Abstract
The Cypricercinae are one of the most speciose subfamilies of non-marine ostracods, with more than 170 described species, mostly from the tropics. Although the identity of the subfamily as such is clear, because of the presence of unifying characters such as the Triebel’s loop in the attachment of the caudal ramus, the supra-specific taxonomy of this group has long been confused because of lack of good generic and tribal characters. Here, the generic characters of the Cypricercinae are revised. Eleven genera are retained in this subfamily, including three new genera: Bradleytriebella n. gen., Nealecypris n. gen. and Pseudostrandesia n. gen. Tanycypris siamensis n. sp. is described from Thailand. In addition, five species [Bradleystrandesia fuscata (Jurine, 1820), Bradleytriebella tuberculata (Hartmann, 1964), Nealecypris obtusa (Klie, 1933), Pseudostrandesia striatoreticulata (Klie, 1932), Spirocypris horrida (Sars, 1926)] are redescribed. A key to the genera is given. We propose three tribes: the nominal tribe Cypricercini McKenzie, 1971, as well as two new tribes, Bradleystrandesiini n. trib. and Nealecypridini n. trib. To evaluate the systematic relationships within this subfamily, phylogenetic analyses, based on morphological characters of valves and soft parts, were conducted. The Neighbour Joining (NJ) tree strongly supports the classification into three independent tribes, whereas the Maximum Parsimony (MP) tree shows that Bradleystrandesiini n. trib is actually a subgroup of the Cypricercini.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ostracods are microcrustaceans which are typically ca. 1 mm in length. They abound in both marine and freshwater and even in (semi-) terrestrial environments. Ostracods have about 2,000 living non-marine species, representing 13 families worldwide (Martens et al., 2008). More than 50% of all non-marine ostracods belong to the family Cyprididae. The Cypricercinae, one of the subfamilies within the Cyprididae, is one of the most common ostracod groups in the tropics, with about 170 described species
At present, the subfamily Cypricercinae comprises nine genera: Astenocypris G.W. Müller, 1912, Bradleycypris McKenzie, 1982, Bradleystrandesia Broodbakker, 1983, Cypricercus Sars, 1895, Diaphanocypris Würdig & Pinto, 1990, Neocypridella Vávra, 1895, Spirocypris Sharpe, 1903, Strandesia Stuhlmann, 1888 and Tanycypris Triebel, 1959. While Strandesia is the most diverse genus with ca. 115 species, there are three monospecific genera in this group. All of these genera are united into the subfamily by the morphology of attachment of the caudal ramus, which bears a loop at the distal end. This loop was first recognized by Triebel (1953), and was later named “Triebel’s loop” by Rome (1969). Based mainly on the presence of the Triebel’s loop in the attachment of the caudal ramus, but also on characters including the shape of the valves and of the caudal ramus, McKenzie (1971) established the tribe Cypricercini in the subfamily Cypridinae. According to him, this tribe at stage comprised four genera (Cypricercus, Spirocypris, Strandesia and Tanycypris). However, Hartmann & Puri (1974) placed Cypricercus, Strandesia and Spirocypris in the Eucypridinae Bronstein, 1947, and Astenocypris and Tanycypris in the Dolerocypridinae Triebel, 1961. In 1979, the tribe Cypricercini McKenzie, 1971, was elevated to the level of subfamily by De Deckker (1979), as many characters of this group were different from the Cypridinae s.s. The validity of the closely related genera Cypricercus and Strandesia was discussed by McKenzie (1982). He established the genus Bradleycypris for European and North American representatives and selected Cypricercus obliquus Brady, 1886, as the type species. One year later, however, Broodbakker (1983) allocated the species Cypricercus obliquus (Brady, 1886) to the subgenus Neocypris of Strandesia s.l. Thus, Bradleycypris became a synonym of Neocypris. In this paper, Broodbakker established the genus Bradleystrandesia and added the genus Astenocypris to subfamily Cypricercinae. This brought the total number of genera of this subfamily to six. Broodbakker (loc. cit.) also placed Acanthocypris Claus, 1892, and Neocypris Sars, 1901, as subgenera of Strandesia s.l. However, this point of view was rejected by Martens (1994), who reported that Neocypris gladiator Sars, 1901, is a synonym of Acanthocypris bicuspis Claus, 1892. In this contribution, Martens also reinstated Bradleycypris as valid genus and transferred at least three Holarctic species to Bradleystrandesia. Karanovic (2005) transferred Bradleycypris obliqua and all South African Cypricercus to Strandesia, as she mentioned that no distinguishable characters between these genera could be found. Two further species (Cypris iheringi Sars, 1901, and Cypricercus mollis Furtos, 1936) were also transferred to Strandesia.
Savatenalinton & Martens (2009) recently redescribed the type species of Strandesia and Cypricercus and reassessed the taxonomic position of species in Strandesia s.l. and Cypricercus s.l. They proposed to retain nine species in Cypricercus s.s and 31 species in Strandesia s.s. The former are restricted to the southern hemisphere, while the latter have wider distributions: southern hemisphere, South and Southeast Asia and the West Indies. Additionally, Bradleycypris, which has B. obliqua as type species, was confirmed as a valid monospecific genus in this paper. Savatenalinton & Martens (2009) also reinstated Cypricercus inermis as a valid species and allocated one Cypricercus and 11 Strandesia species to Bradleystrandesia.
Although taxonomic revisions of several subfamilies within the Cyprididae were already performed, e.g. on Megalocypridinae (Martens, 1986), Herpetocypridinae (Gonzalez Mozo et al., 1996, Martens, 2001a), Cypridinae: Cypridini (Martens, 1990, 1992, 2007), no such studies have thus far been published on the Cypricercinae. Studies on this subfamily have been hampered mainly by the lack of characters and by the insufficient description of most species and genera. To clarify this, both type and other species of the various genera (see Savatenalinton & Martens, 2009 for Strandesia and Cypricercus) are reexamined. Here, we revise the generic characters of the subfamily Cypricercinae. Several extant genera are redescribed and some new genera are proposed. A new tribal taxonomy of the Cypricercinae is proposed and is tested by a phylogenetic analysis based on morphological characters.
Materials and methods
Specimens used for the present paper were sorted from samples collected in Thailand by the first author and from samples in the collection of the second author. New materials of Bradleystrandesia fuscata (Jurine, 1820) and of B. reticulata (Zaddach, 1844) were obtained from the collection of the EU Marie Curie Research and Training Network “SexAsex” (Project no. 512492). Diaphanocypris meridana (Furtos, 1936) and Bradleytriebella trispinosa (Pinto & Purper, 1965) are provided from collections of J. Higuti (Nupelia, Universidade Estadual de Maringá, Brazil). In addition, other specimens from the Cypricercinae, mostly of Strandesia s.l., in the Ostracod Collection of R.B.I.N.Sc (Brussels) were examined.
Specimen preparation was done using a Leica Wild M-10 binocular microscope. Soft parts were dissected in glycerine and sealed on a glass slide. Valves were stored dry in micropalaeontological slides. Drawings of soft parts were made using camera lucida. Carapaces and valves were observed and illustrated using Scanning Electron Microscopy (Philips XL30 SEM).
Phylogenetic analyses of the subfamily Cypricercinae were performed based on 22 morphological characters (see Appendix 1—Electronic supplementary material), using Eucypris virens (Jurine, 1820) as an outgroup. The data matrix (see Appendix 2—Electronic supplementary material) was analysed using PAUP 4.06 (Swofford, 1998). All characters were allocated to the type “unordered”. Characters 6–8 were allocated a weight of 10 (default = 1), as the morphology of valves, caudal ramus attachment and position of Triebel’s loop are deemed to carry a more significant phylogenetic signal than, for example, shape of valves and chaetotaxy of other appendages. Trees were built using Maximum Parsimony (MP) with the Branch-and-Bound routine (furthest taxon input) and with Neighbour Joining (NJ) methods (mean character difference). Bootstrapping used the full heuristic method in MP; for both MP and NJ, 1000 replicates each were performed.
Abbreviation used in text and figures
MSU, Mahasarakham University, Mahasarakham, Thailand; MSU-ZOC, Ostracod Collection of the Natural History Museum, Mahasarakham University, Mahasarakham, Thailand; O.C., Ostracod Collections of the Royal Belgian Institute of Natural Sciences, Brussels; R.B.I.N.Sc., Royal Belgian Institute of Natural Sciences, Brussels. Valves and carapace. H, height of valves; L, length of valves; LV, left valve; RV, right valve; W, width of carapace. Limbs. A1, first antenna; A2, second antenna; Md, mandibula; Mx1, maxillula; T1, first thoracopod; T2, second thoracopod; T3, third thoracopod. Attachment of caudal ramus. db, dorsal branch; vb, ventral branch. Hemipenis. ls, lateral shield; ms, medial shield. Chaetotaxy of the limbs follows the model proposed by Broodbakker & Danielopol (1982), revised for the A2 by Martens (1987).
Results
Eleven genera of the subfamily Cypricercinae are presented here, including three new genera: Bradleytribella n. gen., Nealecypris n. gen. and Pseudostrandesia n. gen. Pseudostrandesia n. gen. is separated from Strandesia while Bradleytriebella n. gen. and Nealecypris n. gen. are separated from Bradleystrandesia and Tanycypris, respectively. The genus Neocypridella is not included in this subfamily as no Triebel’s loop is present on the attachment of the caudal ramus. The taxonomic position of Neocypridella will be discussed elsewhere. The genus Bradleystrandesia is presently divided into two species groups, based mainly on the presence or absence of a groove along the internal LV margin: group I (without groove) and group II (with groove). Thus far, there are two monospecific genera in the subfamily Cypricercinae: Astenocypris and Diaphanocypris. Bradleycypris is no longer a monospecific genus, as we allocate Strandesia vittata (Sars, 1903) to this genus in the present study. Tanycypris siamensis n. sp. is described from Thailand. Additionally, five more species [Bradleystrandesia fuscata (Jurine, 1820), Bradleytriebella tuberculata (Hartmann, 1964), Nealecypris obtusa (Klie, 1933), Pseudostrandesia striatoreticulata (Klie, 1932) and Spirocypris horrida (Sars, 1926)] are redescribed in the present paper.
Generic characters
To clarify the generic characters in Cypricercinae, some characters, e.g. the anterior part of the internal LV, d-setae on T2, attachment of caudal ramus, hemipenis, are broken down into discrete character states. Six diagrammatic patterns of the anterior part of the LV in Cypricercinae are proposed here, based on the presence/absence of a groove and of inner list(s) (Fig. 1). These features are best seen in an oblique inner view of the LV in the stereomicroscope and/or the SEM. The description of each pattern is given in Table 1. In Cypricercinae, the morphology of the anterior part of the LV is diverse. Most genera have type D, while type C, E and F are found only in Strandesia, Cypricercus and Bradleystrandesia group I, respectively. The morphology of the d-setae on T2 is divided into five groups (Table 2). Most genera have type C of this character. There are five types of caudal ramus attachment in Cypricercinae (Fig. 2, Table 3). Type C of the attachment of caudal ramus occurs in most genera of the subfamily. Five types of hemipenis are recognized from this subfamily (Fig. 3). This classification is based on the morphology of the medial and lateral shields and of the postlabyrinthal spermiduct loop(s).
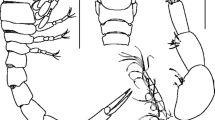
Types of caudal ramus attachment in Cypricercinae. A Astenocypris papyracea (Sars, 1903). B Tanycypris siamensis n. sp. C. Diaphanocypris meridana (Furtos, 1936) D Nealecypris obtusa (Klie, 1933). E Strandesia mercatorum (Vávra, 1895). F Strandesia kraepelini (Müller, 1906) G. Bradleycypris obliqua (Brady, 1868). H Cypricercus cuneatus Sars, 1895. I Cypricercus xhosa Savatenalinton & Martens, 2009 J Pseudostrandesia mamarilorum Victor & Fernando, 1981. K Bradleytriebella tuberculata Hartmann, 1964. L Bradleytriebella decorata (Sars, 1903). M Spirocypris horrida (Sars, 1926). N Bradleystrandesia fuscata (Jurine, 1820). O Bradleystrandesia reticulata (Zaddach, 1844) (A from George & Martens, 1993; C from Broodbakker, 1984; G, O from Meisch, 2000). Not to scale
Types of hemipenis in Cypricercinae. A Nealecypris obtusa (Klie, 1933). B–C Strandesia mercatorum (Vávra, 1895). D Cypricercus cuneatus sars, 1895. E Cypricercus xhosa Savatenalinton & Martens, 2009. F Bradleycypris vittata (Sars, 1903). G Bradleycypris obliqua (Brady, 1868). H Bradleystrandesia fuscata (Jurine, 1820). I Bradleystrandesia splendida (Furtos, 1933) (G from Petkovski, 1964). Not to scale
Based on the classification of characters above, and including other characters, e.g. the presence and shape of the Wouters organ, and the b- and d-setae on T1, the generic diagnoses within the subfamily Cypricercinae are here provided. We propose three tribes (two new) of the subfamily: the nominal tribe Cypricercini and two new tribes, Nealecypridini n. trib. and Bradleystrandesiini n. trib. In addition, a key to the genera is also given.
