Abstarct
Diabetic complications are among the largely exigent health problems currently. Cardiovascular complications, including diabetic cardiomyopathy (DCM), account for more than 80% of diabetic deaths. Investigators are exploring new therapeutic targets to slow or abate diabetes because of the growing occurrence and augmented risk of deaths due to its complications. Research on rodent models of type 1 and type 2 diabetes mellitus, and the use of genetic engineering techniques in mice and rats have significantly sophisticated for our understanding of the molecular mechanisms in human DCM. DCM is featured by pathophysiological mechanisms that are hyperglycemia, insulin resistance, oxidative stress, left ventricular hypertrophy, damaged left ventricular systolic and diastolic functions, myocardial fibrosis, endothelial dysfunction, myocyte cell death, autophagy, and endoplasmic reticulum stress. A number of molecular and cellular pathways, such as cardiac ubiquitin proteasome system, FoxO transcription factors, hexosamine biosynthetic pathway, polyol pathway, protein kinase C signaling, NF-κB signaling, peroxisome proliferator-activated receptor signaling, Nrf2 pathway, mitogen-activated protein kinase pathway, and micro RNAs, play a major role in DCM. Currently, there are a few drugs for the management of DCM and some of them have considerable adverse effects. So, researchers are focusing on the natural products to ameliorate it. Hence, in this review, we discuss the pathogical, molecular, and cellular mechanisms of DCM; the current diagnostic methods and treatments; adverse effects of conventional treatment; and beneficial effects of natural product-based therapeutics, which may pave the way to new treatment strategies.

Graphical Abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic cardiomyopathy (DCM) manifests itself due to myocardial dysfunction in the deficiency of hypertension and coronary artery disease. Hyperglycemia seems to be vital to its pathogenesis and to elicit a series of maladaptive stimuli that result in collagen deposition and myocardial fibrosis. These progressions are thought to be conscientious for altered myocardial relaxation description and are evident as diastolic dysfunction on imaging [1]. DCM was first descriptioned in 1972 by Rubler et al. [2], who reported the autopsy data from four patients with diabetic renal microangiopathy and dilated left ventricles in the absence of other common causes. DCM, most widespread disease due to diabetic complications, is categorized by unusual myocardial structure and performance. Enhanced free radical generation, lipid peroxides, lipid accumulation, energy deficit, mitochondrial dysfunction, advanced glycation end-product (AGEs), activation of isoforms of protein kinase C, imbalance in ATP/O2− consumption ratio, and activation of peroxisome proliferators-activated receptors are its hallmarks.
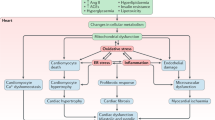
Diabetes mellitus (DM) is associated with demonstrable changes in LV structure and function in the absence of active ischemia. The metabolic milieu associated with DM, such as hyperglycemia, hyperinsulinemia, increased inflammatory cytokines, increased circulating fatty acids, and triacylglycerols, altered multiple molecular pathways in cardiomyocyte, impaired cardiac contractility cause myocyteinjury, dysfunction, and cell death [3]. Numerous molecular mechanisms act in unison to damage the cardiac function and promote cardiomyocyte injury in DM. These are distorted insulin signaling, renin–angiotensin signaling, malformed metabolism and mitochondrial dysfunction, post-translational modifications of signaling and structural proteins, altered cell homeostatic processes such as autophagy, endoplasmic reticulum (ER) stress, apoptosis, and changes in gene regulation (Fig. 1).
Cardiac hypertrophy is established as an important characteristic of DCM. Experimental and clinical research have shown the association between left ventricular remodeling event and pressure burden myocyte hypertrophy in DM. Cardiac myocytes are incurably differentiated myocytes and lose the propensity of proliferation after birth. Such pathological states over burden the heart and postnatal cardiomyocytes endure cardiac hypertrophy, raise the size of individual cardiac myocytes, and result in whole organ swelling. Even though cardiac hypertrophy is primarily compensatory, but once it drawn out may lead to detrimental effects owing to heart failure, stroke, and sudden death [4]. Patients in particular with type 2 DM (T2DM) have higher rates of blood pressure (BP), which contributes to the development of cardiovascular diseases (CVD), a major reason for morbidity and mortality universally and almost 70% of deaths are attributable to it. India faces a daunting task due to the impending burden of DM and its related complications. To surmount these troubles, the introduction of cost-effective advanced and novel therapeutic treatments becomes the most important task to offer a long-term reprieve to DM patients. Hence, this review focuses on creating awareness among readers, researchers, and clinicians about the current state of DCM, its molecular mechanisms, treatment and side effects, and the advantages of natural therapeutics to fight it.
Pervasiveness of DCM
People with T1DM and T2DM had increased heart failures (HF) even after treatment for well-recognized risks for HF such as ischemic heart disease and hypertension [5]. The burden of DM is growing rapidly and has turned into a severe problem globally in both developing and developed countries. The World Health Organization (WHO) estimated that 80% of the DM deaths occur in middle- and low-income countries and that such deaths will grow twofold between 2016 and 2030. It has further been estimated that the burden of T2DM globally will rise to 438 million by 2030 from 285 million currently, and for India, it will go up from 51 million in 2010 to 87 million by 2030 [6]. When the diabetic populace is associated with CVD, the probability of HF raises four fold as compared to non-diabetic heart patients. DM is a lifestyle disease or the disease of modern civilization. Patients, especially those with T2DM, have higher rates of BP, which contributes to the progression of CVD [7].
Progression of DCM
Diabetic patients have a two to four times increased risk of developing HF, one of the greatest contributors to morbidity and mortality. Cardiac fibrosis (CF) is a key characteristic of DCM. In DCM condition, overproduction of extracellular matrix (ECM) protein leads to enlarged myocardial stiffness and subsequent cardiac dysfunction, eventually leading to HF. Therefore, the evaluation of the balance between ECM synthesis and deprivation is a good start to asses the development of DM-induced CF [8]. Prospective contributors to the progression of DCM are increased free fatty acids (FFA), peroxisome proliferator-activated receptor (PPAR)-α signaling, leading to increased transcription of many genes involved in fatty-acid oxidation. Increased fatty-acid oxidation leads to the production of reactive oxygen species (ROS) at the level of electron transport chain. ROS, which also can be produced by extramitochondrial mechanisms such as nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, plays a significant role in numerous pathways involved in the pathogenesis of DCM, together with cell death, tissue damage, and lipotoxicity as well as reduced cardiac efficiency and mitochondrial uncoupling [9].
Several pathophysiological mechanisms are implicated in the stepwise development from DM to HF. These include straight myocardial damage, pressure burden, and volume overload. DM not only strengthens the progression of atherosclerosis in epicardial coronary arteries but also leads to the appearance of functional and structural disarrays in smaller vessels, leading to increased ischemic pressure on the myocardium [9]. On the other hand, the main culprit of DM, hyperglycemia, can harm the myocardium via modified proteins, such as advanced glycation end products (AGEs) and ROS. The AGEs, for instance, formed by glycation of collagen direct to its accretion in the ECM and ultimately in CF, resulting in diastolic dysfunction. Moreover, soluble AGEs associated in related receptors (RAGEs) trigger NADPH oxidase, leading to the fabrication of peroxide and ultimately of ROS, and in turn are the basis to directly hurt the DNA of the myocytes [10].
Experimental DCM models
DCM causes a severe cardiac dysfunction induced by alterations in contractility and structure of the myocardium. This pathology is initiated by alterations in energy substrates and occurs even in the absence of hypertension, atherothrombosis, or other cardiomyopathies. Hypertrophy, steatosis, fibrosis, apoptosis, and inflammation of the myocardium have been premeditated in several DM experimental models in animals, mostly rodents. T1DM and T2DM were induced by pancreatic toxins, fat and sweet diets as also genetic manipulation, and animals confirm the foremost characteristics of human DM and related DCM [11]. Experimental models of both T1DM and T2DM demonstrate modifications in the circulating levels of glucose and lipid. Main T1DM and T2DM animal models show both hyperglycemia and hyperlipidemia, represented by elevated levels of cholesterol, lipoproteins, and tryacylglycerides. More interestingly, these models also show signs of structural, metabolic, and functional irregularities that summarize the human DCM pathology.
DCM development in rodent models like in humans is distinguished by ventricular dilatation and reduced ejection fraction (EF), and later impersonate dilative cardiomyopathy. Investigational T1DM and T2DM animals are prone to progressive systolic and/or diastolic dysfunction as confirmed in plentiful in vivo studies using magnetic resonance imaging (MRI), hemodynamic measurements, and echocardiography [12]. Cardiac dysfunction in T1DM patients could be generated approximately in all conservative models induced by genetic alterations and toxins. In T2DM, diet-induced models may symbolize human pathology more suitably, at least in the superior state of the disease. Maladaptive structural alterations trigger both systolic and diastolic dysfunctions that finally lead to HF. Although the evolution of these actions is not known well, the trademark of changes includes local inflammation, interstitial fibrosis, and cell hypertrophy endorsed by cell death and steatosis routes in the wounded myocardium [13].
Animal models for diabetes and its complications
The streptozotocin model
The most commonly used model of T1DM is the streptozotocin (STZ) model. STZ is a glucosamine-nitrosourea antibiotic that is analogous structurally to glucose and is taken up preferentially by glucose transporter-2 (GLUT2) in insulin-secreting pancreatic β cells. Intraperitoneal administration of STZ leads to β cell toxicity and necrosis, and eventually to deficiency of insulin. Many studies in STZ-induced DM mice showed diastolic and systolic impairments that have savageness in proportion to the period of DM. The most significant compensation of the STZ model is that DM can be induced effortlessly in rats and mice, and that the model allows the assessment of DM on the cardiac in varying genetic background strains. DM can simply be superimposed in genetically transformed mice, which allows the innovative design of complicated mechanistic examination, without the drawn-out waiting periods, if altered mouse strains were crossed with the genetic models of DM. DM can also be induced at different ages, enabling its effects on the heart to be studied at different stages in the lifecycle of the animal [14].
The Zucker fatty rat and Zucker diabetic fatty rat
Zucker fatty (ZF) rats have a homozygous missense transmutation in the Fa (also known as Lepr) gene encoding the rat leptin receptor (Ob-R). ZF rats are hyperphagic, obese, and have extended increased serum insulin levels, triglyceride, and fatty acid but are not hyperglycemic [15]. The Zucker diabetic fatty (ZDF) rats have been investigated from chosen breeding of ZF rats that demonstrate elevated glucose levels, thus ZDF rats are an inherited strain created from the outbred ZF rat. ZDF rats are obese, hyperglycemic, hyperinsulinemic and hyperleptinemic, and have constantly increased serum triglyceride and fatty acid levels. In ZDF rats, damaged cardiac contractility has been noticed more frequently. The diabetic and obese Zucker rats symbolize the useful models to examine the effect of T2DM and/or obesity on the heart [16].
Diet-induced obesity and DM
To overcome prospective problems associated with distorted leptin signaling, many scientists have begun to assess the models of diet-induced obesity and DM. Western diets which contain high sucrose and high fat lead to DM, insulin resistance, and obesity predominantly when supplemented to C57BL/6 mice [17]. After 2 weeks of supplementation of western diet to C57BL/6 mice, they can alter myocardial substrate utilization that expands the severity of insulin resistance and obesity. In particular, the rates of glycolysis and glucose oxidation are condensed, and myocardial fatty acid oxidation and oxygen expenditure are augmented [18]. The commencement of cardiac dysfunction subsequent to western diets is more quickly noticed in Wistar rats, in which high-fat feeding for 7 weeks leads to impaired contractile function, mitochondrial degeneration, and myocardial steatosis. On the other hand, taken together, these examinations suggest that caloric overload might induce metabolic defects associated with DCM. It is important to note that isocaloric high-fat diets, which do not encourage insulin resistance or obesity, emerge to progress cardiac function in rat models of cardiac hypertrophy and HF, implicating an adverse role for hyperinsulinemia and impaired glucose homeostasis in related heart defects that extend following the ingestion of western diet [19].
Using the existing data, the most suitable T1DM model induced by pancreatic toxicity is accomplished by STZ and in T2DM resembles DCM, including both T2DM obesity and T2DM etiologies. Since human T2DM is the result of an unhealthy lifestyle and/or mysterious polygenic mutations, high-fat-fed models can be closer to that point in mutations in lipid storage or on the leptin system.
Diagnosis of DCM
In current clinical practice, the diagnosis of DCM includes distinct functional and structural changes in the LV and searching for evidence to exclude other cardiac diseases and possible etiology for changes in a patient with DM.
Echocardiography
Clinically perceptible DCM may take several years to develop, but echocardiography can identify significant abnormalities well before the onset of the indicative HF. Diverse types of echocardiography are used in diagnosing LV dysfunction.
Conventional echocardiography
Early symptoms are defined by a preserved LV ejection fraction with condensed early diastolic filling, persistence of isovolumetric relaxation and enhanced atrial filling, the presence of which substantiates diastolic dysfunction. A decrease in LV distensibility is differentiated by shorter LV ejection time (LVET) and an increased pre-ejection period (PEP), resulting in an increased LVET/PEP ratio. Such abnormalities have been confirmed in a group of normotensive DM patients without overt macrovascular or microvascular complications [20].
