oil objective and condenser top lens = 1.3 and 1.4, respectively). The diatom taxonomy was based on the references of Krammer and Lange-Bertalot (1986, 1988, 1991a, b), Camburn and Charles (2000), Cumming et al. (1995) and Tanaka (2007). Resting cysts of diatoms were not often encountered and consequently not enumerated.
Statistical analyses
To determine the main directions of variation in the water chemistry and physical variables within the ELA study sites, a principal component analysis (PCA), using inter-sample distances on a correlation matrix of log-transformed values, was run using the computer program CANOCO v. 4.5 (ter Braak and Šmilauer 1998).
Diatom species counts for the ‘top’ and ‘bottom’ samples were converted to relative abundance measures for each lake, and changes in the relative abundance of diatom taxa that achieved >5% relative abundance were examined. To summarize the main direction of variation in the diatom assemblages of the study lakes, a correspondence analysis (CA) with downweighting of rare taxa was performed using CANOCO v. 4.5 for each of the modern and preindustrial diatom assemblages. For summary purposes, diatom taxa were also grouped into functional groups (planktonic, tychoplanktonic and benthic taxa) based on the literature (Krammer and Lange-Bertalot 1986, 1988, 1991a, b). To assess if significant differences in dominant diatom taxa and functional groups existed between all modern versus all pre-industrial samples, ANOVAs and paired t-tests were used.
Multiple correlations with Bonferoni correction were performed to assess if: (1) changes in planktonic diatom species; and (2) changes in the dominant species D. stelligera could be related to the measured limnological variables, or the environmental PCA axis scores of the environmental data. As mentioned previously, the goal of these analyses was to determine if biological changes were related to a unique combination of limnological variables, that could then give insight into possible mechanism for the observed changes (i.e., are the changes larger in lakes that would be more susceptible to lake stratification). Six lakes were eliminated from the PCA and correlation analyses. These lakes included lakes that lacked adequate information on maximum depth (Lake 224, Lake 631 and Lake 651), and/or lakes that were experimentally manipulated (Lake 110, Lake 114, and Lake 226). Experimentally manipulated lakes were also excluded from ANOVAs, because diatom species may reflect changes related to lake recovery after manipulation rather than to natural conditions. ANOVAs, t-tests, and multiple correlations were performed using the program JMP v. 7.0 (SAS Institute Inc.).
Results
Physical and chemical characteristics of study lakes
The study lakes from north-western Ontario (Fig. 1) can be broadly characterized as small (mean depth = 9.3 m; median depth = 8 m; range = 2–30 m), low-conductivity (mean specific conductivity = 18.5 μS/cm; median = 16.4 μS/cm; range = 11–42 μS/cm), low-alkalinity (mean = 5 mg/L, median = 4.5 mg/L; range = 1.5–11.3 mg/L), acidic-to-circumneutral (mean pH = 6.7; median pH = 6.7; range = 5.4–7.2) oligotrophic (mean total phosphorus = 6.9 μg/L; median = 5.1 μg/L, range = ~1–21 μg/L) lakes with moderate to high concentrations of DOC (mean DOC = 7.3 mg/L; median DOC = 6.9 mg/L; DOC range = 3–15 mg/L) (Table 1). A PCA of the water chemistry was used to identify the largest limnological gradients in this dataset. The first two axes explained 78% of the variance in the study lakes, with lake-water pH and DOC being the most important variables contributing to the first axis. Lake depth, nutrients (TP and TKN), and specific conductivity contributed equally to define the first and second axes (Fig. 2). Generally, higher-pH lakes (e.g., lakes 435, 373 and 163) had lower concentrations of DOC, whereas lower-DOC lakes were more acidic (e.g., lakes 661, 470, 115, 131; Fig. 3). Similarly, nutrients were inversely correlated to lake depth (Fig. 2).