Taxonomic descriptions
Class Ostracoda Latreille, 1806
Subclass Podocopa G. W. Müller, 1894
Order Podocopida G. O. Sars, 1866
Suborder Cypridocopina Baird, 1845
Superfamily Cypridoidea Baird, 1845
Family Cyprididae Baird, 1845
Subfamily Cypricercinae McKenzie, 1971
Genera included—Astenocypris, Bradleycypris, Bradleystrandesia, Bradleytriebella n. gen., Cypricercus, Diaphanocypris, Nealecypris n. gen., Pseudostrandesia n. gen., Spirocypris, Strandesia, Tanycypris.
Key to the genera of the Cypricercinae
-
1.
a. Triebel’s loop present on dorsal branch of caudal ramus attachment........................2
b. Triebel’s loop present at middle of distal part of caudal ramus attachment........................4
-
2.
a. d-seta on T1 absent, carapace in lateral view subtriangular, Wouters organ present........................Bradleytriebella n. gen.
b. d-seta on T1 present, carapace in lateral view not subtriangular, Wouters organ absent........................3
-
3.
a. Carapace in dorsal view tumid, valves strongly ornamented with tubercules........................Spirocypris
b. Carapace in dorsal view elliptical, subovate (not tumid), valves weakly or not ornamented, not set with tubercules........................Bradleystrandesia
-
4.
a. b-seta on T1 absent........................5
b. b-seta on T1 present........................6
-
5.
a. no d1- and d2-setae on T2, ventral branch of caudal ramus attachment absent........................Diaphanocypris
b. d1- and d2-setae on T2 present, ventral branch of caudal ramus attachment present........................Astenocypris
-
6.
a. d -eta on T1 absent........................7
b. d-seta on T1 present........................8
-
7.
a. ventral branch of caudal ramus attachment absent, carapace in dorsal view narrow........................Nealecypris n. gen.
b. ventral branch of caudal ramus attachment present, carapace in dorsal view elliptical, subovate........................Pseudostrandesia n. gen.
-
8.
a. caudal ramus attachment with short, stout ventral branch, carapace in dorsal view narrow........................Tanycypris
b. caudal ramus attachment with long, well-developed ventral branch, carapace in dorsal view elliptical, subovate........................9
-
9.
a. anterior part of internal LV without inner list (type B), hemipenis with triangular lateral shield (type D)........................Bradleycypris
b. anterior part of internal LV with 0–2 inner lists (type C, D or E), hemipenis with different lateral shield........................10
-
10.
a. carapace in lateral view elliptical, subovate, elongated (length <2× width), hemipenis with large, wing-like lateral shield (type B), Zenker organ with chitinous sheet on both ends........................Strandesia
b. carapace in lateral view elongated (length >2× width), hemipenis with small, subquadrate lateral shield (type C), Zenker organ with a crown of petal-like structure at distal end plate........................Cypricercus
Remarks. The absence of the Wouters organ on A1 means either that this organ does not exist or that it is so small that it cannot be recognized under a light microscope.
Tribe Nealecypridini new tribe
Diagnosis. Carapace in dorsal view narrow, in lateral view elongated caudal ramus stout, claws strongly serrated; caudal ramus attachment distally with Triebel’s loop in the middle, dorsal and ventral branches not well-developed (short or absent).
Differential diagnosis. This tribe is distinguished from Cypricercini and Bradleystrandesiini n. trib. by the morphology and the position of the Triebel’s loop on the caudal ramus attachment and by a narrow carapace in dorsal view.
Genera included. Astenocypris, Diaphanocypris, Nealecypris n. gen., Tanycypris.
Astenocypris G.W. Müller, 1912
Type species. Astenocypris papyracea (Sars, 1903)
Diagnosis (modified from George & Martens, 1993). Carapace narrow in dorsal view, with thin valves; LV in internal view without a marginal groove; anterior inner lamella relatively wide, without inner list, posterior inner lamella narrower; Wouters organ present; seta d1 on T2 large; caudal ramus stout, strongly serrated; caudal ramus attachment with Triebel’s loop in the middle of the distal part, dorsal branch short, pointed, ventral branch short, stout.
Differential diagnosis. This genus is similar to Diaphanocypris and Nealecypris n. gen. It can be distinguished from both genera by the presence of the ventral branch of the caudal ramus attachment (absent in Diaphanocypris and Nealecypris n. gen.). Additionally, it is different from Diaphanocypris by the presence of d-setae on the T2 (absent in Diaphanocypris) and from Nealecypris n. gen. by the absence of a b-seta on the T1 (present in Nealecypris n. gen.).
Species included. Astenocypris papyracea (Sars, 1903).
Occurrence. Sumatra (Sars, 1903), India (George & Martens, 1993), Thailand (this study).
For redescription of the type species, see George & Martens (1993).
Diaphanocypris Würdig & Pinto, 1990
Type species. Diaphanocypris meridana (Furtos, 1936) (Fig. 4) .
Diagnosis. Carapace narrow in dorsal view, LV in internal view without a groove, anterior inner lamella without an inner list (type A); Wouters organ present; T2 without setae d1 and d2; caudal ramus stout, serrated; caudal ramus attachment with Triebel’s loop in the middle of the distal part, but without ventral branch.
Differential diagnosis. Diaphanocypris is similar to Nealecypris n. gen. It is distinguishable by the absence of b-seta on T1 and the d1- and d2-setae on T2.
Species included. Diaphanocypris meridana (Furtos, 1936)
Occurrence. Mexico (Furtos, 1936), Puerto Rico, La Désirade, Îles des Saintes, Los Testigos, Isla Margarita (Broodbakker, 1984), Brazil, Argentina (Würdig & Pinto, 1990).
Diaphanocypris meridana (Furtos, 1936), female. A LV, internal view (O·C.3119). B RV, internal view (ditto). Scale bar 200 μm for A–B
For description of the type species, see Würdig & Pinto (1990).
Nealecypris n. gen.
Type species. Nealecypris obtusa (Klie, 1933) (here designated).
Etymology. The new genus is named after late John W. Neale (U.K.) in recognition of his large contributions to the study of Ostracoda in general, and especially to the revision of the subfamily Cypricercinae (unpublished).
Differential diagnosis. This genus is superficially similar to Tanycypris in its external morphology. However, these two genera can be distinguished by several aspects: the occurrence of a groove along the internal LV margin (absent in Nealecypris n. gen., present in Tanycypris), the d-seta on T1 (absent in Nealecypris n. gen., present in Tanycypris), the ventral branch of the caudal ramus attachment (absent in Nealecypris n. gen., present in Tanycypris) and the Wouters organ (present in Nealecypris n. gen., absent in Tanycypris).
Species included. The genus is presently monospecific.
Occurrence. South Africa (Hutchinson et al., 1932; Klie, 1933; Martens, 2001b).
Nealecypris obtusa (Klie, 1933) (Figs. 5–7)
Dolerocypris obtusa Klie, 1933
Nealecypris obtusa (Klie, 1933). A–C, F–H female; D–E male. A A1 (O·C.3102). B A2 (ditto). C A2, terminal segment (ditto). D A2, distal end of penultimate segment (O·C.3103). E A2, terminal segment (ditto). F Md-palp (O·C.3102). G Md-palp, terminal segment (ditto). H Md-coxa (O·C.3101). Scale bar 100 μm for A–B, H; 50 μm for C–G
Nealecypris obtusa (Klie, 1933), female. A Mx1 (O·C.3101). B T1 (O·C.3102). C T2 (O·C.3101). D T3 (ditto). E Caudal ramus attachment (ditto). F Caudal ramus (ditto). G Caudal ramus, claws and setae (ditto). Scale bar 50 μm for A–B; 100 μm for C–D, G; 200 μm for E–F
Nealecypris obtusa (Klie, 1933), male. A Hemipenis (O·C.3104). B Left prehensile palp (O·C.3103). C Right prehensile palp (ditto). D Zenker organ (ditto). Scale bar 100 μm for A, D; 50 μm for B–C
Tanycypris siamensis n. sp., female. A LV, internal view (O·C.3099). B RV, internal view (ditto). C LV, external view (ditto). D RV, external view (ditto). E LV, internal view, anterior part (ditto). F LV, internal view, posterior part (ditto). G RV, internal view, anterior part (ditto). H RV, internal view, posterior part (ditto). Scale bar 280 μm for A–D; 200 μm for E–H
Tanycypris obtusa (Klie, 1933)—McKenzie, 1971, p. 172
Material examined. Three females and 3 males from a sample collected from Schoonspruit, South Africa (RSA/93/232) (coordinates: 26° 16′ 30′′ S and 26° 52′ 0′′ E) on 5 November 1993 by K. Martens, E. Skelton & V. Jones.
Diagnosis. Carapace in lateral view elongated, anterior and posterior margins rounded; LV in inner view without groove along inner valve margin; inner lamella wide, without inner list; A1 with Wouters organ, Rome organ short; T1 with a and b-setae, but without d-seta; caudal ramus stout, both claws strongly serrated; caudal ramus attachment with Triebel’s loop at middle of distal part, dorsal branch slim, short; ventral branch absent; hemipenis relatively small, medial shield rounded, lateral shield small, subtriangular (size equal to medial shield); Zenker organ with distal end plate forming a crown of petal-like structures.
Measurements (in μm). LV (n = 3), L = 1100–1200, H = 350–400, RV (n = 3), L = 1100–1150, Carapace (n = 3), L = 1000–1200, W = 200–250.
Remarks. Most species presently allocated to Tanycypris s.l. are only superficially described and the generic characters used here to distinguish between Tanycypris and Nealecypris n. gen. are mostly not given in the original descriptions. It is therefore difficult to deduce the corrected taxonomic position of species in this group. Thus, Nealecypris n. gen. comprises one species (N. obtusa) for the time being. Other species described in Tanycypris are left in this genus until they can be redescribed.
Redescription of female
Carapace in lateral view elongated, with height ca. half the length; anterior and posterior margins rounded; valve surface relatively smooth. Carapace in dorsal view narrow.
LV in inner view without groove along valve margin; anterior inner lamella wide, without inner list.
A1 (Fig. 5A): first segment with small proximal Wouters organ, long dorsal subapical seta, and with two long ventral apical setae. Second segment slightly wider than long, with 1 short dorso-apical seta and a small, ventral Rome organ. Third segment bearing 2 (1 dorsally, 1 ventrally) spine-like apical setae. Fourth segment with dorsally 2 long apical setae, ventrally 2 spine-like, short apical setae. Fifth segment with 2 dorsal apical long setae and 2 (1 long, 1 short) ventral apical setae, the long one reaching the distal end of the next segment. Penultimate segment with 4 long apical setae. Terminal segment with 3 (2 long, 1 short) apical setae and aesthetasc ya, the latter twice as long as the short apical seta.
A2 (Fig. 5B–C): exopodite with 3 (1 long, 2 short) setae, the long one ca. 3/4 of the first endopodal segment. First endopodal segment with natatory setae long, the shortest ca. 1/4 of the length of the penultimate segment, aesthetasc Y long, ventral apical seta long (reaching beyond end of penultimate segment). Penultimate segment undivided, distally with 3 serrated claws and with apical aesthetasc y2 reaching slightly beyond tip of terminal segment; z1–z3 setae long, medially with 2 (1 long, 1 short) dorsal setae (the short seta ca. half the length of the long one) and 4 ventral setae (t1–t4). Terminal segment (Fig. 10C) with 2 serrated claws (GM and Gm), a long g-seta and the aesthetasc y3, the latter ca. half the length of the accompanying seta.
Tanycypris siamensis n. sp., female. A Mx1 (O·C.3100). B T1, protopodite (ditto). C T1 (O·C.3099). D T2 (ditto). E T2, terminal segment (ditto). F T3 (O·C.3100). G T3, terminal segment (ditto). H Caudal ramus attachment (O·C.3099). I Caudal ramus (ditto). Scale bar 38 μm for A–B, E, G; 76 μm for C–D, F; 116 μm for H–I
Md-palp (Fig. 5F–G): first segment with 2 large setae (s1 and s2), 1 slender, long seta and relatively long, smooth α-seta. Second segment dorsally with 3 (2 long, 1 short) apical setae, the short one ca. 1/3 the length of the long one, ventrally with a group of 3 long, hirsute setae, 1 shorter seta and the β-seta, the latter plumose, cone-shape, and with pointed tip. Penultimate segment bearing 3 groups of setae: dorsally with a group of 4 unequal, subapical setae; laterally with the apical γ-seta, 3 smooth apical setae, the former stout, hirsute, long (slightly less than twice the length of the terminal segment); ventrally with 1 long and 1 short (ca. half of terminal segment) apical setae. Terminal segment (Fig. 5G) bearing 3 setae and 3 claws.
Mx1 (Fig. 6A) with 2-segmented palp, three endites and a large branchial plate; basal segment of the palp with a group of 5 unequal long apical setae and 2 (1 long, 1 short) subapical setae; terminal segment elongated, with 3 claws and 3 setae. Two large bristles on third endite serrated. Sideway-directed bristles on first endite unequal, the shorter one ca. 2/3 of the length of the long one.
T1 (Fig. 6B): protopodite with 2 short a-setae, 1 long b-seta, but without d-seta; distally with 14 hirsute, unequal apical setae. Endopodite palp weakly built and set with 3 unequal apical setae.
T2 (Fig. 6C) with 2 hirsute d-setae, d2 ca. 1/3 of the length of d1. Second segment with 1 hirsute, long subapical seta (ca. 3/4 of the length of penultimate segment). Penultimate segment divided in two segments, proximal segment (a) bearing 1 long apical seta, distal segment (b) with a pair of apical setae (1 short, 1 spine-like). Terminal segment with 2 (1 dorsally, 1 ventrally) apical setae and a serrated claw.
