Abstract
This study evaluated the effects of metformin on orthodontic tooth movement in a rat model of type 2 diabetes mellitus. Rats were fed a high-fat diet for 4 weeks to induce fat accumulation and insulin resistance, and then injected with a low dose of streptozotocin (35 mg/kg) intraperitoneally to induce type 2 diabetes. An orthodontic appliance was placed in normoglycemic, type 2 diabetes, and type 2 diabetes with metformin-administrated rats. After 14 days, type 2 diabetes rats exhibited greater orthodontic tooth movement and had a higher number of tartrate-resistant acid phosphatase-positive osteoclasts, stronger cathepsin K expression, and weaker alkaline phosphatase immunostaining than normoglycemic rats. Metformin administration resulted in normalization of osteoclast numbers, cathepsin K immunostaining, and of tooth movement as well as partly recovery of alkaline phosphatase expression in diabetic rats. Metformin also reduced sclerostin expression and improved the immunolocalization of dentin matrix protein 1 in osteocytes of type 2 diabetes rats. These results suggest that metformin administration reversed the adverse effects of diabetes on orthodontic tooth movement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus is a metabolic disorder characterized by hyperglycemia. It is becoming a global epidemic with approximately 6% of the world’s population currently suffering from the condition (Danaei et al. 2011). Chronic hyperglycemia is associated with long-term damage, dysfunction, and failure of various organs. Long-term complications include peripheral and autonomic neuropathy, cardiovascular disease, retinopathy, nephropathy, and an increased prevalence and severity of periodontal disease (Bensch et al. 2003). Diabetes mellitus also adversely affects bone, a dynamic organ that undergoes continuous remodeling to maintain its quantity and quality, resulting in diminished bone-mineral density, osteopenia, osteoporosis, Charcot’s disease, and diffuse idiopathic skeletal hyperostosis (Rakel et al. 2008). It is known that diabetes mellitus is closely associated with many oral, jaw, and facial problems. Giglio and Lama reported that streptozotocin-induced diabetes reduces mandibular growth, resulting in some deformity of the structure (Giglio and Lama 2001). Huh et al. reported that uncontrolled type 2 diabetes mellitus resulted in an uncommon broad maxillary osteonecrosis in a female patient (Huh et al. 2015). Additionally, diabetes is regarded as a risk factor for medication-related osteonecrosis of the jaw (Peer and Khamaisi 2015).
Orthodontic tooth movement is achieved by remodeling surrounding alveolar bone in response to a prolonged application of pressure to the tooth (Fu et al. 2016; Krishnan and Davidovitch 2006). Bone resorption is caused by osteoclast activity on the compression side and by osteoblast-induced new bone formation on the tension side (Wise and King 2008). An alteration in the metabolic state that interferes with bone remodeling can result in a different rate of tooth movement (Verna et al. 2000). The diabetic state up-regulates osteoclast migration and activity, resulting in abnormal orthodontic tooth movement (Braga et al. 2011). These findings suggest that patients with diabetes may respond to orthodontic treatment differently from those without diabetes in terms of how the periodontium and surrounding visceral cranium react to therapy.
Metformin (1-(diaminomethylidene)-3,3-Dimethylguanidine) is an oral anti-hyperglycemia medicine commonly used for the management of type 2 diabetes mellitus. Protective effects against diabetic complications have been found with metformin monotherapy. Vestergaard et al. reported that patients with type 2 diabetes treated with metformin showed decreased risk for bone fractures (Vestergaard et al. 2005). Gao et al. reported that metformin promotes osteoblastic differentiation and inhibits adipogenic differentiation in cultured marrow mesenchymal stem cells of the rat through inhibition of peroxisome proliferator-activated receptor gamma, a nuclear receptor that regulates lipid and glucose metabolism (Gao et al. 2008). Metformin also increases trabecular bone formation through activation of activated mononucleotide protein kinase (AMPK) signaling in osteoblastic cells, a major intracellular pathway that senses energy starvation (Shah et al. 2010). Interestingly, the osteogenic effect of metformin may be specific to bone formation in diabetic mice; it is not seen in mice with normal blood glucose levels (Jeyabalan et al. 2013).
With improvements in dental aesthetic requirements, increasing numbers of adults are seeking orthodontic treatment. This, coupled with the increasing incidence of diabetes each year, means that there are increasing numbers of people with type 2 diabetes seeking orthodontic treatment. As a result, focus has turned to how best to treat orthodontic patients with type 2 diabetes. Metformin has been suggested to be an effective medicine for the treatment of type 2 diabetes. While it is known that such treatment elicits a complex mode of action in bone maintenance, the effect of metformin on orthodontic tooth movement in people with diabetes is unclear (Fig. 1). The aim of this study was to evaluate the effect of metformin treatment on orthodontic tooth movement in a rat model of type 2 diabetes.
