Abstract
An impact of two different flood pulses on phyto- and zooplankton communities of the two largest floodplain lakes of the Daugava River were studied in the spring and summer of 2005. Samples of phyto- and zooplankton were taken at weekly and biweekly intervals. At the end of March, a medium size pulse of spring flood was observed. At the beginning of May, it was followed by an unusually high pulse of flush floods caused by heavy rainstorms in the local drainage area. An overall increase of biomass and the number of taxa of planktonic communities during the filling and drainage phases of the spring floods was stated. The pulse of the flush floods resulted in a lower total biomass and higher species diversity, and can be regarded as a disturbance event. The high species diversity represented by a hump-shaped pattern caused by an intermediate disturbance that was measured by the rate of water level change during the floods according to Intermediate Disturbance Hypothesis (IDH). This study reflected both linear and a slight hump-shaped relationships between the rate of water level change and the number of phytoplankton and zooplankton taxa.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Floodplains are considered as the areas whose existence depends on lateral overflowing of rivers and groundwater seepage (Junk et al., 1989; Tockner et al., 2000; Malard, 2003). According to the Flood Pulse Concept, the flood pulse and/or flow pulse allow the exchange of nutrients and energy between aquatic and terrestrial phases (Junk et al., 1989; Lewis et al., 2000; Tockner et al., 2000; Junk and Watzen, 2003). The degree of connection between a river and its floodplain depends on the water level and floodplain morphology (Junk, 1997; Tockner et al., 1999; Van den Brink et al., 1994; Ward et al., 2002). Hydrological connectivity is an important factor of the structure and productivity of planktonic communities in floodplain lakes. According to Baranyi et al. (2002), the water age in the Danube River floodplain area is an important factor both of biomass and species composition of zooplankton communities.
Flöder and Sommer (1999) define disturbance as an externally forced episodic event. In a natural river-floodplain ecosystem, an annual flooding is a predictable event (Junk et al., 1989; Junk and Watzen, 2003). According to Huszar and Reynolds (1997), regular floods are not disturbing the seasonal development of phytoplankton under natural hydrological conditions of the Central Amazon. On the other hand, they are considered as a disturbance in temporally isolated floodplain lakes of large European rivers (Tockner et al., 1999, 2000; Roozen, 2005). In higher latitudes, a long-lasting ice cover and low temperatures also affect the biota (Tockner et al., 2000; Junk and Watzen, 2003).
According to the Intermediate Disturbance Hypothesis (Connell, 1978), an increase of biological diversity and decrease of total biomass in phytoplankton communities are expected if the flooding acts as a disturbance factor (Reynolds, 1993; Sommer et al., 1993). IDH has been used in numerous theoretical and empirical analyses (Eloranta, 1993; Flöder and Sommer, 1999) and is an interesting theme for discussion (Wilson, 1994; Reynolds, 1995; Collins and Glenn, 1997). For example, Reynolds (1995) suggests that IDH has a considerable potential to explain stable species coexistence. Many IDH tests in freshwater ecosystems are based on phytoplankton communities (Reynolds et al., 1993), whereas applicability of IDH tests for zooplankton communities has been studied in few papers (Eckert and Walz, 1998; Keppeler and Hardy, 2004). Results of these studies are controversial. For example, according to Eckert and Walz (1998), IDH could not be confirmed in its form, and it is necessary to study the influence of a broader range of disturbance frequencies on zooplankton species diversity.
The Daugava is among the largest East-European rivers. It begins in Russia, flows through the East-European Plain, crosses Belarus and Latvia and flows into the Baltic Sea. Its annual flooding is caused by an intensive melting of seasonal snow cover in its drainage area, and is characterised by the ice drift which frequently causes ice jams in shallow sites of the riverbed (Briede et al., 2004). The peak of spring floods is usually observed in April (Briede et al., 2001).