T3 (Fig. 6D) a cleaning limb. First segment with 3 long setae. Second segment with 1 long apical seta (reaching half of the next segment). Third segment with medially 1 short seta (length less than half of the segment). Terminal segment with apical pincer and 1 short reflexed subapical seta (length slightly longer than apical pincer).
Caudal ramus (Fig. 6F–G) stout, considerably serrated along ventral margin, both distal and proximal claws strongly serrated, length of distal claw ca. 2/5 of that of ramus, proximal claw ca. 2/3 as long as the distal claw. Distal seta slightly hirsute, proximal seta long (reaching beyond tip of ramus), slim, with needle-like end.
Caudal ramus attachment (Fig. 6E) with Triebel’s loop distally at middle branch, dorsal branch slim, short, no ventral branch.
Additional redescription of male
Carapace and valves as in female. All limbs as in female, except for A2 (Fig. 5D–E), T1 and reproductive organs. Penultimate segment of A2 with claw-like z1- and z2 setae and with claws G1 and G3 reduced to setae. Terminal segment with reduced Gm claw, being considerably smaller and shorter than in female. T1 endopodites forming asymmetrical, slender prehensile palps (Fig. 7B–C).
Hemipenis (Fig. 7A) relatively small, medial shield rounded, lateral shield small, subtriangular; both shields subequal in size. Postlabyrinthal spermiduct loop small. Zenker organ (Fig. 7D) set with ca. 24 spiny whorls length ca. 2× width, distal end plate forming a crown of petal-like structures.
Tanycypris Triebel, 1959
Type species. Tanycypris madagascarensis (G.W. Müller, 1898).
Diagnosis (modified from Martens, 2001). Carapace elongated in lateral view, narrow in dorsal view; LV internal view with groove along valve margins, anterior inner lamella with or without inner list; Wouters organ absent; caudal ramus stout, serrated; caudal ramus attachment with Triebel’s loop at middle, ventral branch stout, short.
Differential diagnosis. See Nealecypris n. gen.
Species included. Tanycypris madagascarensis (G.W. Müller, 1898), T. siamensis n. sp.
Occurrence. Madagascar (G.W. Müller, 1898), Thailand (this study).
Remarks. As no material of the type species was available to us, we here describe a new species belonging to Tanycypris s.s. The most important characteristics of the genus as described above could be verified in the original description of the type species (Müller, 1898).
Tanycypris siamensis n. sp. (Figs. 8–10)
Material examined. Four females from the type locality and from two other localities (see below).
-
Holotype. Female, soft parts dissected in glycerine in a seal slide, valves stored dry in a micropalaeontological slide (O.C. 3099).
-
Paratypes. Three females (O.C. 3100, MSU-ZOC 018-019) dissected and stored as the holotype.
Repository. The holotype and one paratype are deposited in the Ostracod Collection of the R.B.I.N.Sc. Two further paratypes are curated in the Natural History Museum, MSU (Mahasarakham, Thailand).
Type locality. Maepeum Reservoir, Maejai district, Phayao Province (TH143) (coordinates: 19° 21′28.6′′ N and 99° 51′44.6′′ E), collected by S. Savatenalinton, 7 October 2007. Accompanying ostracod fauna: Bradleystrandesia weberi (Moniez, 1892), Bradleytriebella lineata (Victor & Fernando, 1981), Cypretta sp. 1, Cypretta sp. 2, Cypridopsis s.l. spec. 1, Hemicypris exiqua Broodbakker, 1983, Physocypria sp. 1, Pseudostrandesia mamarilorum (Victor & Fernando, 1981), Stenocypris malayica Victor & Fernando, 1981, Strandesia kraepelini (Müller, 1906), Strandesia perakensis Victor & Fernando, 1981, Strandesia sp. n. sp. 1.
Other localities. Bung Sifai lake, Muang district, Pichit Province (TH130) (coordinates: 16° 25′ 25.7′′ N and 100° 20′ 35.4′′ E), collected by S. Savatenalinton, 13 November 2006. Accompanying ostracod fauna: Bradleystrandesia weberi (Moniez, 1892), Chrissia ceylonica (Daday, 1898), Cypretta sp., Cypridopsis vidua (Müller, 1776), Cypridopsis s.l. spec. 1, Ilyocypris sp., Physocypria sp., Strandesia kraepelini (Müller, 1906), Strandesia sp. n. sp. 1, Thaicythere srisumonae Savatenalinton, Borgonie & Martens, 2008, Vestalenula boteai (Danielopol, 1970).
Nalao natural spring, Konsarn District, Chaiyaphum Province (TH151) (coordinates: 16° 35′ 22.0′′ N and 101° 53′ 49.8′′ E), collected by S. Savatenalinton, 9 October 2007. Accompanying ostracod fauna: Bradleystrandesia weberi (Moniez, 1892), Bradleytriebella lineata (Victor & Fernando, 1981), Chrissia ceylonica (Daday, 1898), Cypretta sp. 1, Cypretta sp. 2, Cypretta sp. 3, Cypridopsis vidua (Müller, 1776), Cypris subglobosa Sowerby, 1840, Physocypria sp., Pseudocypretta maculata Klie, 1932, Pseudostrandesia mamarilorum (Victor & Fernando, 1981), Pseudostrandesia sp. n. sp. 1, Stenocypris malayica Victor & Fernando, 1981, Strandesia kraepelini (Müller, 1906), Strandesia sp. n. sp. 2, Strandesia sp. n. sp. 3, Thaicythere srisumonae Savatenalinton, Borgonie & Martens, 2008, Vestalenula molopoensis (Rossetti & Martens, 1997).
Etymology. The genus is named after “Siam”, the previous name of Thailand, from where the species is described.
Diagnosis. Carapace in lateral view elongated, anterior and posterior margin rounded; carapace in dorsal view narrow; Rome organ long, no Wouters organ and no dorsal subapical seta on A1-first segment; both bristles on third endite of Mx1 serrated; d1 on T2 slim, longer (ca. 2×) than d2; caudal ramus stout, ventral margin strongly serrated, proximal and distal claws strongly serrated.
Differential diagnosis. Tanycypris siamensis n. sp. can be distinguished from T. madagascarensis by several characters. In T. siamensis n. sp., LV and RV are subequal, while the LV is larger in T. madagascarensis. This feature can be easily seen in dorsal view of carapace. Whereas the posterior margin of the LV is evenly rounded in T. siamensis n. sp., it is postero-ventrally pointed in T. madagascarensis. Although both taxa have strongly serrated claws of the caudal ramus, a shorter distal seta and a longer proximal seta are found in T. siamensis n. sp. Additionally, the ventral margin of the caudal ramus is also strongly serrated in T. siamensis n. sp., whereas it is set with ca. seven groups of fine spines in T. madagascarensis.
Measurements (in μm). LV (n = 4), L = 1020–1150, H = 452–498; RV (n = 4), L = 1000–1120, H = 446–525.
Description of female
Valves in lateral view (Fig. 8A–B) elongated, anteriorly and posteriorly rounded, surface of valves relatively smooth; carapace in dorsal view narrow.
LV in inner view (Fig. 8A, C–D) with groove along anterior, ventral and posterior margins, dorsal margin relatively straight, ventral margin curved slightly in front of the mid-length, both anterior and posterior extremities rounded; inner lamella calcified, anteriorly broad, without inner list, posteriorly broad, slightly curved at mid-height.
RV in inner view (Fig. 8B, E–F) without selvage, dorsal margin relatively straight, ventral margin curved slightly in front of the mid-length, inner lamella as in LV.
A1 (Fig. 9A): first segment without Wouters organ and dorsal subapical seta, but with two long ventral apical setae. Second segment slightly wider than long, with 1 short dorsal apical seta and a long, tube-like Rome organ. Third segment bearing 2 (1 dorsally, 1 ventrally) long apical setae, extending beyond the tip of the fourth segment. Fourth segment dorsally with 2 long setae, ventrally with 2 (1 long, 1 short) setae, the long one reaching the middle of the next segment. Fifth segment with 3 (2 dorsal, 1 lateral) long and 1 short ventral seta, the latter reaching beyond end of sixth segment. Sixth segment both with ventrally and dorsally with 2 long setae. Terminal segment with 3 (2 long, 1 short) setae and an aesthetasc ya, the latter slightly shorter than the short apical seta.
A2 (Fig. 9B–C): exopodite with 3 (1 long, 2 short) setae, the long one ca. half the length of the first endopodal segment. Natatory setae on first endopodal segment long, length of the shortest seta ca. 1/4 of the length of the penultimate segment, aesthetasc Y long, ventral apical seta long (reaching the end of the penultimate segment). Penultimate segment undivided, distally with 3 serrated claws and with apical aesthetasc y2 reaching slightly beyond the end of the terminal segment; z1–z3 setae long, this segment medially with 2 (1 long, 1 short) dorsal setae (the short seta slightly more than half the length of the long one) and 4 ventral setae (t1–t4). Terminal segment (Fig. 9C) with 2 serrated claws (GM and Gm), a long g-seta and aesthetasc y3, the latter ca. half the length of the accompanying seta.
Md-palp (Fig. 9D–F): first segment with 2 large setae (s1 and s2), 1 long and relatively long, and a 2-segmented, smooth α-seta. Second segment dorsally with 3 (2 long, 1 short) apical setae, the shortest less than half of the longest one, ventrally with a group of 4 setae (3 hirsute, 1 long) and a β-seta, the latter plumose, cone-shape, with pointed tip. Penultimate segment consisting of 3 groups of setae: dorsally with a group of 4 unequally subapical setae; laterally with an apical γ-seta, and 3 further smooth apical setae, the former stout, hirsute, long (slightly less than twice the length of the terminal segment); ventrally with 1 long and 1 short (less than half the length the terminal segment) apical setae. Terminal segment (Fig. 9E) bearing 3 setae and 3 claws.
Mx1 (Fig. 10A) with 2-segmented palp, 3 endites and large branchial plate. Basal segment of palp with a group of 5 unequal long apical setae and 2 (1 long, 1 short) subapical setae. Terminal segment elongated, with 3 claws and 3 setae. Two large bristles on third endite serrated. Sideways-directed bristles on first endite unequal, length of the short one ca. half of that of the long one.
T1 (Fig. 10B–C): protopodite with 2 short a-setae, 1 long b-seta, 1 long d-seta and with 14 hirsute, unequal apical setae (Fig. 10B). Endopodite palp weakly built and set with 3 unequal apical setae, the longest one in the middle.
T2 (Fig. 10D–E) with 2 slightly hirsute d-setae, d2 ca. half the length of d1. Second segment with 1 long apical seta (more than half the length of penultimate segment). Penultimate segment divided, proximal segment (a) bearing 1 long apical seta, distal segment (b) with a pair of apical setae (1 short, 1 spine-like). Terminal segment with 2 (1 dorsally, 1 ventrally) apical setae and a serrated claw.
T3 (Fig. 10F–G) a cleaning limb. First segment with 3 long setae. Second segment with 1 long apical seta. Third segment medially with 1 short lateral seta (length less than half of that of the segment). Terminal segment with an apical pincer and 1 long reflexed subapical seta.
Caudal ramus (Fig. 10H) stout, strongly serrated along ventral margin, both distal and proximal claws strongly serrated, length of distal claw slightly less than half of that of the ramus, length of proximal claw ca. 2/3 of that of distal claw. Distal seta slightly hirsute, proximal seta long (reaching beyond end of ramus), slim, with needle-like tip.
Caudal ramus attachment (Fig. 10I) stout, with Triebel’s loop at middle of distal part of main branch, dorsal branch slim, short, ventral branch wide and short.
Males unknown.
Remarks. Tanycypris siamensis n. sp. is a rare species. We found four females from three localities. In each locality, samples were collected four times: September 2005, February 2006, November 2006, October 2007. The new species was encountered only in November and October which is the period between the rainy and the cool seasons. Although the macrohabitats of this species seem to be very different (a man-made reservoir, a natural lake and a natural spring), microhabitats are all similar, namely dense macrophyte stands in the littoral zone.
Tribe Cypricercini McKenzie, 1971
Diagnosis. Carapace in dorsal view subovate, elliptical; LV in internal view with a groove, anterior lamella with or without inner list(s); d-seta on T1 absent or present; caudal ramus stout, claws serrated; caudal ramus attachment with Triebel’s loop in the middle of the distal part of the main branch, dorsal and ventral branches both well-developed.
Differential diagnosis. This tribe differs from Tanycypridini n. trib. in the morphology of the caudal ramus attachment, the carapace, especially in dorsal view and the anterior part of LV in internal view. It is distinguishable from Bradleystrandesiini mainly by the position of the Triebel’s loop.
Genera included. Bradleycypris, Cypricercus, Pseudostrandesia n. gen., Strandesia.
Bradleycypris McKenzie, 1982
Type species. Bradleycypris obliqua (Brady, 1868) (Fig. 11)
Diagnosis. Carapace in dorsal view ovate, in caudal view oblique; LV with internal groove along valve margin, inner lamella without inner list (type B); d-seta on T1 present; hemipenis with small, pointed, subtriangular lateral shield and with 1 large postlabyrinthal spermiduct loop; Zenker organ with cone-shaped chitinous sheets at both proximal and distal ends.