Materials and methods
Animal preparation
All animal experiments in this study were conducted according to the Guidelines for Animal Experimentation of Shandong University. 7-week-old male Wistar rats (200 g on average) were obtained from the Laboratory Animal Center of Shandong University (Jinan, China) and kept in plastic cages (3–5 animals per cage) under standard laboratory conditions on a 12-h light/dark cycle and at a constant temperature of 20 °C and humidity of 48%.
Type 2 diabetes mellitus was induced as previously described (Srinivasan et al. 2005). Briefly, rats were given free access to tap water and fed with a high-fat diet (40% fat, 42% carbohydrate, and 18% protein as a total percentage of calories; Huafukang Biotech, Beijing, China) for 4 weeks to induce fat accumulation and insulin resistance, and then injected with a low dose of streptozotocin (Sigma–Aldrich, St Louis, MO, USA) (35 mg/kg) intraperitoneally to induce hyperglycemia. Seventy-two hours after injection, random blood glucose was measured with a glucometer (Roche Diagnostics, Germany) from the tail vein. Twenty rats with random blood glucose ≥16.7 mmol/L were selected for the next experiment.
After raising for 2 months, rats were randomly divided into a type 2 diabetes group (n = 10) and a metformin group (n = 10). The metformin group was given intragastric administration of metformin (100 mg/kg body weight) in phosphate-buffered saline (PBS) every day for 1 month. The type 2 diabetes group was given PBS only. Ten rats fed with standard rodent diet were injected with PBS intraperitoneally and given intragastric administration of PBS as a control group (n = 10).
Orthodontic tooth movement
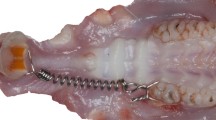
Induction of tooth movement was performed using normoglycemic (NG), type 2 diabetes (DB), and type 2 diabetes with metformin-administrated (MA) rats as previously described (Cui et al. 2016). Briefly, tooth movement was determined by a coil spring delivering a force of 0.5 N (0.012-inch Ni–Ti wire; Grikin Advanced Materials, Beijing, China) anchored to the maxillary right first molar and incisors, resulting in mesial tipping of the first molar (Fig. 2a).
An orthodontic appliance, measurement of tooth movement, and blood glucose level of three groups. a Intra-oral photograph obtained after insertion of the appliance between the upper incisor and the right first molar of a rat. b A digital microscopic photograph of the dental impression of the upper right molars. c The cut surface of the impression with tooth movement. The distance of tooth movement (green arrows) was expressed by the thickness of the impression material between the first and second molars at the level connecting the middle of the distal fissure in the first molar (blue stars in b, c) and the middle of the mesial fissure in the second molar (blue dots in b, c) on the cut surface. d Blood glucose level of three groups. e The distance of tooth movement of three groups at 14 days. (n = 10; **p < 0.01). f Hematoxylin-eosin staining of the upper first molar reveals the area of interest (AOI) of tension side (TS) and compression side (CS) in this experiment. Error bars indicate ± SD. SD standard deviation, AB alveolar bone, PDL periodontal ligament, TR tooth root, TS tension side, CS compression side, AOI area of interest. d × 40. (Color figure online)
For histomorphometric analyses, the left side of the maxilla was used as the control. In addition, the MA group was given intragastric administration of metformin (100 mg/kg body weight) in PBS every day throughout the process of tooth orthodontic movement. The DB and control groups were given PBS only.
Measurement of tooth movement
The distance of tooth movement was measured on day 14 after the application of the tooth movement appliance in each experimental group (n = 10). Rats were anesthetized with an intraperitoneal injection of 10% chloral hydrate (0.4 mL/100 g body weight) and fixed with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) by transcardial perfusion. After fixation, the right and the left halves of the maxillae were dissected. Impressions of the teeth and maxillae were obtained using individual trays containing hydrophilic vinyl polysiloxane dental impression material (Exafine, GC, Tokyo, Japan) (Fig. 2b). Impressions were cut along the line connecting the middle of the distal fissure of the first molar and the middle of the mesial fissure of the second molar (Fig. 2b, c). Tooth movement distance was evaluated by assessing the thickness of the dental impression material between the first and second molars in a cross-sectional surface of the impression observed under a digital microscope (Fig. 2c).
Tissue preparation
Samples were immersed in the same fixative as for tooth movement assessment for an additional 24 h after impressions were taken. Samples were then decalcified with 10% ethylenediaminetetraacetic acid disodium salt solution for 4 weeks at 4 °C. Samples were dehydrated through an ascending ethanol series and embedded in paraffin using standard procedures. Serial sagittal 5-µm-thick sections were prepared for histological analysis using a sliding microtome (Leica SM 2010 R; Leica, Heidelberg, Germany).