Relative abundances of diatom species in modern (solid bars) and pre-industrial (open bars) sediments in 40 study ELA lakes. Diatom taxa and lakes are arranged according to the first-axis species scores and site scores, respectively, of a correspondence analysis (CA). Lake codes are those of the Experimental Lakes Area
Diatom assemblages
From the 40 study lakes, 271 diatom species were identified from both ‘top’ and ‘bottom’ sediment samples. Most of the species were rare and consisted of less than 1% of the total relative abundance of diatom species. Diatom taxa with relative abundance >5% in either a ‘top’ or ‘bottom’ sample are ordered in Fig. 3 according to the ‘present-day’ (top) species and lake scores in a CA analysis. A clear pattern is displayed with planktonic and tychoplanktonic taxa on the left-hand side of the figure (e.g., Urosolenia eriensis (Smith) Round et Crawford ex Round, Crawford et Mann, Cyclotella ocellata Pantocsek, Synedra delicatissima Smith, Aulacosiera taxa, Discostella stelligera (Hustedt) Houk et Klee, Tabellaria floculosa (Roth) Kützing, and Fragilaria tenera (Smith) Lange-Bertalot) and benthic taxa on the right-hand side (e.g., taxa from the genera Achnanthidium, Brachysira, Cymbella, Eunotia, Navicula, Nitzschia, Pinnularia, Staurosira) (Fig. 3). The planktonic D. stelligera complex (including D. glomerata (Bachmann) Houk et Klee, D. stelligera, and D. pseudostelligera (Hustedt) Houk et Klee) was the most abundant taxonomic group, with relative abundances up to 70% in modern sediments and up to 60% in pre-industrial sediments (Fig. 3). The next most abundant taxon, was the tychoplanktonic species Aulacoseira ambigua (Grunow) Simonsen with abundances of ~30% in lakes 164 and 257, in both modern and pre-industrial sediments. Aulacoseira subarctica (Müller) Haworth was less abundant reaching a maximum abundance of 33% in Lake 240. In the remaining lakes, species of Aulacoseira (including A. ambigua, A. subarctica, and the A. distans (Ehrenberg) Simonsen complex) were present at relative abundances of less than 10%. The uppermost sediments of Lake 110 were dominated by U. eriensis (65%) which only reached a maximum of 5% in six other lakes.
The lakes towards the bottom of the figure are dominated by benthic taxa, with smaller abundances of D. stelligera (Fig. 3). In lakes 938, 115, 661, 131, 470, D. stelligera complex was absent or very rare. Diatoms from these lakes were represented by diverse assemblages with benthic species of Achnanthoids, Brachysira brebissonii Ross, B. vitrea (Grunow) Ross, Navicula mandumensis Jørgensen, N. leptostriata Jørgensen, Nitzschia perminuta Grunow, Cymbella spp., Eunotia spp., occasionally Stauroforma exiguiformis (Lange-Bertalot) Flower, Jones et Round, and small abundances of the A. distans complex and T. flocculosa (Fig. 3).
Changes in modern versus pre-industrial diatom assemblages
Over half of the lakes displayed increases in the relative abundance of planktonic taxa since pre-industrial times (Fig. 4). In sixteen lakes the increase in planktonic species (including Discostella spp., Cyclotella spp., Tabellaria spp., Eunotia zasuminensis (Cabejszekowna) Körner, Asterionella formosa Hassal, Fragilaria nanana Lange-Bertalot, F. tenera, S. delicatissima var. angustissima Grunow, and U. eriensis) was between 10 and 40% in modern versus pre-industrial sediments (Fig. 3). A change of less than 5% relative abundance of planktonic species is displayed by the rest of the lakes with the exception of Lake 373 which had a decrease in planktonic taxa of >10%. Based on a pairwise t-tests with Bonferroni correction, the average change in relative abundances of D. stelligera between pre-industrial and modern times was statistically significant (p < 0.05). In other lakes, changes in diatoms species displayed different patterns. For example, in Lake 110, the dominant diatom taxa changed from D. stelligera complex in pre-industrial times to a modern assemblage dominated by U. eriensis (Fig. 3).
Tychoplanktonic Aulacoseira spp. (A. ambigua, A. subarctica, A. distans complex, A. perglabra floriniae (Camburn) Haworth, A. italica (Ehrenberg) Simonsen, A. lacustris (Grunow) Krammer, A. lirata (Ehrenberg) Ross, A. nygaardii Camburn) are less abundant in present-day sediments by 0.3–19% than in pre-industrial sediments with the exception of lakes 257, 240, 651, 384, 938 where abundances increased by more than 5% (Fig. 4). In terms of planktonic diatom species, the highest increase in relative abundance in modern sediments is recorded by the D. stelligera complex while many benthic species, such as Achnanthidium minutissimum (Kützing) Czarnecki, Brachysira neoexilis Lange-Bertalot, Staurosirella pinnata (Ehrenberg) Williams et Round decreased in relative abundance in comparison to pre-industrial sediments (Fig. 3).
In general, the benthic diatom species occurred at higher relative abundances in pre-industrial sediments relative to modern samples. Based on a pairwise t-tests with Bonferroni correction, the average decrease in relative abundances in A. minutissimum, S. pinnata, and S. exiguformis between pre-industrial and modern times were statistically significant (p < 0.05). However, in a small number of samples, benthic species increased in modern sediments (e.g., Staurosira construens var. venter (Ehrenberg) Hamilton in Lake 150, and S. exiguiformis in lakes 115, 127, 436, 383, 470).