Differential diagnosis. This genus is most closely related to Strandesia and can be distinguished from it by the morphology of the anterior part of the LV in internal view, by the shape of the hemipenis and by the shape of the carapace in frontal view, which is strongly oblique in Bradleycypris.
Species included. Bradleycypris obliqua (Brady, 1868), B. vittata (Sars, 1903).
Occurrence. Europe, Africa, South America (see Meisch, 2000), Thailand (this study).
For the morphology of valves and soft parts, as well as for ecology and distribution of the type species, see Meisch (2000).
Cypricercus Sars, 1895
Type species. Cypricercus cuneatus Sars, 1895.
Diagnosis (modified from Savatenalinton & Martens, 2009). Carapace in dorsal view elliptical, in lateral, view elongated (length more than twice the height), in frontal view, LV and RV subequal, not oblique; LV with internal groove along valve margin and with 1 (type D) or 2 (type E) inner lists; d-seta on T1 present; hemipenis with small, subquadrate lateral shield and with 2 postlabyrinthal spermiduct loops; Zenker organ with distal end plate, forming crown of petal-like structure.
Differential diagnosis. Cypricercus is closely related to Strandesia and Bradleycypris. It is distinguished from both genera by the morphology of anterior part of the LV in internal view, of the hemipenis and of the Zenker organ.
Occurrence. South Africa, South America, Australia (See Savatenalinton & Martens, 2009).
For species included and redescription of the type species, see Savatenalinton & Martens (2009).
Pseudostrandesia n. gen.
Type species. Pseudostrandesia striatoreticulata (Klie, 1932) (here designated).
Etymology. The name is the combination of “Pseudo” with “Strandesia”, the genus to which the new genus is superficially similar.
Diagnosis. Carapace in dorsal view elliptical, in lateral view elongated, in caudal view position of LV and RV equal or slightly unequal; LV with internal groove along valve margin, inner lamella with 1 inner list (type D); d-seta on T1 absent; T2 with d1 larger and longer than d2.
Differential diagnosis. This genus is close to Strandesia. It is distinguishable from it by the absence of the d-seta on T1 and the presence of a long Rome organ.
Species included. P. asymmetros (Rome, 1962), P. calapanensis (Tressler, 1937), P. chondropherusa (Rome, 1969), P. mamarilorum (Victor & Fernando, 1981), P. striatoreticulata (Klie, 1932), P. vinciguerrae (Masi, 1905).
Occurrence. Southeast Asia (Klie, 1932, Tressler, 1937, Victor & Fernando, 1981), Taiwan (Klie, 1938), Namibia (Martens, 2001).
Pseudostrandesia striatoreticulata (Klie, 1932) (Figs. 12–15)
Strandesia striatoreticulata Klie, 1932
Pseudostrandesia striatoreticulata (Klie, 1932), female. A Carapace, right lateral view (MSU-ZOC 046). B Carapace, dorsal view (O·C.3123). C Carapace, ventral view (ditto). D LV, internal view (O·C.3122). E RV, internal view (ditto). Scale bar 200 μm for A–E
Pseudostrandesia striatoreticulata (Klie, 1932), female. A A1 (MSU-ZOC 047). B A2 (ditto). C A2, terminal segment (ditto). D Md-coxa (ditto). E Md-palp (ditto). F Md-palp, terminal segment (ditto). G Mx1 (O·C.3122). H T1 (ditto). Scale bar 100 μm for A–D; 50 μm for E–H
Pseudostrandesia striatoreticulata (Klie, 1932), female. A T2 (MSU-ZOC 047). B T3 (ditto). C T3, distal end (ditto). D Caudal ramus attachment (ditto). E Caudal ramus (ditto). Scale bar 100 μm for A–B, D–E; 50 μm for C
Pseudostrandesia striatoreticulata (Klie, 1932), female. A Dorsal view. B Ventral view. Not to scale
Material examined. Three females from a sample collected from Mae Peum Reservoir, Maejai district, Phayao Province, 24 September 2005 (TH030), coordinates: 19° 21′ 28.6′′ N and 99° 51′ 44.6′′ E.
Diagnosis. Carapace in lateral view elongated, anterior and posterior margins evenly rounded, dorsal margin arched, greatest height situated at mid-length; valve surface relatively smooth; carapace in dorsal view subovate, with beak-like anterior and posterior ends; LV with internal groove along valve margin, inner lamella anteriorly wide, without inner list (type C); Wouters and Rome organs long, aesthetasc ya shorter than short apical seta; two large bristles on third endite of Mx1 serrated; d-seta on T1 absent; T2 with d1 larger and longer than d2; caudal ramus slender, distal and proximal claws slender, long, serrated, length of distal claw slightly less than half of that of caudal ramus, length of proximal claw ca. 3/4 of that of distal claw; caudal ramus attachment stout, with Triebel’s loop at middle of distal part of main branch, dorsal branch short, ventral branch well-developed.
Differential diagnosis. Pseudostrandesia striatoreticulata is characterized by the presence of beak-like anterior and posterior ends in dorsal view. It cannot be confused with other species in Pseudostrandesia.
Measurements (in μm). LV (n = 1), L = 773, H = 382, RV (n = 1), L = 745, H = 382, Carapace (n = 2), L = 709–714, W = 373–379.
Redescription of female
Carapace in lateral view (Fig. 12A) elongated, anterior and posterior margin evenly rounded, dorsal margin arched, greatest height situated at mid-length, ventral margin slightly curved; valve surface relatively smooth.
Carapace in dorsal view (Fig. 12B) subovate, with beak-like anterior and posterior ends, greatest width situated slightly behind mid-length; LV overlapping RV, greatest width anterior to the middle.
Carapace in ventral view (Fig. 12C) LV overlapping RV, ventral margin of LV protruding slightly in front of mid-length.
LV in inner view (Fig. 12D) with groove along valve margin, dorsal margin curved, greatest height situated in front of mid-length, sloping down to anterior and posterior margins, the latter both evenly rounded; ventral margin slightly curved; inner lamella calcified, anteriorly wide, without inner list (type C), posteriorly narrower.
RV in inner view (Fig. 12E) without selvages, inner lamella without inner list, anteriorly wider than posteriorly.
A1 (Fig. 13A): first segment with long dorsal subapical seta (reaching tip of segment) and 2 unequal long ventral apical setae, proximal Wouters organ long. Second segment slightly wider than long, with 1 short dorsal apical seta and long Rome organ. Third segment bearing 2 (1 dorsally, 1 ventrally) apical setae, the former longer (reaching beyond distal end of fifth segment), the latter shorter (reaching end of the next segment). Fourth segment with 2 long dorsal setae, ventrally with 2 shorter setae (length of the shortest ca. half of that of the fifth segment). Fifth segment dorsally with 2 long setae, ventrally with 2 (1 long, 1 short) setae, short one reaching the end of penultimate segment. Penultimate segment with 5 (4 long, 1 short) setae, length of short one ca. 2/3 of that of terminal segment. Terminal segment with 3 (2 long, 1 short) apical setae and short aesthetasc ya, the latter shorter than short apical seta.
A2 (Fig. 13B–C): exopodite with 3 (1 long, 2 short) setae, long one reaching end of the first endopodal segment. First endopodal segment with natatory setae long, length of shortest seta ca. half of that of penultimate segment, aesthetasc Y short, ventral apical seta long (reaching beyond tip of terminal segment). Penultimate segment undivided, distally with 3 serrated claws (length ca. 1.3× penultimate segment) and with long aesthetasc y2 (reaching beyond tip of terminal segment), z1–z3 setae long; this segment medially with 2 (1 long, 1 short) dorsal setae (length of short one ca. 4/5 of that of the long one) and 4 ventral setae (t1–t4). Terminal segment (Fig. 13C) with 2 serrated claws (GM and Gm), long g-seta and an aesthetasc y3, length of the latter ca. half of that of accompanying seta.
Md-palp (Fig. 13E–F): first segment with 2 large setae (s1 and s2), 1 slender, long seta and a long, smooth α-seta. Second segment dorsally with 3 unequal long apical setae, length of shortest ca. half of that of longest; ventrally with a group of 3 hirsute setae, 1 shorter seta and the β-seta, the latter plumose, cone-shape, and with pointed tip. Penultimate segment consisting of 3 groups of setae: dorsally with a of 4 unequal long subapical setae; laterally with apical γ-seta and 3 further smooth apical setae, the former stout, hirsute, long (length ca. twice that of the terminal segment); ventrally with two (1 long, 1 short, the latter ca. half length of terminal segment) apical setae. Terminal segment (Fig. 13D) bearing 3 claws and 3 setae.
Mx1 (Fig. 13G) with 2-segmented palp, 3 endites and a large branchial plate; basal segment of palp with a group of 5 unequal long apical setae and 2 (1 long, 1 short) subapical setae, terminal segment elongated, with 3 claws and 3 setae. Two large bristles on third endite distally serrated. Sideways-directed bristles on first endite unequal, length of short one ca. half of that of long one.
T1 (Fig. 13H): protopodite with 2 long a-setae, a long b-seta, but without d-seta, distally with 14 (10 apical, 4 subapical) long, hirsute setae. Endopodite a weakly built palp with 3 unequal apical setae.
T2 (Fig. 14A) with slim d-setae, length of d2 less than half of that of d1. Second segment with 1 long apical seta (reaching beyond half of penultimate segment). Penultimate segment divided proximal segment (a) bearing 1 long apical seta (reaching beyond end of terminal segment), distal segment (b) with a pair of apical setae (1 long, 1 spine-like). Terminal segment with 2 (1 dorsally, 1 ventrally) long apical setae and a serrated claw.
T3 (Fig. 14B–C) a cleaning limb. First segment with 3 long setae. Second segment with 1 long apical seta (slightly more than half of next segment). Third segment with medially 1 long seta (reaching beyond end of segment). Terminal segment with an apical pincer and 1 long reflexed subapical seta.
Caudal ramus (Fig. 14E) slender, with ventral margin serrated, distal and proximal claws slender, long, serrated, length of distal claw slightly less than half of that of ramus, length of proximal claw ca. 3/4 of that of distal claw. Distal seta long (as long as proximal claw), proximal seta also long (reaching beyond end of ramus).
Caudal ramus attachment (Fig. 14D) stout, with Triebel’s loop situated at middle of the distal part of the main branch, dorsal branch short, ventral branch well-developed.
Remarks. This species was synonymized with Strandesia wierzejskii by Victor & Fernando (1981) and Karanovic (2005). After the examination of specimens of S. striatoreticulata from Thailand, the morphology of which was mostly congruent with the original description by Klie (1932), we found that this species is different from S. wierzejskii in several aspects:
-
(1)
While the carapace in dorsal view is beak-like at both anterior and posterior extremities in S. striatoreticulata, it is merely pointed in S. wierzejskii. This significant character is clearly presented in the illustrations of the original description by Klie (1932).
-
(2)
The ornamentation is different between these two species: it is smooth in S. striatoreticulata, but vertically reticulated and sparsely set with tubercles in S. wierzejskii (see Victor & Fernando, 1981). Klie (1932) mentioned in the description that there was reticulated ornamentation in S. striatoreticulata. However, we found that this feature can be seen under light microscope (Fig. 15), but the SEM illustrations of S. striatoreticulata (Fig. 12A–C) clearly show that the valve surface is smooth. The apparent reticulation with transparent light must thus be owing to internal valve structures, covered by a smooth surface.
-
(3)
A comparison on soft part morphology between these two species is not possible, given the incomplete original description of S. wierzejskii.
Here, we reinstate S. striatoreticulata as a valid species and designate it as the type species of Pseudostrandesia n. gen.
Distribution. This species is thus far reported from Java, Sumatra (Klie, 1932) and Thailand (this study).
Strandesia Stuhlmann, 1888
Type species. Strandesia mercatorum (Vávra, 1895).
Diagnosis (modified from Savatenalinton & Martens, 2009). Carapace in lateral view diverse in shape: subovate, elliptical, moderately elongated with length less than twice the height, in frontal view, position of LV and RV equal or slightly unequal; LV with internal groove along valve margin in inner view, inner lamella without inner list (type C) or with 1 inner list (type D); d-seta on T1 present; hemipenis with large, wing-like lateral shield and with 1 postlabyrinthal spermiduct loop; Zenker organ with cone-shaped chitinous sheet at both proximal and distal ends.
Differential diagnosis. Strandesia is similar to Bradleycypris and Pseudostrandesia n. gen. It is distinguished from the former by the morphology of anterior part of the LV in internal view and the hemipenis morphology and from the latter by the presence of a d-seta on T1 and the presence of a short Rome organ.
Occurrence. Africa, South America, Australia, West Indies, Asia (see Savatenalinton & Martens, 2009).
For a redescription of the type species see Savatenalinton & Martens (2009). The latter paper also lists species belonging to Strandesia s.s. (the genus as characterized here) and to Strandesia s.l. (species originally described in Strandesia, possibly belonging to other genera, but presently impossible to reallocate because of insufficient descriptions).
Tribe Bradleystrandesiini new tribe
Diagnosis. Carapace in dorsal view subovate, elliptical; LV internal view with or without groove, anterior lamella with inner list; d-seta on T1 absent or present; Wouters organ absent or present; caudal ramus slender, claws weakly serrated; caudal ramus attachment with Triebel’s loop on dorsal branch, dorsal branch and ventral branch slim.