Tissue Doppler echocardiography
In typical echocardiography, a low-amplitude high-velocity filter looks exclusively at blood flow through the heart to describe the valvular function. Latest methods such as tissue Doppler echocardiographic imaging (TDI) offer hope as they apply a low-amplitude high-velocity filter to the myocardium enabling an evaluation of myocardial tissue velocities. The benefit over usual Doppler echocardiography is that the results are independent of changes in pre-load. This provides a functional tool for defining subtle diastolic and systolic dysfunction. In a recent examination, even though there was a significant reduction in resting peak myocardial systolic velocity (Sm) and early diastolic velocity (Em) in DM patients, the response to dobutamine stress did not differ from control subjects; portentous that ischemia due to small vessel disease may not be significant in premature DCM [21].
Computed tomography
The coronary artery calcification (CAC) score, consequent initially from electron-beam computed tomography (CT) but more in recent times from multislice CT, has been compared strongly with the severity and presence of angiographic and histological proof of conventional coronary heart diseases and coronary atherosclerosis risk factors, in particular C-reactive protein (CRP), reflecting unstable and stable plaques [22].
Magnetic resonance imaging
Myocardial flow reserve is not regularly assessed in myocardial perfusion imaging studies, but has been theorized to influence test accuracy when assessing disease severity by coronary vessel lumenography. Magnetic resonance imaging (MRI) is a promising diagnostic procedure that can perform both myocardial perfusion imaging and assess myocardial flow reserve. In addition, it is also an extremely helpful tool to evaluate the diastolic function perfectly without the shortcomings of echocardiographic detection [23].
Single-photon emission computed tomography
Quantitative myocardial perfusion single-photon emission computed tomography (SPECT) has advanced considerably over the last few decades providing significant benefits to nuclear cardiology contrasted with other high-resolution non-invasive imaging modalities for the recognition of CAD. In particular, gating has provided both functional and perfusion information and reduction correction. SPECT has improved perfusion information [24].
Molecular foundation for DCM
Hyperglycaemia
Hyperglycemia corresponds to one of the most central triggers of metabolic changes in DM. Hyperglycemic people exhibit an 8% enhancement in the risk progression of HF with every 1% rise in glycosylated hemoglobin (HbA1C). Hyperglycemia may cause its destructive effects through a series of secondary transducers. One of the main abnormalities is the excess production of AGEs, which deactivate nitric oxide (NO) and damage coronary vasodilation. Prolonged hyperglycemia leads to excessive formation of mitochondrial ROS, which affects transcription, leading to contractile dysfunction. A raise in ROS diminishes the NO levels, which leads to endothelial dysfunction and myocardial inflammation via poly (ADP-ribose)polymerase (PARP), the inhibition of which has been shown to reverse diabetic endothelial dysfunction [25].
Insulin resistance
Insulin resistance is a risk factor in the development of CAD. On the other hand, HF causes insulin resistance and increases the risk in the development of T2DM. The progression of CAD due to impaired insulin signaling, and the development of insulin resistance (IR) in the HF suggest that the patient is multifactorial. Possible mechanisms by which HF causes insulin resistance include related overactivity, loss of skeletal muscle mass, deskbound lifestyle, and an impending effect of increased circulating cytokines (tumor necrosis factor-α (TNF-α)) on peripheral insulin sensitivity. A vicious cycle is set in motion, in which insulin resistance and HF deteriorate each other [26].
Fatty acids
Increased fatty acid oxidation decreases pyruvate and glucose consumption by inhibiting pyruvate dehydrogenase. Pyruvate oxidation is reduced further by pyruvate dehydrogenase lipoamide kinase isozyme 4 and activated by PPAR. The net result is a surfeit of glycolytic intermediates and amplified synthesis of ceramide leading to apoptosis, which can be inhibited by PPAR-α and γ-agonist troglitazone. Therefore, impaired pyruvate oxidation, glycolysis, lactate uptake, and a greater reliance on fatty acids as a source of acetyl CoA leads to a perturbation of contraction/relaxation coupling and myocardial bioenergetics. Standard pharmacotherapy is intended to restore equilibrium between ATP breakdown and synthesis by increasing oxygen delivery or by declining cardiac power by reducing BP and heart rate [27].
Oxidative stress
Oxidative stress subsists when the production of ROS compensates its deprivation by antioxidant defenses. The consequential elevation of ROS has plentiful of dangerous effects on the cardiovascular system through the disruption of vascular homeostasis by interference with NO, cellular damage by oxidation, and by redox signaling. In a number of animal models of DM and humans with DCM, there is extreme ROS creation from both extramitochondrial and mitochondrial sources, and has been involved in all stages of the improvement of HF, from cardiac hypertrophy to contractile dysfunction, fibrosis, and failure. The raise in ROS causes cardiac dysfunction by directly damaging the DNA and proteins, as well as by promoting apoptosis [28].
Myocardial fibrosis and myocardial hypertrophy
Myocardial fibrosis and collagen accumulation are the crucial structural changes found in DCM. DM locally activates endothelin systems and myocardial rennin angiotens in aldosterone system (RAAS), causative to myocyte fibrosis and necrosis. The allocation of fibrous tissue in the myocardium is perivascular, interstitial, or together, and the pathologic examination shows interstitial fibrosis, capillary endothelial changes, capillary basal laminae thickening, and myocardial hypertrophy. Structural changes in DCM are connected to unpleasant remodeling, consisting of LVH, systolic dysfunction, and diastolic LV dysfunction. LV hypertrophy is a characteristic in the morphologic demonstration of DCM, usually demonstrating a more advanced phase of the DCM. It symbolizes an LV mass excess, which leads to a hardened ventricle and heralds systolic LV dysfunction. The presence of hypertrophy in DCM might not be linked to comprehensible LV diastolic dysfunction by usual echocardiography [29].
Endothelial dysfunction
Endothelial dysfunction is an antecedent to and an effect of atherosclerosis. Functional and anatomical abnormalities of the vascular endothelium are generally coupled with DM. Both chronic dyslipidemia and hyperglycemia contribute to endothelial dysfunction. Hyperglycemia results in the destruction of endothelial cell NO production, amplified production of glycated proteins, endothelium adhesion molecules, vasoconstrictor prostaglandins, and vascular growth factors and platelets, which cumulatively develop vascular permeability and vasomotortone, remodeling, and growth. Endothelial dysfunction also consists of increase in speed of desertion of capillary endothelium, altered protein synthesis, altered expression/production of adhesion glycoproteins, and the deterioration of intercellular junctions on endothelial cells promotes connection of leucocytes and monocytes, as well as their transendothelial migration. In addition, hyperglycemia increases endothelial cell matrix production, which may contribute to cellular membrane thickening. The clinical implications of endothelial dysfunction are not inadequate to increased atherosclerosis. Endothelial cells also help form collateral circulation, which is condensed in patients with DM, and explains the amplified infarct extension and congestive HF after myocardial infraction in DM patients [30].
Myocyte cell death
Myocyte cell death might have happened by necrosis, apoptosis, or mutually. DCM causes higher rates of myocyte death by both necrosis and apoptosis than that of healthy hearts. In a study of DM and diabetic hypertensive hearts, myocyte necrosis was found to be 1.4 times more common in patients with hypertension and DM than with DM alone, whereas myocyte apoptosis was not affected by the addition of hypertension. Hyperglycemia-induced ROS production speeds up apoptosis, some of which is elicited by angiotensin II and glycosylation [31].
Autonomic neuropathy and arterial stiffness
Cardiac autonomic neuropathy (CAN) may alter diastolic function and enhance cardiovascular risk in DM patients. Diabetic autonomic neuropathy is coupled to damaged vasodilator response of coronary resistance vessels to increase sympathetic stimulation. Condensed acquiescence of the large arteries changes the timing of wave reflections and thus is a feature affecting the ventricular load. Ventricular ejection creates a presumptuous pressure wave, which is then reflected back by the arterial tree as a wave traveling back to the cardiac. The net effect of these hemodynamic modifications is ischemia, especially in the sub-endocardium, which, if persistent, can lead to interstitial fibrosis and HF [32].
Altered copper metabolism
Changes in copper metabolism are among the vital contributors to the development of DCM. Higher copper levels have been observed in serum in patients with DM, and the highest levels have been noticed in those with hypertension and microvascular complications. Hyperglycemia can spoil the copper-binding properties of albumin and ceruloplasmin, resulting in amplified copper levels in the ECM. A profusion of copper in the ECM activates the oxidation-reduction system, leading to a higher production of free radicals resulting in increased fibrosis and oxidative stress [33].
Autophagy
Autophagy is a physiological procedure by which long-lived lipids, proteins, ribosomes, and even whole cellular organelles are overwhelmed by double-membrane structures, which are consequently embattled to lysosomes for deprivation. A little level of constitutive autophagy is essential in the heart for sustaining protein and organellar quality control and normal cellular function. Imperfections in this procedure guide to cardiac dysfunction and HF, predominantly when cellular stress is high. Autophagy is implicated in various cardiac disease states, including chronic ischemia, cardiac hypertrophy, ischemia reperfusion, and HF. Modest data is available on the role of autophagy in the pathophysiology of DCM. However, modern research provides convincing support to insulin signaling as a significant regulator of myocardial autophagy [34].
Endoplasmic reticulum stress
Endoplasmic reticulum (ER) stress contributed to myocardial apoptosis in animal models of T1DM and T2DM, as confirmed by the induction of UPR signaling proteins and ER stress-related apoptotic signaling proteins, such as glucose-regulated protein (GRP)-78, GRP-94, cleaved activating transcription factor 6, caspase12, and C/EBP homologous protein and phosphorylated eIF2α. Numerous studies mention that ER stress maybe interceded by enhanced oxidative stress in DCM [35].
Inflammation, innate and adaptive immune responses
Metabolic turbulences provoke subcellular inferior inflammation in the heart. Inflammation is a vital pathogenic feature of DM. The innate immune system inclusive of dendritic cells, mast cells, macrophages, eosinophils, and neutrophils also induces persistent metabolic inflammation. Myocardial inflammation is concerned with the progression of DCM [36]. The nuclear factor, kappa-light-chain enhancer of activated B cells (NF-κB), a principal regulator in inflammatory reactions, is activated in the heart on exposure to glucose or fatty acids. NF-κB induces not only the appearance of proinflammatory cytokines, such as interleukin 6 (IL6), pro-IL18, pro-IL1β, and tumor necrosis factor alpha (TNF-α), but also induces the expression of NLR family pyrin domain-containing 3 (NLRP3) inflammasome. Activated RAGE also mediates an inflammatory reaction by heterodimerizing with toll-like receptor-4 leading to the production of NLRP3, pro-IL1β, and pro-IL18. Activated NLRP3 inflammasome activates caspase-1 and intervenes in the dispensation and release of proinflammatory cytokines IL18 and IL1β resulting in inflammatory cell amplification and infiltration of the inflammatory reaction. Similarly, the diminution of NLRP3 attenuates DCM and inflammation in T2DM rats. Hence, activated inflammasomes play decisive roles in the pathogenesis of HF [37].
Renin-angiotensin-aldosterone system
It is well known that DM is enhanced by the upregulation of the systemic and local enin-angiotensin-aldosterone system (RAAS). Even if the foundation for dysfunction of the RAAS system in the setting of DM remains moderate, its commencement during DM increases oxidative stress, which in turn activates the death pathways concerned with myocardial cell necrosis and apoptosis. These non-myocyte and myocyte alterations in DM hearts resulting from increased activation of RAAS induce mutilation of ventricular function. The repayment of RAAS obstruction in reversing and preventing DCM in DM patients emphasizes the significance of dysregulated RAAS in the pathogenesis of DCM [38].
Molecular signaling pathways of DCM
The perception of DCM is relied on the view that the disease, DM, itself is significant in bringing about changes at the cellular and molecular levels of the myocyte, leading to functional and structural irregularities in the heart. The etiology of DCM is multifactorial and moderately characterized.
Cardiac ubiquitin proteasome system
In cellular mechanisms, ubiquitin proteasome system (UPS) has a pathogenic role, accounting for the continuance of protein equalibrium by mortifying the dented proteins, such as oxidized proteins and terminally misfolded proteins. In vitro and in vivo studies show that UPS dysfunction is an early symptom in DM and it boosts up the pathological remodeling in DM hearts. Research on in vivo UPS functional reporter colligated with other biochemical analyses shows that the escalating activity of the cardiac UPS by overexpression of proteosome activator-28훼 reduced the proteotoxic stress at cardiac level, causing cardiac dysfunction. Therefore, proteasome dysfunction may correspond to an ideal mechanism involved in DCM; nevertheless, the effects of DM on the overall cardiac UPS function and its pathophysiological role in DCM are still to be studied [39].