Patterns in limnological characteristics and relationships to changes in diatom species
Multiple correlations were performed to assess if the amount of change in planktonic, benthic and tychoplanktonic groups between the top and bottom samples, could be significantly related to measured environmental variables (i.e., coring depth, pH, TKN, TP, SiO3, DOC, true colour). The change in planktonic species was positively correlated to lake depth (R = 0.44; p = 0.01). Conversely, the change in benthic species was correlated negatively to lake depth (R = 0.45; p = 0.008) and positively to lake silica concentration, but with very low correlation coefficient and marginally significant. No significant correlation was found between the amount of change of tychoplanktonic species and the measured environmental variables.
Multiple correlations were also used to investigate if the amount of change of dominant diatom species (D. stelligera, Aulacoseira spp., Tabellaria spp., A. formosa and sum of small S. pinnata and S. construens var. venter) could be related to measured environmental variables. The strongest correlation was found between the percent change of D. stelligera and lake depth (R = 0.4; p = 0.01), and true colour (R = −0.5; p = 0.003). The percent change of A. formosa was correlated to lake depth (R = −0.3; p = 0.05) while Tabellaria spp. and S. pinnata and S. construens var. venter were both correlated to TKN (R = −0.5; p = 0.005; and R = 0.48; p = 0.005, respectively). Multiple correlations performed between the amount of change of select diatom species and the PCA axis-one scores, revealed that PCA axis-one site scores is significantly correlated with the change in D. stelligera (R = 0.4; p = 0.01) and Tabellaria spp., (R = −0.35; p = 0.04).
Discussion
Variations in diatom composition and abundance from lacustrine ecosystems have consistently been shown to be strongly related to changes in water physico-chemical properties, such as pH, nutrient concentration, transparency, and lake stratification (Smol and Cumming 2000). Changes in diatom species composition in time and space have been attributed to natural (e.g., geological factors, climate, fire) and anthropogenic factors (e.g., acidification, nutrient enrichment, climate). In this study, we choose a region and study lakes where the impacts from direct watershed disturbance, and the impacts of acidic deposition would be minimal, so that the impact of other potential regional stressors could be assessed. As detailed in the introduction, the ELA lakes used in this study are located in an area that is minimally impacted by human activities (e.g., no agriculture or industrial activities, and limited forestry), and is a region that receives low amounts of acidic deposition (Findlay and Shearer 1992).
The most apparent taxonomic shift that occurred in the ELA study lakes is a significant increase of the relative abundance of planktonic taxa in modern sediments, especially the D. stelligera complex, while the abundance of many benthic species, such as A. minutissimum, S. pinnata, Brachysira spp., Navicula spp., Cymbella spp. sensu lato, Pinnularia spp., have decreased since pre-industrial times (Figs. 3, 4). Large increases in Cyclotella taxa have been reported in lakes from arctic and temperate regions (Rühland et al. 2008). For example, studies across the Arctic have shown marked increases of the small Cyclotella species in the recent lake sediments (Sorvari et al. 2002; Rühland et al. 2003; Rühland and Smol 2005; Rühland et al. 2008). Similar changes have also been reported from temperate regions in Ontario (Forrest et al. 2002) and eastern Canada (Harris et al. 2006) and from high-elevation lakes (Karst-Riddoch et al. 2005). The increase in the relative abundances of the planktonic Discostella and Cyclotella species, especially D. stelligera, has been previously linked to a lengthening of the ice-free season and enhanced lake stratification (Sorvari et al. 2002; Rühland et al. 2003), a phenomenon that can be induced by increasing temperature (Smol et al. 2005; Rühland et al. 2008). Increases in D. stelligera and other planktonic diatom taxa since pre-industrial times from the ELA may be an indication of changes in the thermal characteristics of these lakes. Records of temperature and precipitation are available from the ELA meteorological station since 1970 and from the nearby town of Kenora, Ontario since the beginning of the 20th century (Fig. 5). A strong correlation was found between data recorded at both stations since 1970 (r = 0.98 for mean annual temperature and r = 0.80 for annual precipitation), indicating that the climate record from Kenora is representative of climatic conditions at the ELA (Moos et al. 2005). Mean annual temperatures measured at Kenora display a steady and significant increase of 0.01°C per year over the last century, with a total increase of ~2.5°C since 1899, and the largest increases recorded in the winter months (Dec–Feb: 0.025°C year−1). Concurrently, records of the duration of the ice-free season from Lake 239 show an increasing rate of ~0.3 days year−1 since 1969 (Fig. 5). At ELA the duration of the ice-free season has increased at Lake 239 by ~2 weeks since 1969 (r = 0.35, p < 0.05, Fig. 5), with both earlier ice-off dates and later ice on.
