Abstract
The objective of the present study is to observe the effect of Astragalus polysaccharide (APS) on myocardial glucose and lipid metabolism in diabetes (DM) hamster and to explore its mechanism in intervention of DM cardiomyopathy. Low-dose- streptozotocin-induced hamsters (STZ, 40 mg/kg × 3 days, i.p.) with blood glucose >13.9 mmo/L were considered as type 2 diabetic models. We measure blood glucose, serum lipid, insulin, C-peptide, myocardial enzyme levels, myocardial glycogen staining, myocardial ultrastructure, fluorescence quantitative RT–PCR detection of myocardial PPAR-α and the target genes (FATP, ACS) and GLUT4 mRNA expression in normal control group, DM group and APS treatment group hamsters. There was significant glycolipid metabolic disorders in DM group compared with normal group. Glucose, glycosylated serum protein, myocardial enzymes and lipid levels in APS treatment group decreased significantly than DM group, but insulin and C-peptide levels was no difference. Myocardial glycogen staining and abnormal myocardial ultrastructure in APS treatment group were significantly improved than in DM group. Gene expression of myocardial PPAR-α and its target genes (FATP, ACS) in APS group were significantly lower than in DM group, while gene expression of GLUT4 in APS group was higher than DM group. APS can partially improve myocardial glucose and lipid metabolism disorders in diabetic hamsters and protect myocardium in some extent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a perennial herb, Astragalus membranaceus is a common used centralizer traditional Chinese medicine. Astragalus polysaccharide (APS) is one of the main active ingredients, which regulates cell-mediated immunity, humoral immunity, non-specific immunity, and the activation of cytokines. Our preliminary studies have found APS can prevent or delay the occurrence and development of type 1 diabetes (diabetes mellitus, DM) in non-obese diabetic (NOD) mice [1]. APS protects the ultrastructure of islet cells, impacts β cell gene expressions, and regulates of cytokines gene expressions pattern in T helper cell subsets 1 (T helper cell, Th1)/Th2 that contributes to type 1 DM-related autoimmune reaction. In addition, APS can significantly reduce the deposition of collagen at cardiac myocytes in DM hamster, which in turn inhibits the DM cardiomyopathy [2–4]. This study further explores the APS diabetic hamster myocardial protective effect and its possible mechanism through observing the effect of APS on the DM hamster serum glucose, serum lipid, the level of myocardial enzymes, myocardial glycogen staining, adipose tissue pathology, and cardiomyocytes and myocardial ultrastructure partial peroxisome proliferator-activated (peroxisome proliferator activated receptors-α, PPAR-α) and its downstream gene (fatty acid transporter fatty acid trasport protein, FATP; acetyl coenzyme A synthetase, ACS) and glucose transporter 4 (glucose transporter 4, GLUT4) mRNA expressions.
Materials and methods
Animals and drug
8-week-old male hamsters, weighing 180–200 g, purchased from the Shanghai Institute of Biological Products. APS was provided by Shanghai Physiological Institution of Chinese Academy of Sciences, which was identified by high-performance liquid chromatography. APS was diluted to 1 mg/ml with normal saline when using.
Instruments and reagents
Streptozotocin (STZ): United States Sigma company; rapid Glucometer: Germany Bayer; Insulin, C-peptide RIA kit: Tianjin Sino-foreign DPC Joint Venture Company; Glycosylated serum protein (GSP) enzymatic detection Kit: British Genzyme Corporation; Automatic biochemical analyzer: CL-7300 type, the Hong Kong Bosheng Company; Electron microscope: electron microscope room in Fudan University Shanghai Medical College; Formalin solution, anhydrous ethanol, xylene, Paraffin, hematoxylin, eosin, automatic image analysis system: Department of Pathology Fudan University Shanghai Medical College; 7500 real-time gene amplification Instrument: The United States ABI companies; 377-based sequencer: The United States ABI companies; Automatic Spectrophotometry photometer: BST-310 type, Wanbang Biosystems Inc.; hypothermia centrifuges: Jouan CR-412 type, Promega Corporation; Trizol: GIBCO company; DNA Purification Kit: Biyun days company; RT–PCR kit: Promega Corporation; QIAquick Gel Extraction Kit: United States Sigma company; probe: designed by Primer Express software, synthesis, marker and purified by Shanghai Shengyou bio-engineering company.
