Abstract
Diabetes mellitus impairs angiogenesis and tissue reorganization during orthodontic tooth movement (OTM). Thus, this study evaluated pulpal outcomes in orthodontic tooth movement through metabolic changes in diabetes. Male Wistar rats were used, and the in vivo study design consisted of four groups (n = 10/group): C—non-diabetic animals not subjected to orthodontic tooth movement; D—diabetic animals not subjected to orthodontic tooth movement; OTM—non-diabetic animals subjected to orthodontic tooth movement; and D + OTM—diabetic animals subjected to orthodontic tooth movement. In addition, the pulps of the distovestibular root (DV) and mesiovestibular root (MV) were assessed by histomorphometric analyses and immunoexpression of the RANKL/OPG system. Pulpal analysis of the MV root showed an increase in blood vessels in diabetic animals. Inflammatory infiltrate and fibroblastic cells were elevated in diabetic animals with tooth movement in the DV and MV roots. In the DV and MV roots, diabetic rats with OTM showed a reduction in birefringent collagen fibers. The immunostaining for RANKL was higher in the pulp tissue of OTM in diabetic and non-diabetic animals. It was concluded that the pulp tissue has less adaptive and repair capacity during OTM in diabetes. Orthodontic strength can alter the inflammatory processes in the pulp.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metabolic-compromised patients have been seeking orthodontic treatments for esthetic and functional improvements, changing the reality in the offices, and increasing the professional challenges for safe care [1]. Diabetic patients should not be treated orthodontically until they have this metabolic disease under control [2], as they may have a lower density and greater propensity for bone loss [3]. Type 1 diabetes is related to the destruction of pancreatic β cells and is responsible for 5–10% of diabetics. Conversely, type 2 diabetes is characterized by peripheral insulin resistance [4]. Despite having different origins, their consequences are similar, altering the inflammatory response and vasculogenesis, making tissue repair difficult [5, 6].
Osteoprotegerin (OPG), nuclear factor-kappa B ligand (RANKL), and nuclear factor-kappa B receptor (RANK) are some of the main mediators expressed in the bone remodeling process and are involved in orthodontic movement [7]. The RANK/RANKL/OPG system is responsible for bone remodeling through the activation of osteoclasts and participates in the modulation of the activity of cells that make up the extracellular matrix, such as fibroblasts and osteoblasts. These proteins also regulate the activity of odontoblasts during odontogenesis [8, 9], and their activity may be altered by metabolic changes, such as diabetes.
Patients with diabetes exhibit intense collagenase activity and a decrease in fibroblastic collagen synthesis in gingival tissue [10]. In addition, the inflammatory process stimulates the production of VEGF, one of the most important proangiogenic factors involved in bone angiogenesis, osteoblast differentiation, osteoclast recruitment, and RANKL expression in the periodontal ligament [11]. Inflammatory disorders in diabetes cause vascular and cell differentiation and migration impairment in the early stages of tissue repair [12]. Thus, diabetes may promote poor bone remodeling and pulpal reaction outcomes in orthodontic tooth movement.
Diabetes negatively affects bone remodeling during the application of orthodontic forces [2, 13, 14] and the force application reversibly alters the pulp blood flow under normal metabolic conditions [15, 16]. Metabolic changes in diabetes alter the inflammatory response during orthodontic movement [17]. Root anatomy and orthodontic strength can affect the reactions in the periodontal ligament [18] and can change the pulp response. Due to the negative repercussions of diabetes on dental pulp physiology, we hypothesize that orthodontic tooth movement in experimental diabetes can alter pulp tissue organization and expression of the RANKL/OPG system.
Materials and methods
Sample distribution
The study was approved by the institutional ethics committee (022/2014). Male Wistar rats (Rattus norvegicus), 90 days old, weighing 300 g, were used for the in vivo analysis (n = 10/group): OTM group—non-diabetic animals submitted to OTM and D + OTM group—diabetic animals submitted to OTM. The contralateral side of both groups that were not subjected to OTM was also analyzed; thus, the control groups were established: C group—non-diabetic animals not subjected to OTM and D group—diabetic animals not subjected to OTM. The number of animals was determined according to a study by Santamaria-Jr [17] and adhered to the principles of replacement, reduction, and refinement (the three Rs in animal study) [19].
