Abstract
In aquatic ecosystems, physical disturbances have been suggested to be one of the main factors influencing phytoplankton structure and diversity. To elucidate whether large-scale artificial operation of a hydroelectric reservoir has potential impacts on phytoplankton diversity, the impact on phytoplankton biodiversity of physical disturbances under artificial operation from May 2007 to April 2008 in tributaries of the Three Gorges Reservoir (TGR), China, was analysed. Two disturbance parameters, i.e. the absolute incremental rates of discharge (R d,i ) and precipitation (R p,i ), were created in this study for evaluating physical disturbance intensities during low and high water level periods of the TGR. Results showed that river discharge seemed to be the main factor controlling the phytoplankton diversity in low water level periods (≤151 m), and that precipitation was a potential promoter of the physical disturbance. During the 156-m impoundment process, the species diversity clearly decreased due to the high dilution effect on the phytoplankton communities. At high water level periods (>151 m), the low levels of disturbance eventually allowed the phytoplankton community to approach competitive exclusion in late February 2008. Sharply declining diversity values appeared when the Dinophyta blooms occurred in late March and late April 2008 (Peridinium and Ceratium, respectively).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The ecological impact of large hydroelectric dams on aquatic ecosystems has often been studied (Humborg et al., 1997; Kelly, 2001). The operation of hydroelectric power plants perturbs the integrity of natural rivers and significantly changes the hydrological conditions, including water level, river flow, water velocity, and hydraulic retention times (HRT) (Pringle, 2003; Magilligan & Nislow, 2005). As a result of these hydrological changes, the physical, chemical and biotic characteristics of rivers have been altered, and in many cases, the equilibrium state of the aquatic ecosystem has even been destroyed (Bain et al., 1988; Poff & Ward, 1989). Thus, the ecological assessment of aquatic ecosystems is necessary for the construction and operation of hydroelectric power stations. It is known that phytoplankton are sensitive aquatic organisms, and that their community characteristics are driven by physical environmental variations on a short time scale (Jeong et al., 2007; Cabecinha et al., 2009). The structure, distribution and diversity of phytoplankton communities are often used to assess the impact of large-scale dams on aquatic ecosystems (Bertrand et al., 2001; Nhiwatiwa & Marshall, 2007).
In aquatic ecosystems, physical disturbances have been suggested to be one of the main factors influencing phytoplankton structure and diversity. The Connell’s intermediate disturbance hypothesis (IDH) is an influential conceptual theory that attempts to explain the effects of disturbances on species diversity (Connell, 1978). This hypothesis states that high local diversity can be maintained under intermediate levels of physical disturbance. Low and high levels of disturbance are expected to be less effective for maintaining high diversity because the former enables competitive exclusion and the latter directly eliminates many species (Kimbro & Grosholz, 2006). The IDH has been tested in natural aquatic ecosystems such as lakes (Padisák, 1993; Weithoff, 2003), river floodplains (Davis et al., 2007; Paidere et al., 2007), rivers (Descy, 1993) and reservoirs (Barbiero et al., 1999; Lopes et al., 2009). However, these ecosystems were mainly regulated by natural hydro-meteorological conditions without anthropogenic disturbance. The Three Gorges hydroelectric plant in China provided an opportunity to analyse the impacts of anthropogenic disturbances on a phytoplankton community on the basis of the IDH.
The drastic changes in hydrodynamic conditions in the Three Gorges Reservoir (TGR) played a critical role in transforming the aquatic ecosystem from a riverine-type to a nearly lentic-type system (Hart et al., 2002; Magilligan & Nislow, 2005). Previous studies have shown that the dramatic changes in hydrodynamic conditions that occurred as a result of the Three Gorges Project (TGP) induced a series of ecological problems, including eutrophication and algal blooms in tributaries of the Yangtze River in the TGR (Meng & Zhao, 2007; Li et al., 2009a). To assess variations in the hydrodynamic conditions following the completion of the TGP, some researchers have investigated the influence of hydrodynamic parameters on the aquatic environment and on phytoplankton community characteristics in the main stream and tributaries. Evidence from such studies showed that there were significant differences in phytoplankton communities in the TGR before and after the 156-m impoundment (Kuang et al., 2005; Hu & Cai, 2006). It was supposed that seasonal variations in phytoplankton communities were affected by physical disturbances caused by the operation strategies of the TGR. However, an analysis of phytoplankton biodiversity in the tributaries as a response to variations in the hydrodynamic conditions has not been reported. Moreover, it is not currently clear how the physical processes in the TGR impact the observed phytoplankton biodiversity and the subsequent occurrence of algal blooms. In response to these problems, this study aims to compare and analyse the phytoplankton diversity within different time periods in the Pengxi River backwater area in the TGR. The specific aims of this site-specific study are as follows:
-
(1)
to identify seasonal variations in the phytoplankton species diversity and the main physical environmental parameters in the Pengxi River during operation of the TGR;
-
(2)
to interpret the potential impact on phytoplankton species diversity of different physical disturbances during the low and high water level periods in the Pengxi River applying the IDH theory.