FoxO transcription factors
Forkhead box containing protein-O subfamily (FoxO) proteins is promising as central targets of insulin and other growth factor action in myocardium. In the beginning, known by their association in chromosomal translocations coupled with rhabdomyosarcomas and leukemias, abundant substantiation now proposes that three members of the FoxO subfamily (FoxO1, FoxO3, FoxO4) are central to cardiac stress-responsiveness and to preserve the cardiac function. The straight metabolic effects of FoxO signaling have not yet been completely identified, and the actions of FoxO in non-myocytecellular rudiments of the heart are mostly indefinite. FoxO factors contribute to cardiac function through autophagy, apoptosis, cell cycle control, response to oxidative stress, regulation of metabolism, and remodeling. Despite an array of transcriptional targets, FoxO factors make it possible to retort to changes in the environment through the regulation of energy-dependent proteins and metabolic enzymes. [40].
Hexosamine biosynthesis pathway
Hexosamine biosynthesis pathway (HBP) is hypothesized to be a nutrient sensor that mediates the flux of FFAs, uridine, glutamine, and glucose into the cell. In contrast to other accessory pathways of glycolysis, HBP leads to the glycosylation of nuclear and cytoplasmic proteins. The glycosylation process is synchronized by two key enzymes, N-acetylglucosaminidase (O-GlcNAcase) and O-GlcNAc transferase (OGT) [41]. About 2–5% of glucose carried through HBP is connected with the O-GlcN acylation of transcriptional factors, insulin and cytokine signaling molecules that play an important role in DCM. These in turn upregulate the transcriptional factors like leptin, TGF-α, PAI-1, and TGFβ and decrease phosphorylated Akt and GSK3. Increased HMP flux modified specificity factor (SP1) along with O-linked glucosamine (O-GlcNAc) that connects to assorted promoters aggravates the hyperglycemic state. Such promoter region includes heat shock protein and adipocytokine. SP1 reduces the small heat shock protein 27 expression by uplifting the ROS production important in apoptosis. Further, in DM, SP1 expression has been enhanced to optimistically link with the progression of IR [42].
HBP is an ideal contributor to impaired glycemic control of DCM downstream. The HBP/O-GlcNAc mechanism is distinguished in human myocardium, and its activity is upregulated in investigational models of both T2DM and T1DM. This is imitated in high glucose-induced cardiomyocytes in vitro. In DM heart disease, until recently, very little was known about the long-term consequences of continued activation of HBP/O-GlcNAc pathways in the heart, but these have now emerged as significant mediators of impairment in myocardial excitation contraction coupling, cardiomyocyte viability and LV dysfunction, as well as in insulin receptiveness. Augmented flux through HBP/O-GlcNAc pathways occurred in equivalent to AGEs formation; both symbolize mechanisms identified in the pathogenesis of the DCM downstream of hyperglycemia [43].
Polyol pathway
Polyol pathway is a metabolic pathway, where a part of glucose overload gets metabolized to sorbitol, which is then transformed to fructose. In the polyol pathway, glucose is condensed to sorbitol by aldose reductase (AR), foremost to depletion in cellular stores of nicotinamide adenine dinucleotide phosphate (NAD(P)H) [44]. Reduced NAD(P)H is necessary for the functioning of various endothelial enzymes, including cytochromeP450 and nitri oxide synthase, as well as in the antioxidant activity of glutathione reductase. Sorbitol dehydrogenase oxidizes the sorbitol into fructose. On the other hand, an elevated polyol pathway flux utilizes large amounts of ATP and may thus supply the energy essential for endothelial-derived relaxing factor production. AR is the rate-limiting and vital enzyme in polyol pathway, and glucose and galactose are substrates to it, which get reduced to sorbitol and galactitol, respectivly. Therefore, the activation of the polyol pathway initiates and multiplies numerous mechanisms of cellular damage by interaction and activation of AR and other pathogenetic factors, such as production of AGE, establishment of oxidative-nitrosative stress, protein kinase C (PKC) pathway, and PARP that may lead to the initiation of inflammation and growth factor imbalance. The treatment with S-allylcysteine to STZ-NAD induced DM rats to ameliorate the DM and its complications through polyol pathway. This study suggested that polyol pathway is one of the therapeutic targets to combat DM and its complications. [45].
Protein kinase C signaling
PKC signaling is turned on in the heart in reaction to hyperglycemia, as well as growth factors that are prominent in a state of DM. A build-up of metabolites concerned in the glycolysis pathway drives the production of diacylglycerol, which is a decisive activating cofactor for PKC isoforms. Hyperglycemia-mediated activation of PKC is associated with a host of downstream proteins and gene expression changes, which can contribute to characteristic features of the DM heart including fibrosis (TGF-β, CTGF, and plasminogen activator inhibitor-1), hypertrophy (via activation of MAPKs), cardiac, oxidative stress (via activation of NADPHoxidase), and inflammation (NFκB, TNF-α) [46]. There are more than ten PKC isoforms and at least four (β, ε, δ, α) are connected with mediating cardiac hypertrophy and pathology. Both PKC-β and PKC-α isoforms are upregulated in the DM heart. PKC-α is also associated with prejudiced propensity and contractility toward HF, due to an unpleasant effect on calciumion (Ca2+) handling in cardiomyocytes [47].
NF-κB signaling
NF-κB is a vital transcription factor that controls inflammatory and cardiomyocyte injury processes and consists of five members, including p65 (RelA), RelB, c-Rel, NF-κB1, and NF-κB2. In resting cells, it is dormant by binding to IκBα in the cytoplasm. After elevated glucose stimulation, the IκBα is phosphorylated by IκB kinase (IKK) complex, principal to the translocation of NF-κB to the nucleus and binding to NF-κB response element [48]. AMP-activated protein kinase (AMPK) suppresses NF-κB flow through decreased IκBα degradation and inhibition of IKK. NF-κB cascade may be provoked by the phosphorylation of mitogen-activated protein kinase (MAPK). PPAR has the capability to downregulate NF-κB activity by its association with the p65 subunit or inhibition of MAPK phosphorylation. Sirtuin 1 (SIRT1) inhibits NF-κB by blocking the MAPK or by increasing communication between p65 subunit of NF-κB and PPAR. SIRT1 activation leads to deacetylation of PPAR-γ co-activator-1 (PGC1α) and AMPK activation. NF-κB decreases the activity of PGC-1α indirectly or directly by activating PKB/Akt. The activation of NF-κB triggers an increase in proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6, which contribute to the establishment of pro-fibrotic TGF-β pathway. Under diverse pathological conditions, the human myocardium secretes a variety of proinflammatory cytokines and chemokines, such as IL-6, MCP-1, and TNF-α which wield numerous autocrine pleiotropic effects in heart cells. Nonstop rise in their levels may lead to states that are connected to myocardial inflammation, such as ischemic myocardial injury, dilated cardiomyopathy, and HF. Proinflammatory cytokine expression is beneath the management of inducible and ubiquitous transcription factor NF-κB, which itself is activated in myocarditis, cardiac hypertrophy, and congestive HF. A number of endogenous and exogenous motivations might induce NF-κB transcriptional activity; remarkably, the proinflammatory cytokines themselves elevated free fatty acid levels in plasma, anoxia, ROS, angiotensin II, toll-like receptors, lipoproteins, endothelin 1, and hyperglycemia. A disproportionate formation of proinflammatory cytokines contributes to the pathogenesis of HF [49].
Peroxisome proliferator-activated receptor signaling
Cardiac metabolism is predominantly regulated at the transcriptional level by the peroxisome proliferator-activated receptor (PPAR) transcription factor family, which consists of three isoforms (α, β/δ, and γ). The function of every PPAR subtype depends on ligand binding, tissue distribution, and the recruitment of co-repressors and co-activators. Characteristically, the physiological ligands of PPARs are long-chain fatty acids and their eicosanoid products. Of the three PPAR isoforms, PPAR-α has been studied most part in the cardiac. Metabolic changes are an essential feature of the DM heart. Contrasting with the normal heart, the DM heart develops its energy almost absolutely from fatty-acid metabolism. PPAR-α signaling is activated by prominent FFA, which in turn regulates the expression of genes implicated in fatty-acid uptake and mitochondrial fatty-acid oxidation in the heart [50]. PPARβ/δ overexpression increases myocardial glucose consumption, activates heart glucose transport pathways, and enhances glycolytic gene expression. PPAR-γ is largely expressed in brown and white adipose tissue, and has comparatively low expression in the heart [51]. All the three isoforms of PPARs exert anti-inflammation in the progress of DCM for physical interaction with the p65 subunit of NF-κB and restrain the activation of certain members of the MAPK signaling pathway, have stated that in cardiomyocyte-restricted PPAR-γ knockout mice, protein and transcript levels of significant proteins in fatty-acid uptake and oxidation are concentrated in the heart, and therefore result in depression of cardiac contraction and decreased myocardial fatty acid utilization. In agreement with this, the PPAR-γ underprovided hearts exhibited HF and cardiac hypertrophy [52], suggesting that this subtype is requisite for basal myocardial fatty acid utilization in the heart.
Transcription factor nuclear factor NF-E2-related factor 2 pathways
The NF-E2-related factor 2 (Nrf2) plays an imperative role in ARE-mediated inducible and basal expression of more than 200 genes that can be grouped into many types including phase II detoxifying enzymes and antioxidant genes. Nrf2 is a key regulator of idiom for cytoprotective gene. It is broadly accepted that oxidative stress in Nrf2 plays a remarkable role in the pathogenesis of CVDs [53]. The diminution of Nrf2 activity contributes to mitochondrial dysfunction and extensive oxidative stress in the vasculature, which leads to insulin resistance, abnormal angiogenesis, and endothelial dysfunction noticed in DCM [54]; so, the reduction of Nrf2 was correlated with cardiac damage. Nrf2 system has been anticipated to avert the beginning of DM and to play a momentous job in maintaining glucose metabolism throughout the regulation of glucose consumption and insulin secretion, as well as amendable lipid metabolism [11].
MAPK pathway
MAPKs are a large family of serine/threonine-specific protein kinases characterized into four major subfamilies including p38 MAPK (δ,γ, β, α isoforms), c-Jun N-terminal protein kinase (JNKs), extracellular signal-regulated kinases (ERK1/2), and big mitogen-activated protein kinase (MAPK). They are responsible for the regulation of cardiac remodeling and growth in settings of stress [55]. Experimental evidence confirms that MAPK cascade including p38MAPK, JNK, and extracellular signal-regulated kinase-1/2 are involved in DM complications. p38 MAPK can have four isoforms including p38δ, p38γ, p38β, and p38α. P38α is the most important form articulated in a normal heart, and p38β shows lower expression. p38 MAPK, in particular p38α MAPK, contributes to the progression of DCM due to metabolic abnormalities, oxidative stress, inflammation, hypertrophy, apoptosis, and disordered Ca2+ handling. Excess glucose triggers the expression of PKC in the neonatal rat cardiomyocytes, leading to the upregulation of MAPK, NF-κB, and ROS. ROS activates p38 MAPK, which, in turn, promotes the production of ROS; on the other hand, the supression of p38 MAPK can hold back ROS production and oxidative stress [56].
Micro RNAs (miRNA)
miRNAs are a cluster of ~ 22-nucleotide-length single-stranded non-coding RNA molecules that are extensively expressed in both animals and plants and preserved across mammalian species. They have a decisive function in a range of biological processes such as oncogenesis, apoptosis, and development, where they control gene activity by inhibiting mRNA or by degrading protein production [57]. There is mounting substantiation that a comparatively new class of little non-coding genes, called miRNA, which have philosophical role in several diseases, may contribute to the pathogenesis of DCM. Studies in animal models, cell culture of DCM, and DM HF patients have recognized miRNAs expressed in this syndrome and are demarcating their role in DCM. A change in myocardial miRNA substance is a believable mechanism that could be concurrent to changes in cardiac function. Universal dysregulation of the miRNA biogenesis machinery in the cardiac by cardiomyocyte-specific removal of chopper leads to rapidly progressive DCM and HF. Some studies have revealed related dysregulation of particular miRNAs to the pathophysiology of DCM [58]. Taking into consideration the human genome may be decisive for more than thousand miRNAs and that over 60% of all mammalian genes may correspond to conserve targets of miRNAs, and allowing for persuasive data supporting role for definite miRNAs in the directive of systemic metabolism, specific miRNAs may contribute to molecular defects that symbolize DCM [59]. In recent times, researchers have established that miRNAs have imperative roles in DM and its complications. Jeyabal et al. [60] found that miR-9 expression was considerably reduced in elevated glucose-cultivated cardiomyocytes and human DM heart. Raut et al. [61] found that miR-30c overexpression cured high glucose-induced cardiomyocyte hypertrophy by inhibiting the expression of cell division control in p21-activated kinases and protein 42 homolog Cdc42. Li et al. [62] established that miR-30d endorsed cardiomyocyte pyroptosis in DCM by direct repression of Foxo3a expression.
Current treatment strategies for DCM
The management of DCM will require a multifaceted approach to compact with cellular, metabolic, and molecular factors accountable for the alterations in the function and structure of the myocardium.
Glycemic manage
The glycemic control on DCM has been premeditated in only a limited fashion; excellent glycemic control is advantageous, at least in the premature stages of myocardial dysfunction [63]. Studies also revealed that DCM does not apply to patients with strongly guarded T1DM, and suggest of an imperative role for hyperglycemia in the pathogenesis of DCM [64]. Superior glycemic control is conceivably the most central constituent in its overall supervision. Poor glycemic management has been associated with an amplified risk of cardiovascular deaths. Therefore, recuperating glycemic control must have a favorable impact on cardiovascular mortality and morbidity.