Differential diagnosis. This tribe is characterized by the presence of Triebel’s loop on dorsal branch of caudal ramus attachment.
Genera included. Bradleystrandesia, Bradleytriebella n. gen., Spirocypris.
Bradleystrandesia Broodbakker, 1983
Type species. Bradleystrandesia fuscata (Jurine, 1820).
Diagnosis. Carapace in lateral view subovate, elliptical; LV in internal view with or without groove along valve margin, inner lamella with 1 inner list (type D or F); Wouters organ absent; d-seta on T1 present; caudal ramus slender, claws weakly serrated; caudal ramus attachment slim, with Triebel’s loop on dorsal branch, ventral branch thin, not well-developed; lateral shield of hemipenis elongated, with blunt tip, hemipenis internally with 1 postlabyrinthal spermiduct loop; Zenker organ with distal end plate, forming crown of petal-like structure.
Differential diagnosis. Bradleystrandesia is most closely related to Spirocypris and can be distinguished from it by the absence of the tuberculated ornamentation of the external valve surface and by the morphology of the anterior part of the LV in internal view.
Included species. Group I: Bradleystrandesia fuscata (Jurine, 1820) (Figs. 16–21), B. gigantica (Furtos, 1933) (Figs. 22A–D, 23), B. splendida (Furtos, 1933); group II: B. deltoidea (Delorme, 1970) (Figs. 22E–H, 24), B. reticulata (Zaddach, 1844) (Figs. 25–26).
Bradleystrandesia fuscata (Jurine, 1820), female. A LV, internal view (O·C.3108). B RV, internal view (ditto). C LV, posterior part (ditto). D LV, anterior part (ditto). E RV, anterior part (ditto). F RV, posterior part (ditto). G LV, muscle scars (ditto). Scale bar 500 μm for A–B; 360 μm for C–F; 216 μm for G
Bradleystrandesia fuscata (Jurine, 1820). A–C, E–G female; D male. A A1 (O·C.3110). B A2 (O·C.3111). C A2, terminal segment (ditto). D A2, distal end of penultimate segment and terminal segment (O·C.3112). E Md-palp (O·C.3111). F Md-palp, terminal segment (ditto). G Md-coxa (ditto). Scale bar 100 μm for A–F; 206 μm for G
Bradleystrandesia fuscata (Jurine, 1820), female (A–C), male (D–G) and Bradleystrandesia splendida (Furtos, 1933), male (H). A Caudal ramus (O·C.3110). B Caudal ramus, claws and setae (ditto). C Caudal ramus attachment (ditto). D Zenker organ (O·C.3113). E Right prehensile palp (ditto). F Left prehensile palp (ditto). G Hemipenis (ditto). H Hemipenis (O·C.3114). Scale bar 200 μm for A, C; 100 μm for B, D, G–H; 50 μm for E–F
Bradleystrandesia gigantica (Furtos, 1933), female (A–D), Bradleystrandesia deltoidea (Delorme, 1970), female (E–H). A LV, internal view (O·C.3125). B RV, internal view (ditto). C LV, posterior part (ditto). D LV, anterior part (ditto). E LV, internal view (O·C.3126). F RV, internal view (ditto). G LV, anterior part (ditto). H LV, posterior part (ditto). Scale bar 652 μm for A–B; 566 μm for C–D; 525 μm for E–F; 400 μm for G–H
Bradleystrandesia reticulata (Zaddach, 1844), female. A Carapace, right lateral view (O·C.3117). B Carapace, dorsal view (O·C.3115). C–D Carapace, right lateral view, valve surface (O·C.3117). E LV, internal view (O·C.3116). F RV, internal view (ditto). G LV, anterior part (ditto). H LV, posterior part (ditto). Scale bar 400 μm for A–B, E–F; 200 μm for C, G–H; 100 μm for D
Occurrence. Europe, Turkey, the Middle East and North America (see Meisch, 2000).
Bradleystrandesia fuscata (Jurine, 1820) (Figs. 16–21)
Monoculus fuscata Jurine, 1820
Cypris fuscata (Jurine, 1820)—Desmarest, 1825, p. 385.
Cypricercus fuscata (Jurine, 1820)—Sars, 1925, p. 118.
Material examined. Snell Nook, United Kingdom (Jan 1988) and Carrelage, Soignies, Belgium (SX AT005), 15 April 2008.
Diagnosis. Carapace in lateral view subovate, anterior and posterior margin rounded, dorsal margin arched, highest point situated at ca. mid-length; Carapace in dorsal view elliptical, greatest width situated slightly behind mid-length, posterior end rounded, anterior end pointed; LV in inner view without groove along valve margins, inner lamella calcified, with 1 inner list; A1 with short Rome organ, Wouters organ absent, ya longer than short apical seta on terminal segment; A2 with short aesthetasc Y; 2 large bristles on third endite of Mx1 serrated; d-seta on T1 present; d1 and d2 on T2 slim, d1 longer than d2; caudal ramus slender, claws slim, insertion of proximal seta far from proximal claw; caudal ramus attachment slim, Triebel’s loop on dorsal branch, ventral branch thin; hemipenis with elongated lateral shield; Zenker organ with distal end plate forming a crow of petal-like structure.
Differential diagnosis. Bradleystrandesia fuscata is similar to B. gigantica and B. splendida. It can be distinguished from them by the more ovate carapace in lateral view and by the length of various setae. For example, the shortest natatory seta and the apical seta on the second segment of T2 in B. fuscata are shorter than those of B. gigantica; the proximal and distal setae of the caudal ramus in B. fuscata are shorter than those of B. splendida.
Measurements (in μm). LV (n = 2), L = 1520–1540, H = 911.45–919, RV (n = 2), L = 1510–1540, H = 903.36–919, Carapace (n = 2), L = 1450–1550, W = 809.
Redescription of female
Carapace in lateral view (Fig. 16A) subovate, anterior and posterior margin rounded, dorsal margin arched, greatest height situated ca. mid-length, ventral margin straight; surface of valves (Fig. 16C–D) with 2 kinds of setae (with and without rimmed pore), anteriorly and posteriorly with tiny tubercles.
Carapace in dorsal view (Fig. 16B) elliptical, greatest width situated slightly behind mid-length, posterior end rounded, anterior end pointed.
LV in inner view (Fig. 17A, C–D, G) without groove along valve margins, dorsal margin curved, ventral margin straight; inner lamella calcified, with 1 inner list.
RV in inner view (Fig. 17B, E–F) with large selvage.
A1 (Fig. 18A): first segment unusually large, with dorsal apical seta long (reaching distal end of next segment) and 2 unequal long ventral apical setae, proximal Wouters organ absent. Second segment slightly wider than long, with 1 short, dorsal subapical seta and a short Rome organ. Third segment bearing 2 (1 dorsally, 1 ventrally) apical setae, the former longer, the latter shorter (reaching distal end of next segment). Fourth segment dorsally with 2 long setae, ventrally with 2 short setae. Fifth segment dorsally with 2 long setae, ventrally 2 (1 long, 1 short) setae, short one reaching beyond the end of next segment. Penultimate segment with 4 (3 long, 1 short) setae, the latter slightly more than half of the terminal segment. Terminal segment with 3 (2 long, 1 short) apical setae and aesthetasc ya, the latter longer than short apical seta.
A2 (Fig. 18B–C) elongated: exopodite with 3 (1 long, 2 short) setae, long one reaching beyond the distal end of first endopodal segment. First endopodal segment with natatory setae long, length of shortest seta ca. 1/4 of that of penultimate segment; aesthetasc Y short, ventral apical seta long (reaching the tip of penultimate segment). Penultimate segment undivided, distally with 3 serrated claws and with long apical aesthetasc y2, reaching beyond the tip of terminal segment), z1–z3 setae long; this segment medially with 2 (1 long, 1 short) dorsal setae (length of short one ca. 3/4 of that of long one) and 4 ventral setae (t1–t4). Terminal segment (Fig. 18C) with 2 serrated claws (GM and Gm), a short g-seta and aesthetasc y3, the latter ca. 2/3 of length of accompanying seta.
Md-palp (Fig. 18E–F): first segment with 2 large setae (s1 and s2), 1 slender, long seta and a considerably long and smooth α-seta. Second segment dorsally with 3 unequal but long setae, ventrally with a group of 4 hirsute setae (3 long, 1 short) and a β–seta, the latter plumose, cone-shape, and with pointed tip. Penultimate segment consisting of 3 groups of setae: dorsally with a group of 4 unequal but long subapical setae; laterally with the apical γ-seta and 3 smooth apical setae, the former stout, hirsute and long (ca. 2.5× the length of the terminal segment); ventrally with 1 long, 1 short (ca. half of terminal segment) apical setae. Terminal segment (Fig. 18F) with 3 claws and 3 setae.
Mx1 (Fig. 19A) with 2-segmented palp, 3 endites and a large respiratory plate; basal segment of the palp with a group of 5 unequal long apical setae and 2 (1 long, 1 short) subapical setae; terminal segment elongated, with 3 claws and 3 setae. Two large bristles on third endite serrated. Sideways-directed bristles on first endite unequal, length of short one ca. half of that of long one.
T1 (Fig. 19B–C): protopodite with 2 short a-setae, 1 long b-seta and 1 long d-seta and with 14 (10 apical and 4 subapical) hirsute, long setae (Fig. 19C). Endopodite palp weakly built and set with 3 unequal apical setae.
T2 (Fig. 19D) with 2 slim d-setae, d2 ca. half the length of d1. Second segment with 1 long apical seta (slightly more than half the length of penultimate segment). Penultimate segment divided, proximal segment (a) bearing 1 long apical seta, distal segment (b) with a pair of apical setae (1 short, 1 spine-like). Terminal segment with 2 (1 dorsally, 1 ventrally) apical setae and a serrated claw.
T3 (Fig. 19E) a cleaning limb. First segment with 3 long setae. Second segment with 1 long apical seta. Third segment with medially 1 short seta (length ca. 1/3 of that of segment). Terminal segment with an apical pincer and 1 long reflexed subapical seta.
Caudal ramus (Fig. 20A–B) slender, slightly serrated along ventral margin, both distal and proximal claws slim, long, slightly serrated, length of distal claw ca. half of that of ramus, length of proximal claw ca. 3/4 of that of distal claw, insertion of proximal claw slightly distant from that of distal claw. Distal seta thin, with length less than half of that of distal claw; proximal seta thin, long (reaching beyond tip of ramus), insertion of proximal seta at a considerably distance from proximal claw.
Caudal ramus attachment (Fig. 20C) slim, with Triebel’s loop forming an eyelet on dorsal branch, ventral branch thin.
Redescription of male
Carapace and valves as in female. All limbs as in female, except for A2 (Fig. 18D), T1 and reproductive organs. Penultimate segment of A2 with claw-like z1 and z2 setae and with claws G1 and G3 reduced to setae. Terminal segment with reduced Gm claw, being considerably smaller and shorter than that in female. T1 with asymmetrical palp (endopodite); right prehensile palp (Fig. 20E) anteriorly with large subquadrate lobe and two unequal apical spines; left prehensile palp (Fig. 20F) anteriorly bearing slightly curved, elongated lobe, two tubercles (one large, one small) and two apical spines.
Hemipenis (Fig. 19G) large, medial shield large, lateral shield elongated, with 1 postlabyrinthal spermiduct loop. Zenker organ (Fig. 20D) set with ca. 24 spiny whorls, length ca. 2.6× the width, distal end a plate forming a crown of petal-like structures.
Bradleytriebella n. gen.
Type species. Bradleytriebella tuberculata (Hartmann, 1964) (here designated).
Etymology. The new species is named after Erich Triebel (Germany), who recognized the loop on the caudal ramus attachment for the first time. The name is a combination of “Bradley”, for P.C. Sylvester-Bradley (U.K.), after whom Bradleystrandesia was named, from which the new genus is separated, with “Triebel”.
Diagnosis. Carapace in lateral view subtriangular, in dorsal view subovate; LV in inner view with groove, inner lamella with 1 inner list (type D); Wouters organ present; d-seta on T1 absent; caudal ramus slender, claws weakly serrated; caudal ramus attachment slim, with Triebel’s loop on dorsal branch, ventral branch well-developed.
Differential diagnosis. Bradleytriebella n. gen. is closely related to Bradleystrandesia, yet there are several characters that distinguish these two genera: d-seta on T1 (absent in Bradleytriebella n. gen., present in Bradleystrandesia), Wouters organ (present in Bradleytriebella n. gen., absent in Bradleystrandesia) and caudal ramus attachment (more stout and better-developed ventral branch in Bradleytriebella n. gen.).
Species included. Bradleytriebella lineata (Victor & Fernando, 1981), B. trispinosa (Pinto & Purper, 1965), B. tuberculata (Hartmann, 1964), B. decorata (Sars, 1903).
Occurrence. Southeast Asia (Victor & Fernando, 1981), India (Hartmann, 1964), China (Sars, 1903), Brazil (Pinto & Purper, 1965), West Indies (Broodbakker, 1983).