Materials and methods
Study area
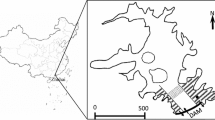
The Three Gorges Dam (TGD) on the Yangtze River in China is one of the world’s largest dams, with a height of 185 m and a length of 2,335 m (Wu et al., 2003). The TGR, with a water surface of up to 1,080 km2 located in the middle of the Yangzte River, was formed after the construction of the dam (Fig. 1). The TGR is located at 29°16′–31°25′N, 106°–111°10′E and has a catchment of the typical gorge type (Zeng et al., 2006). In 2006, the impoundment of the TGR resulted in a water level increase in the Yangtze River from 145 to 156 m, at which point the TGR began performing its multiple functions. According to the operational strategies of the TGR, the water level in the TGR would be permitted to rise during drought seasons for power generation. Conversely, a low water level would be maintained during the summer flood season to prevent floods downstream.
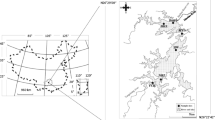
The Pengxi River is one of the largest tributaries in the central part of the TGR and is located about 247 km upstream from the TGD (Li et al., 2009b). The Pengxi River covers a watershed area of 5,172.5 km2 located at 30°49′–31°42′N, 107°56′–108°54′E. It has a main stream length of 182 km and an average slope of 1.25‰. The area has warm, humid and subtropical weather conditions. The average annual precipitation in the watershed is 1,100–1,500 mm, and the flood season is from July to September. The average annual flow is 118 m3 s−1 with a runoff of 3.41 billion m3.
After the 156-m impoundment, the Pengxi River formed a backwater area approximately 60 km from its confluence with the Yangzte River that extended upstream from Shuangjiang Town to Qukou Town (Fig. 2). In this study, five sampling sites were established in the centre of the main channel of the Pengxi River. The spatial coordinates of the sampling sites (from upstream to downstream) were as follows: Quma (QM, 31°07′50.8″N, 108°37′13.9″E), Gaoyang (GY, 31°07′50.5″N, 108°40′29.5″E), Huangshi (HS, 31°03′38.6″N, 108°41′36.2″E), Shuangjiang (SJ, 30°56′51.1″N, 108°41′37.5″E) and Hekou (HK, at the confluence with the Yangtze River, 30°57′03.8″N, 108°39′30.6″E).
Sampling methods
Phytoplankton samples were collected twice a month at the five sampling sites from May 2007 to April 2008. Sampling was conducted between 9:30 AM and 16:30 PM. Phytoplankton samples for qualitative identification were collected from the surface water with 25-μm phytoplankton nets and immediately preserved with formaldehyde (Heise, 1949; Zeng et al., 2006). Samples for quantitative analysis were collected from each sampling site at different water depths (0.5, 1, 2, 3, 5, and 8 m) using 250-ml polyethylene bottles. The quantitative samples were fixed by adding Lugol’s solution (Zhang & Huang, 1991; Donk & Hessen, 1993). After mixing equal amounts of the above six samples, 1,000 ml was set aside for sedimentation for approximately 48 h before counting according to Utermöhl’s method (Utermöhl, 1958). Phytoplankton were counted with a standard light microscope (XSP-8CA), and all algal taxa were identified to species level according to the classifications of Hu & Wei (2006). Algal cells were counted across 100-graticule areas with a compound microscope at ×400 magnification. All the algal individuals, including colonial and filamentous algae, were counted and sized according to standard methodology (Hu & Wei, 2006). A maximum counting error of 25% was accepted for the abundance estimates of each major algal group (Venrick, 1978). The biovolume of each species was calculated on the basis of cell size and number and then converted to carbon biomass units of mg ml−1 (Strathmann, 1967; Montagnes et al., 1994).
Data processing
Biodiversity depends on two different measures: (1) community structure or richness, i.e., the number of species in the community, and (2) evenness or the degree of similarity in abundance among species (Krebs, 1999; Scrosati & Heaven, 2007). In this study, the Shannon–Weaver diversity index (H index), Margalef richness index (d index) and Pielou evenness index (J index) were applied to comprehensively estimate the characteristics of the phytoplankton community. The formulae are as follows (Shannon & Weaver, 1949; Pielou, 1966; Margalef, 1975):
where S is the number of phytoplankton species, N is the total phytoplankton biomass in the same water sample, and N i is the biomass of i species.
The daily precipitation in the watershed was downloaded from the China Meteorological Data Sharing System (http://cdc.cma.gov.cn/). The water level at HK in the Pengxi River was estimated according to the water surface gradients between the TGD dam site (http://www.ctgpc.com.cn/) and the Wanxian hydrology monitoring station (http://www.cqwater.gov.cn). The daily water level data from both of those sites can be downloaded online. The daily discharge of the Pengxi River was calculated using a distributed hydrologic model of the Pengxi watershed, which was calibrated and validated by historical measurements of 52 years of monthly discharge (Long et al., 2009; Wu et al., 2010). The HRT was calculated based on the Hydrologic Engineering Centres River Analysis System (HEC-RAS, v.4.1) 1D hydrodynamic model of the Pengxi River (Li et al., 2012).