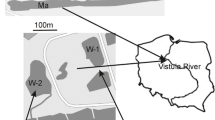
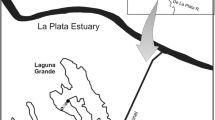
A cascade of three large hydroelectric power stations and man-made reservoirs transform the lower course of the Daugava, whereas its upper and middle courses are not yet regulated. Floodplains of the Middle Daugava (South-Eastern Latvia) are among the largest intact floodplains in European Union. Their most diverse reach is located in Daugavpils region (South-Eastern Latvia), where the Nature Park “Dvietes paliene” (Natura-2000 site) was founded in 2004. It consists of about 50 km2 of floodplain meadows and wetlands as well as two largest floodplain lakes of the Daugava River. The lakes are located in the middle of the River Daugava basin with the drainage area of the Lake Skuku being 190 km2 and that of the Lake Dvietes 230 km2. Both lakes are small and shallow eutrophic macrophyta-type lakes of the glacial origin (Gruberts, 2003), which are drained by the River Dviete (Fig. 1). The Lake Skuku surface area is 1.1 km2 and its largest depth in summer is 1.1 m, whereas the Lake Dvietes surface area is 0.8 km2 and its largest depth is only 0.9 m.
Depending on the location of lakes above the Daugava riverbed they are inundated not only at the mean spring flood level, but also at the highest summer (autumn) flush flood level after heavy rainfalls. The relative height of the Lake Skuku above the Daugava riverbed is 3.0 m and mean amplitude of water level fluctuation is about 3.5 m year−1 whereas for the Lake Skuku these figures are 2.9 m and 3.6 m year−1 respectively (Gruberts et al., 2006, in the process of publication).
An impact of the spring floods on phytoplankton communities and aquatic chemistry of the Lake Skuku was studied already by Gruberts and Druvietis in 1999 (Gruberts and Druvietis, 2001). Planktonic communities of 24 floodplain lakes of the Middle Daugava located in Daugavpils region were also explored in the summer of 2004. As a result, floodplain lakes of the Middle Daugava were divided in 6 hydrological groups according to the frequency of flooding. The authors also stated that not only the number of zooplankton taxa significantly correlated to the long-term annual flooding frequency and seasonal amplitude of water level fluctuation but also the highest biological diversity of phytoplankton was observed in the lakes with intermediate amplitude of water level fluctuation (Gruberts et al., 2005, 2006). However, seasonal dynamics of zooplankton communities in these floodplain lakes have not been studied until now.
The main aim of the present study was to find out whether, and under what circumstances, floods could be regarded as an intermediate disturbance in the ecology of phyto- and zooplankton communities of the high latitude floodplain lakes. It was assumed that such disturbance should result in higher species diversity and a lower biomass in phytoplankton communities what are central assumption of IDH. It was also assumed that the annual spring flooding could not be regarded as a disturbance factor in phytoplankton ecology of floodplain lakes located in higher latitudes because it usually starts before the growth season (Roozen, 2005). In contrast, flush floods caused by heavy rainstorms could have a disruptive impact. It was believed that the same is also true for zooplankton communities. In this study, the intensity of disturbance was measured by the rate of water level change per day during the floods.
Materials and methods
Zooplankton and phytoplankton communities of the floodplain lakes Skuku and Dvietes were studied from March to June 2005. Samples of phyto- and zooplankton were taken at weekly and biweekly intervals. Phytoplankton samples were collected in the pelagic region of the lakes (Fig. 1) at the 0.5 m depth by a Ruttner type sampler, and fixed with Lugol’s solution. A systematic analysis of phytoplankton samples was performed by an inverted microscope according to the method of Utermőhl (1958) and taxonomic literature of the Middle Europe freshwater flora. The counting unit was the cell, except for cyanobacteria and green algae, for which it was a filament or a colony. Their mean biovolumes were converted into the fresh-weight biomass, assuming that the specific density of cells is equal to that of water. Species diversity of phytoplankton communities was calculated according to Shannon’s equation, in which data concerning the biomass were used.
Zooplankton samples were concentrated by filtering 100 l of water through a of the 65-μm mesh size net at the surface in the pelagic region of the lakes. Samples were immediately preserved in the 4% solution of sucrose-formalin. Zooplankton communities were identified and evaluated in 6 ml sub-samples by a compound microscope. Individual weights of rotifers were calculated from their average body lengths, according to Ruttner-Kolisko (1977). At least 20 individuals of every species were measured in each sample. Biomass data were used for the calculation of Shannon’s equation.