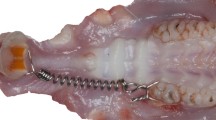
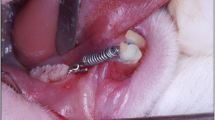
The diabetes induction procedures and subsequent installation of the orthodontic device were performed under intraperitoneal anesthesia with xylazine hydrochloride (20 mg/kg) and ketamine hydrochloride (60 mg/kg). The experimental protocol is shown in Fig. 1.
Diabetes induction and orthodontic tooth movement protocol
Diabetes induction was performed with alloxan monohydrate at a single dose of 150 mg/kg (Sigma Chemical Co., St Louis, MO, USA) diluted in 0.9% saline. The solution was administered intraperitoneally while fasting. After the induction of diabetes, the animals received a glucose solution (80%) orally for 24 h to prevent alloxanic hypoglycemia [3]. Blood samples were collected weekly for 30 days (Accu-Chek Advantage, Boehringer Mannheim, IN, USA), and animals with a blood glucose level > 200 mg/dL were considered diabetic [3, 17].
Sixty days after diabetes induction, an orthodontic device was installed in the upper first molar, with a 4-mm nickel–titanium spring, releasing a force of 0.4 N [20, 21], for 10 days [22]. At the end of the experimental period, the animals were euthanized with a lethal dose of xylazine hydrochloride and ketamine hydrochloride, and the epithelial and muscular components surrounding the maxilla were removed. The effect of orthodontic strength was analyzed on the larger sized mesiovestibular root (MV) and the smaller sized distovestibular root (DV) [18] (Fig. 1).
Histomorphometric analysis
The samples were fixed in a 10% formaldehyde-buffered solution for 72 h and demineralized in EDTA for 2 months. Transverse paraffin sections with a thickness of 5 µm were cut from the specimens to permit visualization of all roots of the first molars until the cervical region of the periodontal tissue and roots were reached to visualize the pulp [21, 22]. In the histomorphometric analysis, three pulp fields were made in the five sections obtained from each animal in which three images were captured (n = 15 images/animal) from the root cervical level. The measurements were made from the original image documented at 400× magnification by a single calibrated observer. The agreement from both assessments was tested using the intraclass correlation test (intra-rater consistency, IC = 0.8).
The histological sections were stained with 0.025% toluidine blue (TB) solution (pH 4.0) for 30 min at room temperature for fibroblast and blood vessel determination (n/104 μm2). The inflammatory infiltrate (n/104 μm2) was quantified using the Dominici method (0.5% acid fuchsin, orange G, and toluidine blue) for 10 min. The percentage of the area of birefringent collagen fibers in the pulp and dentin tissue (% of the total area) was determined using Picrosirius-hematoxylin (PH) staining, with 0.1% Sirius red for 10 min and visualized in a bright field (TM) under polarized light (pol) [21, 22]. The images were obtained using a Leica DM2000 photomicroscope.
Immunohistochemical analysis
For immunohistochemical staining, 5.0-μm sections were placed on silanized slides. The samples were incubated with primary antibodies: anti-OPG (sc-390518, 1:200) and anti-RANKL (sc-377079, 1:200) (Santa Cruz Biotechnology, Dallas, USA), and the detection reaction was performed using DAB (Novolink™ Max Polymer Detection System, RE7280-K, Leica Biosystems Newcastle Ltd., UK), according to the manufacturer’s specifications [23, 24]. The number of pulp cells that were positive for RANKL and OPG was determined in three fields of the pulp tissue in five cuts per animal (400×).
Statistical analysis
The results were expressed as mean ± standard deviation (SD). All data were subjected to the Kolmogorov–Smirnov normality test, adjusted to the normality curve. The different parameters were analyzed within the same MV or DV root, using ANOVA and Tukey’s post-test, with significance level set at *p < 0.05, **p < 0.01, and ***p < 0.001. Student’s t test was used to compare the same group in the two different roots, DV and MV (•p < 0.05).