Mean annual temperature (°C) (solid line) and total annual precipitation (mm) (dotted line) recorded at Kenora airport, Ontario, Canada. Also shown is the duration of the ice-free period in days recorded at ELA Lake 239. Climate data are from http://www.cccma.bc.ec.gc.ca/hccd
Changes in seasonality are also now occurring in larger lakes in north-western Ontario. In a nearby, but much larger waterbody (Whitefish Bay of Lake of the Woods), there has been a reported increase of ice-free days (Rühland et al. 2008). Evidence of the effects of climatic change on small lakes in the Northern Hemisphere is clearly shown with ice-on and ice-off data (Magnuson et al. 2000). The length of the ice-free season is increasing in many small, mid-latitude lakes, as well as some of the Great Lakes (Austin and Colman 2007), due to later freezing and earlier breakup dates. Changes in seasonality can have significant impacts on the thermal regime of lakes. In a number of the Great Lakes, summer stratification has been increasing largely due to an earlier onset of stratification (Austin and Colman 2007). Changes to the thermal structure of a lake have implications for many in-lake processes, including nutrient recycling, primary production, and late-summer hypolimnetic oxygen levels, which in turn have biological impacts for many aquatic organisms.
Long-term monitoring of lakes from ELA has documented limnological changes due to climate in the 1980s. Prolonged dry periods combined with generally higher temperatures in the late 1980s resulted in a small decrease in lake depth, and increased lake transparency and light transmission in a number of ELA lakes (Schindler et al. 1996). The rapid spring warming and decreases in DOC inputs resulted in deeper thermoclines and increased thermal stability in the ELA reference lakes (Schindler 2001). Examination of algal communities from four oligotrophic lakes from the ELA between 1968 and 1998 revealed noticeable shifts in phytoplankton species composition and abundance during drought periods, including an increase in the biomass of diatoms (Findlay et al. 2001), providing further evidence that ELA lakes are climatically sensitive. Long-term monitoring data from Lake Tahoe, USA, provide additional evidence that climate warming may favour smaller diatoms over larger forms during periods of enhanced stratification (Winder et al. 2008). Winder et al. (2008) argues that reduced vernal mixing and increased thermal stability that occurs with climatic warming may alter cell sinking velocities, and the rate at which nutrients are redistributed in the water column, ultimately favouring smaller taxa such as small species of Cyclotella.
The results of the multiple correlation analysis are also consistent with some of the variation in diatom assemblages being related to a recent change in climate. They suggest that lake depth is the most important variable correlated with changes in planktonic, benthic and D. stelligera between the ‘pre-industrial’ and ‘modern’ sediment samples. According to our results, lake depth may be a strong predictor explaining the increase of D. stelligera among lakes. Shallow lakes generally displayed a smaller increase in D. stelligera, and they exhibited increases in the relative abundance of other planktonic taxa (e.g. A. formosa, Lake 240) or benthic species (e.g., Lake 127). The role of lake characteristics in modulating a climate signal is also supported by the correlation between PCA axis one scores of the environmental data, representing environmental gradients of pH, DOC, True Colour, and lake depth (Fig. 2), and variation associated with changes in the relative abundance of D. stelligera and Tabellaria species. The multiple correlation results also show that increases in A. formosa occurred more commonly in shallower lakes (<10 m maximum depth), while deeper lakes recorded shifts towards increases in D. stelligera complex. In general, shallower lakes were more highly coloured and higher in nutrient concentrations than the deeper, dimictic lakes. Thus, it appears that the specific biological response to climate warming varies among lakes, depending on lake morphometry and pre-existing water quality. Changes in planktonic taxa were not just limited to increases in D. stelligera. In Lake 110, D. stelligera decreased dramatically in favour of an abrupt increase of U. eriensis; however, dissolution of U. eriensis, a lightly-silicified taxon, in deeper sediments cannot be ruled out.
The modulation of lake response by lake morphometry in response to climate warming has been reported elsewhere (Adrian et al. 1999; Gerten and Adrian 2001). Gerten and Adrian (2001) demonstrated that the influence of climatic warming can vary substantially among lake types and that water temperature regimes are expected to show more impact in deep, dimictic lakes. In contrast, shallow, well-mixed lakes have a low heat-storage capacity with relatively short-term effects on water temperature regimes, and implicitly with a reduced impact on the plankton community (Adrian et al. 1999). Similar findings have also been reported from arctic and subarctic regions where deeper, stratified lakes displayed the highest increase in D. stelligera and decrease of benthic species, whereas changes in isothermal lakes were typically among benthic species (Sorvari et al. 2002; Rühland et al. 2003; Smol et al. 2005). Changes in diatom assemblages reported at ELA suggest that deeper lakes may be the most responsive to recent climatic warming, with a longer growing season, increased water transparency, and more persistent thermal stability. However, D. stelligera and other planktonic species also increased in some of our shallow study lakes (e.g., ELA lakes 114, 99, 132, 129). A possible explanation is that the seasonal position of the thermocline is not only dependent climate conditions, but also on other variables such as lake area, fetch, and water clarity (Fee et al. 1996; Mazumder et al. 1990; Xenopoulos and Schindler 2001).