To discuss the variation of phytoplankton biodiversity in response to different hydrodynamic conditions, the year was divided into two periods: (1) the low water level period (before impoundment) when the water level was less than 151 m, from May to September 2007, and (2) the high water level period (after impoundment) when the water level was higher than 151 m, from October 2007 to April 2008 (Fig. 3). The disturbance intensities during the two different water level periods were calculated from the absolute incremental rates of discharge (R d,i ) and precipitation (R p,i ) using the following formulae:
where D i is the discharge of the current day (m3 s−1), D i−1 is the discharge during the previous day (m3 s−1), P i is the precipitation of the current day (mm), P i−1 is the precipitation of the previous day (mm) and Δt is the interval time (1 day).
The rate data were then divided into three groups sorted by incremental size (0–30, 30–70, and 70–100%); these three groups represented low, intermediate and high intensities of disturbance, respectively, and are referred to hereafter as levels 1, 2 and 3, respectively (Table 1). To analyse the relationship between disturbance and diversity, the average rate from all sampling data within every sampling interval was used to express the intensity of disturbance that occurred during every sampling interval.
The statistical analyses in this study were conducted using SPSS® 16.0 and ORIGIN® 8.0. To analyse the differences among the five sampling sites during the two water level periods, both t test and one-way ANOVA were performed. Coefficient of variance (CV) was also applied to quantify the level of variance within specific datasets. In addition, Spearman correlation analyses were applied to analyse the relationships between the hydrodynamic conditions during the two different water level stages.
Results
Hydrologic conditions and physical disturbance
There were significant differences in the water level, HRT, discharge and precipitation before and after the 156-m impoundment in the Pengxi River (P < 0.05). Before the 156-m impoundment in October, the water level changed from 144.58 to 150.60 m (mean = 146.60 m; CV = 0.77%). The water level varied between 146.44 and 156.10 m (mean = 154.14 m; CV = 1.2%) after impoundment (Fig. 3). The significant difference in the HRT between the two stages was caused by the artificial water scheduling in the TGR. Before the impoundment, the river had a short HRT and was similar to a riverine-type system (mean = 38.07 days; CV = 57.65%); after the 156-m impoundment, it shifted to a near lentic-type system with a prolonged HRT (mean = 83.77 days; CV = 25.72%). During the low water level period, the HRT was significantly negatively correlated with the river flow (r = −0.950; P < 0.01), while during the high water stage, the HRT was strongly correlated with the water level (r = 0.851, P < 0.05) and negatively correlated with the river flow (r = −0.771, P < 0.01). The precipitation data showed characteristic local seasonal patterns in the TGR. The rainy and dry seasons occurred in the low and high water level periods, respectively (mean = 5.05 mm vs. 0.85 mm; CV = 223.52% vs. 273.44%). Before the impoundment, the river flow changed dramatically from 50.90 to 907.09 m3 s−1 (mean = 199.87 m3 s−1; CV = 88.9%); this change was attributed mainly to the variability in precipitation (r = 0.676, P < 0.05). After the impoundment, the river discharge stayed at a relatively stable level between 50.80 and 423.15 m3 s−1 during the drought season.
During the study, river discharge and precipitation regulated the hydrodynamic features of the Pengxi River and acted as the main factors influencing the physical disturbance. Before the impoundment, the daily absolute incremental rate of discharge (R d,i ) ranged from 0 to 440.18 m3 s−1 day−1, and the daily incremental rate of precipitation (R p,i ) ranged from 0 to 71.36 mm day−1 (Fig. 4). The fluctuations in R d,i and R p,i induced different intensities of disturbances in the river system. The average disturbances of discharge and precipitation were mainly of high intensity, but the disturbances reached an intermediate intensity in early May, late August and late September (Fig. 5). After the impoundment, the variations in R d,i and R p,i ranged from 0 to 288.48 m3 s−1 day−1 and 0 to 47.68 mm day−1, respectively, and were much less than the variations during the low water level stage. The average disturbances in the discharge were mainly of low and intermediate intensity with several changes, but the disturbances from precipitation were in the intermediate and high intensity range with few fluctuations. The discharge maintained a low intensity of disturbance from January to February 2008, when the precipitation caused an intermediate intensity of disturbance.
Phytoplankton biomass and community composition
Throughout the year, the phytoplankton biomass showed great seasonal variation (Fig. 6). The highest biomass of 16.75 mg l−1 occurred in late May, while the lowest value, 0.23 mg l−1, occurred in mid-January 2008. The biomass during spring and summer (February–August) was higher than that during the autumn and winter (September–January). This seasonal difference was significant (P < 0.01). Throughout the year, Chlorophyta was the dominant group (28.98%) followed by Bacillariophyta (19.28%) and Cyanophyta (17.27%). Chlorophyta dominated from May to November except for the dominance of Cyanophyta (73.68%) in late May, when the first algal bloom occurred before the impoundment. The Cyanophyta bloom, which was dominated by Anabaena flos-aquae, lasted for 20 days, and the relative biomass abundance reached 69.7 ± 3.1% in the Pengxi River (Table 2).