Bradleytriebella tuberculata (Hartmann, 1964) (Figs. 27–29)
Strandesia tuberculata Hartmann, 1964
Bradleytriebella tuberculata (Hartmann, 1964), female. A Carapace, right lateral view (O·C.3121). B Carapace, dorsal view (ditto). C Carapace, ventral view (MSU-ZOC 030). D LV, internal view (O·C.3120). E RV, internal view (ditto). Scale bar 200 μm for A–E
Bradleytriebella tuberculata (Hartmann, 1964), female. A A1 (MSU-ZOC 029). B A2 (ditto). C Md-palp (ditto). D Md-palp, terminal segment (ditto). E Md-coxa (ditto). Scale bar 100 μm for A–B, E; 50 μm for C–D
Bradleytriebella tuberculata (Hartmann, 1964), female. A Mx1 (MSU-ZOC 029). B T1, protoprodite (O·C.3120). C T1-palp (ditto). D T2 (MSU-ZOC 029). E T3 (MSU-ZOC 048). F T3, terminal end (ditto). G Caudal ramus attachment (MSU-ZOC 029). H Caudal ramus (ditto). Scale bar 50 μm for A–C, F; 100 μm for D–E; 87.2 μm for G–H
Material examined. Several females from 3 samples: (1) rice field, Muang district, Phitsanulok Province, 27 September 2005 (TH045), coordinates: 16° 45′ 30.5′′ N and 100° 14′ 3.2′′ E; (2) rice field, Wiangsa district, Nan Province, 26 September 2005 (TH038), coordinates: 18° 36′ 51.8′′ N and 100° 44′ 29.5′′ E; (3) rice field, Kosumpee Nakhon district, Kamphaeng Phet Province, 22 September 2005 (TH016), coordinates: 16° 31′ 52.8′′ N and 99° 28′ 25.3′′ E.
Diagnosis. Carapace in lateral view subtriangular, with submarginal tubercle at ca. mid-height along rounded anterior margin, posterior margin bluntly pointed; surface of valves slightly reticulated and set with dispersedly setae; carapace in dorsal view ovate, anterior end with bluntly angulated point on both valves (situated at ca. 1/8 of length); LV in inner view with groove along valve margin; A1 with long proximal Wouters organ, long Rome organ, aesthetasc ya longer than short apical seta; 2 large bristles on third endite of Mx1 serrated; T1 without d-seta; caudal ramus slender, claws slim, proximal claw as long as distal seta; caudal ramus attachment slim, Triebel’s loop an eyelet on dorsal branch, ventral branch long, well-developed.
Differential diagnosis. A tubercle at ca. mid-height on the anterior part of the external LV and RV provides an easy diagnosis of Bradleytriebella tuberculata. It cannot be confused with any of its congener.
Measurements (in μm). LV (n = 2), L = 760–769, H = 470–475, RV (n = 2), L = 733–744, H = 462–465, Carapace (n = 3), L = 759–762, W = 501–503.
Redescription of female
Carapace in lateral view (Fig. 27A) subtriangular, with submarginal tubercle at ca. mid-height on anterior part, anterior margin rounded, posterior margin pointed, dorsal margin arched, greatest height situated at ca. mid-length, ventral margin straight; surface of valve slightly reticulated and set with dispersed setae.
Carapace in dorsal view (Fig. 27B) ovate, anterior end with bluntly angulated tubercles on both valves (situated ca. 1/8 of length), posterior end broadly rounded; LV overlapping RV anteriorly.
Carapace in ventral view (Fig. 27C) ovate, ventral margin slightly protruded at mid-length.
LV in inner view (Fig. 27D) subtriangular, with groove along valve margin, dorsal margin arched, ventral margin straight, anterior margin rounded, posterior margin slightly pointed; anterior inner lamella calcified, with 1 inner list (type D).
RV in inner view (Fig. 27E) without selvage; inner lamella without inner list, anteriorly wider than posteriorly, ventral margin slightly sinuous in front of mid-length.
A1 (Fig. 28A): first segment with dorsal subapical seta long (reaching tip of segment) and 2 unequal long ventral apical setae, proximal Wouters organ long. Second segment slightly wider than long, with 1 short dorsal apical seta and long Rome organ. Third segment bearing 2 (1 dorsally, 1 ventrally) apical setae, the former longer, the latter shorter (reaching half of fifth segment). Fourth segment dorsally with 2 long setae, ventrally with 2 short setae (the shortest one reaching tip of next segment). Fifth segment dorsally with 2 long setae, ventrally with 2 (1 long, 1 short) setae, short one reaching half the length of terminal segment. Penultimate segment with 5 (4 long, 1 short) setae, the latter reaching the tip of the terminal segment. Terminal segment with 3 (2 long, 1 short) apical setae and aesthetasc ya, length of the short apical seta ca. 3/4 of that of aesthetasc ya).
A2 (Fig. 28B): exopodite with 3 (1 long, 2 short) setae, long one reaching tip of first endopodal segment. First endopodal segment with natatory setae long, shortest one reaching ca. 3/4 of penultimate segment, aesthetasc Y long, ventral apical seta long (reaching beyond tip of penultimate segment). Penultimate segment undivided, distally with 3 serrated claws and with a long apical aesthetasc y2 (reaching slightly beyond tip of terminal segment), z1–z3 setae long; this segment medially with 2 (1 long, 1 short) dorsal setae (length of short one ca. 2/3 of that of long one) and 4 ventral setae (t1–t4). Terminal segment with 2 serrated claws (GM and Gm), a short g-seta and aesthetasc y3, length of the latter ca. 2/3 of that of accompanying seta.
Md-palp (Fig. 28C–D): first segment with 2 large setae (s1 and s2), 1 slender, long seta and a long and smooth α-seta. Second segment dorsally with 3 unequal, but long setae, length of shortest ca. 1/3 of that of longest; ventrally with a group of hirsute setae (3 long, 1 short) and the β-seta, the latter plumose, cone-shape and with a pointed tip. Penultimate segment bearing 3 groups of setae: dorsally with a group of 4 unequal long subapical setae; laterally with the apical γ-seta and 3 smooth apical setae, the former stout, hirsute, and long (ca. 2× of length of terminal segment); ventrally with 1 long, 1 short (ca. half of terminal segment) apical setae. Terminal segment (Fig. 28D) bearing 3 claws and 3 setae.
Mx1 (Fig. 29A) with a 2-segmented palp, 3 endites and a large respiratory plate; basal segment of palp with a group of 5 unequal long apical setae and 2 (1 long, 1 short) subapical setae; terminal segment elongated, with 3 claws and 3 setae. Two large bristles on third endite serrated. Sideways-directed bristles on first endite unequal, short one ca. 2/3 of length of long one.
T1 (Fig. 29B–C): protopodite with 2 short a-setae, long b-seta, without d-seta, distally with 14 (10 apical, 4 subapical) hirsute, long setae. Endopodite palp (Fig. 29C) weakly built and set with 3 unequal apical setae, shortest ca. 1/3 of longest.
T2 (Fig. 29D) with slender d-setae, d2 ca. half the length of d1. Second segment with 1 long apical seta (reaching slightly beyond half the length of penultimate segment). Penultimate segment divided, proximal segment (a) bearing 1 long apical seta, distalsegment (b) with a pair of apical setae (1 short, 1 spine-like). Terminal segment with 2 (1 dorsal, 1 ventral) apical setae and a serrated claw.
T3 (Fig. 29E–F) a cleaning limb. First segment with 3 long setae. Second segment with 1 long apical seta (reaching slightly beyond half the length of next segment). Third segment with 1 short medial seta (reaching slightly beyond the tip of the segment). Terminal segment with an apical pincer and 1 short reflexed subapical seta (slightly longer than half of penultimate segment).
Caudal ramus (Fig. 29H) slender, weakly serrated along ventral margin, both distal and proximal claws slim, long, slightly serrated; length of distal claw ca. half of that of the ramus, length of proximal claw ca. half of that of distal claw. Distal seta thin, as long as proximal claw; proximal seta thin, long (reaching slightly beyond the tip of the ramus).
Caudal ramus attachment (Fig. 29G) slender, with Triebel’s loop an eyelet on the dorsal branch, ventral branch long, well-developed.
Remarks. Bradleytriebella tuberculata was described from India by Hartmann (1964). Thus far, this species has been recorded from India (Hartmann, 1964) and Thailand (this study). The present records, all from rice fields, are the first for Southeast Asia.
Spirocypris Sharpe, 1903
Type species. Spirocypris passaica Sharpe, 1903
Diagnosis. Carapace in lateral view subovate, in dorsal view tumid; valves with clear external tuberculated ornamentation; LV in internal view with a groove along valve margins, inner lamella with 1 inner list (type D); d-seta on T1 present; caudal ramus slender; caudal ramus attachment slim, with Triebel’s loop on dorsal branch, dorsal branch and ventral branch thin, not well-developed.
Differential diagnosis. Spirocypris can be distinguished from Bradleystrandesia by the morphology of the anterior inner part of the LV, the tumid carapace and characteristic tuberculated valve surface.
Species included. Spirocypris horrida (Sars, 1926), S. passaica Sharpe, 1903, S. tuberculata Sharpe, 1908.
Occurrence. America (Sharpe, 1903), Canada (Sars, 1926).
Remarks. Since no material of the (fossil) type species was available to us, the more common S. horrida (Sars, 1926) is here redescribed.
Spirocypris horrida (Sars, 1926) (Figs. 30–32)
Cypricercus horrida Sars, 1926
Spirocypris horrida (Sars, 1926), female. A LV, external view (SS573). B RV, external view (ditto). C LV, internal view (ditto). D RV, internal view (ditto). Scale bar 200 μm for A–D
Spirocypris horrida (Sars, 1926), female. A A1 (O·C.3118). B A2 (ditto). C A2, terminal segment (ditto). D Md-palp (ditto). E Md-palp, terminal segment (ditto). F Md-palp, showing α, β, γ setae (ditto). G Md-coxa (ditto). Scale bar 100 μm for A–C, F–G; 50 μm for D–E
Spirocypris horrida (Sars, 1926), female. A Mx1 (O·C.3118). B T1, distal end of protopodite (ditto). C T1 (ditto). D T2 (ditto). E T3 (ditto). F Caudal ramus attachment (ditto). G Caudal ramus (ditto). Scale bar 50 μm for A–B; 100 μm for C–G
Material examined. Several females in a sample (T34) collected from Churchill, Manitoba, Canada, between 1 July and 30 August in both 1987 and 1988, coordinates 59º N and 94º W. More details on this locality can be found in Havel et al. (1990).
Diagnosis. Carapace in lateral view subovate, anterior and posterior margins rounded, LV overlapping RV; surface of valves tuberculated, with a seta on each tubercle; carapace in dorsal view tumid; LV in inner view with groove; A1 with short Rome organ, aesthetasc ya longer than short apical seta, no Wouters organ; A2 with short aesthetasc Y; 2 large setae on third endite of Mx1 serrated; d-seta on T1 present; caudal ramus slim, claws slim, weakly serrated, insertion of proximal seta at a distance from proximal claw; caudal ramus attachment slim, Triebel’s loop an eyelet on dorsal branch, dorsal and ventral branches thin.
Differential diagnosis. Spirocypris horrida resembles S. tuberculata Sharpe, 1908. It can be distinguished mainly by the morphology of the caudal ramus and the overlap of the valves: LV overlaps RV (anteriorly wider than posteriorly) in S. horrida, RV slightly overlaps LV anteriorly in S. tuberculata.
Measurements (in μm). LV (n = 2), L = 970–985, H = 549–555; RV (n = 2), L = 950–966, H = 534–549.
Redescription of female
Carapace in lateral view (Fig. 30A–B) subovate (length ca. 1.82× height), anterior and posterior margins rounded, dorsal margin slightly arched, ventral margin almost straight; LV overlapping RV anteriorly, ventrally and posteriorly; surface of valves tuberculated, with a seta on each tubercle. Carapace in dorsal view tumid.
LV in inner view (Fig. 30C) with groove along valve margins, anterior margin rounded, posterior margin more narrowly rounded; inner lamella calcified, anteriorly wider than posteriorly, and with 1 inner list (type D); dorsal margin arched, ventral margin slightly sinuated at mid-length.
RV in inner view (Fig. 30D) with marginal selvage, without groove; inner lamella without inner list; dorsal and ventral margins as in LV.
A1 (Fig. 31A): first segment with 1 dorsal subapical seta and 2 long ventral apical setae, no Wouters organ present. Second segment wider than long, with 1 short dorsal apical seta and a short, small ventral subapical Rome organ. Third segment bearing 2 (1 dorsally, 1 ventrally) long apical setae, extending beyond tip of the next segment. Fourth segment dorsally with 2 long setae, ventrally with 2 short setae, the latter reaching to mid-length of fifth segment. Fifth segment dorsally with a group of 3 long setae and ventrally with 1 shorter seta, the latter extending slightly beyond middle of terminal segment. Penultimate segment dorsally with 3 (2 long, 1 short) setae, length of short seta ca. half of that of terminal segment, ventrally with 2 long setae. Terminal segment with 3 (2 long, 1 short) apical setae and the aesthetasc ya, the latter slightly longer than short apical seta.
A2 (Fig. 31B–C): exopodite with 3 (1 long, 2 short) setae, longer seta extending beyond tip of first endopodal segment. First endopodal segment with long natatory setae, length of shortest less than half of that of penultimate segment, aesthetasc Y short, dorsal apical seta long. Penultimate segment undivided, distally with 3 serrated claws and with apical aesthetasc y2 reaching beyond tip of terminal segment, z1–z3 long, this segment medially with 2 unequal dorsal setae and 4 (t1–t4) ventral setae, shortest one reaching tip of segment. Terminal segment (Fig. 31C) with 2 serrated claws (GM and Gm), a g-seta and aesthetasc y3, length of the latter ca. 2/3 of that of accompanying seta.