At the same time, planktonic communities, hydrophysical and hydrochemical parameters as well as hydrological conditions of the lakes were monitored. The water level in the Daugava floodplain was measured from two bridges across the River Dviete on the day of water sampling (Fig. 1). Water samples were collected from pelagic regions of the lakes by a Rutner type sampler and analysed for NH4, NO3, NO2, PO4 and total Si (mg l−1) on the same day or on the day following sampling, according to the standard methods accredited in Latvia (LVS ISO 7150/84; LVS ISO 7890-3:2001; LVS ISO 6777:1984 and LVS EN 1189:2000). Water temperature (°C), conductivity (μS cm−1), pH, turbidity (NTU), total dissolved solids (g l−1) and dissolved oxygen (mg l−1) were measured in the field by a Hydrolab 4 minisonde. In addition, meteorological observations were performed by an automatic weather station Vantage Pro 2, located in the Nature Park “Dvietes paliene” at Bebrene (Fig. 1).
All statistical analyses were performed by the SPSS 11.5 for Windows software package. Normal distributions of physical, chemical, phytoplankton and zooplankton parameters were tested. Spearman’s rank correlation was used in order to find out the relationship between seasonal variations in the main physical and chemical parameters, water level and the rate of its change. The regression was used to analyze relationships between limnological and biological variables. Variations of water level, dissolved oxygen, conductivity, pH, total dissolved solids, NH4, PO4 were used for Principal Component Analysis (PCA) in order to reduce the number of factors that may correlate with biological parameters—total zooplankton and phytoplankton biomass, biomass of Rotifera and Copepoda, Shannon’s index, and number of taxa. Correlations were used in order to analyse patterns of limnological and biological variations along the two PCA axes.
Results
It was observed in 2005 that two different flood pulses in the floodplain of the Middle Daugava (Gruberts and Druvietis, 2006) depended on the water level and its fluctuation. Total amplitude of water level fluctuation reached 3.4–3.5 m in both lakes. At the end of March, a medium-size pulse of the spring flood was observed. At the beginning of May, it was followed by an unusually high pulse of flush floods caused by heavy rainstorms in the local drainage area. On May 10, for example, the amount of daily precipitation reached 49 mm. The May of flush floods reached their maximum height shortly after this rainstorm and significantly raised the water level in the floodplain. The height of this flood pulse exceeded that of the first one by 0.6–1.0 m (Gruberts and Druvietis, 2006).
Physical and chemical parameters of these lakes varied widely and correlated with the water level fluctuation in the floodplain. Conductivity and dissolved oxygen concentration significantly correlated with the water level, shoved a linear trend and substantial dependency on the water level (r = −0.99, r 2 = 0.97, P < 0.001; r = 0.89, r 2 = 0.73, P < 0.01). Breaking of ice and rise of the water level in both lakes during the spring floods resulted in a considerable increase of the water temperature and concentration of dissolved oxygen, and in the decrease of conductivity (Fig. 2). In contrast, the water temperature dropped to about 2°C, and conductivity decreased whereas dissolved oxygen concentration increased in both lakes at the beginning of the flush floods (Fig. 2).
The concentration of phosphate was high at the peak of the spring flood, but dropped sharply afterwards and remained below the detection level during the rest of the flooding period. Although the concentration of silica also decreased during the drainage phase of the spring flood, it peaked again during the flush floods in early May. Ammonium showed an opposite pattern, and obviously increased at the beginning of June (Fig. 2).
In total, 83 taxa of phytoplankton were recorded, mostly of the Bacillariophyceae, Chlorophyceae and Euglenophyceae groups (35, 15 and 14, respectively). In general, when compared to the Lake Dvietes the Lake Skuku showed a higher number of algae taxa. The number of taxa gradually increased in both sites during the spring floods. In the Lake Dvietes, it also significantly increased at the beginning of May, during the rising phase of the flush flood (Tables 3, 4).