While lake depth is an important variable in predicting differences in the response of diatoms to climatic change across the landscape, it is unlikely that the biological changes we observe within lakes are a result of increasing lake depth since pre-industrial times. For example, changes in lake level have been shown to be minimal since the mid-to-late 1800s to present at the ELA (Laird and Cumming 2008). Furthermore, the frequency of drought, an important determinant of changes in lake level, have not increased significantly since AD 1783 in the Winnipeg River Basin, according to reconstructions from a network of 54 tree-ring sites (St. George et al. 2008). Finally, marked increases in Cyclotella sensu lato species have been shown in a sediment core from Lake of the Woods, a large international lake ~100 km from the ELA (Rühland et al. 2008), during a period of minimal change in lake level (1980-present) (Lake of the Woods Control Board, http://www.lwcb.ca). Thus, the changes in diatom species from the ELA study lakes are consistent with recent warming, but the influence on individual lakes is modulated by lake-specific factors.
Acidic deposition can be ruled out as a factor responsible for the observed changes. As mentioned earlier, the ELA region is also located in an area with a low-deposition of sulphate. Although there are significant changes in diatom species composition over time, circumneutral taxa dominate both pre-industrial and present-day assemblages. The primary shift to planktonic taxa, such as D. stelligera (a circumneutral taxon; Findlay and Shearer 1992), is not consistent with biological changes resulting from lake acidification (Battarbee et al. 2010).
The observed changes in diatom taxa since pre-industrial times at ELA are also not consistent with known watershed disturbances, including logging and fires. Although some forest harvesting has occurred, it has been of limited intensity. The observed changes are also not consistent with increases in eutrophic taxa, as might be expected with more severe or sustained changes in land-use (Hall and Smol 2010). The main, albeit small, watershed disturbance has been some forest harvesting, but only from a small subset of the study lakes. Previous studies have shown that the impact from logging is minimal on diatom species if forests are allowed to regenerate (Laird and Cumming 2001; Laird et al. 2001), or may be masked by regional changes in climate (Paterson et al. 1998, 2000).
Long-term changes in nutrient deposition (e.g., nitrogen) warrant further examination, but are generally not consistent with the observed changes in diatom assemblages in the study lakes. Several studies have shown that increases in D. stelligera abundances are not necessarily coeval with changes in nutrient deposition (e.g., increasing N deposition), or to changes in nutrient concentrations in lakes (Fritz et al. 1993; Sorvari and Korhola 1998; Rühland et al. 2003, 2008; but see Wolfe et al. 2001, which shows abrupt decrease in D. stelligera in the post-industrial era). Rühland et al. (2008) examined the relationship between long-term changes in diatom species and inorganic N deposition in Lake of the Woods and concluded that there was no significant relationship between long-term diatom species changes and inorganic N deposition. However, Rühland et al. (2008) note that changes in inorganic N deposition may be correlated to climatic changes, indirectly through changes in precipitation. Thus, a synergistic relationship between climate and nutrient dynamics may exist in the ELA region, and may provide a partial explanation of the observed biological changes.
Changes in temperature and seasonality are likely explanatory factors for the observed trends in diatom taxa in the ELA region, recognizing that the ‘top’/’bottom’ approach is limited, and does not allow for a full assessment of causes of the observed species changes. Future studies of dated sediment cores should be applied to assess the timing of changes in planktonic taxa, synchronicity of the changes among lakes, and to more fully assess the biological changes relative to measured changes in climate and other regional stressors.
In conclusion, we believe that the changes we observe in the modern diatom assemblages from the ELA lakes, punctuated with an increase in the relative abundance of D. stelligera complex in deeper lakes, are consistent with enhanced summer stratification, a longer ice-free season and increased thermal stability in recent decades. The ELA lakes have been minimally impacted from anthropogenic disturbances in comparison to lakes in other regions of the Precambrian Shield (e.g., south-central Ontario; Yan et al. 2008), and thus we conclude that recent warming provides the strongest explanation for the observed species changes. The biological changes were greater in deeper, thermally stratified, and nutrient poor lakes, although a few of the shallower lakes also showed similar changes.
Detailed paleolimnological studies are necessary from the ELA region to further assess when planktonic taxa increased, so that the relationship between climate change and other potentially important regional factors can be evaluated further.