Animal experiments
8-week-old male hamsters, intraperitoneal injection of STZ 40 mg/kg × 3 days, DM hamsters with blood glucose >13.9 mmol/L were randomly divided into two groups: (1) APS treatment of DM Group (APS group) included 15 hamsters, APS 2 g/kg/day, orally for 10 weeks; (2) DM control group included 15 hamsters, gavaged with normal saline for 10 weeks. At the same time, 15 normal hamsters at the same age were recruited as normal control group. At the end of 10 weeks, hamsters were killed to collect blood specimens and heart tissue specimens, which was stored by cryopreservation.
Measure index
All animals were tested for the blood sugar levels, GSP, insulin (Ins), C-peptide (C-P), triglycerides (TG), total cholesterol (CHO), low density lipoprotein (LDL), high density lipoprotein (HDL), myocardial enzymes [aspartate aminotransferase (AST), creatine phosphokinase (CPK) and its isoenzyme CK-MB, lactate dehydrogenase (LDH)].
Myocardial glycogen staining
Myocardial tissues were fixed, embedded, sliced, then followed by hematoxylin–eosin staining, dehydration, transparency, mount, observation by light microscopy.
Electron microscopic examination of cardiac myocardial tissue
Glutaraldehyde fixation, dehydration, embedding, slicing, observation under the electron microscope after double-staining.
Fluorescence quantitative PCR method (FG-PCR) detection of myocardial PPAR-α, FATP, ACS and ACE mRNA expressions
Target gene amplification and purification total RNA extraction, RNA samples quantitative, reverse transcriptase–polymerase chain reaction (RT–PCR); PCR amplification products of cloning ligation, transformation, screening of recombinant plasmid; fluorescence quantitative PCR primer synthesis, plasmid extraction and quantitative, the establishment of standard curve.
PPAR-αProbe | Name: PPAR-α loaded-21F |
Sequence: 5′-TGAACACGATCTGGAAGATTCG-3′ | |
Name: PPAR-α loaded-91R | |
Sequence: 5′-GGTAGGCCTCGTGGATTCTCT-3′ | |
Taqman probe loaded-44T | |
Sequence: 5′-FAM-AAACTGCCGACCTCAAATCTCTGGCC-TAMRA-3′ | |
GLUT4 probe | Name: GLUT4 loaded-310F |
Sequence: 5′-CAATGGTTGGGAAGGAAAAGG-3′ | |
Name: GLUT4 loaded-372R | |
Sequence: 5′-GGCGCCCCCCAACA-3′ | |
Taqman probe loaded-333T | |
Sequence: 5′-FAM-TATGCTGGCCAACAATGTCTTGG-TAMRA-3′ | |
FATP probe | Name: FATP loaded-778F |
Sequence: 5′-ATTGTGGTGCACAGCAGGTACTA-3′ | |
Name: FATP loaded-841R | |
Sequence: 5′-CCCGCATGCTGTAGGAATG-3′ | |
Taqman probe loaded-804T | |
Sequence: 5′-FAM-CATCGCAGCCTTCGGCCA-TAMRA-3′ | |
ACS probe | Name: ACS loaded-1732F |
Sequence: 5′-GATGGTGAGGAGCGGGTAGTC-3′ | |
Name: ACS loaded-1796R | |
Sequence: 5′-AGGTCGGGCTGGAAAGTGT-3′ | |
Taqman probe loaded-1754T | |
Sequence: 5′-FAM-TGTTTCTGAAGATGGCCTCTGG-TAMRA-3′ |
Statistical analysis
The data was analysis by SPSS 10.0 statistical software. All data were performed with normality test, ANOVA was used for comparing multiple sets of samples. Data were presented with mean ± SD, All p values were two-sided, and statistical significance was defined as p < 0.05.
Result
General features of hemodynamics
At the end of experimental period, LV end-diastolic pressure (LVEDP) showed a marked elevation in the untreated diabetic group. This elevation was significantly attenuated in APS treatment groups. LV systolic pressure (LVSP) and LV maximum ± dP/dt were significantly lower in the untreated diabetic group than in the normal control group. However, LVSP and LV maximum ± dP/dt significantly improved after APS treatment for 10 weeks (Fig. 1).
Hemodynamic parameters of 19-week-old male hamsters in normal control, diabetic, APS-treatment diabetic (2 g/kg/day) groups. LVEDP LV end-diastolic pressure, LVSP LV systolic pressure, ± dP/dt = LV maximum ± dP/dt. Data are expressed as mean ± SD. Comparisons of parameters between two groups were made with unpaired student t test. Comparisons of parameters among three groups were made with ANOVA, followed by the Scheffe multiple-comparison test. *p < 0.05 vs. normal control group; † p < 0.05 vs. untreated diabetic group
Levels of glucose, glycosylated serum protein, insulin, C-peptide, blood lipid and enzymogram
Serum glucose in diabetic group and APS treatment group were significantly higher than the normal control group soon after model established. And the glucose levels between the groups were no difference. 10 weeks later, glucose and GSP levels in APS treatment group were significantly lower than the diabetic group. 10 weeks after the experiment, Ins and C-P in APS treatment group and DM group were significantly lower than the normal control group, while there is no significant difference between the APS group and the DM group (Fig. 2).