The total biomass of phytoplankton was very low (<0.1 mg l−1) in both lakes at the end of the ice cover period. It increased significantly during the rising phase of spring floods and reached its maximum at the end of April and beginning of May (Tables 3, 4). In both lakes, the biomass dropped during the filling phase of flush floods, slightly increased at the beginning of their drainage phase, and continued to decrease later on alongside the fall of the water level. In the Lake Dvietes, it also remained low during the low water period, whereas in the Lake Skuku it reached its maximum in June after the end of the flush flood.
During the flooding period, species diversity (Shannon’s index) of phytoplankton communities varied similarly in both lakes, except for the very beginning of this study. In general, diversity decreased simultaneously in both sites during the spring floods and increased during the flush floods and, also, during the low water period (Tables 3, 4). According to the rank correlation analysis, correlation between the species diversity of phytoplankton communities and other parameters was not significant. On the other hand, the total biomass significantly and negatively correlated to the concentration of silica in both lakes (r = −0.90, P < 0.01 in the Lakes Dvietes, r = −0.96, P < 0.01 in the Lake Skuku).
Small planktonic Bacillariophyceae or Chlorophyceae species dominated in the total biomass of both lakes during the ice cover period (Table 1). Cryptomonas sp. and other small unidentified flagellates replaced them almost completely during the drainage phase of spring floods. At the beginning of flush floods, there was an increase in the percentage of Nitzschia acicularis in both lakes. Later on, Cryptomonas sp. dominated in the phytoplankton community of the Lake Dvietes, whereas the Lake Skuku was dominated by Pandorina morum just as during the first drainage phase. In June, at the beginning of the low water period, both lakes were almost entirely dominated by euglenoids.
At first, the pattern of zooplankton development was similar to that of phytoplankton. During the spring floods, the total biomass of zooplankton increased significantly in both lakes. Zooplankton biomass in the Lake Skuku continued increasing and reached its maximum at the beginning of the flush flood drainage phase, decreasing at the end of flush floods drainage phase In the Lake Dvietes, zooplankton biomass decreased at the maximum of flush floods, increased at the beginning of the flush floods drainage phase and decreased again at the end of it (Tables 3, 4).
Totally 43 taxa of zooplankton were recorded at the period of spring and flush floods, dominated by the Rotifera taxa. In the Lake Dvietes, the number of zooplankton taxa was not too large at the maximum of spring floods but slightly increased and was invariable at the drainage phase of spring floods. In the Lake Skuku, the number of zooplankton taxa decreased at the end of the spring flood drainage phase. The number of zooplankton taxa continued to increase later and reached its maximum at the end of flush floods (Tables 3, 4). The relationships between the number of zooplankton taxa and water temperature was significant (r 2 = 0.88, P < 0.006 in the Lakes Dvietes, r 2 = 0.69, P < 0.03 in the Lake Skuku).
During the maximum of spring floods the zooplankton community was characterized by the presence of Copepoda and Bdelloidea species in both lakes. Copepoda dominated in the total biomass of zooplankton communities (92% in the Lake Skuku; 83% in the Lake Dvietes) at the beginning of the spring flood drainage phase, particularly, nauplii that formed 76% of the total biomass in the Lake Skuku and 68% in the Lake Dvietes. At the end of the spring flood drainage phase, they were largely replaced by the Rotifera species, such as Synchaeta oblonga. This species dominated mostly during the spring floods drainage phase (48% of the total biomass in the Lake Skuku and 54% of the total biomass in the Lake Dvietes) whereas Synchaeta sp.—at the maximum and the beginning of the drainage phase of flush floods (33% and 80% respectively of the total biomass in the Lake Skuku, and 66% and 62% respectively of the total biomass in the Lake Dvietes) (Table 2).
The species diversity of zooplankton communities showed similar values during the spring floods in both lakes. The species diversity values were higher at the maximum of spring floods and at the end of the spring flood drainage phase, whereas at the beginning of the spring flood drainage phase species diversity was low. After the spring floods, species diversity of zooplankton communities varied differently in each lake. The Shannon’s index of zooplankton communities decreased in the lake Dvietes and increased in the lake Skuku during the filling phase of flush floods. Later on, the opposite pattern was observed (Tables 3, 4). The highest species diversity in zooplankton communities was observed in both lakes at the end of flush floods when the rate of the water level change was low (0.10–0.16 m per day).