References
Adrian R, Waltz N, Hintze T, Hoeg S, Rusche R (1999) Effects of ice duration on plankton succession during spring in a shallow polymictic lake. Freshw Biol 41:621–632
Austin JA, Colman SM (2007) Lake Superior summer water temperatures are increasing more rapidly than regional air temperatures: a positive ice-albedo feedback. Geophys Res Lett 34:L06604. doi:10.1029/2006GL02921
Battarbee RW, Charles DF, Bigler C, Cumming BF, Renberg I (2010) Diatoms as indicator of lake-water acidity. In: Stoermer EF, Smol JP (eds) The diatoms: applications for the environmental and earth sciences. Cambridge University Press, Cambridge, pp 98–121
Blenkner T, Adrian R, Livingstone DM, Jennings E, Weyhenmeyer GA, George DG, Jankowski T, Järvinen M, Aonghusa CN, Nõges T, Straile D, Teubner K (2007) Large-scale signatures in lakes across Europe: a meta—analysis. Glob Change Biol Postprint. doi:10.1111/j.1365-2486.2007.01364.x
Blumberg AF, Di Toro DM (1990) Effects of climate warming on dissolved oxygen concentrations in Lake Erie. Am Fish Soc 119:210–223
Bradbury JP, Cumming BF, Laird KR (2002) A 1500-year record of climatic and environmental change in Elk Lake, Minnesota III: measures of past primary productivity. J Paleolimnol 27:321–340
Camburn KE, Charles DF (2000) Diatoms of low-alkalinity Lakes in the Northeastern United States. The Academy of Natural Sciences Philadelphia, Philadelphia, U.S.A, p 152
Cumming BF, Smol JP, Kingston JC, Charles DF, Birks HJB, Camburn KE, Dixit SS, Uutala AJ, Selle AR (1992) How much acidification has occurred in Adirondack region (New York, USA) lakes since pre-industrial times? Can J Fish Aquat Sci 49:128–141
Cumming BF, Davies KE, Smol JP, Birks HJB (1994) When did acid sensitive Adirondack (New York, USA) lakes begin to acidify and are they still acidifying? Can J Fish Aquat Sci 51:1550–1568
Cumming BF, Wilson SE, Hall RI, Smol JP (1995) Diatoms from British Columbia (Canada) Lakes and their relationship to salinity, nutrients and other limnological variables (with 248 figures, 6 tables and 1041 photos on 60 plates). Bibl Diatomol 31. Stuttgart, Germany, pp 207
Fee EJ, Hecky RE, Kassian SEM, Cruikshank DR (1996) Effects of lake size, water clarity, and climatic variability on mixing depths in Canadian Shield lakes. Limnol Oceanogr 41:912–920
Findlay DL, Shearer JA (1992) Relationships between sedimentary diatom assemblages and lakewater pH values in the experimental Lakes Area. J Paleolimnol 7:145–156
Findlay DL, Kasian SEM, Stainton MP, Beaty K, Lyng M (2001) Climatic influences on algal populations of boreal forest lakes in the Experimental Lakes Area. Limnol Oceanogr 46:1789–1793
Forrest F, Reavie E, Smol JP (2002) Comparing limnological changes associated with 19th century canal construction and other catchment disturbances in four lakes within the Rideau Canal system, Ontario, Canada. J Limnol 61:183–197
Fritz SC, Kingston JC, Engstrom DR (1993) Quantitative trophic reconstruction from sedimentary diatom assemblages: a cautionary tale. Freshw Biol 30:1–23
Fritz SC, Cumming BF, Gasse F, Laird KR (2010) Diatoms as indicators of hydrologic and climatic change in saline lakes. In: Stoermer EF, Smol JP (eds) The diatoms: applications for the environmental and earth sciences. Cambridge University Press, Cambridge, pp 186–208
Gerten D, Adrian R (2001) Differences in the persistency of the North Atlantic oscillation signal among Lakes. Limnol Oceanogr 46:448–455
Ginn B, Cumming BF, Smol JP (2007a) Assessing pH changes since pre-industrial times in 51 low-alkalinity lakes in Nova Scotia, Canada. Can J Fish Aquat Sci 64:1043–1054
Ginn B, Cumming BF, Smol JP (2007b) Long-term acidification trends in high- and low-sulphate deposition regions from Nova Scotia, Canada. Hydrobiologia 586:261–275
Glew JR, Smol JP, Last WM (2001) Sediment core collection and extrusion. In: Last WM, Smol JP (eds) Tracking environmental change using lake sediments. Vol 1: basin analysis, coring, and chronological techniques. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 73–105
Hall RI, Smol JP (2010) Diatoms as indicators of lake eutrophication. In: Stoermer EF, Smol JP (eds) The diatoms: application for the environmental and earth sciences. Cambridge University Press, Cambridge, pp 122–151