General features of 19-week-old male hamsters in normal control, diabetic, APS-treatment diabetic (2 kg/kg/day) groups. C-P C-peptide, Ins insulin, BG blood glucose, GSP glycosylated serum protein, AST plasma aspartate aminotransferase, CPK creatine phosphokinase, CPK-MB the isoenzyme of creatine kinase; LDH lactate dehydrogenase, TG triacylglycerol, CHO total cholesterol, LDL-C low density lipoprotein cholesterol, HDL-C high density lipoprotein cholesterol. Data are expressed as mean ± SD. Comparisons of parameters between two groups were made with unpaired student t test. Comparisons of parameters among three groups were made with ANOVA, followed by the Scheffe multiple-comparison test. *p < 0.05 vs normal control group; † p < 0.05 vs. untreated diabetic group
At the end of experimental period, TG, CHO and LDL-C levels in diabetic group hamster were significantly higher than the normal control group. HDL-C levels were no significant difference between groups. TG levels in APS treatment group were significantly lower than the DM group (Fig. 2).
At the end of experimental period, enzymogram CPK, CK-MB and AST levels in DM group were significantly higher than the normal control group. LDH levels were no significant difference between groups. Enzymogram CPK, CK-MB levels in APS treatment group were significantly decreased than DM group, but still higher than normal group (Fig. 2).
Myocardial glycogen staining
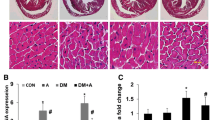
10 weeks after the experiment, myocardial glycogen staining results in three groups of hamsters showed that myocardial glycogen in diabetes group stained strongly positive when compared with normal control group, while only part of myocardial tissues were stained in the APS treatment group (Fig. 3a–c).
Myocardial ultrastructure
10 weeks after the experiment, myocytes electron microscope results in three groups of hamsters showed that compared with normal control group, a large number of collagen fibers distributed among myocardial cells, myocardial fibers were disordered, mitochondria was seriously damaged, many lipid droplets and glycogen granules were distributed within myocardial cells in diabetes group. While, APS treatment group myocardial cells arranged neatly, collagen fibers were much less between myocardial cells, mitochondria were swelled, mitochondrial membrane and crests were intact, lipid droplets and glycogen particles in myocardial cells decreased significantly (Fig. 4).
Myocardial ultrastructure. a Myocardial ultrastructure of normal control group (×3,000): There were no lipid droplets and glycogen in myocardial cells, morphology of mitochondrial was order and its structural was integrity, myocardial fibers were arranged regularly b. Myocardial ultrastructure of diabetes group (×3,000): There were great amount of collagen fibers distributing in intercellular substance of myocardial cells, numbers of mitochondrial deceased with their irregular shape, arrangement of myocardial fibers was irregular surrounding with the a large number of lipid droplets. c Myocardial ultrastructure of APS treatment group (×3,000): Amount of the collagen fibers distributing in intercellular substance of myocardial cells obviously decreased, mitochondrial became swelling, myocardial fibers were arranged regularly, amount of lipid droplets significantly reduced. d Myocardial ultrastructure of diabetes group (×6,000): Myocardial fibers were arranged irregularly, morphology mitochondrial was irregular and partially damaged, surrounding with lipid droplets and a large amount of glycogen granules. e Myocardial ultrastructure of APS treatment group (×6,000): part of mitochondrial was swelling, the structure was still complete, myocardial fibers were arranged regularly, glycogen and lipid droplets decreased significantly
Gene expressions of GLUT4, PPAR-α, FATP and ACS in myocardial cells
10 weeks after the experiment, fluorescence quantitative RT–PCR results showed that myocardial PPAR-α gene (and its downstream gene FATP and ACS) gene copy number of values in diabetic hamster were significantly higher than the normal control group, while GLUT4 gene was significantly lower; and myocardial PPAR-α gene (and its downstream gene FATP and ACS) gene copy number in APS treatment group hamster were significantly lower than that of diabetic group, while GLUT4 gene was significantly higher (Fig. 5).