When the number of taxa of phyto- and zooplankton communities was plotted against the intensity of external disturbance (the rate of water level change per day), characteristic hump-shaped curves were obtained (Fig. 3). In both lakes, the highest species diversity of planktonic communities coincided with the intermediate intensity of disturbance as predicted by IDH and was not significant (Table 5), except for the linear relationship (Spearman rank correlation, r = 0.63, P > 0.1) between the rate of water level change and the number of phytoplankton taxa in the Lake Dvietes.
According to the results of the Principal Component Analysis, seasonal variation in species diversity of phytoplankton and zooplankton communities in these floodplain lakes was affected by two different factors. The axes identified by the PCA ordination correlated to similar variables in both lakes (Tables 6, 7). In the lake Skuku, the PC1 axe strongly positively correlated to the total of dissolved solids and conductivity, and negatively to the water level. In the Lake Dvietes, the PC1 axe was also strongly positively correlated to conductivity, concentration of ammonium, and the total of dissolved solids, and negatively to the water level and dissolved oxygen. It could be related to the decomposition of organic material when water level is low (Junk, 1997; Scheffer, 2004).
In the Lake Skuku, the PC2 axe was strongly positively correlated to pH and dissolved oxygen, and negatively to the concentration of phosphates. In the Lake Dvietes, it correlated to dissolved oxygen and pH. This suggests that a higher value of pH during spring months, especially during flush floods, depends on the effects of photosynthesis, and that phosphorus is the limiting factor of phytoplankton development (Lampert and Sommer, 1997).
In order to examine how the biological variables are related to environmental conditions, PCA axes were correlated to the total zooplankton and phytoplankton biomasses, number of taxa and species diversity, and the biomass of Rotifera and Copepoda. The phytoplankton biomass and Rotifera biomass were demonstrating a significant positive correlation to the PC2 in both lakes. The number of phytoplankton taxa in the Lake Skuku was correlating to this axe (Table 8). The relationship between the phytoplankton biomass and PC2 axe seems to shows biotic relationships. In this case, the biomass of Rotifera increased continuously along the phytoplankton growth during flush floods maximum and beginning of the drainage phase, when more stable hydrological conditions established.
The Copepoda biomass and zooplankton species diversity were showing the highest positive correlation to the PC1 axe in the Lake Dvietes, whereas not a single biological parameter in the Lake Skuku was correlating to it (Table 8). More stable conditions, especially at the end of the flush flood drainage phase, were obviously favourable for higher zooplankton species diversity among Cladocera and littoral, periphytic or benthic genera of Rotifera. When more stable conditions with low water level were prevailing, nauplii and copepodite become most dominant in the zooplankton community. The highest species diversity and significance of Copepoda in the zooplankton communities was stated at the beginning of June.
Discussion
Beginning of spring floods could not be regarded as a disturbance in the development of phytoplankton communities in lakes. On the other hand, at the end of March and beginning of April 2005, an interaction between the flood pulse and the ice cover in the lakes resulted in a fast development of their phytoplankton communities characterised by simultaneous increase in the total biomass and number of taxa (Tables 1–3). Such changes that immediately follow the ice cover degradation, were also observed in other East Latvian lakes (Trifonova, 1993), and are regarded as the initial stage of phytoplankton development.
Further development of phytoplankton communities in these lakes was obviously not interrupted by the water level stabilisation and its gradual decrease at the end of spring floods. Moreover, it was marked for a further stabilisation or ecological equilibrium state in which a decrease in species diversity (Shannon’s index) was observed, and most species were replaced by one or few dominants (Tables 3, 4).