Oral anti-hyperglycaemic medication
It is therefore very important to reduce the level of hyperglycemia to avert the incidence and shrink the savagery of DCM. There is a large group of oral antidiabetic medications, which include the classes of dipeptidyl peptidase 4 (DPP-4) inhibitors, sulfonylureas, biguanides, thiazolidinediones, meglitinides, and alpha glucosidase inhibitors.
Insulin and insulin-secreting agents
Insulin-synthesizing components impede the assimilation of sugars from the gastrointestinal tract. In general, two types of insulin-secreting medications exist in the market, such as non-sulfonylureas and sulfonylureas. Sulfonylureas motivate the pancreatic β cell to enhance the discharge of insulin, such as glipizide, tolbutamide, chlorpropamide, glimepiride, and glibenclamide (glyburide). Non-sulfonylureas are fast-acting modern agents that also excite insulin secretion [65]. Sulfonylureas amplify insulin secretion by escalating Ca2+ permeability from voltage-gated Ca2+ channels. On the other hand, sulfonylureas restrain emission of glucagon and focus on tissues that are sensitized by the accomplishment of insulin. Second-generation sulfonylureas, such as glimepiride, glibornuride, glibenclamide, gliclazide, and glipizide, are more powerful than the first-generation ones like chlorpropamide, carbutamide, and tolbutamide and are effective even at lower doses [66].
α-Glucosidase inhibitors
Alpha-glucosidase inhibitor proceeds by slowing carbohydrate assimilation, and reducing postprandial climb in the levels of blood glucose. They do not reduce plasma glucose level during fasting, but rather cause a gentle decline in HbA1c. Miglitol, voglibose, and acarbose are the familiar ones in this group. In gut membrane, bound α-glucosidases hydrolyze starch residues to disaccharides and oligosaccharides, and release glucose in the intestine. This hydrolysis is important for the formation of digestive carbohydrates in the form of monosaccharides; inhibition of α-glucosidases by medication, such as miglitol, voglibose, emiglitate, or acarbose delays; and diminishes the hydrolysis and absorption of carbohydrates and decreases postprandial rise in blood glucose level [67].
Biguanides
Biguanides (metformin) are a broadly used medication in the management of T2DM, in particular among overweight and obese people because of their capability to inhibit hepatic gluconeogenesis. Even though they are contraindicated in HF due to the progression of lactic acidosis, numerous studies show that they are extensively used under the management of DM and reduce CVD and deaths associated with DM [68]. Metformin is considered to be the most broadly prescribed antidiabetic drug on the globe. It cures peripheral insulin sensitivity and decreases hepatic glucose harvest, and therefore helps in managing hyperglycemia [69].
Thiazolidinedione
Thiazolidinediones (TZD) are a class of compounds for the management of patients with T2DM, which act by escalating insulin sensitivity in adipose tissue and skeletal muscle through activation and binding of PPAR-δ. TZD (e.g., Rosiglitazone and Pioglitazone), distant from insulin-sensitizing fat and skeletal muscle, raises the expression and function of glucose transporters in the cardiac, enhances glucose metabolism, and reduces NEFA utilization by the myocardium. It defends against myocardial injury associated with ischemia and improves the recuperation of function subsequent to ischemia [70].
β-blockers
Beta-blockers (β-blockers) are the paradigm of interest in patients with systolic HF. There has been disinclination in the use of β-blockers in DM patients out of trepidation of unpleasant effects on insulin resistance and a lack of knowledge of hypoglycemia. On the other hand, with advances in HF and of the significance of SNS in the discharge of vasoactive substances, β-blockers have turned out to be indispensable in the management of HF. They have been able to avert and even arrest cardiac remodeling, resulting in improved LV function and decrease in deaths [71].
Angiotensin converting enzyme inhibitors
Angiotensin converting enzyme inhibitors (ACEI) is a medication prescribed basically to deal with high BP (hypertension) and congestive HF. This class of medications relaxes blood vessels as well as decreases blood volume, which leads to lower BP and diminished oxygen demand from the heart. ACEI can be classified into three main groups based on their molecular structures. These include phosphonate-containing agents, dicarboxylate, and sulfhydryl. Experimental data support the application of ACEI in preventing interstitial fibrosis, cardiac hypertrophy, and coronary perivascular and myocardial mechanical dysfunction associated with DCM [71]. ACEI can also help recover insulin action at the molecular level. ACEI improves the symptoms and signs of DCM by mounting insulin-induced glucose uptake in skeletal muscle cells. ACEI also encourage correcting the position of GLUT-4 and post-receptor insulin signaling in the cell membrane. Since ACEI lessen the arteriolar resistance and enlarge venous capacity, they consequently increase the cardiac index and cardiac output. ACEI are predominantly functional in suppressing renovascular resistance. This will lead to augmented natriuresis and reduce the workload on the heart. As per this, ACEI are the first line of medications in the management of LV dysfuntion and congestive HF, all part of signs of DCM [72].
Angiotensin II receptor antagonists
Angiotensin II (Ang II) is considered to be a main player in increasing cardiac dysfunction. Ang II-blockers (ARB) have provided cardiovascular protection to DM patients. Candesartan enhances the echocardiographic constraints of decreased collagen synthesis, diastolic dysfunction, and augmented collagen dilapidation in asymptomatic DM subjects [73]. Ang II receptor competitors, conversely, obstruct the action of Ang II at the receptor level. The two groups of medications share parallel functions. Ang II receptor antagonists, such as condensed LV dysfunction and irbesartan, normalized the matrix metalloproteinase and cardiac fibrosis in C57/bl6 mice, an animal model of DCM evaluated with DM animals [74]. Ang II receptor antagonists are used when jointed has advanced to either agent unaccompanied. An amalgamation of ACEI may be of advantage for the treatment of persistant HF, resulting from DCM, and might lower the disease and ventricular remodeling associated with DCM [75].
Calciumion channel antagonists
Calciumion (Ca2+) blockers can overturn the intracellular Ca defects and avert DCM. Verapamila Ca2+ blocker significantly develops the condensed rate of reduction and the rate of relaxation, lowers peak LV systolic pressure, and raises the left ventricular diastolic pressure [76]. Ca2+ blockers can be classified into three major classes: A: phenylalkylamines, B: benzothiazepines, and C: dihydropyridines. Dihydropyridines, such as amlodipine, clinidipine, and azelnidipine, are a cluster of Ca2+ blockers used to decrease arterial pressure and systemic vascular resistance, but hardly have ever been used to treat angina [77].
Hydroxymethylglutaryl CoA reductase inhibitors (Statins)
Statins are largely antihyperlipidemic drugs, which act as the rate-limiting factor in the synthesis of cholesterol, and play an important role in the diminution of ischemic heart disease. Statins like fluvastatin and rosuvastatin are important in the management of DCM as they re-establish endothelial function and reduce platelet aggregation and inflammatory mediators [78].
Limitations or adverse effects of synthetic drugs
Inspite of increasing frequency of DM and CVD, at present not enough safe and effective medications are available in the market and even the existing ones have substantial adverse effects (Table 1). Some of these medications have even been withdrawn from the market owing to side effects. Despite the huge selection of pharmacological agents at the disposal of the practitioners, they are uncertain to identify the fundamental causes of DM, specifically, insulin resistance and the progressive weakness in pancreatic β cell function. The death rates from the syndrome are nothing short of disquieting, with DM and its associated complications, especially DCM, causing one death every 6 s. DM places enormous economic pressure on already overstretched health care budgets both in the developing as well as developed countries. The number and class of oral hypoglycemic agents permitted for human use in T2DM have grown drastically over the past two decades, yet such interferences, which mainly aspire to accomplish and maintain euglycemic levels, are yet to reach the quality levels required clinically. This is reproduced by their limited efficiency, which translates to lowering glycemic control exposing the patients to a broad range of macrovascular or microvascular complications, eventually leading to early death [87].
Natural products to combat DCM
Because of huge costs involved and adverse effects associated, there is an increasing shift to natural product-based drugs, where phytochemicals function effectively. Dietary connections and plant-based remedies belonging to the native systems of medicine have higher attractiveness for treating DM. On the other hand, plants are a source of plentiful of medicines, are cost-effective, and have no or limited side effects when compared with artificial drugs. Thus, there is a shift in favor of plant-based medicines to treat all diseases, including DM. As medicinal plants have turned out to be the future sources of a number of medications and formulations, researchers propose to isolate pharmaceutically active composites to find out a superior stratagy for the treatment and counteractive alleviation of DM with diet control and exercise [88]. In ancient Indian and Chinese systems of medicine, many herbal and plant-based formulations have been cited to have the power to provide cardioprotective and cardiotonic effects and are used to cure arterial, stroke, dyslipidemia, and hypertension.
S-Allylcysteine
S-Allylcysteine (SAC) is a sulfur-containing amino acid derived from garlic (Allium sativum) and has abundance of favorable effects, such as antilipidemic action, antioxidant function, and radical scavenging. SAC is produced from the amino acid, cysteine, in which an allyl group has been added to the sulfur atom [89]. SAC upregulates the activities of fructose-6-phosphatase and glucose-6-phosphatase enzymes in hepatic tissues through insulin release and thus improves the deployment of glucose for cellular biosynthesis, which is noticeable by the significant decrease in plasma glucose. Saravanan et al. [90] reported that SAC has antihyperglycemic effect similar to insulin in the STZ-NAD-induced DM rats and also reversed the alterations in the levels of glucose metabolism in liver by altering the metabolic enzymes. SAC has ameliorated hyperglycemia-induced oxidative stress and endothelial dysfunction by its oral supplementation (150 mg/kgBW) by making NO bioavailable. In addition, SAC restored the changes in glucose, insulin, insulin resistance, and antioxidant levels of aorta and mRNA expression of NOS, argininosuccinate lyase, and argininosuccinate synthase in experimental DM rats. Consequently, SAC is proven to have antidiabetic capability by mitigating hyperglycemia, varying insulin resistance by assuaged endothelial dysregulation in both tissues and plasma. So, SAC might prevent DCM associated with DM [30].
Alpha-lipoic acid
1,2-Dithiolane-3-pentanoic acid or thioctic acid (ALA) is a naturally occurring constituent implicated in mitochondrial dehydrogenase reactions, is highly concentrated, and is an ideal antioxidant. Experimental studies have confirmed that intraperitoneal supplementation of ALA to STZ-induced DM Wistar rats normalized the levels of thiobarbituric acid reactive substances in pancreas, retina, liver, and plasma. In addition, in CVD, dietary administration with ALA has been effectively used in diverse in vivo models: HF, hypertension, and ischemia reperfusion [91]. Chun et al. reported that the administration of ALA to STZ-induced DCM rats has shown increased heart rate, decreased cardiac dysfunction, and collagen deposition in heart tissues; they also found the support of augmented cardiac fibrosis, as suggested by increased tissue inhibitor of metalloproteinases 2, α-smooth muscle actin, TGF-β, and squashed matrix mettallo protein-2 activity in the DCM rats, all these being alleviated by ALA supplementation. The study revealed that attenuated MPAK signaling pathway activation after ALA treatment established that abridged MPAK signaling may contribute to defending the outcome of ALA. The studies suggest that ALA supplementation may correspond to a sensible approach for limiting the development of DCM. In addition, these results, together with excellent tolerability and safety profile of ALA in humans, reveal that ALA may have potential benefits in the management of DCM by attenuating extracellular matrix remodeling, mitochondrial oxidative stress, JNKs, and p38 MAPK activation [92].
Resveratrol (RL)
RL is an innate polyphenol that occurs in several plant-based beverages and foods normally found in our diets. As a non-flavonoid polyphenolic ingredient, RL is a powerful free radical scavenger and antioxidant [93], and has favorable effect in CVD, including ischemic heart diseases, atherosclerosis, hypertension, and HF [94]. Shuang et al. found that when RLwas applied to the cell coverage to high glucose, apoptotic cell injury and death were significantly minimized. The amplified ratio of Bax/Bcl-2 has been upturned by the addition of RL to cardiomyocytes exposed to high glucose. In addition, endeavors have been made to identify the pathways involved in the beneficial effect of RL on cardiacmyocytes in high glucose. RL prevented high glucose-induced apoptosis by restraining NADPH-derived ROS formation and ameliorating the diminution of cardiac antioxidant enzyme activities. These effects were perhaps regulated by AMPK-associated signaling pathway establishing the remedial potential of RL in the management of DCM [95].
Curcumin
Curcumin is a constituent of Curcuma longa (turmeric), and yellow curry pigment extracted from turmeric powder. It has several beneficial effects, such as scavenging of free radicals, as an antioxidant, as a delmucent; it also can reverse hyperglycemia, hyperlipidemia, insulin resistance, and obesity [96]. Wei et al. show decline in systolic and diastolic myocardial concert in 16-week diabetic rats with cardiac hypertrophy and surplus collagen accumulation. In addition, there is accumulation of amplified AGEs with prominent expression of RAGE, improved myocardial expression of NADPH oxidase isoforms p47phox, Rac1, and gp91phox activity, and attenuated antioxidant defense with inactivation of pro-survival pathways of Akt/GSK-3b, increased myocardial lipid peroxidation eventually concluding in cell death, and increased inflammation and cardiac fibrosis in DM heart. Supplementation of curcumin (200 mg/kg BW/day) prevented the growth of the above characteristic changes in DCM [97].