During the high water level stage, Cryptophyta dominated in December (33.33 and 26.70% abundance at the two sampled times during the month), but was then replaced by Euglenophyta (24.07%) in early January 2008. Bacillariophyta became the dominant taxonomic group from late January to early March (36.37, 76.75, and 47.75%, respectively). The Bacillariophyta bloom, dominated by Asterionella formosa, appeared in late February and lasted for about 15 days. At the end of the high water level stage, Dinophyta dominated from late March to April, and two Dinophyta blooms occurred within that short period. A Peridinium spp. bloom occurred in late March, and a Ceratium spp. bloom occurred in late April 2008. The Ceratium bloom lasted for about 50 days, during which time the relative biomass abundance reached 84.3 ± 9.1%, attaining a steady state (Sommer et al., 1993).
Species diversity, richness and evenness indices
The seasonal variations in phytoplankton species indicated by the three diversity indices are shown in Fig. 7. There were no significant differences in any of the diversity indices among the five sites (P > 0.05). The maximum and minimum Shannon–Weaver indices appeared in the low and high water level periods, with 3.77 ± 0.06 (mean ± SE) in July and 1.58 ± 0.26 in March, respectively. Both the Margalef and Pielou indices reached their maximum and minimum values during the high water level stage. The maximum of the Margalef index was attained in November with a value of 3.31 ± 0.17, and the minimum occurred in January (1.79 ± 0.17). The maximum Pielou index was 1.23 ± 0.08 in January, and its minimum value of 0.51 ± 0.18 occurred in March. However, t tests showed that there were no significant differences for the three diversity indices between the low and high water level periods (P > 0.05).
Before the impoundment, the only dramatic drop in the three indices occurred in May when the Cyanophyta bloom appeared. During the bloom, the Shannon–Weaver index declined from 2.72 ± 0.27 to 1.79 ± 0.23, and the Pielou index dropped from 0.88 ± 0.19 to 0.58 ± 0.15. After the bloom, the three indices continued to increase and maintained high values during the rest of the low water level period.
At the beginning of the impoundment, the three indices all declined in October, when the water level rose from 145 to 156 m within 1 month. The Shannon–Weaver index decreased from 3.43 ± 0.08 to 3.06 ± 0.17. The Margalef index decreased from 2.76 ± 0.14 to 2.43 ± 0.09. After the impoundment, the biodiversity indices decreased dramatically when the algal blooms occurred. During the Bacillariophyta bloom, the Shannon–Weaver index declined to 2.37 ± 0.13, and Margalef index dropped to its minimum in February. During the Peridinium bloom, the Shannon–Weaver and Pielou indices both reached their minimum values in March. Moreover, during the last Ceratium bloom, the Shannon–Weaver and Pielou indices decreased to 1.60 ± 0.40 and 0.52 ± 0.28 respectively in late April.
Discussion
According to the IDH, species diversity should reach its maximum at intermediate intensities and frequencies of disturbance within certain time scales (Sommer et al., 1993). Because disturbances are random events in natural ecosystems, the principal difficulty in the application of IDH is the recognition and measurement of disturbances (Reynolds et al., 1993). Many events can induce physical disturbances, including wind, precipitation and discharge (Muylaert et al., 1999). In this study, river flow and precipitation with strong fluctuations appeared to be the major factors influencing physical disturbance and phytoplankton community dynamics (Jones & Barrington, 1985; Paidere et al., 2007). Qualitative analyses of the response of phytoplankton biodiversity to physical disturbances were undertaken during two water level periods.
Low water level stage
At the low water level period in the Pengxi River, the river flow appeared to be a major factor regulating the phytoplankton biomass in the river system because water movement determined the turbulence in the system (Becker et al., 2009; Centis et al., 2010). Ács & Kiss (1993) found that the numbers of individuals and phytoplankton diversity in the Danube River at Göd reached a maximum when the flow was in the range of 100–400 m3 s−1 under an intermediate level of disturbance. In the Moselle River, the maximum phytoplankton diversity also occurred when the fluctuations in the discharge were within 100–400 m3 s−1, and large discharge variations resulted in low diversity (Descy, 1993). Our analysis failed to confirm the range of water discharge during the intermediate disturbance. Nevertheless, the R d,i range between 0.04 and 19.46 m3 s−1 day−1 was considered to be an intermediate disturbance by reference to the statistical analyses.
It should be noted that a late spring bloom occurred in the Pengxi River during late May 2007. The bloom, with abundant Cyanophyta, especially A. flos-aquae, resulted in a sharp decrease in the three diversity indices (Li et al., 2009a). It is frequently mentioned in the literature that diversity indices decline rapidly when algal blooms develop (Jacobsen & Simonsen, 1993; Figueredo & Giani, 2001). The steep decline in the biodiversity indices in the Pengxi River during 2007 coincided with a sharp increase in disturbance intensity; during this period, the disturbance transformed from an intermediate level with an average R d,i to a high intensity disturbance, and the average R p,i thereafter maintained a high intensity. The bloom occurred 1 week after the last major rainfall in mid-May, and this rainfall event was considered to be a potential promoter of the physical disturbance (Beyruth, 2000). During this week, the R d,i increased and the discharge declined from 296.62 to 92.46 m3 s−1. The Cyanophyta bloom occurred immediately after the decline in discharge. In addition, the irradiance and temperature were increasing, and both factors favoured the dominance of A. flos-aquae through interspecific competition (Guo et al., 2008; Li et al., 2010). Similar results were reported in the Nakdong River, South Korea, and the Meuse River, Belgium; in each case, a sharp drop in discharge and an increase in water temperature triggered the bloom (Gosselain et al., 1994; Ha et al., 1998).