Expression of ACS, FATP, PPAR-α and GLUT4 by quantitative real-time RT–PCR data are expressed as mean ± SD. Comparisons of parameters between two groups were made with unpaired Student t test. Comparisons of parameters among three groups were made with ANOVA, followed by the Scheffe multiple-comparison test. *p < 0.05 vs normal control group; † p < 0.05 vs. untreated diabetic group
Discussion
Astragalus, a leguminous perennial herb, is a commonly used centralizer Chinese medicine. APS is the one of main active ingredients, which is a homogeneous polysaccharide isolated by water extraction from the roots of Astragalus mongholicus Bunge. Three subtypes of APS are defined phytochemically; i.e., APS I, II and III. APS I consists of d-glucose, d-galactose and l-arabinose, whereas both APS II and APS III contain d-glucose (dextrans). APS I is a heteropolysaccharide, which is composed by d-glucose, d-galactose and l-arabinose, molar ratio is 1.75:1.63:1, average molecular weight is about 36,300, specific rotation is positive. APS II and III are dextran, average molecular weight is 12,300 and 34,600 respectively, specific rotation is positive. The main chains are α-(1→4)-d-glucose condensation chains except for a small amount of α-(1→6)-d-glucose condensation chains. Our preliminary study indicated that APS can prevent or delay the occurrence and development of type 1 diabetes DM in NOD mice, considerably inhibit diabetic cardiomyopathy by decreasing myocardial collagen deposition in DM hamster [1–4]. In this study, serum myocardial enzymes (CPK, CK-MB) levels in diabetic hamsters were significantly higher than normal hamsters, mitochondrial were damaged in myocardial ultrastructure, myocardial fibers were disordered, and interstitial collagen fibers were dysplasia. After treatment with APS, serum myocardial enzymes (CPK, CK-MB) decreased significantly, abnormal myocardial ultrastructure was improved, mitochondria was intact, myocardial fibers arranged orderly, and interstitial collagen fibers reduced; moreover, hemodynamics disorder had also been significantly improved in diabetic hamsters after treatment with APS; which indicated APS treatment can protect cardiomyocytes in some extent in diabetic hamsters.
In this study, there was an obvious glucose metabolism disorders in myocardial tissues in diabetic hamsters. Serum glucose, GSP levels in diabetes hamsters were significantly higher than normal hamsters, myocardial glycogen staining were strongly positive, there were a large number of glycogen-rich granules in myocardial ultrastructure. Ten weeks after APS treatment in diabetic hamsters, serum glucose, glycosylated serum protein decreased significantly, glycogen staining was positive in part of myocardial tissues, glycogen granules in myocardial ultrastructure decreased, which indicated that APS treatment can significantly alleviate prompt myocardial glucose metabolism disorders in diabetic hamsters.
GLUT4 is a transmembrane protein, only lies in insulin-sensitive skeletal muscle, myocardial and adipose tissue. Its function is to promote the glucose transportation to the insulin-sensitive organs to maintain normal cell physiological functions. Currently, it is pointed that GLUT4 potential defects in type 2 diabetes include GLUT4 translocation is blocked, GLUT4 rich vesicles cannot fuse with membrane, hidden GLUT4 cannot expose to extracellular environment, and activity of GLUT4 drops although the GLUT4 vesicles can fuse [5, 6]. Fluorescence quantitative RT–PCR results in our study showed that GLUT4 mRNA of myocardial tissues in diabetic hamsters were significantly lower than the normal group, and increased after treatment with APS, suggesting APS can ameliorate myocardial glucose metabolism disorders in diabetic hamster, which may be due to the promotion of myocardial GLUT4 gene expressions. But the actual mechanisms have yet to be studied further.
In this study, diabetic hamsters showed an obvious dyslipidemia: in diabetic hamsters, lipid levels (TG, CHO and LDL-C) were significantly higher than that of normal hamsters; ultrastructural observation with electron microscopy showed that DM hamsters’ myocardial cells were rich in a large number of lipid droplets. While in diabetes hamsters given with APS treatment after 10 weeks, serum triglyceride levels decreased significantly, myocardial ultrastructure showed the lipid droplets significantly reduced; which indicated that prompt treatment of APS can considerably alleviate dyslipidemia in diabetic cardiomyopathy of hamsters.