Oleanolic acid
Oleanolic acid (OL) is a pentacyclic triterpenoid ((3P)-3-hydroxy-olean-12-en-28-oic acid) with an innate chemical compound formed from combined or free glycosides. It occurs extensively in plants, such as white Hedyotis diffusa, Crataegus monogyna fruit, Syzygium aromaticum, Carica papaya, Prunella vulgaris, and Ligustrum lucidum. Studies have confirmed that OL has effective kidney and liver protective properties, blocks platelet aggregation, and has hypoglycemic, anti-inflammatory, anti-microbial, anti-cancer, hypolipidemic, anti-stress, and anti-ulcer properties [98]. Wei-Fang et al. [99] mention the protective effects of OL against DCM through the inhibition of DCM-induced heart rate alterations and body weight, enhanced hemodynamic and echocardiographic measurements, and condensed blood glycogen levels in DCM rats. HO-1/Nrf2 signaling pathway also has a significant role in OL fortification against cardiac injury through insulin modulation of GS/GP signaling pathway with DCM. These results suggest that OL can be an ideal restorative management solution for DCM.
Astragalus polysaccharides
Astragalus polysaccharides (APS) are isolates of Astragalus membranaceus and are considered the foremost constituent with antidiabetic action. Several studies show that they can lower lipid and blood glucose levels, and reverse insulin resistance in DM rats [100]. APS could alter lipid and glucose metabolism through the PPAR-α pathway and diminish cardiac fibrosis through containment of local cardiac chymase-ang II system [101]. APS also plays a role in the management of DM complications such as DCM. Shuqin et al. documented that pretreatment with APS reduced the apoptotic rate and amplified cell viability in high glucose-stimulated H9C2 cells, and suggested that APS might have an anti-apoptotic role in cardiomyocytes. After pretreatment of H9C2 cells with APS, the release of cytochrome C into cytoplasm and expression of caspase 3, 8, 9 diminished. It was also reported that APS confined cells from apoptosis by amending anti (pro)-apoptotic proteins of both extrinsic and intrinsic pathways. Excess glucose could encourage apoptosis in H9C2 cells by reducing the ratio of Bcl-2to Bax and upregulating Bax in mitochondria. After pretreatment of cells with APS, the expression of Bcl-2 in mitochondria augmented the expression of Bax in mitochondria reduced and the ratio of Bcl-2 to Bax enhanced. These data point out that APS could restrain high glucose-induced apoptosis [102]. Hence, the anti-apoptotic effect of APS could be a significant curative agent in the management of DCM.
Troxerutin
Troxerutin is a flavonol, a kind of flavonoid and exactly a hydroxyethylrutoside, and can be secluded from Sophora japonica. Yongzhi and Guanzhong [103] reported the prospective use of troxerutin to fight DCM on a rat model of T2DM, through changes to NF-κB expression. Troxerutin (150 mg/kg BW) supplementation notably reduced BP, heart rate, plasma triglyceride, and blood glucose levels athwart all calculated time points. In addition, troxerutin considerably condensed ROS levels, NF-κB expression, and covert phosphorylated forms of AKT, JNK, and insulin receptor substrate 1. These results suggest that troxerutin protects against DCM through alterations to AKT, insulin receptor substrate 1 signaling, and NF-κB.
Sulforaphane
Sulforaphane (SFN) is a molecule of isothiocyanate group of organosulfur compounds from cruciferous vegetables, such as sprouts broccoli or Brussel cabbage. Broccoli is an edible green plant in the cabbage group whose giant flowering head is consumed as a vegetable. Among all the cruciferous vegetables, broccoli stands out as the widely held concentrated source of vitamin C, plus the flavonoids essential for vitamin C to reprocess powerfully [104]. SFN is an efficient Nrf2 activator and has successfully prevented various DM complications in many animal models. Supplementation of SFN for 3 months might significantly ward off the pathological development of DCM in T2DM, and also considerably increases the Nrf2 function and expression to prevent DM-induced cardiac oxidative stress and inflammation. Zhang et al. discuss about the strength provided by SFN adjoining DCM via upregulation of Nrf2 transcription expression and activation, and subsequently avert oxidative stress and damage in both STZ-induced T1DM and T2DM models [105].
Dietary phenolic acids
Phenolic acids are a group of phenolic compounds that are comprehensively distributed in the food, principally in vegetables, whole grains, beverages, and fruits. Epidemiological examination has suggested association between the consumption of phenolic acid-rich foods or beverages and deterrence to numerous diseases [106]. These compounds display greater in vitro antioxidant and chemoprotective activities. Different naturally existing phenolic acids and analogs, namely ferulic and gallic acids, demonstrate a wide array of innate functions, in addition to their essential antioxidant property [107]. Leung et al. [108] discovered the molecular mechanisms essential to phenolic acid-regulated chages of antioxidant phase II enzymes in the heart tissue of the rat. It was shown that Nrf2 activates the ARE and in turn mediates the expression of antioxidant phase II detoxifying enzymes. Further, importantly, Nrf2 knockout mice have much poorer levels of phase II detoxifying enzymes and are additionally susceptible to oxidative stress than wild-type animals. The quantum of cardiac Nrf2 expression is considerably increased with phenolic acid supplementation, suggesting that enhanced expression of Nrf2 can be associated with phenolic acid-induced HO-1 gene establishment. Therefore, the administration of natural phenolic acids through an independent diet containing sufficient fruits and vegetables might be the most ideal practice for inducing cardiac phase II antioxidant enzymes and might assist in the management of DCM.
Polyphenols
Polyphenols occur in plant-derived beverages and foods. Experimental studies point out that innate polyphenols have several defensive effects against CVD. In addition, they divide into different sub-groups to improve fibrosis following cardiac injury and cardiac dysfunction [109]. Quercetin, one of the most extensively circulated flavonols, is rich in citrus fruits, grains, red onions, and many other edible stuff of plant base. Quercetin found in nutritional bioflavonoids co-exists with its glycoside-derived rutin. Research has shown that in the animal model of cardiac fibrosis, the supplementation of rutin and quercetin or lone quercetin alleviated myocardial injury and cardiac dysfunction induced by isoproterenol and prevented cardiac fibrosis by inhibiting TGF-β1, ECM, and connective tissue growth factor accumulation [110]. Panchal et al. found that a mold of an obese rat being fed high-fat diet supplemented with quercetin prevented cardiac remodeling by promoting Nrf-2 and reverse the NF-κB signaling pathway [111]. Taxifolin, a quercetin derivative, has the capability to rounded cardiac fibrosis provoked by excess pressure, and the mechanism fundamental to anti-fibrotic effect of taxifolin relies on the inhibition of TGF-β/Smad signaling pathway [112]. Isorhamnetin, another constituent of flavonols, cures cardiac fibrosis and hypertrophy caused by aortic banding [113]. Luteolin is a flavone isolated from onion, broccoli, cauliflower, and thyme. It inhibits cardiac fibrosis propagation through the diminution of oxidative stress, the mechanism underlying the inhibition of NOX4 and NOX2 in cardiac hypertrophy, thus decreasing the phosphorylation of TGF-β1 and JNK expression and ameliorating cardiac fibrosis [114]. Accordingly, these studies recommend the intake of polyphenols to alleviate DCM.
Nutraceuticals
Nutraceuticals are in early stages of use in the management of DM, a challenging ailment responsible for a large number of deaths all over the globe. In modern times, attention is increasing over nutraceuticals for their health benefits and substitution role to contemporary medicine. Nutrients and dietary and herbal supplements are major ingredients in nutraceuticals, which make them effective in maintaining health, improve the qulity of life, and act against a range of diseases [115,116,117,118,119].
Sesbania grandiflora
Ghanshyam et al. [120] found that S. grandiflora leaves (400 mg/kg BW) have effective antihyperglycemic activity in glucose-induced hyperglycemic rats. Nandi et al. [121] found that the S. grandiflora fruit (400 mg/kg BW) extract has hypoglycemic agents, which considerably reduced the levels of blood glucose, triglyceride, cholesterol, and LDL; lipid peroxidation drastically reduced; and catalase and superoxide dismutase considerably increased. Sangeetha et al. [122] have shown that S. grandiflora leaves (300 mg/kg BW) have antidiabetic activity in STZ-induced DM rats, which restored all the biochemical parameters, such as glucose, blood urea nitrogen, glycosylated hemoglobin, creatinine uric acid, aspartate and alkaline phosphatase, alanine transaminase, and glycogen content. S. grandiflora has significant hypoglycaemic, antilipidimic, and antioxidants effectiveness, and might alleviate DCM.
Abroma augusta
Abroma augusta, an evergreen bush, is grown throughout the wet and warm parts of India. Its seeds and leaves are considered safe to consume in New Guinea and India. It has a comprehensive account in traditional Ayurvedic system. Its leaves are used as a remedy for headache, inflammation, rheumatic pain of joints, uterine disorders, and DM. The entire plant has flavonoids, steroids, triterpenes, megastigmanes, phenylethanoid glycosides, and alkaloids [123]. Ritu et al. found that its oral supplementation (100 and 200 mg/kg BW/day) could reduce membrane disintegration, hyperlipidemia, oxidative stress, vascular inflammation, hyperglycemia, and prevent the activation of oxidative stress-induced signaling pathways [124]. Thus, its administration decreased related inflammatory responses and oxidative stress of the heart providing cardioprotection in T2DM rats. This might be due to its antioxidant effect concurrent to the presence of flavonoids and phenolic compounds, while the anti-inflammatory effect might be appropriate to taraxerol. Moreover, A. augusta can reduce the NF-κB, mitochondria-dependent apoptotic signaling cascades, and PKCs activation. With these benefits and lack of any unfavorable effects, A. augusta may be used as a supplement to cure T2DM and DCM.
Panax quinquefolius (North American ginseng)
Ginseng is a conventional therapeutic plant with several pharmacological activities. Ginsenosides are its main bioactive secondary metabolites. Its bioactives possess scavenging free radicals and anti-antioxidants, inhibiting lipid peroxidation, and protecting LDL from oxidation [125]. Subhrojit et al. [126] reported that oral administration of alcoholic ginseng root (200 mg/kg BW/day) extract to both T1DM and T2DM mice over 2–4 months significantly ameliorated dysmetabolic state in the DM mice. In the heart of DM animals, ginseng supplementation drastically reduced vasoactive factors, DM-induced upregulation of ECM proteins and oxidative stress. Ginseng supplementation to DM animals created an improvement in ejection fraction, cardiac output, stroke volume, and LV pressure during diastole and systole and a reduction in stroke work. On the other hand, mRNA expression of brain natriuretic factor and atrial natriuretic factor were appreciably reduced in ginseng-supplemented DM mice. These data support that ginseng can ameliorate diabetes-induced DCM perhaps through the inhibition of oxidative stress. Therefore, ginseng may potentially be a reasonably inexpensive and safe adjuvant for the management of DCM.
Allium sativum (garlic) and aged garlic
Garlic is a variety in the onion species and is inhabitant of Central Asia and has long been a frequent seasoning globally, with a history of many centuries of human consumption and use as a food ingredient. Utilization of garlic and its constituents has been documented to have countless beneficial properties together with antidiabetic and antilipidemic activities. Its antidiabetic property is well known in miscellaneous animal models of T1DM andT2DM [127]. There is evidence of effectiveness of unrefined garlic in lowering hyperglycemia and for treating cardiac complications in T2DM animals. Garlic treatment enhanced the myocardial Nrf2 expression and this associated with amplified myocardial Mn-superoxide dismutase expression. Along with Mn-superoxide dismutase gene expression, garlic administration significantly improved the myocardial superoxide dismutase, glutathione peroxidase, glutathione levels, and catalase activity. Effects of garlic on (A) immunoblotting of Nrf2 and keap1 and (B) gene expression of Nrf2 and endogenous antioxidant after garlic supplementation in fructose-fed rats may be responsible for the attenuation of oxidative stress and cardiac hypertrophy [128]. Administartion of raw garlic homogenate in T2DM rat turned on myocardial Nrf2 through H2S and PI3K/AKT pathway supporting the view that garlic ameliorates DCM.
Among a diversity of garlic preparations available, aged garlic extract (AGE) has a distinguishing method to extract the enrichment of water-soluble cysteinyl moieties and it has no toxic side effects. AGE and its components have been acknowledged to have beneficial pharmacological effects [129]. AGE increases the expression of antioxidant enzymes through the Nrf2–ARE pathway in human umbilical vein endothelial cells (HUVECs) [130]. Sun et al. [131] reported that AGE develops the expression of both HO-1 and glutamate-cysteine ligase modifier genes in HUVECs. Diallyl sulfide and allicin, the ingredients of raw garlic, activate Nrf2 and increase NQO1 and HO-1 expression via the ERK/p38 pathway. AGE contains water-soluble sulfur-containing compounds, such as S-allyl mercaptocysteine and SAC. Till date, garlic and its preparations have mostly been known as a beneficial dietary agent in the management of DCM [132].