Results
Pulp microstructural analysis
Figure 2a shows the pulp of the DV and MV roots of the first molar. In all the evaluated groups, the pulp cavity content was divided into an acellular region, which was in close contact with the dentin, a layer of odontoblasts, and the central area, rich in fibroblastic cells and blood vessels. The acellular area showed no apparent differences between the groups. On the other hand, the odontoblast layer presented a more juxtaposed strata in the moved tooth groups that, with their nuclei presenting hypertrophic chromatin and more basophilic cytoplasm.
Morphometric analysis of the dental pulp. a Photomicrographs of the transverse sections of the distovestibular and mesiovestibular root of the first molar. b Total number of blood vessels and c inflammatory cells. Force direction (F—arrow); D: dentin; (*) blood vessels; (→) odontoblasts; (►) fibroblastic cells. Bar = 200 µm. Statistical significance, *p < 0.05; **p < 0.01, ***p < 0.001, and •p < 0.05; difference in the same group in the different roots
Pulp histomorphometric analysis
Distovestibular root
In the analysis of the pulp of the DV root, it was observed that the number of blood vessels during orthodontic movement was equal (p > 0.05) in the OTM (1.8 ± 0.6) and D + OTM groups (2.1 ± 0.9) (Fig. 2b). The inflammatory infiltrate was higher (p < 0.01) in diabetic animals with movement in D + OTM (10.9 ± 1.5) in relation to the other groups, C (8.6 ± 1.7), D (8.8 ± 1.9), and OTM (8.8 ± 1.6) (Fig. 2c).
The number of fibroblastic cells present in the pulp area was higher (p < 0.001) in diabetics with and without movement, D (20.2 ± 2.1) and D + OTM (22.9 ± 1.6), in relation to non-diabetic animals with and without orthodontic movement, C (16.2 ± 2.8) and OTM (18.6 ± 2.4) (Fig. 3b). The area of birefringent collagen fibers was greater (p < 0.001) in the OTM group (14.1 ± 1.6) than in the D + OTM group (11.7 ± 2.34) (Fig. 3a, c).
Dental pulp tissue organization. a Picrosirius sections of the distovestibular root of the first molar of rats with 10 days of OTM. b Total number of fibroblastic cells and c area of birefringent collagen fibers in the pulp region of the distovestibular and mesiovestibular root. Force direction (F—arrow); D: dentin. The sections were treated by the picrosirius-hematoxylin method and analyzed in bright field and under polarized light (pol). (►) Collagen fibers. Bar = 200 µm. Statistical significance, *p < 0.05; **p < 0.01, ***p < 0.001, and •p < 0.05, difference in the same group in the different roots
Mesiovestibular root
In the analysis of the pulp of the MV root, it was observed that the number of blood vessels in the D (4.2 ± 1.0) and D + OTM groups (4.4 ± 0.8) was higher (p < 0.001) in relation to the C (2.6 ± 0.7) OTM group (2.9 ± 0.7) (Fig. 2b). The inflammatory infiltrate was higher (p < 0.001) in diabetic animals with movement, D + OTM (9.1 ± 0.9), in relation to C (6.9 ± 0.2), D (7.3 ± 1.2), and OTM (7.4 ± 1.0) groups (Fig. 2c).
The number of fibroblasts in the D (38.2 ± 3.9) and D + OTM groups (37.2 ± 3.3) was higher (p < 0.001) than in the control groups C (29.2 ± 2.3) and OTM (30.1 ± 1.6) (Fig. 3b). The area of birefringent collagen fibers was greater (p < 0.001) in the C group (19.6 ± 1.6) than in the D + OTM group (17.6 ± 1.7) (Fig. 3c).
The number of blood vessels, fibroblasts, and collagen fibers was higher in the MV root than in the DV root; however, the inflammatory infiltrate was greater in the DV root (p < 0.05).
Pulp immunohistochemical analysis
Distovestibular root
In the analysis of the pulp of the DV root, an increase (p < 0.001) of the cells immunostained for RANKL was observed in D + OTM (3.5 ± 0.6) compared to D (2.1 ± 0.6) and C (2.0 ± 0.7). Significant differences (p < 0.05) were found between the OTM (2.9 ± 0.7), C (2.0 ± 0.7), and D (2.1 ± 0.6) groups (Fig. 4a, b). There was no difference in the expression of OPG in any of the groups analyzed (p > 0.05) (Fig. 4c).