The process of impoundment
The 156-m impoundment was the main characteristic feature of the TGR under artificial operation. In October, the water level of the Pengxi River increased rapidly to a maximum of 156 m. It is worth noting that the Shannon–Weaver and Margalef indices clearly decreased during that month. The impoundment process was similar to the “flood pulse” in river-floodplain systems; the increase in the water level that occurs due to this pulse can impact the phytoplankton composition and diversity and can therefore be considered a disturbance factor (Huszar & Reynolds, 1997). In river-floodplain systems, the quantitative relationship between water level changes and turbulence has been established effectively from the long-term annual flooding frequency (Gruberts et al., 2007), but could not be established using the rate of water level change (Paidere et al., 2007). An quantitative relationship between the water level variation and phytoplankton diversity was not obvious in the Pengxi River because the diversity index was not sensitive to physical forcing on a daily time scale in the river reservoirs (Pannard et al., 2008). In addition, the sharp rise in the dilution induced by the water level affected the river phytoplankton communities (Ha et al., 1998; Mihaljević et al., 2009). The high dilution forced a decline in the phytoplankton density, which occurred in parallel with a decline in diversity values (Carvajal-Chitty, 1993).
High water level period
The Pengxi River resembled a lentic-type system after the winter impoundment, after which the water level, flow and HRT were maintained at stable values. From January to February, the average R d,i consistently maintained the lowest intensities of disturbance, and the R d,i was at an intermediate disturbance intensity without any fluctuations. The IDH states that the lowest biodiversity appears at the lowest levels of disturbance because only the best competitors can persist under these conditions (Sommer et al., 1993). However, in the Pengxi River, the diversity indices did not decline in January immediately after the winter impoundment; instead, they decreased in February in response to the low disturbance intensity. Despite this delay, it is evident that low levels of disturbance eventually create a tendency toward competitive exclusion (Padisák, 1994). It was found in earlier studies that competitive exclusion in phytoplankton community occurs when conditions of ecosystem stability persist for about 20 generations (Harris, 1986; Train & Rodrigues, 1997). As suggested by Reynolds, the succession process may well require 12–16 generations and may therefore require periods of 35–60 days (Reynolds et al., 1993; Padisák, 1994). In this study, the time required for succession was approximately 45 days, from January to late February 2008, at which time the dominant phytoplankton species appeared. A. formosa, a eurythermal species, became the dominant species under the low levels of hydrodynamic disturbance (low variability in hydrologic conditions and long retention times) that prevailed during the cold season (Suzuki & Takahashi, 1995; Bertrand et al., 2003). In later February 2008, after the 45-day succession time, the winter Bacillariophyta bloom of Asterionella occurred in the backwater area of the Pengxi River, with a concomitant decrease in the biodiversity values.
At the end of the high water level stage, a clear decline in phytoplankton diversity occurred in late March and late April 2008, when two Dinophyta blooms occurred sequentially (Peridinium spp. and Ceratium spp.). Peridinium dominated in the lentic water system under warmer conditions, determined by the hydrological features and the eutrophic state of the system (Kishimoto et al., 2001; Canion & Ochs, 2005). Ceratium was able to undertake significant diel vertical migrations (DVM), apparently for its optimal exploitation of light and nutrients in temperate conditions (Hart & Wragg, 2009). Ceratium blooms frequently appear in lakes and reservoirs during late summer across the trophic status range (Padisák & Reynolds, 1998). During the two Dinophyta blooms, the tremendous precipitation and flow resulted in high levels of disturbance. The average R p,i maintained a high disturbance intensity during March, while the R d,i switched from a low to intermediate disturbance intensity before the blooms. The high levels of disturbance were the major reason that the biodiversity decreased and the Dinophyta blooms occurred. Moreover, the water transparency was lower during the spring rainy season as a result of the higher influx of sawdust particles into the Pengxi River (Fang et al., 2010; Zhang et al., 2010). The water transparency was correlated with daily photosynthetic rates, available nutrients and growth rate, and ultimately impacted the variations in phytoplankton density and species composition, and may be completely responsible for the decrease in phytoplankton diversity (Figueredo & Giani, 2001).
References
Ács, É. & K. T. Kiss, 1993. Effects of the water discharge on periphyton abundance and diversity in a large river (River Danube, Hungary). Hydrobiologia 249: 125–133.
Bain, M. B., J. T. Finn & H. E. Booke, 1988. Streamflow regulation and fish community structure. Ecology 69: 382–392.
Barbiero, R. P., W. F. James & J. W. Barko, 1999. The effects of disturbance events on phytoplankton community structure in a small temperate reservoir. Freshwater Biology 42: 503–512.