Seasonal development of zooplankton communities in these lakes reveals similar patterns (Tables 3, 4). During spring floods, zooplankton biomass of in both lakes increased. The number of taxa continued to increase exponentially along with a rising temperature until the beginning of June in the both lakes. High zooplankton species diversity occurred during the periods of mid-stable conditions at the beginning of June, when the co-existence of Copepoda, Rotifera and Cladocera was observed, competitive exclusion processes were minimized, and diversity maintained (Sommer et al., 1993). This finding is confirmed by the results of PCA, and suggests that water level changes more significantly affected on the zooplankton species diversity (Shannon’s index) than on the number of taxa (Tables 3, 4, 8).
Floodplain lakes were obviously affected by the flush flood event. The flush flood pulse repeatedly altered hydrological and physical conditions in the lakes as well as nutrients exchange. Plankton biomass was obviously decreased at the maximum of flush floods (Baranyi et al., 2002; Keckeis et al., 2003), except for the Lake Skuku where the biomass of zooplankton continued to increase. In addition, high species diversity or an increase in diversity of phytoplankton occurred in compositionally disturbed communities in the Lake Dvietes, where phytoplankton composition was characterized by Cryprophyceae and Bacillariophyceae taxa. Many species of the Bacillariophyceae class are typical R-strategists. They are quite resistant to grazing, specifically adapted to conditions characterised by high frequency of physical variability, reduced light, low temperatures and plentiful nutrients while Cryprophyceae is characterised by a high growth rate; they benefit from high concentration of nutrients (Reynolds, 1993). More stable conditions at the beginning of the flush flood drainage phase were obviously favourable for an increase in phytoplankton biomass and Rotifera biomass (Tables 3, 4) (Tockner et al., 1999; Keckeis et al., 2003). However, only Synchaeta sp. with a short development times was able to develop important populations (Baranyi et al., 2002). On the other hand, as far as the Lake Skuku is concerned, these conditions were characterised not only by a high biomass, but also by increasing species diversity (Table 3). Accumulation of biomass after a sudden disturbance revert the plankton community to its early successional phase (Lampert and Sommer, 1997; Baranyi et al., 2002; Muylaert and Vyverman, 2006).
The response of plankton communities to the flush floods also differs depending on the location of the lakes within the floodplain or their connectivity to the river. As a result, phytoplankton and zooplankton communities in the Lake Dvietes, which is located lower and closer to the Daugava main course bed, was more affected than those of the Lake Skuku (Tables 3, 4) (Van den Brink et al., 1994; Tockner et al., 1999; Baranyi et al., 2002; Keckeis et al., 2003).
Conclusions
Under natural hydrological conditions, annual spring floods act as a stimulating factor rather than a disturbance in the seasonal development of phyto- and zooplankton communities of the largest floodplain lakes of the Daugava River. It is clearly demonstrated by an overall increase of biomass and the number of taxa of planktonic communities during the filling and drainage phases of spring floods, and could be explained by an improving underwater lighting conditions and nutrient availability, a higher water temperature, etc., when compared to the ice cover period. Flush floods, on the other hand, act as a disturbance, especially in seasonal development of phytoplankton communities of these lakes. The flush flood pulse caused by heavy local rainstorms resulted in a lower total biomass and higher species diversity, and can be regarded as a disturbance. High species diversity and the hump-shaped pattern of species in relation to the intensity of disturbance measured by the rate of water level change during floods corresponding to the Intermediate Disturbance Hypothesis.
References
Baranyi, C., T. Hein, C. Holarek, S. Keckeis & F. Schiemer, 2002. Zooplankton biomass and community structure in a Danube River floodplain system: effects of hydrology. Freshwater Biology 47: 473–482.
Briede, A., M. Kļaviņš, V. Rodinovs, & L. Lizuma, 2004. Ice regime in Latvian rivers. Ģeogrāfiski raksti XII: 49–55.
Briede, A., M. Kļaviņš, V. Rodinovs & I. Kokorīte, 2001. Upju noteces sezonālās un ilglaicīgās izmaiņas Latvijā. Ģeogrāfiski raksti IX: 20–32.
Collins, L. S. & S. M. Glenn, 1997. Intermediate Disturbance and its relationship to within—and between- patch dynamics. New Zealand Journal of Ecology 21(1): 103–110.
Connell, J., 1978. Diversity in tropical rain forests and coral reefs. Science 199: 1304–1310.