Conclusion and future perceptions
DCM, attributed by functional and structure alterations, is a complex disease. Widespread preclinical studies examined the molecular targets for its pathogenesis. Modern research in animals and humans has offered novel insights into fundamental pathophysiological and molecular mechanisms that raise the susceptibility of the DM heart to malfunction. As the mechanisms accounting for DCM are explicated, they will provide ideal treatments to diminish the risk of HF in patients with DM. Treatment of DCM is based on the common remedial rules of HF and no specific treatment have yet been approved. For that reason, additional research is necessary to advance our awareness of this multifaceted syndrome. Some medications have been produced to treat DCM, but most of them have significant adverse effects and some have even been recalled from the markets. In view of this, there is a mounting need for natural product-based remedies. Several epidemiological experiments have found that the use of natural products reduces the risk of suffering from chronic diseases. Increased intake of vegetables and fruits may influence the carbohydrate and lipid metabolisms and regulate the genes related to pathophysiology of DCM which results in alleviating CVD, myocardial infraction, and cardiovascular death. A nutritional increment of natural compounds affords a suitable mode to avert the incidence of DCM. Some developments have been made in natural product research in animal models to control DCM. On the other hand, the major shortcoming with therapeutic plants and products is that several of them require convincing scientific proof and clinical validation. Primarily, clinical trials must be conducted to assess their beneficial effect, toxicity, dose, bioavailability, and absorption of natural products. Therefore, an understanding of the anti-DCM targets of natural products and improved screening methods, their structure-activity relationship, and clinical trials are essential to identify the prospective way for novel drug development.
References
Aneja A, Tang W, Bansilal S et al (2008) Diabetic cardiomyopathy: insights into pathogenesis, diagnostic challenges, and therapeutic options. Am J Med 121:748–757. https://doi.org/10.1016/j.amjmed.2008.03.046
Rubler S, Dlugash J, Yuceoglu YZ (1972) New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol 30:595–602. https://doi.org/10.1016/0002-9149(72)90595-4
Bugger H, Abel ED (2014) Molecular mechanisms of diabetic cardiomyopathy. Diabetologia 57:660–671. https://doi.org/10.1007/s00125-014-3171-6
Kohli S, Chhabra A, Jaiswal A, Rustagi Y, Sharma M, Rani V (2013) Curcumin suppresses gelatinase B mediated norepinephrine induced stress in H9C2 cardiomyocytes. PLoS One 8:76519. https://doi.org/10.1371/journal.pone.0076519
Alonso N, Moliner P, Mauricio D (2018) Pathogenesis, clinical features and treatment of diabetic cardiomyopathy. Adv Exp Med Biol 1067:197–217. https://doi.org/10.1007/5584_2017_105
WHO (2016) Global report on diabetes, ISBN 978 92 4 156525 7
Goyal BR, Mehta AA (2012) Diabetic cardiomyopathy: pathophysiological mechanisms and cardiac dysfunction. Hum Exp Toxicol 32:1–20. https://doi.org/10.1177/0960327112450885
Li B, Zheng Z, Wei Y, Wang M, Peng J, Kang T, Huang X, Xiao J, Li Y, Li Z (2011) Therapeutic effects of neuregulin-1 in diabetic cardiomyopathy rats. Cardiovasc Diabetol 10:69. https://doi.org/10.1186/1475-2840-10-69
Saravanan G, Ponmurugan P, Sathiyavathi M, Vadivukkarasi S, Sengottuvelu S (2013) Cardioprotective activity of Amaranthus viridis Linn: effect on serum marker enzymes, cardiac troponin and antioxidant system in experimental myocardial infarcted rats. Int J Cardiol 165:494–498. https://doi.org/10.1016/j.ijcard.2011.09.005
Bodiga VL, Eda SR, Bodiga S (2014) Advanced glycation end products: role in pathology of diabetic cardiomyopathy. Heart Fail Rev 19:49–63. https://doi.org/10.1007/s10741-013-9374-y
Sathibabu Uddandrao VV, Brahmanaidu P, Nivedha PR, Vadivukkarasi S, Saravanan G (2018) Beneficial role of some natural products to attenuate the diabetic cardiomyopathy through Nrf2 pathway in cell culture and animal models. Cardiovasc Toxicol 18:199–205. https://doi.org/10.1007/s12012-017-9430-2
Khan JN, Wilmot EG, Leggate M (2014) Subclinical diastolic dysfunction in young adults with type 2 diabetes mellitus: a multiparametric contrast-enhanced cardiovascular magnetic resonance pilot study assessing potential mechanisms. Eur Heart J Cardiovasc Imaging 15:1263–1269. https://doi.org/10.1093/ehjci/jeu121
Fuentes-Antras J, Ioan AM, Tunon J, Egido J, Lorenzo O (2014) Activation of toll-like receptors and inflammasome complexes in the diabetic cardiomyopathy-associated inflammation. Int J Endocrinol 2014:847827. https://doi.org/10.1155/2014/847827
Heiko Bugger DAE (2009) Rodent models of diabetic cardiomyopathy. Dis Model Mech 2:454–466. https://doi.org/10.1242/dmm.001941
Coort SL, Hasselbaink DM, Koonen DP et al (2004) Enhanced sarcolemmal FAT/CD36 content and triacylglycerol storage in cardiac myocytes from obese zucker rats. Diabetes 53:1655–1663. https://doi.org/10.2337/diabetes.53.7.1655
Wang P, Lloyd SG, Zeng H, Bonen A, Chatham JC (2005) Impact of altered substrate utilization on cardiac function in isolated hearts from Zucker diabetic fatty rats. Am J Physiol Heart Circ Physiol 288:2102–2110. https://doi.org/10.1152/ajpheart.00935.2004
Symons JD, McMillin SL, Riehle C, Tanner J, Palionyte M et al (2009) Contribution of insulin and Akt1 signaling to endothelial nitric oxide synthase in the regulation of endothelial function and blood pressure. Circ Res 104:1085–1094. https://doi.org/10.1161/CIRCRESAHA.108.189316
Wright JJ, Kim J, Buchanan J, Boudina S, Sena S, Bakirtzi K, Ilkun O, Theobald HA, Cooksey RC, Kandror KV, Abel ED (2009) Mechanisms for increased myocardial fatty acid utilization following short-term high-fat feeding. Cardiovasc Res 82:351–360. https://doi.org/10.1093/cvr/cvp017
Rennison JH, McElfresh TA et al (2009) Prolonged exposure to high dietary lipids is not associated with lipotoxicity in heart failure. J Mol Cell Cardiol 46:883–890. https://doi.org/10.1016/j.yjmcc.2009.02.019
Ahmed SS, Jafer GA, Narang RM et al (1975) Preclinical abnormality of left ventricular function in diabetes mellitus. Am Heart J 89:153–158. https://doi.org/10.1016/0002-8703(75)90039-3
Fang ZY, Najos-Valencia O, Leano R, Marwick TH (2003) Patients with early diabetic heart disease demonstrate a normal myocardial response to dobutamine. J Am Coll Cardiol 42:46–53. https://doi.org/10.1016/S0735-1097(03)00654-5
Thompson GR, Partridge J (2004) Coronary calcification score: the coronary-risk impact factor. Lancet 363:557–559. https://doi.org/10.1016/S0140-6736(04)15544-X
Ficaro EP, Corbett JR (2004) Advances in quantitative perfusion SPECT imaging. J Nucl Cardiol 11:62–70. https://doi.org/10.1016/j.nuclcard.2003.10.007
Schinkel AF, Bax JJ, Valkema R, Elhendy A, van Domburg R, Vourvouri EC, Bountioukos MA, Krenning EP, Roelandt JR, Poldermans D (2003) Effect of diabetes mellitus on myocardial 18F-FDG SPECT using acipimox for the assessment of myocardial viability. J Nucl Med 44:877–883
Soriano FG, Pacher P, Mabley J, Liaudet L, Szabo C (2001) Rapid reversal of the diabetic endothelial dysfunction by pharmacological inhibition of poly(ADP-ribose) polymerase. Circ Res 89:684–691. https://doi.org/10.1161/hh2001.097797
Coats AJ, Anker SD (2000) Insulin resistance in chronic heart failure. J Cardiovasc Pharmacol 35:9–14. https://doi.org/10.1016/j.jacc.2008.03.044
Stanley WC, Marzilli M (2003) Metabolic therapy in the treatment of ischaemic heart disease: the pharmacology of trimetazidine. Fundam Clin Pharmacol 17:133–145. https://doi.org/10.1046/j.1472-8206.2003.00154.x
Turan B (2010) Role of antioxidants in redox regulation of diabetic cardiovascular complications. Curr Pharm Biotechnol 11:819–836. https://doi.org/10.2174/138920110793262123
Brunner F, Bras-Silva C, Cerdeira AS, Leite-Moreira AF (2006) Cardiovascular endothelins: essential regulators of cardiovascular homeostasis. Pharmacol Ther 111:508–531. https://doi.org/10.1016/j.pharmthera.2005.11.001
Brahmanaidu P, Sathibabu Uddandrao VV, Saravanan G (2017) Reversal of endothelial dysfunction in aorta of streptozotocinnicotinamide-induced type-2 diabetic rats by S-Allylcysteine. Mol Cell Biochem 432:25–32. https://doi.org/10.1007/s11010-017-2994-0
Fiordaliso F, Leri A, Cesselli D, Limana F, Safai B, Nadal-Ginard B, Anversa P, Kajstura J (2001) Hyperglycemia activates p53 and p53-regulated genes leading to myocyte cell death. Diabetes 50:2363–2375. https://doi.org/10.2337/diabetes.50.10.2363
Vinereanu D, Nicolaides E, Boden L, Payne N, Jones CJ, Fraser AG (2003) Conduit arterial stiffness is associated with impaired left ventricular subendocardial function. Heart 89:449–451. https://doi.org/10.1136/heart.89.4.449
Argirova MD, Ortwerth BJ (2003) Activation of protein-bound copper ions during early glycation: study on two proteins. Arch Biochem Biophys 420:176–184. https://doi.org/10.1016/j.abb.2003.09.005
Riehle C, Wende AR, Sena S, Pires KM, Pereira RO, Zhu Y, Bugger H, Frank D, Bevins J, Chen D, Perry CN, Dong XC, Valdez S, Rech M, Sheng X, Weimer BC, Gottlieb RA, White MF, Abel ED (2013) Insulin receptor substrate signaling suppresses neonatal autophagy in the heart. J Clin Invest 123:5319–5333. https://doi.org/10.1172/JCI71171
Lakshmanan AP, Harima M, Suzuki K, Soetikno V, Nagata M, Nakamura T, Takahashi T, Sone H, Kawachi H, Watanabe K (2013) The hyperglycemia stimulated myocardial endoplasmic reticulum (ER) stress contributes to diabetic cardiomyopathy in the transgenic nonobese type 2 diabetic rats: a differential role of unfolded protein response (UPR) signaling proteins. Int J Biochem Cell Biol 45:438–447. https://doi.org/10.1016/j.biocel.2012.09.017
Frieler RA, Mortensen RM (2015) Immune cell and other noncardiomyocyte regulation of cardiac hypertrophy and remodeling. Circulation 131:1019–1030. https://doi.org/10.1161/CIRCULATIONAHA.114.008788
Li J, Ma W, Yue G, Tang Y, Kim IM, Weintraub NL, Wang X, Su H (2017) Cardiac proteasome functional insufficiency plays a pathogenic role in diabetic cardiomyopathy. J Mol Cell Cardiol 102:53–60. https://doi.org/10.1016/j.yjmcc.2016.11.013
Asghar O, Al-Sunni A, Khavandi K, Withers S et al (2009) Diabetic cardiomyopathy. Clin Sci 116:741–760. https://doi.org/10.1042/CS20080500
Tang M, Huang Li J et al (2010) Proteasome functional insufficiency activates the calcineurin-NFAT pathway in cardiomyocytes and promotesmaladaptive remodelling of stressed mouse hearts. Cardiovasc Res 88:424–433. https://doi.org/10.1093/cvr/cvq217
Ferdous A, Battiprolu PK, Ni YG, Rothermel BA, Hill JA (2010) FoxO, autophagy, and cardiac remodeling. J Cardiovasc Transl Res 3:355–364. https://doi.org/10.1007/s12265-010-9200-z
Marsh SA, DellItalia LJ, Chatham JC (2011) Activation of the hexosamine biosynthesis pathway and protein O-GlcNAcylation modulate hypertrophic and cell signaling pathways in cardiomyocytes from diabetic mice. Amino Acids 40:819–828. https://doi.org/10.1007/s00726-010-0699-8