Becker, V., V. L. M. Huszar & L. O. Crossetti, 2009. Responses of phytoplankton functional groups to the mixing regime in a deep subtropical reservoir. Hydrobiologia 628: 137–151.
Bertrand, C., V. Siauve, S. Fayolle & A. Cazaubon, 2001. Effects of hydrological regime on the drift algae in a regulated Mediterranean river (River Verdon, southeastern France). Regulated Rivers: Research & Management 17: 407–416.
Bertrand, C., S. Fayolle, E. Franquet & A. Cazaubon, 2003. Responses of the planktonic diatom Asterionella formosa Hassall to abiotic environmental factors in a reservoir complex (south-eastern France). Hydrobiologia 501: 45–58.
Beyruth, Z., 2000. Periodic disturbances, trophic gradient and phytoplankton characteristics related to cyanobacterial growth in Guarapiranga Reservoir, São Paulo State, Brazil. Hydrobiologia 424: 51–65.
Cabecinha, E., P. J. Van, J. A. den Brink, R. Cabral, M. Cortes, M. Lourenco & M. Pardal, 2009. Ecological relationships between phytoplankton communities and different spatial scales in European reservoirs: implications at catchment level monitoring programmes. Hydrobiologia 628: 27–45.
Canion, A. K. & C. Ochs, 2005. The population dynamics of freshwater armored dinoflagellates in a small lake in Mississippi. Journal of Freshwater Ecology 20: 617–626.
Carvajal-Chitty, H. I., 1993. Some notes about the intermediate disturbance hypothesis and its effects on the phytoplankton of the middle Orinoco River. Hydrobiologia 249: 117–124.
Centis, B., M. Tolotti & N. Salmaso, 2010. Structure of the diatom community of the River Adige (North-Eastern Italy) along a hydrological gradient. Hydrobiologia 639: 37–42.
Connell, J., 1978. Diversity in tropical rain forests and coral reefs. Science 199: 1302–1310.
Davis, S. R., A. G. Brown & M. H. Dinnin, 2007. Floodplain connectivity, disturbance and change: a palaeoentomological investigation of floodplain ecology from south-west England. Journal of Animal Ecology 76: 276–288.
Descy, J. P., 1993. Ecology of the phytoplankton of the River Moselle: effects of disturbances on community structure and diversity. Hydrobiologia 249: 111–116.
Donk, E. & D. O. Hessen, 1993. Grazing resistance in nutrient-stressed phytoplankton. Oecologia 93: 508–511.
Fang, F., H. Zhou, Z. Li, M. Long, J. Guo & J. Sheng, 2010. Spatiotemporal variations of euphotic depth and its causing factors in Xiaojiang River backwater area of Three Gorges. Advances in Water Science 21: 113–119.
Figueredo, C. C. & A. Giani, 2001. Seasonal variation in the diversity and species richness of phytoplankton in a tropical eutrophic reservoir. Hydrobiologia 445: 165–174.
Gosselain, V., J. P. Descy & E. Everbecq, 1994. The phytoplankton community of the River Meuse, Belgium: seasonal dynamics (year 1992) and the possible incidence of zooplankton grazing. Hydrobiologia 289: 179–191.
Gruberts, D., I. Druvietis, E. Parele, J. Paidere, A. Poppels, J. Prieditis & A. Skute, 2007. Impact of hydrology on aquatic communities of floodplain lakes along the Daugava River (Latvia). Hydrobiologia 196: 223–237.
Guo, J., C. Zhang, F. Fang, Z. Li, J. Chen & G. Tian, 2008. Study on features of phytoplankton community in Xiaojiang River backwater area in Three Gorges Reservoir during spring and summer time. Science & Technology Review 26: 70–75.
Ha, K., H. W. Kim & G. J. Joo, 1998. The phytoplankton succession in the lower part of hypertrophic Nakdong River (Mulgum), South Korea. Hydrobiologia 369–370: 217–227.
Harris, G. P., 1986. Phytoplankton Ecology: Structure, Function and Fluctuation. Chapman and Hall, London.
Hart, R. C. & P. D. Wragg, 2009. Recent blooms of the dinoflagellate Ceratium in Albert Falls Dam (KZN): history, causes, spatial features and impacts on a reservoir ecosystem and its zooplankton. Water SA 35: 455–468.
Hart, D. D., T. E. Johnson, K. L. Bushaw-Newton, R. J. Horwitz, A. T. Bednarek, D. F. Charles, D. A. Kreeger & D. J. Velinsky, 2002. Dam removal: challenges and opportunities for ecological research and river restoration. BioScience 52: 669–682.
Heise, H. A., 1949. Symptoms of hay fever caused by algae. Journal of Allergy 20: 383–385.
Hu, Z. & Q. Cai, 2006. Preliminary report on aquatic ecosystem dynamics of the Three Gorges Reservoir before and after impoundment. Acta Hydrobiologica Sinica 30: 1–6.
Hu, H. & Y. Wei, 2006. The Freshwater Algae of China: Systematics, Taxonomy and Ecology. Science Press, Beijing.