RANKL/OPG expression in the dental pulp. a Transverse sections of the distovestibular root of the first molar of rats with 10 days of OTM. b Total number of pulp cells immunostained for RANKL and c OPG in the pulp region of the distovestibular and mesiovestibular root. Force direction (F—arrow); D: dentin. (►) Cells with positive reaction. Bar = 350 µm. Statistical significance, *p < 0.05; **p < 0.01, ***p < 0.001, and •p < 0.05, difference in the same group in the different roots
Mesiovestibular root
In the analysis of the pulp of the MV root, a significant increase (p < 0.05) in cells immunostained for RANKL was observed in D + OTM (1.8 ± 0.7) compared to that in the C group (1.0 ± 0.8) (Fig. 4b). RANKL levels were higher in the DV root than in the MV root (p < 0.05). In addition, there was no significant difference in the OPG between the groups (p > 0.05) (Fig. 4c).
Discussion
Orthodontic movement promotes bone remodeling through resorption and apposition, activating inflammatory cells essential for the process [25]. As a modifying factor of the inflammatory response, diabetes mellitus negatively affects bone remodeling in orthodontic movement [14]. Diabetes is a metabolic disorder with a high incidence worldwide [26]. Symptoms of type 1 or 2 diabetes are similar [27, 28], causing major vascular and repair changes in the connective tissues [5, 6]. Therefore, as the dental pulp is a highly vascularized connective tissue, it can be directly affected by diabetes.
In vivo microscopic analysis has demonstrated orthodontic movement in periodontal tissues [17, 18, 21, 22]. The presence of a hyaline area in the periodontal ligament [29] and root resorption indicates the application of inappropriate forces [30, 31]. Moreover, the anatomy and size of the roots have proven to be an important factor in the intensity of orthodontic strength [32], where the DV, due to its smaller dimensions, may be subjected to higher orthodontic force while the MV, because its larger size, may be subjected to a lower orthodontic force [18, 32]. In this study, we used an in vivo model to elucidate pulp changes after orthodontic movement in the presence of this metabolic disorder. Pupal analysis showed differences between the moved and non-moved teeth. During orthodontic movement, the odontoblast layer presented more juxtaposed strata and nuclei with hypertrophic chromatin and more basophilic cytoplasm. Under these conditions, cells and blood vessels were clustered more and had larger diameters. In all groups, the extracellular matrix was predominantly fibrillar with sparse collagen fibers.
Under normal metabolic conditions, the dentinopulpar complex, when subjected to orthodontic movement, presents reversible pulp inflammation when its biological limit is not exceeded. In addition, after movement, no degeneration can be detected by optical microscopy [33, 34]. However, diabetes can lead to a pulp inflammatory process with a consequent increase in the number of blood vessels [17]. The results of the present study reveal that this increase occurs, especially in the MV root, of greater volume and with a greater density of vessels.
In vivo studies indicate that after the first hours of the start of orthodontic movement, the pulp tissue presents an increase in vascular volume, returning to the initial values in the first 72 h [20]. The risk of irreversible pulp inflammation during orthodontic treatment is related to dental trauma [15], and these changes can be enhanced in diabetic patients who have less control of the inflammatory process [35,36,37]. In this study, the inflammatory infiltrate was greater in diabetic animals with orthodontic movement (D + OTM) than in the other groups (C, D, and OTM), both in the DV and MV root. In the distovestibular root, this process occurred more intensely because of its lower volume in relation to the mesiovestibular root, demonstrating that the inflammatory process was more present in the application of higher orthodontic strength.