Harris MA, Cumming BF, Smol JP (2006) Assessment of recent changes in New Brunswick (Canada) Lakes based on paleolimnological shifts in diatom species assemblages. Can J Bot 84:151–163. doi:10.1139/B05-157
Karst-Riddoch TL, Pisaric MFJ, Smol JP (2005) Diatom responses to 20th century climate-related environmental changes in high-elevation lakes of the northern Canadian Cordillera. J Paleolimnol 33:265–282
Krammer K, Lange-Bertalot H (1986) Bacillariophyceae. 1. Teil: Naviculaceae. In: Ettl H, Gärtner G, Gerloff J, Heynig H, Mollenhauer D (eds) Süßwasserflora von Mitteleuropa, Band 2/1. Gustav Fischer Verlag, Stuttgart/New York, p 876
Krammer K, Lange-Bertalot H (1988) Bacillariophyceae. 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae. In: Ettl H, Gärtner G, Gerloff J, Heynig H, Mollenhauer D (eds) Süßwasserflora von Mitteleuropa, Band 2/2. Gustav Fischer Verlag, Stuttgart/New York, p 596
Krammer K, Lange-Bertalot H (1991a) Bacillariophyceae. 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. In: Ettl H, Gärtner G, Gerloff J, Heynig H, Mollenhauer D (eds) Süßwasserflora von Mitteleuropa, Band 2/3. Gustav Fischer Verlag, Stuttgart/Jena, p 576
Krammer K, Lange-Bertalot H (1991b) Bacillariophyceae. 4. Teil: Achnanthaceae Kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema. In: Ettl H, Gärtner G, Gerloff J, Heynig H, Mollenhauer D (eds) Süßwasserflora von Mitteleuropa, Band 2/4. Gustav Fischer Verlag, Stuttgart/Jena, p 437
Laird KR, Cumming BF (2001) A regional paleolimnological assessment of the impact of clearcutting on lakes from the central interior of British Columbia. Can J Fish Aquat Sci 58:492–505
Laird KR, Cumming BF (2008) Reconstruction of Holocene lake level from diatoms, chrysophytes, and organic matter in a drainage lake from the Experimental Lakes Area (northwestern Ontario, Canada). Quat Res 69:292–305
Laird KR, Cumming BF, Nordin R (2001) A regional paleolimnological assessment of the impact of clearcutting from the west coast of Vancouver Island, British Columbia. Can J Fish Aquat Sci 58:479–491
Magnuson JJ, Webster KE, Assel RA, Bowser CJ, Dillon PJ, Eaton JG, Evans HE, Fee EJ, Hall RI, Mortsch LR, Schindler DW, Quinn FH (1997) Potential effects of climate changes on aquatic ecosystems: Laurentian Great Lakes and Precambrian Shield region. Hydrol Process 11:825–871
Magnuson JJ, Robertson DM, Benson BJ, Wynne RH, Livingstone DM, Arai T, Assel AR, Barry RG, Card V, Kuusisto E, Granin NG, Prowse TD, Stewart KM, Vuglinski VS (2000) Historical trends in lake and river ice cover in northern hemisphere. Science 289:1743–1746
Mazumder A, Taylor WD, McQueen DJ, Lean DRS (1990) Effects of fish and plankton on lake temperature and mixing depth. Science 247:312–315
Mills RB, Paterson AM, Blais JM, Lean DRS, Smol JP, Mierle G (2009) Factors influencing the achievement of steady state in mercury contamination among lakes and catchments of south-central Ontario. Can J Fish Aquat Sci 66:187–200
Moos MT, Laird KR, Cumming BF (2005) Diatom assemblages and water depth in Lake 239 (Experimental Lakes Area, Ontario): implications for paleoclimatic studies. J Paleolimnol 34:217–227
Ontario Ministry of the Environment (1983) Handbook of analytical methods for environmental samples, vol 1 and 2. Laboratory Services Branch, Ontario Ministry of the Environment and Energy, Sudbury, ON
Paterson AM, Cumming BF, Smol JP, Blais JM, France R (1998) Assessment of the effects of logging, forest fires and drought on lakes in northwestern Ontario: a 30-year paleolimnological perspective. Can J For Res 28:1546–1556
Paterson AM, Cumming BF, Smol JP, Blais JM, France R (2000) A paleolimnological assessment of the effects of logging on two lakes in northwestern Ontario, Canada. Verh Internat Verein Limnol 27:1214–1219
Paterson AM, Morimoto DS, Cumming BF, Smol JP, Szeicz JM (2002) A paleolimnological investigation of the effects of forest fire on lake water quality in northwestern Ontario over the past ca. 150 years. Can J Bot 80:1329–1336
Rühland K, Smol JP (2005) Diatom shifts as evidence for recent Subarctic warming in a remote tundra lake, NWT, Canada. Paleogeogr, Paleoclimatol, Palaeoecol 226:1–16
Rühland K, Priesnitz A, Smol JP (2003) Paleolimnological evidence from diatoms for recent environmental changes in 50 Lakes across Canadian Arctic Treeline. Arct Antarct Alp Res 35:110–123