Humborg, C., V. Ittekkot, A. Cociasu & B. Bodungen, 1997. Effect of Danube River dam on Black Sea biogeochemistry and ecosystem structure. Nature 386: 385–388.
Huszar, V. L. M. & C. S. Reynolds, 1997. Phytoplankton periodicity and sequences of dominance in an Amazonian flood-plain lake (Lago Batata, Pará, Brasil): responses to gradual environmental change. Hydrobiologia 346: 169–181.
Jacobsen, B. A. & P. Simonsen, 1993. Disturbance events affecting phytoplankton biomass, composition and species diversity in a shallow, eutrophic, temperate lake. Hydrobiologia 249: 9–14.
Jeong, K. S., D. K. Kim & G. J. Joo, 2007. Delayed influence of dam storage and discharge on the determination of seasonal proliferations of Microcystis aeruginosa and Stephanodiscus hantzschii in a regulated river system of the lower Nakdong River (South Korea). Water Research 41: 1269–1279.
Jones, R. I. & R. J. Barrington, 1985. A study of the suspended algae in the River Derwent, Derbyshire, UK. Hydrobiologia 128: 255–264.
Kelly, V. J., 2001. Influence of reservoirs on solute transport: a regional-scale approach. Hydrological Processes 15: 1227–1249.
Kimbro, D. L. & E. D. Grosholz, 2006. Disturbance influences oyster community richness and evenness, but not diversity. Ecology 87: 2378–2388.
Kishimoto, N., Y. Ohnishi, I. Somiya & M. Ohnishi, 2001. Behavior of Peridinium bipes (Dinophyceae) resting cysts in the Asahi Reservoir. Limnology 2: 101–109.
Krebs, C. J., 1999. Ecological Methods. Benjamin Cummings, Menlo Park, CA.
Kuang, Q., Y. Bi, G. Zhou, Q. Cai & Z. Hu, 2005. Study on the phytoplankton in the Three Gorges Reservoir before and after sluice and the protection of water quality. Acta Hydrobiologica Sinica 29: 353–358.
Li, Z., F. Fang, J. Guo, J. Chen, C. Zhang & G. Tian, 2009a. Spring algal bloom and nutrients characteristics in Xiaojiang River backwater area, Three Gorge Reservoir, 2007. Journal of Lake Sciences 21: 36–44.
Li, Z., J. Guo, M. Long, F. Fang, J. Sheng & H. Zhou, 2009b. Seasonal variation of nitrogen and phosphorus in Xiaojiang River—a tributary of the Three Gorges Reservoir. Frontiers of Environmental Science & Engineering in China 3: 334–340.
Li, Z., J. Guo, F. Fang, X. Gao, J. Sheng, H. Zhou & M. Long, 2010. Seasonal variation of cyanobacteria and its potential relationship with key environmental factors in Xiaojiang backwater area, Three Gorges Reservoir. Huan Jing Ke Xue 31: 301–309.
Li, Z., J. Guo, F. Fang, M. Long, Z. Liu & X. Gao, 2012. A HEC-RAS based 1D hydrodynamic model on Pengxi (Xiaojiang) River Backwater Area in the Three Gorges Reservoir. Journal of Chongqing University (in press).
Long, T., L. Wu, L. Liu & C. Li, 2009. The simulation of adsorbed phosphorus pollution load based on GIS and the theory of blind number in Xiaojiang River watershed. Journal of Agro-Environment Science 28: 1880–1887.
Lopes, M. R. M., C. Ferragut & C. E. M. Bicudo, 2009. Phytoplankton diversity and strategies in regard to physical disturbances in a shallow, oligotrophic, tropical reservoir in Southeast Brazil. Limnetica 28: 159–174.
Magilligan, F. J. & K. H. Nislow, 2005. Changes in hydrologic regime by dams. Geomorphology 71: 61–78.
Margalef, R., 1975. Perspectives in Ecological Theory. University of Chicago Press, Chicago.
Meng, C. & B. Zhao, 2007. Study on the trend of eutrophication after impounding in Three Gorges Reservoir. Journal of Agro-Environment Science 26: 863–867.
Mihaljević, M., F. Stević, J. Horvatić & B. H. Kutuzović, 2009. Dual impact of the flood pulses on the phytoplankton assemblages in a Danubian floodplain lake (Kopački Rit Nature Park, Croatia). Hydrobiologia 618: 77–88.
Montagnes, D. J. S., J. A. Berges, P. J. Harrison & F. Taylor, 1994. Estimating carbon, nitrogen, protein, and chlorophyll a from volume in marine phytoplankton. Limnology and Oceanography 39: 1044–1060.
Muylaert, K., V. J. Wichelen, K. Sabbe & W. Vyverman, 1999. Chapter 7. Irregular flushing events regulate phytoplankton dynamics in a freshwater tidal estuary. In Distribution and dynamics of protist communities in a freshwater tidal estuary. PhD dissertation, University of Gent, Gent, Belgium.
Nhiwatiwa, T. & B. E. Marshall, 2007. Water quality and plankton dynamics in two small dams in Zimbabwe. African Journal of Aquatic Science 32: 139–151.