Histological examination and image analysis
Hematoxylin and eosin staining were performed to identify the morphology of maxillae in both groups. Stained sections were observed and digital images were taken with a light microscope (Olympus BX-53; Olympus, Tokyo, Japan).
To evaluate osteoclasts, tartrate-resistant acid phosphatase (TRAP) staining was performed as previously described (Li et al. 2013). Briefly, dewaxed paraffin sections were submerged in a mixture of 3.0 mg naphthol AS-BI phosphate, 18 mg fast red violet LB salt, and 100 mM L(+) tartaric acid (0.36 g) diluted in 30 mL 0.1 M sodium acetate buffer (pH 5.0) for 15 min at 37 °C. Sections were then faintly counterstained with methyl green for assessment by light microscopy (Olympus BX-53; Olympus, Tokyo, Japan). Osteoclast numbers were counted in three randomly selected nonoverlapping microscopic fields in each section using Image-Pro Plus 6.2 software (Media Cybernetics, Silver Spring, MD, USA).
Immunohistochemistry for alkaline phosphatase, cathepsin K, sclerostin, and dentin matrix protein 1
Prepared 5-µm-thick paraffin sections were used for alkaline phosphatase (ALP), cathepsin K (CK), sclerostin (SOST), and dentin matrix protein 1 (DMP-1) immunolabeling. Briefly, after xylene treatment, dewaxed paraffin sections were pretreated with 0.3% hydrogen peroxide for 30 min and then with 1% bovine serum albumin (BSA) (Serologicals Proteins, Kankakee, IL, USA) in PBS (1% BSA-PBS) for 20 min to reduce nonspecific staining. Sections were then incubated for 2 h at room temperature with: (1) rabbit antiserum for rat tissue nonspecific ALP (Niigata University, Niigata, Japan) (Oda et al. 1999) at a dilution of 1:100; (2) anti-CK antibody (Abcam, Cambridge, MA, USA) at a dilution of 1:100; (3) anti-SOST antibody (R&D Systems, Minneapolis, MN, USA) at a dilution of 1:50; and (4) anti-DMP-1 antibody (Millipore, Boston, MA, USA) at a dilution of 1:50 in 1% BSA-PBS. After rinsing with PBS, sections were incubated with horseradish peroxidase-conjugated swine anti-rabbit IgG (Dako, Glostrup, Denmark) for ALP and CK; rabbit anti-goat IgG (Jackson ImmunoResearch Laboratories, Baltimore, MD, USA) for SOST; and goat anti-mouse IgG (Jackson ImmunoResearch Laboratories, Baltimore, MD, USA) for DMP-1 at a dilution of 1:100 for 1 h at room temperature. The immunoreaction was visualized with diaminobenzidine (Sigma–Aldrich, St. Louis, MO, USA). All sections were counterstained faintly with methyl green and observed under a light microscope (Olympus BX-53; Olympus, Tokyo, Japan). Immunostaining intensities (optical density) were analyzed using Image-Pro Plus 6.2 software. Positive reaction areas of ALP, CK, SOST, and DMP-1 were manually selected in a color cube-based manner. At least 10 sections from each sample were analyzed.
Statistical analysis
Statistical analyses were performed using SPSS17.0 software. Values are presented as mean ± standard deviation (SD). Differences among the type 2 diabetes group, the type 2 diabetes with metformin administrated group, and the control group were assessed using one-way analysis of variance. A p value of *p < 0.05 or **p < 0.01 was considered statistically significant.
Results
Blood glucose levels
The fasting blood glucose level of NG rats was 5.2 ± 0.5 mmol/L and the fasting blood glucose level of DB rats was 18.1 ± 0.8 mmol/L. The hyperglycemic state was maintained during the experimental course in the DB group. Metformin treatment significantly reversed the diabetic state (blood glucose: 5.3 ± 0.6 mmol/L; p < 0.01). The normal blood glucose level of the MA group was maintained during the experimental course (Fig. 2d).
Tooth movement alterations
A greater amount of tooth movement was observed in the DB group at day 14 of mechanical loading compared with normal rats and MA rats at the same time point (Fig. 2e) (p < 0.01). Sagittal sections are shown of the periodontium around the distobuccal root of the first molar at day 14 after mechanical loading. Areas enclosed by white dotted lines were chosen for further evaluation (Fig. 2f).
Immunolocalization for ALP, CK, and TRAP staining
ALP staining showed weaker ALP expression in the DB group compared with the control group (Fig. 3a, b), and the MA group showed stronger ALP expression than the DB group (Fig. 3b, c). Statistical analysis revealed significant differences in optical densities of ALP between the MA and DB groups (NG group: 0.122 ± 0.01 vs. DB group: 0.032 ± 0.007, p < 0.01; DB group: 0.032 ± 0.007 vs. MA group: 0.105 ± 0.009, p < 0.01; NG group: 0.122 ± 0.01 vs. MA group: 0.105 ± 0.009, p < 0.05) (Fig. 3j).