In diabetes, the pulp response is deficient in injuries [35] and its repair is impaired [36]. The pulp alterations caused by diabetes were evident because the entire organizational structure was affected, including the fibroblasts and collagen fibers, as demonstrated in this study. This condition modifies all processes of defense and repair of pulp tissue [37]. Morphometric analysis revealed differences between the control and diabetic groups. The number of fibroblasts in diabetic rats with orthodontic movement (D + OTM) and without tooth movement (D) was similar and significantly higher than in the control group with OTM and without tooth movement (C), both in the distovestibular root and in the mesiovestibular root. Despite the increase in the number of fibroblasts in diabetes, when associated with orthodontic movement, the area of birefringent collagen fibers was smaller in the pulp and dentin, demonstrating the difficulty in organizing and repairing the pulp. Tissue disorganization may have been aggravated by an increase in the inflammatory process associated with tooth movement with metabolic alteration, independent of the intensity of the applied force.
The study showed a significant increase in RANKL in the moved tooth groups (OTM and D + OTM) compared to the non-moved groups (C and D). The greater expression of this marker indicates that odontoblastic cells showed less dentinogenesis [9, 38], signaling a down-regulation in pulp metabolism and its homeostasis process. We can conclude that orthodontic movement was a determining factor for this increase since the non-moved groups (C and D) presented similar results and were statistically smaller before the application of the force. As a result of this increase, there was a compensatory increase in OPG levels in the same groups. Although the difference was not statistically significant, it could be a counter-regulatory mechanism for pulp homeostasis. In addition, the entire process was greater in the action of higher orthodontic strength.
Although the results show evident metabolic changes in the face of orthodontic forces and diabetes, this study is an experimental protocol, in which animals have uncontrolled diabetes and the time to assess orthodontic movement has been limited. Further molecular and clinical studies are needed to better understand the pulp changes in metabolic disorders and orthodontic treatment.
Conclusion
The pulp tissue has a less adaptive and repair capacity during orthodontic movement in diabetes mellitus. Higher orthodontic strength may promote a greater inflammatory process and disorganization of pulp tissue.
References
Hassan AH, Al-Fraidi AA, Al-Saeed SH. Corticotomy–assisted orthodontic treatment: review. Open Dent J. 2010;4:159–64. https://doi.org/10.2174/1874210601004010159.
Bensch L, Braem M, Van Acker K, Willems G. Orthodontic treatment considerations in patients with diabetes mellitus. Am J Orthod Dentofacial Orthop. 2003;123(1):74–8. https://doi.org/10.1067/mod.2003.53.
Ferreira CL, da Rocha VC, da Silva Ursi WJ, De Marco AC, Santamaria M, Santamaria MP, et al. Periodontal response to orthodontic tooth movement in diabetes-induced rats with or without periodontal disease. J Periodontol. 2018;89(3):341–50. https://doi.org/10.1002/JPER.17-0190.
Camargo WA, de Vries R, van Luijk J, Hoekstra JW, Bronkhorst EM, Jansen JA, et al. Diabetes mellitus and bone regeneration: a systematic review and meta-analysis of animal studies. Tissue Eng Part B Rev. 2017;23(5):471–9. https://doi.org/10.1089/ten.TEB.2016.0370.
Fatehi F, Nafissi S, Basiri K, Amiri M, Soltanzadeh A. Chronic inflammatory demyelinating polyneuropathy associated with diabetes mellitus. J Res Med Sci. 2013;18(5):438–41 (PMID: 24174953).
Gilbert RE. Endothelial loss and repair in the vascular complications of diabetes: pathogenetic mechanisms and therapeutic implications. Circ J. 2013;77(4):849–56 (PMID: 23503045).
Li Y, Jacox LA, Little SH, Ko CC. Orthodontic tooth movement: the biology and clinical implications. Kaohsiung J Med Sci. 2018;34(4):207–14. https://doi.org/10.1016/j.kjms.2018.01.007.
Kohli SS, Kohli VS. Role of RANKL–RANK/osteoprotegerin molecular complex in bone remodeling and its immunopathologic implications. Indian J Endocrinol Metab. 2011;15(3):175–81. https://doi.org/10.4103/2230-8210.83401.
Calsa B, Masiero BC, Esquisatto MAM, Catisti R, Santamaria M Jr. Gestational protein restriction alters the RANKL/OPG system in the dental germ of offsprings. J Oral Biol Craniofac Res. 2020;10(4):743–6. https://doi.org/10.1016/j.jobcr.2020.10.007.