Md (Fig. 31D, F): first segment of palp with 2 large subapical setae (s1 and s2), 1 long seta and a long, slender and smooth α seta. Second segment dorsally with a group of 3 unequally long apical setae, ventrally with 3 hirsute, long setae, 1 shorter seta and the β seta, the latter stout, plumose, cone-shaped, and with needle-like tip. Penultimate segment with 3 groups of setae: dorsally with a subapical group of 4 unequal long setae; laterally with apical γ seta and 3 smooth subapical setae, the former long (ca. 2× length of terminal segment) and hirsute; ventrally with 2 (1 long, 1 short) setae, short seta just not reaching tip of terminal segment. Terminal segment (Fig. 31E) with 3 claws and 3 shorter setae.
Mx1 (Fig. 32A) with a 2-segmented palp, three endites and a larger respiratory plate. Basal segment of palp with an apical group of 5 long setae and 2 (1 long, 1 short) subapical setae, shorter seta extending beyond tip of terminal segment; terminal segment elongated, with 3 claws and 3 setae. Two large bristles on third endite serrated. Sideways-directed bristles on first endite unequal, length of short one ca. 2/3 of that of long one.
T1 (Fig. 32B–C): protopodite with 2 short a-setae, a long b- and d-setae, apically with a group of 14 hirsute apical setae (10 apical, 4 subapical). Endopodite a weakly built palp with 3 unequal apical setae, longest situated in the middle, shortest with a length ca. 1/4 of that of longest.
T2 (Fig. 32D) with d-setae slender, d2-seta ca. half the length of d1-seta. Second segment with 1 long apical seta (length ca. 2/3 of that of penultimate segment). Penultimate segment divided, proximal segment (a) with 1 long apical seta, proximal segment (b) with a pair (1 long, 1 spine-like) of apical setae. Terminal segment with apically serrated claws and 2 subequal setae (length ca. 1/3 of that of claw).
T3 (Fig. 32E) a cleaning limb. First segment with 3 long setae. Second segment with 1 long apical seta (length more than half of that of penultimate segment). Penultimate segment with medially 1 long lateral seta, extending beyond tip of segment. Terminal segment with apical pincer and 1 reflexed subapical seta.
Caudal ramus (Fig. 32F) slender, with slim, slightly serrated distal and proximal claws, distal claw ca. half length of ramus. Distal seta slim, short (ca. 1/3 of distal claw), proximal seta slim, reaching end of ramus.
Caudal ramus attachment (Fig. 32G) slender, with Triebel’s loop on dorsal branch, vb thin, long, not well-developed.
Phylogenetic analysis
Phylogenies of the genera of the Cypricercinae are analysed using morphological characters of valves and soft parts in order to evaluate the relationships within this subfamily. Twelve species, each representing one genus except for two species of the genus Bradleystrandesia, were used. The topology of the Neighbour Joining (NJ) tree (Fig. 33) comprises three major clusters. In Group 1, Diaphanocypris and Nealecypris n. gen. cluster with Astenocypris, and this in its turn clusters with Tanycypris. This group constitutes the Nealecypridini n. trib., with a bootstrap value of almost 80. In Group 2, Strandesia and Cypricercus cluster with Bradleycypris, and this clade subsequently joins with Pseudostrandesia n. gen. Together, they form the nominal tribe Cypricercini, but with a relatively weak bootstrap support. In Group 3, Bradleystrandesia group II (B. reticulata) and Spirocypris cluster with Bradleystrandesia group I (B. fuscata), and this in turn clusters with Bradleytriebella n. gen. These form the Bradleystrandesiini n. trib., with a bootstrap support of more than 90. The NJ analysis thus supports the division of the Cypricercinae into three tribes.
Distance tree (rooted phylogram) of the Cypricercinae, computed with the Neighbour Joining algorithms. Abbreviations: Aspap, Astenocypris papyracea (Sars, 1903); Bcob, Bradleycypris obliqua (Brady, 1868); Bsfus, Bradleystrandesia fuscata (Jurine, 1820); Bsret, Bradleystrandesia reticulata (Zaddach, 1844); Btub, Bradleytriebella tuberculata (Hartmann, 1964); Ccun, Cypricercus cuneatus Sars, 1895; Dimer, Diaphanocypris meridana (Furtos, 1936); Evir, Eucypris virens (Jurine, 1820); Neob, Nealecypris obtusa (Klie, 1933); Pstri, Pseudostrandesia striatoreticulata (Klie, 1932); Shor, Spirocypris horrida (Sars, 1926); Smer, Strandesia mercatorum (Vávra, 1895); Tasia, Tanycypris siamensis n. sp
However, with Maximum Parsimony (MP, Fig. 34), the strict consensus tree differs in a number of aspects. Two major clusters are shown in this tree. Astenocypris, Diaphanocypris and Nealecypris n. gen. form Group 1; while Tanycypris s.s. now is in Group 2. Group 2 comprises two subgroups. Bradleystrandesia, Bradleytriebella n. gen. and Spirocypris are in the first subgroup in Group 2, with Pseudostrandesia n. gen. as the closest clade.
A strict consensus tree (cladogram) of parsimonious tree of the Cypricercinae, computed with Full heuristic option. Abbreviations of taxa, see caption of Fig. 33
Discussion
Generic characters of Cypricercinae
The most significant morphological feature uniting the subfamily Cypricercinae is the presence of the Triebel’s loop in the caudal ramus attachment, which was thus far never found in other ostracod groups. This unique character was recognized by Triebel (1953) and mentioned as diagnostic character by Rome (1969), McKenzie (1982) and Broodbakker (1983). Here, we use several additional morphological characters in this subfamily, in order to clarify the generic taxonomy.
-
(1)
Morphology of the LV in internal view:
The presence and position of the groove and the inner list in the anterior part of the LV can be divided into six different patterns in the Cypricercinae. The groove along the anterior margin of the LV is present in all genera, except in Astenocypris, Diaphanocypris, Nealecypris n. gen. and Bradleystrandesia species group I. The calcified inner lamella can have 0–2 inner lists. While the absence of inner lists (type C) and the presence of two inner lists (type E) are found only in Strandesia and Cypricercus, respectively, the occurrence of one inner list is common in the subfamily Cypricercinae, and is encountered in several genera.
-
(2)
The presence/absence and length ratio of setae d1 and d2 on T2 is mostly characteristic either at subfamily or at genus level in several Cyprididae. For example, in the Cyprinotinae, only a d1-seta is present (Savatenalinton & Martens, 2008). In the Eucypridinae, the length ratio of these setae is thus far considered to be a good generic character (Martens et al., 2002). Also, in the Cypricercinae, the ratio of the d-setae on the T2 appears to show a phylogenetic signal.
-
(3)
In Cypricercinae, the position of the Triebel’s loop in the caudal ramus attachment was reported as a supra-generic character by McKenzie (1982) and Martens (1994). Here, we classify the morphology of the caudal ramus attachment into five groups, based not only on the position of the Triebel’s loop, but also on the presence and the morphology of dorsal and ventral branches. Most genera in the Cypricercinae have the Triebel’s loop in the middle of the distal part of the main branch, and have a well-developed ventral branch (type C).
-
(4)
The presence of the c-seta on the T1 has been used as a diagnostic character for the subfamily Eucypridinae, as it is absent in all other subfamilies within the Cyprididae (Martens et al., 2002). In the Cypricercinae, the presence or absence of the b- and d-setae on this limb are significant. Apart from Astenocypris and Diaphanocypris, all cypricercinid genera have the b-seta, while the d-seta is absent in four genera (Astenocypris, Diaphanocypris, Pseudostrandesia n. gen. and Bradleytriebella n. gen.). Similar characters are also used in the taxonomy of the Candoninae. For example, the presence/absence of the proximal seta on caudal ramus and of setae d1, d2, dp and f on T3 are used to identify genera in this subfamily (Meisch, 2000).
-
(5)
Also the morphology of the hemipenis and of the Zenker organ can in some cases be regarded as generic characters, although the incidence of males can be very low in some genera. Savatenalinton & Martens (2009) described the different aspects of the hemipenes and the Zenker organs between Strandesia and Cypricercus. Here, we additionally report the morphology of these male reproductive organs for Nealecypris obtusa, Bradleycypris vittata and Bradleystrandesia (B. fuscata, B. splendida). Five groups of hemipenes can be recognized within the Cypricercinae. Type A of the hemipenis, which is found in Nealecypris obtusa, has a small, subovate lateral shield. Strandesia has large, wing-like lateral shields and 1 loop of the postlabyrinthal spermiduct (type B), while small, subquadrate lateral shields and 2 postlabyrinthal spermiduct loops are found in Cypricercus (type C). The hemipenis of Bradleycypris is characterized by a triangular lateral shield and a single, large postlabyrinthal spermiduct loop (type D). Type E hemipenes occur in Bradleystrandesia. This hemipenis type is distinguished by an elongated lateral shield.
The morphology of the Zenker organ in Cypricercinae can be divided into two groups: the Strandesia type and the Cypricercus type. The former has a cone-shaped chitinous sheet at both ends, whereas the latter has a distal end plate forming a crown of petal-like structures (for illustrations, see Savatenalinton & Martens, 2009). The Strandesia type Zenker organ is found in Strandesia and Bradleycypris, while the Cypricercus type Zenker organ can be recognized from Cypricercus, Nealecypris n. gen., Bradleystrandesia and Spirocypris. Of course, investigations in males of other Cypricercinae species will test if these types are indeed genus-specific as is postulated here.
-
(6)
The size of the Rome organ can be typical at the subfamily level. For example, it is large in all Herpetocypridinae (Martens, 2001a). However, this aspect is different in Cypricercinae. Most genera in Cypricercinae have a small Rome organ, except for Tanycypris, Pseudostrandesia n. gen. and Bradleytriebella n. gen.
-
(7)
The Wouters organ is the third sensory organ of the cypridoidean A1. This organ can also be diagnostic at the subfamily level (e.g. present in the Cyclocypridinae, absent in the Herpetocypridinae, Ilyocypridinae, Notodromadidae) or at the generic level (e.g. in Cyprinotinae: present in Hemicypris and Homocypris, absent in Cyprinotus and Heterocypris) (Smith & Matzke-Karasz, 2008). In Cypricercinae, we found this organ in all genera except for Tanycypris, Spirocypris, and Bradleystrandesia.
We are aware of the fact that some of the characters described above may appear to be of too little significance at the generic level. However, these are thus far the only morphological features known that are constant within groups of species, and thus the only characters on which genera can be based. The alternative would be to reintroduce nearly all species back into Strandesia, a solution which is favoured by some authors (e.g. Karanovic, 2005). However, such a conservative solution would mean the loss of a great deal of phylogenetic signal within the Cypricercinae, even when based on a limited set of characters. Future research on other species of Cypricercinae, new or at present insufficiently described, will test the validity of this phylogenetic signal, and hence of the taxonomy proposed here.
Tribes within the Cypricercinae
Based on the characters above, we propose to divide this subfamily into three tribes. The Nealecypridini n. trib. has a narrow carapace in dorsal view, stout caudal rami with strongly serrated claws, a Triebel’s loop situated at the middle of the distal part of the main caudal ramus attachment and a weakly developed ventral branch (short or absent). The Cypricercini, the nominal tribe, have a subovate carapace in dorsal view, stout caudal rami with strongly to moderately serrated claws, a Triebel’s loop at the middle of the distal part of the main caudal ramus attachment and a well-developed ventral branch. The Bradleystrandesiini n. trib., finally, are characterized by a Triebel’s loop, which is situated on the dorsal branch of the caudal ramus attachment and a slender caudal ramus with weakly serrated claws. In each of these tribes, the features used to distinguish between genera are the morphology of the anterior inner part of the LV, including the presence or absence of the groove along the inner margin, the b- and d-setae on the T1, the length ratio of setae d1 and d2 on T2, the Wouters organ and the caudal ramus attachment.
Taxonomic position of selected species
At present, two genera in the Cypricercinae remain monospecific (Astenocypris and Diaphanocypris). Strandesia vittata (Sars, 1903) is here allocated to Bradleycypris, because of the similarities in valve morphology (carapace shape, anterior part of internal LV) and in soft parts (the presence of b- and d-setae on T1, features of the caudal ramus attachment and of the hemipenis). In this way, Bradleycypris is no longer a monospecific genus.
Tanycypris siamensis n. sp. is here placed in Tanycypris s.s. which has T. madagascarensis (Müller, 1898) as type species. In the present study, we also examined specimens of Tanycypris obtusa collected from South Africa. The morphology of both valves and soft parts of this species are not congruent to characters typical of Tanycypris s.s. Significant differences between these two taxa are in the occurrence of the anterior inner groove on the LV (absent in T. obtusa, present in Tanycypris), the d-seta on T1 (absent in T. obtusa, present in Tanycypris), the Wouters organ (present in T. obtusa, absent in Tanycypris), and the morphology of the caudal ramus attachment (type B in T. obtusa, type A in Tanycypris) as well as in other features of the anterior internal part of the LV (type A in T. obtusa, type B in Tanycypris). Therefore, we create a new genus for T. obtusa: Nealecypris n. gen.