Immunolocalization of ALP and CK, TRAP staining for osteoclasts and statistical analysis. Upper line (a control group; b diabetes group; c metformin administration group) revealed the immunostaining of ALP at the tension side; middle line (d control group; e diabetes group; f metformin administration group) showed TRAP staining for osteoclasts at the compression side. Lower line (g control group; h diabetes group; i metformin administration group) exhibited the immunostaining of CK at the compression side. The osteoclasts in control group were flat and slender with 1–2 nuclei (high-magnification image of white boxed area of d, g); whereas in diabetes group the osteoclasts were round and polygon with many nuclei (high-magnification image of white boxed area of e, h); however, the osteoclasts in the metformin group recovered to flat and slender shape with few nuclei (high-magnification image of white boxed area of f, i). In addition, the dental root was seriously absorbed in diabetes group (showed by red circles e, h); but, a light sorption was found in the metformin group (showed by green circles f, i). j Mean optical density of ALP immunoreactivity were assessed at the tension side for three groups (n = 10; *p < 0.05, **p < 0.01). k TRAP-positive osteoclasts were counted at the compression side for three groups (n = 10; **p < 0.01). l Mean optical density of ALP immunoreactivity were assessed at the tension side for three groups (n = 10; **p < 0.01). All corresponding numerical information is found in "Results". Error bars indicate ± SD. SD standard deviation; a–i × 200; Cells: × 400. (Color figure online)
Compared with the control group, the number of TRAP-positive osteoclasts significantly increased in rats of the DB group, and the number of TRAP-positive osteoclasts decreased in rats of the MA group. Osteoclasts in the NG group were flat and slender with 1–2 nuclei, whereas osteoclasts in the DB group were round or polygonal with many nuclei (Fig. 3d–f). Statistical analysis returned significant differences in the numbers of osteoclasts between the DB and control groups as well as the DB and MA groups (NG group: 10 ± 1 vs. DB group: 24 ± 2, p < 0.01; DB group: 24 ± 2 vs. MA group: 12 ± 2, p < 0.01; NG group: 10 ± 1 vs. MA group: 12 ± 2, p > 0.05) (Fig. 3k).
There was stronger CK expression in the DB group compared with the control group (Fig. 3g, h). The MA group showed weaker CK expression than the DB group (Fig. 3h, i). Statistical analysis returned significant differences in optical densities of CK between the MA and DB groups (NG group: 0.140 ± 0.024 vs. DB group: 0.32 ± 0.035, p < 0.01; DB group: 0.32 ± 0.032 vs. MA group: 0.15 ± 0.027, p < 0.01) (Fig. 3l).
SOST and DMP-1 immunolocalization
Compared with the control group, on the tension side, the DB group showed more SOST-positive osteocytes (Fig. 4a, b) and decreased DMP-1-positive osteocytes (Fig. 4d, e). The MA group showed slighter SOST expression (Fig. 4b, c) and stronger DMP-1 expression than the DB group (Fig. 4e, f). Statistical analysis returned significant differences in the numbers of SOST-positive and DMP-1-positive osteocytes between the DB and control groups (SOST: 23.00 ± 1.90 cells/mm2 in the DB group vs. 11.00 ± 1.30 cells/mm2 in the NG group, p < 0.01 (Fig. 4g); DMP-1: 6.00 ± 1.40 cells/mm2 in the DB group vs. 23.00 ± 2.00 cells/mm2 in the NG group, p < 0.01 (Fig. 4h) and between the DB and MA groups (SOST: 23.00 ± 1.90 cells/mm2 in the DB group vs. 12.00 ± 1.40 cells/mm2 in the MA group, p < 0.01 (Fig. 4g); DMP-1: 6.00 ± 1.40 cells/mm2 in the DB group vs. 24.00 ± 1.80 cells/mm2 in the MA group, p < 0.01 (Fig. 4h). There was no significant difference in SOST expression or DMP-1 expression between the MA and control groups (SOST: 12.00 ± 1.40 cells/mm2 in the MA group vs. 11.00 ± 1.30 cells/mm2 in the NG group, p > 0.05 (Fig. 4g); DMP-1: 24.00 ± 1.80 cells/mm2 in the MA group vs. 23.00 ± 2.00 cells/mm2 in the NG group, p > 0.05 (Fig. 4h).