PPAR belongs to the nuclear hormone receptor superfamily, which is a ligand-activating transcription factor. PPAR-α is one of its subtypes, which is highly expressed in the mitochondria and β-oxidation activity rich organizations, such as liver, kidney cortex, intestinal mucosa and the heart. PPAR-α mainly regulates β-oxidation of peroxisome, gene transcription of the enzymes related to lipid metabolism, such as ACS, acyl-coenzyme A oxidase, FATP, 3-hydroxy-methyl-3 coenzyme A synthetic enzymes. It also regulates fatty acid oxidation, synthesis of fatty acids and TG. In addition, PPAR-α is also involved in mediation of expressions in apolipoprotein AI, AII, the promotion the expressions in FATP and synthesis in lipoprotein lipase, catalytication of lipolytic TG into free fatty acids, effect on the fatty acid intake, binding and lipid transfer. Therefore, PPAR-α is a key factor in regulation of energy metabolism and lipid peroxidation, plays a pivotal role in maintaining the dynamic balance of lipid metabolism. Recent studies have found that PPAR-α regulatory pathway is over-activated in diabetic myocardium [7–9]. Fluorescence quantitative RT–PCR results in our study showed that myocardial PPAR-α mRNA in diabetic hamsters is significantly higher than the normal group, and increased after given with APS treatment, suggesting that alleviation of diabetic myocardial lipid metabolism disorders with APS treatment in hamsters may be related to the descent of PPAR-α gene expressions.
FATP and ACS is one of the main target genes of PPAR-α. After PPAR-α binds with peroxisome proliferator responsive elements, expressions of FATP and fatty acid translocase is upregulated, expressions of ACS increases, facilitates the intake of fatty acids and converse to acyl-coenzyme A, which controls the transport of fatty acids to the mitochondria. In addition, PPAR-α can also regulate lipid metabolism in mitochondria through regulation of mitochondrial fatty acid transporter protein, expressions of fatty acids catalase, promotion of mitochondrial fatty acid β-oxidation process, which decreases the synthesis of fatty acids and triglyceride [10, 11]. Fluorescence quantitative RT–PCR results in our study showed that myocardial FATP and ACS gene expressions in diabetic hamsters more increased than normal group, but significantly decrease to near normal after treatment with APS, suggesting that improvement of myocardial lipid metabolism with APS treatment in diabetic hamster may be related to the descent of myocardial tissue FATP and ACS gene expressions. While, specific mechanisms have still need to be studied further.
References
Chen W, YU M-H, LI Y-M et al (2008) Astragalus polysaccharides: an effective treatment for diabetes prevention in NOD mice. Exp Clin Endocrinol Diabetes 116(8):468–474
CHEN W, YU M-H, LI Y-M et al (2007) Effects of Astragalus polysaccharides on chymase, angiotensin-converting enzyme and angiotensin II in diabetic cardiomyopathy in hamsters. J Int Med Res 35(6):873–877
CHEN W, YU M-H, LI Y-M et al (2010) Astragalus polysaccharides inhibited diabetic cardiomyopathy in hamsters depending on suppression of heart chymase activation. J Diabetes Complications 24(3):199–208
Chen W, YU M-H, LI Y-M et al (2009) Beneficial effects of astragalus polysaccharides treatment on cardiac chymase activities and cardiomyopathy in diabetic hamsters. Acta Diabetol 47(suppl 1):535–546
Mohammad A, Sharma V, Mcneill JH (2002) Vanadium increases GLUT4 in diabetic rat skeletal muscle. Mol Cell Biochem 233(1):139–143
Watson RT, Pessin J (2001) Intracellular organization of insulin signaling and GLUT4 translocation. Recent Prog Horm Res 56:175–193
Suden MC, Bulmer K, Gibbons GF et al (2002) Peroxisome-proliferator activated receptor alpha deficiency leads to dysregulation of hepatic lipid and carbohydrate metabolism by fatty acids and insulin. Biochem J 364:361–368
Lee SS, Chan WY, Lo CK et al (2004) Requirement of PPAR-alpha in maintaining phospholipid and triacylglycerol homeostasis during energy deprivation. J Lipid Res 45:2025–2037
Farrell GC, Robertson G, Auwerx J et al (2003) Central role of PPAR-alpha-dependent hepatic lipid turnover in dietary steatohepatitis in mice. Hepatology 38:123–132
Laukkanen Johanna, Yla-Herruala Seppo (2002) Genes involved in Atherosclerosis. Exp Nephrol 10:150–163
Sundvold H, Lien S (2001) Identification of a novel peroxisome proliferator-activated receptor (PPAR) promoter in man and transactivation by the nuclear receptor ROR alpha1. Biochem Biophys Res Commun 287(2):383–390
Acknowledgments
This work was supported by Chinese State Natural Science Funds Commission (NSFC), Grant No: 81001568.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Wei Chen and Yan-Ping Xia contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, W., Xia, YP., Chen, WJ. et al. Improvement of myocardial glycolipid metabolic disorder in diabetic hamster with Astragalus polysaccharides treatment. Mol Biol Rep 39, 7609–7615 (2012). https://doi.org/10.1007/s11033-012-1595-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-1595-y