Eckert, B. & N. Walz, 1998. Zooplankton succession and thermal stratification in the polymictic shallow Müggelsee (Berlin, Germany): a case for the intermediate disturbance hypothesis? Hydrobiologia 387/388: 199–206.
Eloranta, P., 1993. Diversity and succession of the phytoplankton in a small lake over a two-year period. Hydrobiologia 249: 25–32.
Flöder, S. & U. Sommer, 1999. Diversity in planktonic communities: an experimental test of the intermediate disturbance hypothesis. Limnology and Oceanography 44(4): 1114–1119.
Gruberts, D., 2003. The four largest floodplain lakes in Latvia: hydrology, hydrochemistry and hydrobiology. In Järvet A. & E. Lode (eds), Ecohydrological Processes in Northern Wetlands. Selected Papers of International Conference and Educational Workshop. Tartu University Press, Tallinn: 196–202.
Gruberts, D. & I. Druvietis, 2001. Phytoplankton periodicity in floodplain lake Grīvas, Latvia—Baltic State. In Partnerships for Sustainable Life in Lake Ecosystems. Proceedings of 9th international conference on the conservation and management of lakes. Shiga, BIWAKO, 54–57.
Gruberts, D. & I. Druvietis, 2006. Impact of floods on composition, biomass and diversity of phytoplankton communities of the Middle Daugava, in Latvia. In Ács E., Kiss K. T., Padisak J. & Szabo K. E. (eds), Proceedings of 6th International Symposium on Use of Algae for Monitoring Rivers. Hungary, Baltonfüred, 12–16 Sept. 2006. 54–59.
Gruberts, D., I. Druvietis, L. Enģele, J. Paidere, E. Parele, J. Priedītis, A. Poppels & A. Škute, 2006. Biodiversity of the largest floodplain lake ecosystems in Latvia. In Proceedings of the 11th World Lake Conference, Nairobi, Kenya, 31 October to 4th November 2005. Vol. 2: 225–229.
Gruberts, D., J. Paidere, J. Priedītis, A. Škute, I. Druvietis, A. Poppels, E. Parele & L. Enģele, 2005. Biodiversity of the Daugava’s floodplain lakes in South-East Latvia. Acta Biologica Universitatis Daugavpiliensis 5(2): 137–153.
Gruberts, D., I. Druvietis, E. Parele, J. Paidere, A. Poppels & A. Škute, 2006. Impact of hydrology on aquatic communities of floodplain lakes along the Middle Daugava (Latvia). Hydrobiologia, in the process of publication.
Huszar, V. L. D. & C. S. Reynolds, 1997. Phytoplankton periodicity and sequences of dominance in an Amazonian flood-plain lake (Lago-Batata, Para, Brazil)—responses for gradual environmental change. Hydrobiologia 346: 169–181.
Junk, W. J. & K. M.Wantzen, 2003. The flood pulse concept: new aspects, approaches and applications—an update. Max-Planck-Institute for Limnology, Working Group Tropical Ecology, P.O. Box 165, 24302.
Junk, W. J. (eds), 1997. The central Amazon floodplain. Springer-Verlag, New York.
Junk, W. J., P. B. Bayley & R. E. Sparks, 1989. The flood-pulse concept in river-floodplain systems. Canadian Journal of Fisheries and Aquatic Sciences 106: 110–127.
Keckeis, S., C. Baranyi, T. Hein, C. Holarek, P. Riedler & F. Schiemer, 2003. The significance of zooplankton grazing in a floodplain system of the River Danube. Journal of Plankton Research 25(3): 243–253.
Keppeler, C. E. & E. R. Hardy, 2004. Vertical distribution of zooplankton in the water column of Lago Amapa, Rio Branco, Acre, Brazil. Revista Brasileira de Zoologia 21(2): 169–177.
Lampert, W. & U. Sommer, 1997. Limnoecology: the ecology of lakes and streams. Oxford University Press, New York, USA.
Lewis, M.W. Jr., S. K. Hamilton, M. A. Lasi, M. Rodríguez & J. F. Saunders III, 2000. Ecological determinism on the Orinoco floodplain. BioScience 50(8): 681–692.