Immunolocalization of SOST and DMP-1 at the tension side and statistical analysis. Upper line (a control group; b diabetes group; c metformin administration group) revealed the immunostaining of SOST at the tension side; lower line (d control group; e diabetes group; f metformin administration group) showed immunostaining of DMP-1 for osteocytes at the tension side. Comparing to the control group (a), stronger expressions of SOST were showed in the diabetes group (b); however, weaker expressions of SOST were revealed in metformin administration group (c). Comparing to the control group (d), weaker expressions of DMP-1 were showed in the diabetes group (e); however, stronger expressions of DMP-1 were revealed in metformin administration group (f). g SOST-positive osteocytes were counted at the tension side and compression side for three groups (n = 10; **p < 0.01). h DMP-1-positive osteocytes were counted at the tension side and at the compression side for three groups (n = 10; **p < 0.01). All corresponding numerical information is found in the "Results" section. Error bars indicate ± SD. SD standard deviation, AB alveolar bone; a–f × 400
Discussion
We investigated the effects of metformin on orthodontic tooth movement in a rat model of type 2 diabetes mellitus. We found that diabetic rats had increased numbers and enhanced activity of osteoclasts compared with the control group, resulting in a greater amount of tooth movement. The administration of metformin resulted in normalization of tooth movement in these diabetic rats. We found that SOST and DMP-1 from osteocytes may be involved in this process (Fig. 5).
Orthodontic movement of teeth under mechanical force depends on the balance of alveolar bone remodeling (Li et al. 2015; Lv et al. 2015; Wise and King 2008), which can be disturbed in diabetes (He et al. 2004; Mishima et al. 2002). Braga et al. reported that diabetic mice show increased bone resorption and abnormal tooth movement (Braga et al. 2011). In agreement, an increased number of TRAP-positive osteoclasts, augmented CK expression of osteoclasts, and a greater amount of tooth movement were observed in diabetic rats under orthodontic tooth movement in the current study. Alkaline phosphatase (ALP), as a main marker of osteoblast activity, has been detected to be decreased in the diabetic rats by immunostaining in the present study. This result suggested that diabetes obviously decreased the activity of osteoblasts. Similarly, previous studies have reported that diabetes diminishes bone formation as a result of decreased osteoblastic activity (Botolin et al. 2005; He et al. 2004; Hie et al. 2007).
Interestingly, metformin administration resulted in normalization of osteoclast numbers, CK immunostaining, and of tooth movement as well as partly recovery of ALP expression in diabetic rats in the current study. In support of these findings, it is reported that control of glucose blood levels with metformin prevented disturbances in bone turnover in other models. Tolosa et al. reported that partially insulin-deficient diabetes induces deleterious effects on long-bone microarchitecture, which is associated with a decrease in bone marrow progenitor cell osteogenic potential; however, these diabetes-induced alterations can be totally or partially prevented by oral administration of metformin (Tolosa et al. 2013).
Metformin stimulates osteoprotegerin expression and reduces receptor activator of nuclear factor κB ligand (RANKL) in osteoblasts, inhibits osteoclastogenesis, and prevents bone loss in ovariectomized rats (Gao et al. 2010; Mai et al. 2011), which is also evidenced by a significant reduction in serum bone resorption marker (−12.7%) in metformin-treated male diabetic patients (Zinman et al. 2010). Metformin also increases trabecular bone formation through activation of AMPK signaling in osteoblastic cells, a major intracellular pathway that senses energy starvation (Shah et al. 2010). Cortizo et al. reported that metformin promoted osteoblastic differentiation and increased the formation of mineralization nodules in 3-week-old MC3T3E1 cultures via activating and redistributing phosphorylated extracellular signal-regulated kinase in a transient manner (Cortizo et al. 2006). We therefore suggest that RANKL/receptor activator of nuclear factor κB/osteoprotegerin pathway, AMPK signaling, and the extracellular signal-regulated kinase signaling pathway may be involved in the process by which metformin was able to reverse the adverse effects of diabetes on orthodontic tooth movement.
Osteocytes, the most abundant cells in bone, are regarded as mechanosensory cells, which send signals to other bone cells to initiate bone formation and remodeling (Matsumoto et al. 2013; Yang et al. 2005) as well as playing a vital role in monitoring the mechanical environment within bone tissue under orthodontic force (Jiang et al. 2015). Sclerostin is an osteocyte-specific cysteine knot-secreted glycoprotein that plays a key negative regulatory role in mechanotransduction by inhibiting osteoblastic bone formation (Inagaki et al. 2016; Lin et al. 2009; Zagrodna et al. 2016). DMP-1 is reported to be a promoter of bone mineralization, and the DMP-1 gene is activated in response to mechanical loading in osteocytes in alveolar bone in response to tooth movement (Gluhak-Heinrich et al. 2003). In the current study, after metformin administration, osteocytes on all sides of alveolar bone showed slighter SOST expression and stronger DMP-1 expression than the DB group. Consistent with our findings, van Lierop et al. (van Lierop et al. 2012)reported that metformin decreased serum sclerostin levels in patients with type 2 diabetes mellitus, and Yee CS et al. (Yee et al. 2016) reported that sclerostin antibody treatment improves DMP-1 signaling along the surface of bone in a mouse model of type 1 diabetes through the Wnt/β-catenin signaling pathway.