Popescu MR, Surlin P, Rauten AM, Dragomir L, Olteanu M. Histological analysis of collagen fibers in patients with diabetes mellitus and periodontal disease. J Cytol Histol. 2014;S4:008. https://doi.org/10.4172/2157-7099.S4-008.
Park HJ, Baek KH, Lee HL, Kwon A, Hwang HR, Qadir AS, et al. Hypoxia inducible factor-1α directly induces the expression of receptor activator of nuclear factor-κB ligand in periodontal ligament fibroblasts. Mol Cells. 2011;31(6):573–8. https://doi.org/10.1007/s10059-011-1055-x.
Neves LMG, Matheus RL, Santos GMT, Esquisatto MAM, Amaral MEC, Mendonça FAS. Effects of microcurrent application and 670 nm InGaP low-level laser irradiation on experimental wound healing in healthy and diabetic Wistar rats. Laser Phys. 2013. https://doi.org/10.1088/1054-660X/23/3/035604.
Villarino ME, Lewicki M, Ubios AM. Bone response to orthodontic forces in diabetic Wistar rats. Am J Orthod Dentofacial Orthop. 2011;139(4 Suppl):S76-82. https://doi.org/10.1016/j.ajodo.2010.06.021.
Najeeb S, Siddiqui F, Qasim SB, Khurshid Z, Zohaib S, Zafar MS. Influence of uncontrolled diabetes mellitus on periodontal tissues during orthodontic tooth movement: a systematic review of animal studies. Prog Orthod. 2017;18(1):5. https://doi.org/10.1186/s40510-017-0159-z.
Javed F, Al-Keraif AA, Romanos EB, Romanos GE. Influence of orthodontic forces on human dental pulp: a systematic review. Arch Oral Biol. 2015;60(2):347–56. https://doi.org/10.1016/j.archoralbio.2014.11.011.
Von Böhl M, Ren Y, Kuijpers-Jagtman AM, Fudalej PS, Maltha JC. Age-related changes of dental pulp tissue after experimental tooth movement in rats. PeerJ. 2016;4:e1625. https://doi.org/10.7717/peerj.1625.
Santamaria-Jr M Jr, Bagne L, Zaniboni E, Santamaria MP, Jardini MAN, Felonato M, et al. Diabetes mellitus and periodontitis: inflammatory response in orthodontic tooth movement. Orthod Craniofac Res. 2020;23:27–34. https://doi.org/10.1111/ocr.12340.
Zaniboni E, Vedovello Filho M, Santamaria MP, Jardini MAN, Martins-Ortiz MF, Consolaro A, et al. Root morphology can be a risk factor for periodontal damage and root resorption in orthodontic movement. Braz J Oral Sci. 2017;16:1–8. https://doi.org/10.20396/bjos.v16i0.8651188.
Tannenbaum J, Bennett BT. Russell and Burch’s 3Rs then and now: the need for clarity in definition and purpose. J Am Assoc Lab Anim Sci. 2015;54:120–32 (PMID: 25836957).
Santamaria M Jr, Milagres D, Stuani AS, Stuani MBS, Ruellas ACO. Initial changes in pulpal microvasculature during orthodontic tooth movement: a stereological study. Eur J Orthod. 2006;28:217–20. https://doi.org/10.1093/ejo/cji117.
Spadari GS, Zaniboni E, Vedovello SA, Santamaria MP, do Amaral ME, Dos Santos GM, et al. Electrical stimulation enhances tissue reorganization during orthodontic tooth movement in rats. Clin Oral Investig. 2017;21:111–20. https://doi.org/10.1007/s00784-016-1759-6.
Franzoni JS, Soares FMP, Zaniboni E, Filho MV, Santamaria MP, Dos Santos GMT, et al. Zoledronic acid and alendronate sodium and the implications in orthodontic movement. Orthod Craniofac Res. 2017;20:164–9. https://doi.org/10.1111/ocr.12192.
Gonzalez-Suarez E, Jacob AP, Jones J, Miller R, Roudier-Meyer MP, Erwert R, et al. RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature. 2010;468(7320):103–7. https://doi.org/10.1038/nature09495.