Malard, F., 2003. Groundwater-surface water interaction. In Ward J. V. & U. Uehlinger (eds), Ecology of a Glacial Flood Plain: Aquatic Ecology Series. Kluwer Academic Publishers, The Netherlands: 37–55.
Muylert, K. & W. Vyverman, 2006. Impact of a flood event on the planktonic food web of the Schelde estuary (Belgium) in spring 1998. Hydrobiologia 559: 385–394.
Reynolds, C. S., 1993. Scales of disturbance and their role in plankton ecology. In Padisak, J., C. S. Reynolds & U. Sommer (eds), Intermediate Disturbance Hypothesis in Phytoplankton Ecology. Developments in Hydrobiology 81. Kluwer Academic Publishers, Belgium.
Reynolds, C. S., 1995. The intermediate disturbance hypothesis and its applicability to panktonic communities: comments on the views of Padisák of Wilson. New Zealand Journal of Ecology 19(2): 219–225.
Reynolds, C. S., J. Padisák & U. Sommer, 1993. Intermediate disturbance in the ecology of phytoplankton and the maintenance of species diversity: a synthesis. In Padisák J., C. S. Reynolds & U. Sommer (eds), Intermediate Disturbance Hypothesis in Phytoplankton Ecology. Kluwer Academic Publishers, Dordrecht: 183–188.
Roozen, F. C. J. M., 2005. Transparency of floodplain lakes; a study of plankton and suspended mater along the lower Rhine. Doctoral thesis. Wageningen Universiteit (http://library.wur.nl/wda/dissertations/dis3839.pdf).
Scheffer, M., 2004. Ecology of shallow lakes. Kluwer Academic Publishers, The Netherlands.
Sommer, U., J. Padisak, C. S. Reynolds & P. Juház- Nagy, 1993. Hutchinson’s heritage: he diversity-disturbance relationship in phytoplankton. In Padisak J., C. S. Reynolds & U. Sommer (eds), Intermediate Disturbance Hypothesis in Phytoplankton Ecology. Developments in Hydrobiology 81. Kluwer Academic Publishers, Belgium.
Trifonova, I., 1993. Seasonal succession of phytoplankton and its diversity in two highly eutrophic lakes with different conditions of stratification. Hydrobiologia 249: 93–100.
Tockner, K., F. Malard & J. V. Ward, 2000. An extension of the flood pulse concept. Hydrological Processes 14: 2861–2883.
Tockner, K., N. P. D. Reiner, F. Schiemer & J. V. Ward, 1999. Hydrological connectivity and the exchange of organic matter and nutrients in a dynamic river floodplain system (Danube, Austria). Freshwater Biology 41: 521–535.
Utermöhl, H., 1958. Zur Vervollkomnug der quantitativen Phytoplankton-Methodik. Mitteilungen Internationale Vereiningung für Theoretische und Angewandte Limnologie 19: 100–124
Van den Brink, F. W., M. M. Van Katwijk & G. Van der Velde, 1994. Impact of hydrology on phyto- and zooplankton community composition in floodplain lakes along the Lower Rhine and Meuse. Journal of Plankton Research 16: 351–373.
Ward, J. V., C. T. Robinson & K. Tockner, 2002. Applicability of ecological theory to riverine ecosystems. Verhandlungen Internationale Vereinigung für Theoretische und Angewandte Limnologie 28: 443–450.
Wilson, B. J., 1994. The ‘Intermediate Disturbance Hypothesis’ of species coexistence is based on patch dynamics. New Zealand Journal of Ecology 18(2): 176–181.
Acknowledgements
The study was supported by the VPD1/ESF/PIAA/04/NP/3.2.3.1/0003/0065 project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: S. I. Dodson
Rights and permissions
About this article
Cite this article
Paidere, J., Gruberts, D., Škute, A. et al. Impact of two different flood pulses on planktonic communities of the largest floodplain lakes of the Daugava River (Latvia). Hydrobiologia 592, 303–314 (2007). https://doi.org/10.1007/s10750-007-0770-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-007-0770-y