It has been recently reported that osteocytes themselves are the major bone source for RANKL and osteoprotegerin molecules (Kramer et al. 2010; Nakashima et al. 2011; Xiong et al. 2011). Several previous studies have reported that altered sclerostin levels regulate the expression of RANKL/osteoprotegerin (Wijenayaka et al. 2011; You et al. 2008), and proteins encoded by ‘mineralization-related genes’, such as DMP-1, phosphate-regulating neutral endopeptidase (X-linked), and probably fibroblast growth factor 23 (Atkins et al. 2011; Gluhak-Heinrich et al. 2003; Rendenbach et al. 2014). Therefore, the Wnt/β-catenin signal pathway and RANKL/receptor activator of nuclear factor κB/osteoprotegerin pathway may play important roles in the ability of metformin to reverse the detrimental effects of diabetes on osteocytes during orthodontic tooth movement.
In conclusion, our results suggest that in this diabetic model, diabetes enhanced the number and activity of osteoclasts under orthodontic force, resulting in a greater amount of tooth movement. However, metformin administration decreased the number and activity of osteoclasts, improved osteoblast activity, and rescued the function of osteocytes in these diabetic rats, resulting in normalization of tooth movement. These potentially bone-protecting effects of metformin may be relevant in clinical settings of diabetic orthodontic tooth movement.
References
Atkins GJ, Rowe PS, Lim HP, Welldon KJ, Ormsby R, Wijenayaka AR, Zelenchuk L, Evdokiou A, Findlay DM (2011) Sclerostin is a locally acting regulator of late-osteoblast/preosteocyte differentiation and regulates mineralization through a MEPE-ASARM-dependent mechanism. J Bone Miner Res 26:1425–1436. doi:10.1002/jbmr.345
Bensch L, Braem M, Van Acker K, Willems G (2003) Orthodontic treatment considerations in patients with diabetes mellitus. Am J Orthod Dentofac Orthoped 123:74–78. doi:10.1067/mod.2003.53
Botolin S, Faugere MC, Malluche H, Orth M, Meyer R, McCabe LR (2005) Increased bone adiposity and peroxisomal proliferator-activated receptor-gamma2 expression in type I diabetic mice. Endocrinology 146:3622–3631. doi:10.1210/en.2004-1677
Braga SM, Taddei SR, Andrade I Jr., Queiroz-Junior CM, Garlet GP, Repeke CE, Teixeira MM, da Silva TA (2011) Effect of diabetes on orthodontic tooth movement in a mouse model. Eur J Oral Sci 119:7–14. doi:10.1111/j.1600-0722.2010.00793.x
Cortizo AM, Sedlinsky C, McCarthy AD, Blanco A, Schurman L (2006) Osteogenic actions of the anti-diabetic drug metformin on osteoblasts in culture. Eur J Pharmacol 536:38–46. doi:10.1016/j.ejphar.2006.02.030
Cui J, Li J, Wang W, Han X, Du J, Sun J, Feng W, Liu B, Liu H, Amizuka N, Li M (2016) The effect of calcitriol on high mobility group box 1 expression in periodontal ligament cells during orthodontic tooth movement in rats. J Mol Histol 47:221–228. doi:10.1007/s10735-016-9669-0
Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M, Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating (2011) National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 378:31–40. doi:10.1016/S0140-6736(11)60679-X
Fu HD, Wang BK, Wan ZQ, Lin H, Chang ML, Han GL (2016) Wnt5a mediated canonical Wnt signaling pathway activation in orthodontic tooth movement: possible role in the tension force-induced bone formation. J Mol Histol 47:455–466. doi:10.1007/s10735-016-9687-y
Gao Y, Xue J, Li X, Jia Y, Hu J (2008) Metformin regulates osteoblast and adipocyte differentiation of rat mesenchymal stem cells. J Pharm Pharmacol 60:1695–1700. doi:10.1211/jpp.60/12.0017
Gao Y, Li Y, Xue J, Jia Y, Hu J (2010) Effect of the anti-diabetic drug metformin on bone mass in ovariectomized rats. Eur J Pharmacol 635:231–236. doi:10.1016/j.ejphar.2010.02.051
Giglio MJ, Lama MA (2001) Effect of experimental diabetes on mandible growth in rats. Eur J Oral Sci 109:193–197
Gluhak-Heinrich J, Ye L, Bonewald LF, Feng JQ, MacDougall M, Harris SE, Pavlin D (2003) Mechanical loading stimulates dentin matrix protein 1 (DMP1) expression in osteocytes in vivo. J Bone Miner Res 18:807–817. doi:10.1359/jbmr.2003.18.5.807