In Bradleystrandesia, some species have a groove along the inner valve margin of the LV, while others do not show this feature. While the latter is the case for B. fuscata (Jurine, 1820) and B. gigantica (Furtos, 1933), the former is found in B. deltoidea (Delorme, 1970) and B. reticulata (Zaddach, 1844). This character is the only main difference between these species, as the morphology of the soft parts is typical of the entire genus. Therefore, we maintain these species groups as informal groups within the genus: group I (without groove on internal LV) and group II (with groove on internal LV).
Based on the present revision of the generic characters, several species are shifted from Strandesia s.l. (see Savatenalinton & Martens, 2009) to other genera. Six species are here allocated to Pseudostrandesia n. gen. (S. asymmetros Rome, 1962, S. chondropherusa Rome, 1965, S. mamarilorum Victor & Fernando, 1981, S. striatoreticulata Klie, 1932, S. vinciguerrae (Masi, 1905), S. calapanensis Tressler, 1937). Two species can be confirmed within Strandesia s.s (S. kraepelini (Müller, 1906), S. sexpunctata Klie, 1932). Four species of Bradleystrandesia [B. lineata (Victor & Fernando, 1981), B. trispinosa (Pinto & Purper, 1965), B. tuberculata (Hartmann, 1964), B. decorata (Sars, 1903)] are transferred to Bradleytriebella n. gen. Other species presently in Strandesia s.l. and in Bradleystrandesia s.l. are insufficiently described to determine their correct taxonomic position.
Phylogeny of the subfamily Cypricercinae
The three tribes proposed here are at least partly supported by our phylogenetic analyses. In the NJ tree, the three tribes are clearly separated, but in the MP tree, Bradleystrandesiini n. trib. forms a subcluster of the Cypricercini. The difference between NJ and MP trees lies also in the position of Tanycypris, which belongs to the Nealecypridini n. trib. in the NJ tree, but to the Cypricercini in the MP tree. This uncertain position of Tanycypris is owing to the fact that it shares characters with both these two tribes. Tanycypris is similar to other members of the Nealecypridini n. trib. by the narrow carapace in dorsal view and the caudal ramus attachment type A. At the same time, it is similar to Cypricercini by the presence of a groove along the inner margin of the LV and by the morphology of the d-setae on T2. At present, we place this genus in Nealecypridini n. trib., because of the external morphology of the carapace which is used for identification and because the morphology of the caudal ramus attachment is a significant character in the whole subfamily. At least the topology and bootstrap values in the NJ tree support that the three tribes of Cypricercinae proposed here are possible phyletic units.
References
Broodbakker, N. W., 1983. The genus Strandesia and other Cypricercini (Crustacea, Ostracoda) in the West Indies. Part I. Taxonomy. Bijdragen tot de Dierkunde 53(2): 327–368.
Broodbakker, N. W., 1984. The genus Tanycypris (Crustacea, Ostracoda) in the West Indies. Bijdragen tot de Dierkunde 54(1): 15–24.
Broodbakker, N. W. & D. L. Danielopol, 1982. The chaetotaxy of Cypridacea (Crustacea, Ostracoda) limbs: proposals for a descriptive model. Bijdragen tot de Dierkunde 52(2): 103–120.
De Deckker, P., 1979. Evaluation of features distinctive in the taxonomy of the Cypridacea, above the generic level. In Krstic, N. (ed.), Proceedings of the VII International Symposium on Ostracods: Taxonomy, Biostratigraphy and Distribution of Ostracods. Serbian Geological Society, Beograd (Serbia): 9–17.
Furtos, N. C., 1936. On the Ostracoda from the Cenotes of Yucatan and vicinity. Publications of the Carnegie Institution of Washington 457: 89–115.
George, S. & K. Martens, 1993. Redescription of Astenocyris papyracea (Sars, 1903) (Crustacea, Ostracoda) in Kerala, India. Zoological Journal of the Linnean Society 109: 27–34.
Gonzalez Mozo, M. E., K. Martens & A. Baltanas, 1996. A taxonomic revision of European Herpetocypris Brady & Norman, 1889 (Crustacea, Ostracoda). Bulletin van het Koninklijk Belgisch Instituut voor Natuurwetenschappen, Biologie 66: 93–132.
Hartmann, G., 1964. Asiatische Ostracoden. Systematische und zoogeographische Untersuchungen. Internationale Revue der gesamten Hydrobiologie, Systematische Beihefte 3: 1–155.
Hartmann, G. & S. Puri, 1974. Summary of Neontological and Paleontological classification of Ostracoda. Mitteilungen aus dem Hamburgischen Zoologischen Museum und Institut 70: 7–73.
Havel, J. E., P. D. N. Hebert & J. D. Delorme, 1990. Genetics of sexual Ostracoda from a low Arctic site. Journal of Evolutionary Biology 3: 65–84.
Hutchinson, G. E., G. E. Pickford & J. F. M. Schuurman, 1932. A contribution to the hydrobiology of pans and other inland waters of South Africa: an ecological study of natural and artificial waters in the southern Transvaal and Southwestern Cape Province, of material collected by miss E.L. Stephens from coastal pans in Portuguese East Africa, and of certain miscellaneous localities elsewhere. Archiv für Hydrobiologie 24: 1–54.
Karanovic, I., 2005. On the genus Strandesia Stuhlmann, 1888 (Crustacea, Ostracoda, Cyprididae) with description of Strandesia kimberleyi n. sp. and a key to the extant species of the genus. Contributions to Zoology 74(1/2): 77–95.
Klie, W., 1932. Die Ostracoden der Deutschen Limnologischen Sundra-Expedition. Archiv für Hydrobiologie, supplementband 11: 447–502.
Klie, W., 1933. Drei neue Süßwasser-Ostracoden aus Südafrika. Zoologischer Anzeiger 102(3/4): 65–74.
Martens, K., 1986. Taxonomic revision of the subfamily Megalocypridinae Rome, 1965 (Crustacea, Ostracoda). Academie voor Wetenschappen, Letteren en Schone Kunsten van Belgie, Brussel: 146 pp.
Martens, K., 1987. Homology and functional morphology of the sexual dimorphism in the Antenna of Sclerocypris Sars, 1924 (Crustacea, Ostracoda, Megalocypridinae). Bijdragen tot de Dierkunde 57(2): 183–190.
Martens, K., 1990. Taxonomic revision of African Cypridini. Part I. The genera Cypris O.F. Müller, Pseudocypris Daday and Globocypris Klie (Crustacea, Ostracoda). Bulletin van het Koninklijk Belgisch Instituut voor Natuurwetenschappen, Biologie 60: 127–172.
Martens, K., 1992. Taxonomic revision of African Cypridini. Part II. Description of Ramotha gen. nov. (Crustacea, Ostracoda). Annals of the South African Museum 102(2): 91–130.
Martens, K., 1994. Towards a revision of the Cypricercinae (Crustacea, Ostracoda): on the validity of the genera Neocypris Sars, 1901 and Bradleycypris McKenzie, 1982. Bulletin van het Koninklijk Belgisch Instituut voor Natuurwetenschappen, Biologie 64: 231–233.
Martens, K., 2001a. Taxonomy of the Herpetocypridinae (Ostracoda, Cyprididae). Crustaceana 74(3): 295–308.
Martens, K., 2001b. Ostracoda. In Day, J. A., I. J. Moor, B. A. Stewaet & A. E. Louw (eds), Guides to the freshwater invertebrates of southern Africa. Volume 3: crustacean II. Ostracoda, Copepoda and Branchiura. Water Research Commission, The Republic of South Africa: 9–77.
Martens, K., 2007. On a new species and genus in the Cypridini (Crustacea, Ostracoda, Cyprididae) from South Africa, with a phylogenetic analysis of the tribe and a discussion on the genus concept in this group. Journal of Natural History 41(5–8): 381–399.
Martens, K., S. Schwartz, C. Meisch & L. Blaustein, 2002. The non-marine Ostracoda (Crustacea) of Mount Carmel (Israel), with taxonomic notes on Eucypridinae and circum Mediterranean Heterocypris. Israel Journal of Zoology 48: 53–70.
Martens, K., I. Schön, C. Meisch & D. J. Horne, 2008. Global diversity of ostracods (Ostracoda, Crustacea) in freshwater. Hydrobiologia 595: 185–193.
McKenzie, K. G., 1971. Species list of South African freshwater Ostracoda with an appendix listing museum collections and some further determinations. Annals of the South African Museum 57(9): 157–213.
McKenzie, K. G., 1982. Homoeomorphy: persistent joker in taxonomic pack, with the description of Bradleycypris gen. nov. In Bate, R. H., E. Robinson & L. M. Sheppard (eds), Fossil and Recent Ostracods. Ellis Horwood Ltd., Chichester: 407–438.
Meisch, C., 2000. Freshwater Ostracoda of Western and Central Europe. Spektrum Akademischer Verlag GmbH, Heidelberg, Berlin: 522 pp.
Müller, G. W., 1898. Ergebnisse einer zoologischen forschungsreise in Madagaskar und ost-afrika 1889–1895 von Dr. A. Voeltzkow: Die Ostracoden. Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft 21(2): 255–296.
Müller, G. W., 1912. Crustacea: Ostracoda. Das Tierreich 31: 1–434.
Petkovski, T. K., 1964. Bemerkenswerte Entomostraken aus Jugoslavien. Acta Musei Macedonici Scientiarum Naturalium 9(7): 147–181.
Rome, D. R., 1969. Morphologie de l’attache de la furca chez les Cyprididae et son utilisation en systématique. In Neale, J. W. (ed.), The Taxonomy, Morphology and Ecology of Recent Ostracoda. Oliver & Boyd Ltd., Edinburgh: 168–193.
Sars, G. O., 1903. Freshwater Entomostraca from China and Sumatra. Archiv for Mathematik of Naturvidenskab 25(8): 3–44.
Sars, G. O., 1926. Freshwater Ostracoda from Canada and Alaska. Report of the Canadian arctic expedition 1913–18. Vol. VII: Crustacea, part I: Ostracoda. Ottawa: 24 pp.
Savatenalinton, S. & K. Martens, 2008. Redescription of Hemicypris mizunoi Okubo, 1990 (Crustacea, Ostracoda) from Thailand, with a reassessment of the validity of the genera Hemicypris and Heterocypris. Bulletin van het Koninklijk Belgisch Instituut voor Natuurwetenschappen, Biologie 78: 17–27.
Savatenalinton, S. & K. Martens, 2009. Redescription of the type species of Strandesia Stuhlmann, 1888 and Cypricercus Sars, 1895 (Crustacea, Ostracoda, Cypricercinae), with a description of a new species of Cypricercus from South Africa. Zootaxa 2007: 1–42.
Sharpe, R. W., 1903. Report on the freshwater Ostracoda of The United States National Museum, including a revision of the subfamilies and genera of the family Cyprididae. Proceedings of the U.S. National Museum 26: 969–1001.
Smith, R. & R. Matzke-Karasz, 2008. The organ on the first segment of the cypridoidean (Ostracoda, Crustacea) antennule: morphology and phylogenetic significance. Senckenbergiana lethaea 88(1): 127–140.
Swofford, D. L., 1998. PAUP: Phylogenetic Analysis Using Parsimony, Version 4.0. Computer Program; United States National Museum of Natural History, Smithsonian Institution, Washington D.C.
Triebel, V. E., 1953. Genotypus und schalen-Merkmale der Ostracoden-Guttung Stenocypris. Senckenbergiana 34(1/3): 5–14.
Vávra, W., 1895. Die von Dr. F. Stuhlmann gesammelten Süsswasser-Ostracoden Zanzibar’s. Jahrbuch der Hamburgischen Wissenschaftlichen Anstalten 12: 1–23.
Victor, R. & C. H. Fernando, 1981. Freshwater ostracods (Crustacea: Ostracoda) of the genus Strandesia Vavra, 1895 from Malaysia, Indonesia and the Philippines. Archiv für Hydrobiologie, Supplements 58(4): 469–522.
Acknowledgements
Sampling trips in Thailand were financially supported by the Royal Belgian Institute of Natural Sciences (R.B.I.N.Sc., Brussels). The Staff Development Fund, Mahasarakham University (Mahasarakham, Thailand), supported the PhD scholarship grant to the first author. Karel Wouters (R.B.I.N.Sc., Brussels) kindly loaned cypricercinid material from the Ostracod Collection of the R.B.I.N.Sc (Brussels). Dunja Lamatsch (Mondsee, Austria) provided specimens of Bradleystrandesia fuscata and B. reticulata, and from sample collections of the EU Marie Curie Research and Training Network “SexAsex” (project no. 512492). Material of Diaphanocypris meridana and of Bradleytriebella trispinosa was provided by Janet Higuti (Universidade Estadual de Maringá, Brazil). Robin J. Smith (Lake Biwa Museum, Japan) and Claude Meisch (Musée national d’Histoire Naturelle, Luxembourg) suggested important improvements to an earlier version of the manuscript. Julien Cillis and Claudine Behen (R.B.I.N.Sc., Brussels) offered technical assistance with the SEM illustrations and with the line drawings, respectively.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Luigi Naselli-Flores
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Savatenalinton, S., Martens, K. Generic revision of Cypricercinae McKenzie, 1971 (Crustacea, Ostracoda), with the description of three new genera and one new species and a phylogenetic analysis of the subfamily. Hydrobiologia 632, 1–48 (2009). https://doi.org/10.1007/s10750-009-9826-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-009-9826-5