Wollaston-Hayden EE, Harris RB, Liu B, Bridger R, Xu Y et al (2015) Global O-GlcNAc levels modulate transcription of the adipocyte secretome during chronic insulin resistance. Front Endocrinol (Lausanne) 5:223. https://doi.org/10.3389/fendo.2014.00223
Rajamani U, Essop MF (2010) Hyperglycemia-mediated activation of the hexosamine biosynthetic pathway results in myocardial apoptosis. Am J Physiol Cell Physiol 299:139–147. https://doi.org/10.1152/ajpcell.00020.2010
Gabbay KH (1973) The sorbitol pathway and the complications of diabetes. N Engl J Med 288:831–836. https://doi.org/10.1056/NEJM197304192881609
Brahmanaidu P, Sathibabu Uddandrao VV, Pothani S, Saravanan G et al (2016) Effects of S-allylcysteine on biomarkers of polyol pathway in experimental type II diabetes in rats. Can J Dia 40:442–448. https://doi.org/10.1016/j.jcjd.2016.03.006
Huynh K, Bianca C et al (2014) Diabetic cardiomyopathy: mechanisms and new treatment strategies targeting antioxidant signaling pathways. Pharmacol Ther 142:375–415. https://doi.org/10.1016/j.pharmthera.2014.01.003
Bernardo BC, Weeks KL, Pretorius L, McMullen JR (2010) Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther 128:191–227. https://doi.org/10.1016/j.pharmthera.2010.04.005
Palomer XL, Salvado E, Barroso Vazquez-Carrera M (2013) An overview of the crosstalk between inflammatory processes and metabolic dysregulation during diabetic cardiomyopathy. Int J Cardiol 168:3160–3172. https://doi.org/10.1016/j.ijcard.2013.07.150
Turner NA, Mughal RS, Warburton P et al (2007) Mechanism of TNFalpha-induced IL-1alpha, IL-1beta and IL-6 expression in human cardiac fibroblasts: effects of statins and thiazolidinediones. Cardiovasc Res 76:81–90. https://doi.org/10.1016/j.cardiores.2007.06.003
Duncan JG (2011) Peroxisome proliferator activated receptor-alpha (PPAR alpha) and PPAR gamma coactivator-1alpha (PGC-1 alpha) regulation of cardiac metabolism in diabetes. Pediatr Cardiol 32:323–328. https://doi.org/10.1007/s00246-011-9889-8
Burkart EM, Sambandam N, Han X, Gross RW, Courtois M, Gierasch CM, Shoghi K, Welch MJ, Kelly DP (2007) Nuclear receptors PPAR beta/delta and PPAR alpha direct distinct metabolic regulatory programs in the mouse heart. J Clin Invest 117:3930–3939. https://doi.org/10.1172/JCI32578
Luo J, Wu S, Liu J, Li Y, Yang H, Kim T, Zhelyabovska O, Ding G, Zhou Y, Yang Y, Yang Q (2010) Conditional PPARgamma knockout from cardiomyocytes of adult mice impairs myocardial fatty acid utilization and cardiac function. Am J Transl Res 3:61–72
Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, Kevil CG, Lefer DJ (2009) Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res 105:365–374. https://doi.org/10.1161/CIRCRESAHA.109.199919
Zhang Z, Wang S, Zhou S, Yan X, Wang Y, Chen J, Mellen N, Kong M, Gu J, Tan Y, Zheng Y, Cai L (2014) Sulforaphane prevents the development of cardiomyopathy in type 2 diabetic mice probably by reversing oxidative stress-induced inhibition of LKB1/AMPK pathway. J Mol Cell Cardiol 77:42–52. https://doi.org/10.1016/j.yjmcc.2014.09.022
Marber MS, Rose B, Wang Y (2011) The p38 mitogen-activated protein kinase pathway-a potential target for intervention in infarction, hypertrophy, and heart failure. J Mol Cell Cardiol 51:485–490. https://doi.org/10.1016/j.yjmcc.2010.10.021
Min W, Bin ZW, Quan ZB, Hui ZJ, Sheng FG (2009) The signal transduction pathway of PKC/NF-κB/c-fos may be involved in the influence of high glucose on the cardiomyocytes of neonatal rats. Cardiovasc Diabetol 8(1):8. https://doi.org/10.1186/1475-2840-8-8
Bernardo BC, Charchar FJ, Lin RC, McMullen JR (2012) A microRNA guide for clinicians and basic scientists: background and experimental techniques. Heart Lung Circ 21:131–142. https://doi.org/10.1016/j.hlc.2011.11.002
Kartha RV, Subramanian S (2010) MicroRNAs in cardiovascular diseases: biology and potential clinical applications. J Cardiovasc Transl Res 3:256–270. https://doi.org/10.1007/s12265-010-9172-z
Friedman RC, Farh KK, Burge CB, Bartel DP (2009) Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19:92–105. https://doi.org/10.1101/gr.082701.108
Jeyabal P, Thandavarayan RA, Joladarashi D, Suresh Babu S, Krishnamurthy S, Bhimaraj A, Youker KA, Kishore R, Krishnamurthy P (2016) Micro- RNA-9 inhibits hyperglycemia-induced pyroptosis in human ventricular cardiomyocytes by targeting ELAVL1. Biochem Biophys Res Commun 471:423–429. https://doi.org/10.1016/j.bbrc.2016.02.065
Raut SK, Kumar A, Singh GB, Nahar U, Sharma V, Mittal A, Sharma R, Khullar M (2015) miR-30c mediates upregulation of Cdc42 and Pak1 in diabetic cardiomyopathy. Cardiovasc Ther 33:89–97. https://doi.org/10.1111/1755-5922.12113
Li X, Du N, Zhang Q et al (2014) MicroRNA-30d regulates cardiomyocyte pyroptosis by directly targeting foxo3a in diabetic cardiomyopathy. Cell Death Dis 5:1479. https://doi.org/10.1038/cddis.2014.430
Von Bibra H, Siegmund T, Hansen A et al (2007) Augmentation of myocardial function by improved glycemic control in patients with type 2 diabetes mellitus. Dtsch Med Wochenschr 132:729–734. https://doi.org/10.1055/s-2007-973608
Konduracka E, Gackowski A, Rostoff P, Galicka-Latala D, Frasik W, Piwowarska W (2007) Diabetes-specific cardiomyopathy in type 1 diabetes mellitus: no evidence for its occurrence in the era of intensive insulin therapy. Eur Heart J 28:2465–2471. https://doi.org/10.1093/eurheartj/ehm361
Nguyen NDT, Le LT (2012) Targeted proteins for diabetes drug design. Adv Nat Sci Nanosci Nanotechnol 3:1–9. https://doi.org/10.1088/2043-6262/3/1/013001
Tiwari N, Thakur AK, Kumar V, Dey A, Kumar V (2014) Therapeutic targets for diabetes mellitus: an update. Pharmacol Biopharm 3:117. https://doi.org/10.4172/2167-065X.1000117
Lebovitz HE (1997) Alpha-Glucosidase inhibitors. Endocrinol Metab Clin N Am 26:539–551. https://doi.org/10.1016/S0889-8529(05)70266-8
Andreadis EA, Katsanou PM, Georgiopoulos DX, Tsourous G, Yfanti G, Gouveri E, Diamantopoulos E (2009) The effect of metformin on the incidence of type 2 diabetes mellitus and cardiovascular disease risk factors in overweight and obese subjects the Carmos study. Exp Clin Endocrinol Diabetes 117:175–180. https://doi.org/10.1055/s-0028-1087177
Xie Z, Lau K, Eby B, Lozano P, He C, Pennington B, Li H, Rathi S, Dong Y, Tian R, Kem D, Zou MH (2011) Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes 60:1770–1778. https://doi.org/10.2337/db10-0351
Young LH (2003) Insulin resistance and the effects of thiazolidinediones on cardiac metabolism. Am J Med 115:75–80. https://doi.org/10.1016/j.amjmed.2003.09.013
Zaman AK, Fujii S, Goto D, Furumoto T, Mishima T, Nakai Y, Dong J, Imagawa S, Sobel BE, Kitabatake A (2004) Salutary effects of attenuation of angiotensin ii on coronary perivascular fibrosis associated with insulin resistance and obesity. J Mol Cell Cardiol 37:525–535. https://doi.org/10.1016/j.yjmcc.2004.05.006
Adeghate E, Kalasz H, Veress G, Teke K (2010) Medicinal chemistry of drugs used in diabetic cardiomyopathy. Curr Med Chem 17:517–551. https://doi.org/10.2174/092986710790416281
Kawasaki D, Kosugi K, Waki H, Yamamoto K, Tsujino T, Masuyama T (2007) Role of activated renin-angiotensin system in myocardial fibrosis and left ventricular diastolic dysfunction in diabetic patients–reversal by chronic angiotensin ii type 1a receptor blockade. Circ J 71:524–529. https://doi.org/10.1253/circj.71.524
Westermann D, Rutschow S, Jager S, Linderer A, Anker S, Riad A, Unger T, Schultheiss HP, Pauschinger M, Tschope C (2007) Contributions of inflammation and cardiac matrix metalloproteinase activity to cardiac failure in diabetic cardiomyopathy: the role of angiotensin type 1 receptor antagonism. Diabetes 56:641–646. https://doi.org/10.2337/db06-1163
Solomon SD, Skali H, Anavekar NS, Bourgoun M, Barvik S, Ghali JK, Warnica JW, Khrakovskaya M, Arnold JMO, Schwartz Y, Velazquez EJ, Califf RM, McMurray JV, Pfeffer MA (2005) Changes in ventricular size and function in patients treated with valsartan, captopril, or both after myocardial infarction. Circulation 111:3411–3419. https://doi.org/10.1161/CIRCULATIONAHA.104.508093
Fang ZY, Prins JB, Marwick TH (2004) Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev 25:543–567. https://doi.org/10.1210/er.2003-0012
Veeraveedu PT, Watanabe K, Ma M, Gurusamy N, Palaniyandi SS, Wen J, Prakash P, Wahed MII, Kamal FA, Mito S, Kunisaki M, Kodama M, Aizawa Y (2006) Comparative effects of pranidipine with amlodipine in rats with heart failure. Pharmacology 77:1–10. https://doi.org/10.1159/000091746
Rajanikant GK, Zemke D, Kassab M, Majid A (2007) The therapeutic potential of statins in neurological disorders. Curr Med Chem 14:103–112. https://doi.org/10.2174/092986707779313462
Nisbet JC, Sturtevant JM, Prins JB (2004) Metformin and serious adverse effects. Med J Aust 180:53–54
Lund A, Knop FK (2012) Worry vs. knowledge about treatment-associated hypoglycaemia and weight gain in type 2 diabetic patients on metformin and/or sulphonylurea. Curr Med Res Opin 28:731–736. https://doi.org/10.1185/03007995.2012.681639
Han S, Hagan DL, Taylor JR, Xin L, Meng W, Biller SA, Wetterau JR, Washburn WN, Whaley JM (2008) Dapagliflozin, a selective SGLT2 inhibitor, improves glucose homeostasis in normal and diabetic rats. Diabetes 57:1723–1729. https://doi.org/10.2337/db07-1472
Nicolle LE, Capuano G, Ways K, Usiskin K (2012) Effect of canagliflozin, a sodium glucose co-transporter 2 (SGLT2) inhibitor, on bacteriuria and urinary tract infection in subjects with type 2 diabetes enrolled in a 12-week, phase 2 study. Curr Med Res Opin 28:1167–1171. https://doi.org/10.1185/03007995.2012.689956
fa V d l, Lucassen PL, Akkermans RP et al (2005) Alpha-glucosidase inhibitors for patients with type 2 diabetes: results from a Cochrane systematic review and meta-analysis. Diabetes Care 28:154–163. https://doi.org/10.2337/diacare.28.1.154
Cobble M (2012) Differentiating among incretin-based therapies in the management of patients with type 2 diabetes mellitus. Diabetol Metab Syndr 4:8. https://doi.org/10.1186/1758-5996-4-8
Ferwana M, Firwana B, Hasan R, al-Mallah MH, Kim S, Montori VM, Murad MH (2013) Pioglitazone and risk of bladder cancer: a meta-analysis of controlled studies. Diabet Med 30:1026–1032. https://doi.org/10.1111/dme.12144
Campbell RK, Cobble ME, Reid TS, Shomali ME (2010) Distinguishing among incretin-based therapies. Safety, tolerability, and nonglycemic effects of incretin-based therapies. J Fam Pract 59:20–27
Kokil GR, Veedu RN, Ramm GA, Prins JB, Parekh HS (2015) Type 2 diabetes mellitus: limitations of conventional therapies and intervention with nucleic acid-based therapeutics. Chem Rev 115:4719–4743. https://doi.org/10.1021/cr5002832
Saravanan G, Ponmurugan P (2012) Antidiabetic effect of S-allylcysteine: effect on thyroid hormone and circulatory antioxidant system in experimental diabetic rats. J Diab Comp 26:280–285. https://doi.org/10.1016/j.jdiacomp.2012.03.024
Sathibabu Uddandrao VV, Brahmanaidu P, Saravanan G (2017) Therapeutical perspectives of S-allylcysteine: effect on diabetes and other disorders in animal models. Cardiovasc Hematol Agents Med Chem 15:71–77. https://doi.org/10.2174/1871525714666160418114120