Rühland K, Paterson AM, Smol JP (2008) Hemispheric-scale patterns of climate-related increases in planktonic diatoms from North American and European lakes. Glob Change Biol. doi:10.1111/j.1365-2486.2008.01670.x
Schindler DW (2001) The cumulative effects of climate warming and other human stresses on Canadian freshwaters in the new millennium. Can J Fish Aquat Sci 58:18–29
Schindler DW, Beaty KG, Fee EJ, Cruikshank DR, DeBruyn ED, Findlay DL, Linsey GA, Shearer JA, Stainton MP, Turner MA (1990) Effects of climatic warming on lakes of the central boreal forest. Science 250:967–970
Schindler DW, Bayley SE, Parker BR, Beaty KG, Cruikshank DR, Fee EJ, Schindler EU, Stainton MP (1996) The effects of climate warming on the properties of boreal lakes and streams at the Experimental Lakes Area, northwestern Ontario. Limnol Oceanogr 41:1004–1017
Smol JP (2008) Pollution of Lakes and Rivers: a paleoenvironmental perspective, vol 2. Oxford University Press, New York, USA
Smol JP, Cumming BF (2000) Tracking long-term changes in climate using algal indicators in lake sediments. J Phycol 36:986–1011
Smol JP, Douglas MSV (2007) Crossing the final ecological threshold in high Arctic ponds. PNAS 104:12395–12397
Smol JP, Wolfe AP, Birks HJB, Douglas MSV, Jones VJ, Korhola A, Pienitz R, Rühland K, Sorvari S, Antoniades D, Brooks SJ, Fallu M-A, Hughes M, Keatley BE, Laing TE, Michelutti N, Nazarova L, Nyman M, Paterson AM, Perren B, Quinlan R, Rautio M, Saulnier-Talbot E, Siitonen S, Solovieva N, Weckström J (2005) Climate-driven regime shifts in the biological communities of arctic lakes. PNAS 102:4397–4402
Snucins E, Gunn J (2000) Interannual variation in thermal structure of clean and colored lakes. Limnol Oceanogr 45:1639–1646
Sorvari S, Korhola A (1998) Recent diatom assemblage change in subarctic Lake Saanajärvi, NW Finland Lapland. J Paleolimnol 20:205–215
Sorvari S, Korhola A, Thompson R (2002) Lake diatom response to recent Arctic warming in Finnish Lapland. Glob Change Biol 8:171–181. doi:10.1046/j.1365-2486.2002.00463.x
St. George S, Meko DM, Evans MN (2008) Regional tree growth and inferred summer climate in the Winnipeg River basin, Canada since AD 1783. Quat Res 70:158–173
Tanaka H (2007) Taxonomic studies of the genera Cyclotella (Kützing) Brébisson, Discostella Houk et Klee and Puncticulata Håkansson in the family Sephanodiscaceae Glezer et Makarova (Bacillariophyta) in Japan. Bibl Diatomol 53. Stuttgart, Germany, pp 205
ter Braak CJF, Šmilauer P (1998) CANOCO reference manual and user’s guide to CANOCO for windows: software for Canonical community ordination (version 4). Microcomputer Power, Ithaca, USA, p 352
Winder M, Schindler DE (2004) Climatic effects on the penology of lake processes. Glob Change Biol 10:1844–1856. doi:10.1111/j.1365-2486.2004.00849.x
Winder M, Reuter JE, Schladow SG (2008) Lake warming favours small-sized planktonic diatom species. Proc R Soc Lond B Biol Sci. doi:10.1098/rspb.2008.1200
Wolfe AM, Baron JS, Cornett J (2001) Anthropogenic nitrogen deposition induces rapid ecological changes in alpine lakes of the Colorado Front Range (USA). J Paleolimnol 25:1–7
Xenopoulos MA, Schindler DW (2001) The environmental control of near-surface thermoclines in boreal lakes. Ecosystems 4:699–707
Yan ND, Paterson AM, Somers KM, Scheider WA (2008) Introduction to the Dorset special issue: transforming understanding of the factors that regulate aquatic ecosystems on the southern Canadian Shield. Can J Fish Aquat Sci 65:781–785
Acknowledgments
We acknowledge the assistance of Christina Clarke, Chris Lorenz, Erin MacMillan, and Shelley Wilkinson for providing assistance with logistics and coring. This manuscript was improved with comments from Drs. K. Laird and K. Rühland. Funding for analyses and some of the fieldwork was provided by a grant to BFC under the Best in Science program of the Ontario Ministry of the Environment, with additional funding for fieldwork provided by a NSERC Discovery Grant to BFC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Enache, M.D., Paterson, A.M. & Cumming, B.F. Changes in diatom assemblages since pre-industrial times in 40 reference lakes from the Experimental Lakes Area (northwestern Ontario, Canada). J Paleolimnol 46, 1–15 (2011). https://doi.org/10.1007/s10933-011-9504-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10933-011-9504-2