Padisák, J., 1993. The influence of different disturbance frequencies on the species richness, diversity and equitability of phytoplankton in shallow lakes. Hydrobiologia 249: 135–156.
Padisák, J., 1994. Identification of relevant time-scales in non-equilibrium community dynamics: conclusions from phytoplankton surveys. New Zealand Journal of Ecology 18: 169–176.
Padisák, J. & C. S. Reynolds, 1998. Selection of phytoplankton associations in Lake Balaton, Hungary, in response to eutrophication and restoration measures, with special reference to the cyanoprokaryotes. Hydrobiologia 384: 41–53.
Paidere, J., D. Gruberts, A. Škute & I. Druvietis, 2007. Impact of two different flood pulses on planktonic communities of the largest floodplain lakes of the Daugava River (Latvia). Hydrobiologia 592: 303–314.
Pannard, A., M. Bormans & Y. Lagadeuc, 2008. Phytoplankton species turnover controlled by physical forcing at different time scales. Canadian Journal of Fisheries and Aquatic Sciences 65: 47–60.
Pielou, E. C., 1966. The measurement of diversity in different types of biological collections. Journal of Theoretical Biology 13: 131–144.
Poff, N. L. & J. V. Ward, 1989. Implications of streamflow variability and predictability for lotic community structure: a regional analysis of streamflow patterns. Canadian Journal of Fisheries and Aquatic Sciences 46: 1805–1818.
Pringle, C., 2003. What is hydrologic connectivity and why is it ecologically important? Hydrological Processes 17: 2685–2689.
Reynolds, C. S., J. Padisák & U. Sommer, 1993. Intermediate disturbance in the ecology of phytoplankton and the maintenance of species diversity: a synthesis. Hydrobiologia 249: 183–188.
Scrosati, R. & C. Heaven, 2007. Spatial trends in community richness, diversity, and evenness across rocky intertidal environmental stress gradients in eastern Canada. Marine Ecology Progress Series 342: 1–14.
Shannon, C. E. & W. Weaver, 1949. The Mathematical Theory of Information, Vol. 97. University of Illinois Press, Urbana: 111–128.
Sommer, U., J. Padisák, C. Reynolds & P. Juhász-Nagy, 1993. Hutchinson’s heritage: the diversity–disturbance relationship in phytoplankton. Hydrobiologia 249: 1–7.
Strathmann, R. R., 1967. Estimating the organic carbon content of phytoplankton from cell volume or plasma volume. Limnology and Oceanography 12: 411–418.
Suzuki, Y. & M. Takahashi, 1995. Growth responses of several diatom species isolated from various environments to temperature. Journal of Phycology 31: 880–888.
Train, S. & L. C. Rodrigues, 1997. Temporal fluctuations of the phytoplankton community of the Baía River, in the upper Paraná River floodplain, Mato Grosso do Sul, Brazil. Hydrobiologia 361: 125–134.
Utermöhl, H., 1958. Zur vervollkommnung der quatitativen phytoplankton-methodik. Mitteilungen-Internationale Vereinigung Für Theoretische und Angewandte Limnologie 9: 1–38.
Venrick, E. L., 1978. How many cells to count? In Sournia, A. (ed.), Phytoplankton Manual. UNESCO, Paris: 167–180.
Weithoff, G., 2003. The concepts of ‘plant functional types’ and ‘functional diversity’ in lake phytoplankton – a new understanding of phytoplankton ecology? Freshwater Biology 48: 1669–1675.
Wu, J., J. Huang, X. Han, Z. Xie & X. Gao, 2003. Three-Gorges Dam – experiment in habitat fragmentation? Science 300: 1239–1240.
Wu, L., T. Long & C. Li, 2010. The simulation research of dissolved nitrogen and phosphorus non-point source pollution in Xiao-Jiang watershed of Three Gorges Reservoir area. Water Science and Technology 61: 1601–1616.
Zeng, H., L. Song, Z. Yu & H. Chen, 2006. Distribution of phytoplankton in the Three-Gorge Reservoir during rainy and dry seasons. Science of the Total Environment 367: 999–1009.
Zhang, Z. & X. Huang, 1991. Research Methods for Freshwater Plankton. Science Press, Beijing: 335–348.
Zhang, C., J. Guo, Z. Li, Q. Pu, F. Fang, M. Long & X. Gao, 2010. Seasonal variation of transparency and its regulating factors in the Xiaojiang backwater area, Three Gorges Reservoir. Journal of Lake Sciences 22: 189–194.
Acknowledgments
This work was supported by the National Critical Patented Projects on Water Pollution Control and Management Program (Program No. 2009ZX07104), the National Natural Science Foundation (Program No. 51009155) and the Fundamental Research Funds for the Central Universities (Program No. CDJXS10211153).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Judit Padisak
Rights and permissions
About this article
Cite this article
Li, Z., Wang, S., Guo, J. et al. Responses of phytoplankton diversity to physical disturbance under manual operation in a large reservoir, China. Hydrobiologia 684, 45–56 (2012). https://doi.org/10.1007/s10750-011-0963-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-011-0963-2