He H, Liu R, Desta T, Leone C, Gerstenfeld LC, Graves DT (2004) Diabetes causes decreased osteoclastogenesis, reduced bone formation, and enhanced apoptosis of osteoblastic cells in bacteria stimulated bone loss. Endocrinology 145:447–452. doi:10.1210/en.2003-1239
Hie M, Shimono M, Fujii K, Tsukamoto I (2007) Increased cathepsin K and tartrate-resistant acid phosphatase expression in bone of streptozotocin-induced diabetic rats. Bone 41:1045–1050. doi:10.1016/j.bone.2007.08.030
Huh S, Lee CY, Ohe JY, Lee JW, Choi BJ, Lee BS, Kwon YD (2015) Chronic maxillary sinusitis and diabetes related maxillary osteonecrosis: a case report. J Korean Assoc Oral Maxillofac Surg 41:332–337. doi:10.5125/jkaoms.2015.41.6.332
Inagaki Y, Hookway ES, Kashima TG, Munemoto M, Tanaka Y, Hassan AB, Oppermann U, Athanasou NA (2016) Sclerostin expression in bone tumours and tumour-like lesions. Histopathology. doi:10.1111/his.12953
Jeyabalan J, Viollet B, Smitham P, Ellis SA, Zaman G, Bardin C, Goodship A, Roux JP, Pierre M, Chenu C (2013) The anti-diabetic drug metformin does not affect bone mass in vivo or fracture healing. Osteoporos Int 24:2659–2670. doi:10.1007/s00198-013-2371-0
Jiang F, Xia Z, Li S, Eckert G, Chen J (2015) Mechanical environment change in root, periodontal ligament, and alveolar bone in response to two canine retraction treatment strategies. Orthod Craniofac Res 18(Suppl 1):29–38. doi:10.1111/ocr.12076
Kramer I, Halleux C, Keller H, Pegurri M, Gooi JH, Weber PB, Feng JQ, Bonewald LF, Kneissel M (2010) Osteocyte Wnt/beta-catenin signaling is required for normal bone homeostasis. Mol Cell Biol 30:3071–3085. doi:10.1128/MCB.01428-09
Krishnan V, Davidovitch Z (2006) Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofacial Orthop 129(469):e461–e432. doi:10.1016/j.ajodo.2005.10.007
Li M, Hasegawa T, Hogo H, Tatsumi S, Liu Z, Guo Y, Sasaki M, Tabata C, Yamamoto T, Ikeda K, Amizuka N (2013) Histological examination on osteoblastic activities in the alveolar bone of transgenic mice with induced ablation of osteocytes. Histol Histopathol 28:327–335
Li J, Feng W, Liu, Sun B, Han B, Du X, Sun J, Yimin J, Cui J, Guo J, Kudo J, Amizuka A, Li M (2015) Altered distribution of HMGB1 in the periodontal ligament of periostin-deficient mice subjected to Waldo’s orthodontic tooth movement. J Mol Histol 46:303–311. doi:10.1007/s10735-015-9619-2
Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J, Li Y, Feng G, Gao X, He L (2009) Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res 24:1651–1661. doi:10.1359/jbmr.090411
Lv S, Li J, Feng W, Liu H, Du J, Sun J, Cui J, Sun B, Han X, Oda K, Amizuka N, Xu X, Li M (2015) Expression of HMGB1 in the periodontal tissue subjected to orthodontic force application by Waldo’s method in mice. J Mol Histol 46:107–114. doi:10.1007/s10735-014-9606-z
Mai QG, Zhang ZM, Xu S, Lu M, Zhou RP, Zhao L, Jia CH, Wen ZH, Jin DD, Bai XC (2011) Metformin stimulates osteoprotegerin and reduces RANKL expression in osteoblasts and ovariectomized rats. J Cell Biochem 112:2902–2909. doi:10.1002/jcb.23206
Matsumoto T, Iimura T, Ogura K, Moriyama K, Yamaguchi A (2013) The role of osteocytes in bone resorption during orthodontic tooth movement. J Dent Res 92:340–345. doi:10.1177/0022034513476037
Mishima N, Sahara N, Shirakawa M, Ozawa H (2002) Effect of streptozotocin-induced diabetes mellitus on alveolar bone deposition in the rat. Arch Oral Biol 47:843–849
Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, Penninger JM, Takayanagi H (2011) Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med 17:1231–1234. doi:10.1038/nm.2452
Oda K, Amaya Y, Fukushi-Irie M, Kinameri Y, Ohsuye K, Kubota I, Fujimura S, Kobayashi J (1999) A general method for rapid purification of soluble versions of glycosylphosphatidylinositol-anchored proteins expressed in insect cells: an application for human tissue-nonspecific alkaline phosphatase. J Biochem 126:694–699
Peer A, Khamaisi M (2015) Diabetes as a risk factor for medication-related osteonecrosis of the jaw. J Dent Res 94:252–260. doi:10.1177/0022034514560768
Rakel A, Sheehy O, Rahme E, LeLorier J (2008) Osteoporosis among patients with type 1 and type 2 diabetes. Diabetes Metab 34:193–205. doi:10.1016/j.diabet.2007.10.008