Saravanan G, Ponmurugan P, Senthil kumar GP, Rajarajan T (2009) Modulatory effect of S-allylcysteine on glucose metabolism in streptozotocin induced diabetic rats. J Funct Foods 1:336–340. https://doi.org/10.1016/j.jff.2009.09.001
Ghibu S, Richard C, Vergely C, Zeller M, Cottin Y, Rochette L (2009) Antioxidant properties of an endogenous thiol: alpha-lipoic acid, useful in the prevention of cardiovascular diseases. J Cardiovasc Pharmacol 54:391–398. https://doi.org/10.1097/FJC.0b013e3181be7554
Li C-j, Lv L, Li H, Yu D-m (2012) Cardiac fibrosis and dysfunction in experimental diabetic cardiomyopathy are ameliorated by alpha-lipoic acid. Cardiovasc Diabetol 11:73. https://doi.org/10.1186/1475-2840-11-73
Li H, Xia N, Forstermann U (2012) Cardiovascular effects and molecular targets of resveratrol. Nitric Oxide 26:102–110. https://doi.org/10.1016/j.niox.2011.12.006
Dolinsky VW, Dyck JR (2011) Calorie restriction and resveratrol in cardiovascular health and disease. Biochem Biophys Acta 1812:1477–1489. https://doi.org/10.1016/j.bbadis.2011.06.010
Guo S, Yao Q, Ke Z, Chen H, Wu J, Liu C (2015) Resveratrol attenuates high glucose-induced oxidative stress and cardiomyocyte apoptosis through AMPK. Mol Cell Endocrinol 412:85–94. https://doi.org/10.1016/j.mce.2015.05.034
Shehzad A, Ha T, Subhan F, Lee YS (2011) New mechanisms and the anti-inflammatory role of curcumin in obesity and obesity-related metabolic diseases. Eur J Nutr 50:151–161. https://doi.org/10.1007/s00394-011-0188-1
Wei Y, Jiliang W, Fei C et al (2012) Curcumin alleviates diabetic cardiomyopathy in experimental diabetic rats. PLoS One 7(52013):e52013. https://doi.org/10.1371/journal.pone.0052013
Xu K, Chu F, Li G, Xu X, Wang P, Song J, Zhou S, Lei H (2014) Oleanolic acid synthetic oligoglycosides: a review on recent progress in biological activities. Pharmazie 69:483–495. https://doi.org/10.1691/ph.2014.3933
Li WF, Wang P, Li H, Li TY, Feng M, Chen SF (2017) Oleanolic acid protects against diabetic cardiomyopathy via modulation of the nuclear factor erythroid 2 and insulin signaling pathways. Exp Ther Med 14:848–854. https://doi.org/10.3892/etm.2017.4527
Agyemang K, Han L, Liu E, Zhang Y, Wang T, Gao X (2013) Recent advances in Astragalus membranaceus anti-diabetic research: pharmacological effects of its phytochemical constituents. Evid Based Complement Alternat Med 2013:654643. https://doi.org/10.1155/2013/654643
Chen W, Xia P et al (2012) Improvement of myocardial glycolipid metabolic disorder in diabetic hamster with Astragalus polysaccharides treatment. Mol Biol Rep 39:7609–7615. https://doi.org/10.1007/s11033-012-1595-y
Sun S, Yang S, Dai M, Jia X, Wang Q, Zheng Z, Mao Y (2017) The effect of Astragalus polysaccharides on attenuation of diabetic cardiomyopathy through inhibiting the extrinsic and intrinsic apoptotic pathways in high glucose -stimulated H9C2 cells. BMC Complement Altern Med 17:310. https://doi.org/10.1111/j.1432-1033.1974.tb03714.x
Yu Y, Zheng G (2017) Troxerutin protects against diabetic cardiomyopathy through NF κB/AKT/IRS1 in a rat model of type 2 diabetes. Mol Med Rep 15:3473–3478. https://doi.org/10.3892/mmr.2017.6456
Hamblin M, Friedman DB, Hill S, Caprioli RM, Smith HM, Hill MF (2007) Alterations in the diabetic myocardial proteome coupled with increased myocardial oxidative stress underlies diabetic cardiomyopathy. J Mol Cell Cardiol 42:884–895. https://doi.org/10.1016/j.yjmcc.2006.12.018
Zheng H, Whitman SA, Wu W, Wondrak GT, Wong PK, Fang D, Zhang DD (2011) Therapeutic potential of Nrf2 activators in streptozotocin-induced diabetic nephropathy. Diabetes 60:3055–3066. https://doi.org/10.2337/db11-0807
Tsuda H, Ohshima Y, Nomoto H, Fujita KI, Matsuda E, Iigo M, Takasuka N, Moore MA (2004) Cancer prevention by natural compounds. Drug Metab Pharmacokinet 19:245–263. https://doi.org/10.2133/dmpk.19.245
Yeh CT, Ching LC, Yen GC (2009) Inducing gene expression of cardiac antioxidant enzymes by dietary phenolic acids in rats. J Nut Biochem 20:163–171. https://doi.org/10.1016/j.jnutbio.2008.01.005
Leung L, Kwong M, Hou S, Lee C, Chan JY (2003) Deficiency of the Nrf1 and Nrf2 transcription factors results in early embryonic lethality and severe oxidative stress. J Biol Chem 278:48021–48029. https://doi.org/10.1074/jbc.M308439200
Niu L, He XH, Wang QW, Fu MY, Xu F, Xue Y, Wang ZZ, An XJ (2015) Polyphenols in regulation of redox signaling and inflammation during cardiovascular diseases. Cell Biochem Biophys 72:485–494. https://doi.org/10.1007/s12013-014-0492-5
Li M, Jiang Y, Jing W, Sun B, Miao C, Ren L (2013) Quercetin provides greater cardioprotective effect than its glycoside derivative rutin on isoproterenol-induced cardiac fibrosis in the rat. Can J Physiol Pharmacol 91:951–959. https://doi.org/10.1139/cjpp-2012-0432
Panchal SK, Poudyal H, Brown L (2012) Quercetin ameliorates cardiovascular, hepatic, and metabolic changes in diet-induced metabolic syndrome in rats. J Nutr 142:1026–1032. https://doi.org/10.3945/jn.111.157263
Guo H, Zhang X, Cui Y, Zhou H, Xu D, Shan T, Zhang F, Guo Y, Chen Y, Wu D (2015) Taxifolin protects against cardiac hypertrophy and fibrosis during biomechanical stress of pressure overload. Toxicol Appl Pharmacol 287:168–177. https://doi.org/10.1016/j.taap.2015.06.002
Gao L, Yao R, Liu Y, Wang Z, Huang Z, du B, Zhang D, Wu L, Xiao L, Zhang Y (2017) Isorhamnetin protects against cardiac hypertrophy through blocking PI3K-AKT pathway. Mol Cell Biochem 429:167–177. https://doi.org/10.1007/s11010-017-2944-x
Nakayama A, Morita H, Nakao T, Yamaguchi T, Sumida T, Ikeda Y, Kumagai H, Motozawa Y, Takahashi T, Imaizumi A, Hashimoto T, Nagai R, Komuro I (2015) A food-derived flavonoid luteolin protects against angiotensin II-induced cardiac remodeling. PLoS One 10(0137106):e0137106. https://doi.org/10.1371/journal.pone.0137106
Naidu PB, Uddandrao S, Naik RR et al (2016) Ameliorative potential of gingerol: promising modulation of inflammatory factors and lipid marker enzymes expressions in HFD induced obesity in rats. Mol Cell Endocrinol 419:139–147. https://doi.org/10.1016/j.mce.2015.10.007
Kalaivani A, Uddandrao VVS, Parim B et al (2018) Reversal of high fat diet-induced obesity through modulating lipid metabolic enzymes and inflammatory markers expressions in rats. Arch Physiol Biochem. https://doi.org/10.1080/13813455.2018.1452036
Kalaivani A, Sathibabu Uddandrao VV, Brahmanaidu P, Saravanan G, Nivedha PR, Tamilmani P, Swapna K, Vadivukkarasi S (2017) Anti obese potential of Cucurbita maxima seeds oil: effect on lipid profile and histoarchitecture in high fat diet induced obese rats. Nat Prod Res. https://doi.org/10.1080/14786419.2017.1389939
Meriga B, Parim B, Chunduri VR, Naik RR, Nemani H, Suresh P, Ganapathy S, Uddandrao S (2017) Antiobesity potential of Piperonal: promising modulation of body composition, lipid profiles and obesogenic marker expression in HFD-induced obese rats. Nut Met 14:76. https://doi.org/10.1186/s12986-017-0228-9
Sathibabu Uddandrao VV, Brahmanaidu P, Meriga B, Saravanan G (2016) The potential role of S-allylcysteine as antioxidant against various disorders in animal models. Oxid Antioxid Med Sci 5:79–86. https://doi.org/10.5455/oams.240716.rv.025
Ghanshyam P, Chhayakanta P, Shankar MU et al (2012) Investigation of possible hypoglycemic and hypolipidemic effect of methanolic extract of Sesbania grandiflora. Int Res J Pharm 3:275–280
Nandi MK, Garabadu D, Krishnamurthy S, Singh TD, Singh VP (2014) Methanolic fruit extract of Sesbania grandiflora exhibits anti-hyperglycemic activity in experimental type-2 diabetes mellitus model. Ann Biol Res 5:50–58
Sangeetha A, Sriram Prasath G, Subramanian S (2014) Antihyperglycemic and antioxidant potentials of Sesbania grandiflora leaves studies in STZ induced experimental diabetic rats. Int J Pharm Sci Res 5:2266–2275. https://doi.org/10.13040/IJPSR.0975-8232.5(6).2266-75
Islam T, Rahman A, Islam AU (2013) In vitro effect of aqueous extract of fresh leaves of Abroma augusta L on the diffusion of glucose. Bangladesh Pharm J 16:21–26. https://doi.org/10.3329/bpj.v16i1.14486
Khanra R, Dewanjee S, Dua T, Sahu R, Gangopadhyay M, De Feo V, Zia-Ul-Haq M (2015) Abroma augusta L. (Malvaceae) leaf extract attenuates diabetes induced nephropathy and cardiomyopathy via inhibition of oxidative stress and inflammatory response. J Transl Med 13:6. https://doi.org/10.1186/s12967-014-0364-1
Wang C, Aungi HH, Zhang B et al (2008) Chemopreventive effects of heat-processed Panax quinquefolius root on human breast cancer cells. Anticancer Res 28:2545–2552
Sen S, Chen S, Wu Y, Feng B, Lui E, Chakrabarti S (2012) Preventive effects of north American ginseng (Panax quinquefolius) on diabetic retinopathy and cardiomyopathy. Phytother Res 27:290–298. https://doi.org/10.1002/ptr.4719
Padiya R, Banerjee SK (2013) Garlic as an anti-diabetic agent: recent progress and patent reviews. Recent Pat Food Nutr Agric 5:105–127. https://doi.org/10.2174/18761429113059990002
Erejuwa OO, Sulaiman SA, Ab Wahab MS, Sirajudeen KN, Salleh S, Gurtu S (2012) Honey supplementation in spontaneously hypertensive rats elicits antihypertensive effect via amelioration of renal oxidative stress. Oxidative Med Cell Longev 2012:374037. https://doi.org/10.1155/2012/374037
Amagase H, Petesch BL, Matsuura H, Kasuga S, Itakura Y (2001) Intake of garlic and its bioactive components. J Nut 131:955–962. https://doi.org/10.1093/jn/131.3.955S
Hiramatsu K, Tsuneyoshi T, Ogawa T, Morihara N (2016) Aged garlic extract enhances heme oxygenase-1 and glutamate–cysteine ligase modifier subunit expression via the nuclear factor erythroid 2-related factor 2-antioxidant response element signaling pathway in human endothelial cells. Nut Res 36:143–149. https://doi.org/10.1016/j.nutres.2015.09.018
Sun Z, Chin YE, Zhang DD (2009) Acetylation of Nrf2 by p300/CBP augments promoter-specific DNA binding of Nrf2 during the antioxidant response. Mol Cell Biol 29:2658–2672. https://doi.org/10.1128/MCB.01639-08
Khatua TN, Adela R, Banerjee SK (2013) Garlic and cardioprotection: insights into the molecular mechanisms. Can J Physiol Pharma 91:448–458. https://doi.org/10.1139/cjpp-2012-0315
Acknowledgements
The authors are thankful to Innovation in Science Pursuit for Inspired Research (INSPIRE), Department of Science & Technology (DST). Govt. of India for providing the financial assistance and faculty fellowship (Grant No: DST/INSPIRE/04/2016/000893). The authors are also thankful to Indian Council of Medical Research, Government of India for providing laboratory and library facilities. The authors are also thankful to Dr. Mir Irfan and Dr. Syed S.Y.H. Qadri, Dr. Harishankar Nemani for helpful comments, and Mr. Srinivas for technical help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Parim, B., Sathibabu Uddandrao, V.V. & Saravanan, G. Diabetic cardiomyopathy: molecular mechanisms, detrimental effects of conventional treatment, and beneficial effects of natural therapy. Heart Fail Rev 24, 279–299 (2019). https://doi.org/10.1007/s10741-018-9749-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-018-9749-1