Zaniboni E, Bagne L, Camargo T, do Amaral MEC, Felonato M, de Andrade TAM, et al. Do electrical current and laser therapies improve bone remodeling during an orthodontic treatment with corticotomy? Clin Oral Investig. 2019;23(11):4083–97. https://doi.org/10.1007/s00784-019-02845-9.
Toms A, Gannon B, Carati C. The immunohistochemical response of the rat periodontal ligament endothelium to an inflammatory stimulus. Aust Orthod J. 2000;16(2):61–8 (PMID: 11201966).
Murphy A, Biringanine M, Roberts B, Stringer B, Perel P, Jobanputra K. Diabetes care in a complex humanitarian emergency setting: a qualitative evaluation. BMC Health Serv Res. 2017;17(1):431. https://doi.org/10.1186/s12913-017-2362-5.
Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–53. https://doi.org/10.2337/diacare.27.5.1047.
Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103(2):137–49. https://doi.org/10.1016/j.diabres.2013.11.002.
Hellsing E, Hammarström L. The hyaline zone and associated root surface changes in experimental orthodontics in rats: a light and scanning electron microscope study. Eur J Orthod. 1996;18(1):11–8. https://doi.org/10.1093/ejo/18.1.11.
Cuoghi OA, Aiello CA, Consolaro A, Tondelli PM, Mendonça MR. Resorption of roots of different dimension induced by different types of forces. Braz Oral Res. 2014;28(1):1–7. https://doi.org/10.1590/1807-3107BOR-2014.vol28.0013.
Maltha JC, van Leeuwen EJ, Dijkman GE, Kuijpers-Jagtman AM. Incidence and severity of root resorption in orthodontically moved premolars in dogs. Orthod Craniofac Res. 2004;7(2):115–21. https://doi.org/10.1111/j.1601-6343.2004.00283.x.
Weltman B, Vig KWL, Fields HW, Shanker S, Kaizar EE. Root resorption associated with orthodontic tooth movement: a systematic review. Am J Orthod Dentofacial Orthop. 2010;137(4):462–76. https://doi.org/10.1016/j.ajodo.2009.06.021 (discussion 12A).
Santamaria M Jr, Milagres D, Iyomasa MM, Stuani MBS, Ruellas ACO. Initial pulp changes during orthodontic movement: histomorphological evaluation. Braz Dent J. 2007;18(1):34–9 (PMID: 17639198).
Massaro CS, Consolaro RB, Santamaria M Jr, Consolaro MF, Consolaro U. Analysis of the dentin-pulp complex in teeth submitted to orthodontic movement in rats. J Appl Oral Sci. 2009;17(Suppl):35–42. https://doi.org/10.1590/S1678-77572009000700007.
Amatyakul S, Chakraphan D, Chotpaibulpan S, Patumraj S. The effect of long-term supplementation of vitamin C on pulpal blood flow in streptozotocin-induced diabetic rats. Clin Hemorheol Microcirc. 2003;29(3–4):313–9 (PMID: 14724356).
Garber SE, Shabahang S, Escher AP, Torabinejad M. The effect of hyperglycemia on pulpal healing in rats. J Endod. 2009;35(1):60–2. https://doi.org/10.1016/j.joen.2008.09.010.
Moraru AI, GheorghiŢa LM, Dascălu IT, Bătăiosu M, Manolea HO, Agop Forna D, et al. Histological and immunohistochemical study on the dental pulp of patients with diabetes mellitus. Rom J Morphol Embryol. 2017;58(2):493–9 (PMID: 28730235).
Tyrovola JB. The “mechanostat theory” of frost and the OPG/RANKL/RANK system. J Cell Biochem. 2015;116(12):2724–9. https://doi.org/10.1002/jcb.25265.
Acknowledgements
This work was supported by the National Council for Scientific and Technological Development–CAPES/PNPD (Process no 23038.008192/2013‐01) and the Hermínio Ometto Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors state that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Santamaria-Jr, M., do Nascimento, E.R.A., Bagne, L. et al. Pulpal outcomes in orthodontic tooth movement in diabetes mellitus. Odontology 109, 921–929 (2021). https://doi.org/10.1007/s10266-021-00609-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10266-021-00609-0