Rendenbach C, Yorgan TA, Heckt T, Otto B, Baldauf C, Jeschke A, Streichert T, David JP, Amling M, Schinke T (2014) Effects of extracellular phosphate on gene expression in murine osteoblasts. Calcif Tissue Int 94:474–483. doi:10.1007/s00223-013-9831-6
Shah M, Kola B, Bataveljic A, Arnett TR, Viollet B, Saxon L, Korbonits M, Chenu C (2010) AMP-activated protein kinase (AMPK) activation regulates in vitro bone formation and bone mass. Bone 47:309–319. doi:10.1016/j.bone.2010.04.596
Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P (2005) Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res 52:313–320. doi:10.1016/j.phrs.2005.05.004
Tolosa MJ, Chuguransky SR, Sedlinsky C, Schurman L, McCarthy AD, Molinuevo MS, Cortizo AM (2013) Insulin-deficient diabetes-induced bone microarchitecture alterations are associated with a decrease in the osteogenic potential of bone marrow progenitor cells: preventive effects of metformin. Diabetes Res Clin Pract 101:177–186. doi:10.1016/j.diabres.2013.05.016
van Lierop AH, Hamdy NA, van der Meer RW, Jonker JT, Lamb HJ, Rijzewijk LJ, Diamant M, Romijn JA, Smit JW, Papapoulos SE (2012) Distinct effects of pioglitazone and metformin on circulating sclerostin and biochemical markers of bone turnover in men with type 2 diabetes mellitus. Eur J Endocrinol/Eur Fed Endocr Soci 166:711–716. doi:10.1530/EJE-11-1061
Verna C, Dalstra M, Melsen B (2000) The rate and the type of orthodontic tooth movement is influenced by bone turnover in a rat model. Eur J Orthod 22:343–352
Vestergaard P, Rejnmark L, Mosekilde L (2005) Relative fracture risk in patients with diabetes mellitus, and the impact of insulin and oral antidiabetic medication on relative fracture risk. Diabetologia 48:1292–1299. doi:10.1007/s00125-005-1786-3
Wijenayaka AR, Kogawa M, Lim HP, Bonewald LF, Findlay DM, Atkins GJ (2011) Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS One 6:e25900. doi:10.1371/journal.pone.0025900
Wise GE, King GJ (2008) Mechanisms of tooth eruption and orthodontic tooth movement. J Dent Res 87:414–434
Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA (2011) Matrix-embedded cells control osteoclast formation. Nat Med 17:1235–1241. doi:10.1038/nm.2448
Yang W, Lu Y, Kalajzic I, Guo D, Harris MA, Gluhak-Heinrich J, Kotha S, Bonewald LF, Feng JQ, Rowe DW, Turner CH, Robling AG, Harris SE (2005) Dentin matrix protein 1 gene cis-regulation: use in osteocytes to characterize local responses to mechanical loading in vitro and in vivo. J Biol Chem 280:20680–20690. doi:10.1074/jbc.M500104200
Yee CS, Xie L, Hatsell S, Hum N, Murugesh D, Economides AN, Loots GG, Collette NM (2016) Sclerostin antibody treatment improves fracture outcomes in a Type I diabetic mouse model. Bone 82:122–134. doi:10.1016/j.bone.2015.04.048
You L, Temiyasathit S, Lee P, Kim CH, Tummala P, Yao W, Kingery W, Malone AM, Kwon RY, Jacobs CR (2008) Osteocytes as mechanosensors in the inhibition of bone resorption due to mechanical loading. Bone 42:172–179. doi:10.1016/j.bone.2007.09.047
Zagrodna A, Jozkow P, Medras M, Majda M, Slowinska-Lisowska M. (2016) Sclerostin as a novel marker of bone turnover in athletes. Biol Sport 33:83–87. doi:10.5604/20831862.1194125
Zinman B, Haffner SM, Herman WH, Holman RR, Lachin JM, Kravitz BG, Paul G, Jones NP, Aftring RP, Viberti G, Kahn SE (2010) Effect of rosiglitazone, metformin, and glyburide on bone biomarkers in patients with type 2 diabetes. J Clin Endocrinol Metab 95:134–142. doi:10.1210/jc.2009-0572
Acknowledgements
This study was partially supported by the National Nature Science Foundation of China (Nos. 81271965; 81470719; 8151101150) to Li M. The Shandong Province Science and Technique Foundation, China (No. 2014GSF118093) to Guo J.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Rights and permissions
About this article
Cite this article
Sun, J., Du, J., Feng, W. et al. Histological evidence that metformin reverses the adverse effects of diabetes on orthodontic tooth movement in rats. J Mol Hist 48, 73–81 (2017). https://doi.org/10.1007/s10735-016-9707-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-016-9707-y