Abstract
Orthodontic tooth movement (OTM) is associated with bone remodeling mediated by orthodontic mechanical loading. Increasing studies reported that Wnt signaling played crucial roles in mechanical stimuli induced bone remodeling. However, little is known about the involvement of Wnt signaling in orthodontic force–induced bone formation during OTM. In virtue of the OTM mice model as we previously reported, where new bone formation was determined by micro-CT and immunoreactivity of osteocalcin and osterix, we explored the activation of Wnt signaling pathway during OTM. Our results proved the nuclei translocation of β-catenin, suggesting the activation of canonical Wnt signaling pathway in the periodontal ligament cells (PDLCs) near the alveolar bone at the tension site (TS). Moreover, the immunoreactivity of Wnt5a, but not Wnt3a in PDLCs indicated the activation of canonical Wnt pathway might be mediated by Wnt5a, but not Wnt3a as in most cases. The co-location of Wnt5a and β-catenin that was evidenced by double labeling immunofluorescence staining further supported the hypothesis. In addition, the high expression of FZD4 and LRP5 in PDLCs at TS of periodontium suggested that the activation of Wnt signaling pathway was mediated by these receptors. The negligible expression of ROR2 also indicated that canonical but not non-canonical Wnt signaling pathway was activated by Wnt5a, since previous studies demonstrated that the activation of canonical/non-canonical Wnt signaling pathway was largely dependent on the receptors. In summary, we here reported that Wnt5a mediated activation of canonical Wnt signaling pathway might contribute to the orthodontic force induced bone remodeling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During orthodontic treatment, alveolar bone and periodontal ligament remodeling after the constant application of mechanical loading to the tooth realize the expected orthodontic tooth movement (OTM) (Krishnan and Davidovitch 2006). Periodontal ligament (PDL), which connects the cementum of tooth to the alveolar bone, is a layer of mechanosensitive fibrous soft tissue that transduces the mechanic loading from tooth to alveolar bone (Mabuchi et al. 2002; Pavlin and Gluhak-Heinrich 2001) and thus provides necessary microenvironment for cells participating in the alveolar bone remodeling (Krishnan and Davidovitch 2006). When the orthodontic force exerts to a tooth, one side of the alveolar bone and the PDL is compressed (compression site), while the opposite side is stretched [tension site (TS)] (Garlet et al. 2007; Sprogar et al. 2008). Different mechanic stimuli initiates different biological remodeling of periodontal tissues, allowing compression force-associated bone resorption and tension force-associated bone formation, which together causes OTM (Lv et al. 2015; Masella and Meister 2006). The bone formation at the TS is extremely important for the long-term stability of the moved tooth (Novackova et al. 2011). However, the mechanism by which tension force induces bone formation is far from clear.
Evidenced by its high level of alkaline phosphatase (ALP) activity, PDL is reported to play a pivotal role in the tension force-induced bone formation during OTM (Cui et al. 2016; Li et al. 2015; Yamashita et al. 1987). The heterogeneous cells residing in PDL, including osteoblasts, osteoclasts, fibroblasts, cementoblasts, progenitor/stem cells, etc., may contribute to the alveolar bone remodeling (Lee et al. 2015). It has been reported that under the tension loading, some PDL cell populations are stimulated and switched to osteoblast differentiation state, thus responsible for following osteogenesis (Takimoto et al. 2015). The up-regulation of the multiple osteogenic differentiation related factors, such as bone morphogenetic protein-2 (BMP2), osteocalcin (OCN), osteopontin (OPN), runt-related transcription factor 2 (Runx2), osterix (OSX) in PDL exposed to tension force, demonstrates that these osteogenic factors might be involved in the alveolar bone formation (Feller et al. 2015; Kim et al. 2012; Taddei et al. 2012, 2013).
Recently, Wnt signaling has been evidenced to play a crucial role in mechanical stimuli induced bone remodeling by regulating the osteogenic differentiation via the up-regulation of multiple osteogenic genes (Huang and Ogawa 2010; Kang and Robling 2014). According to the activation status of β-catenin, Wnt signaling pathway can be divided into two types: canonical β-catenin dependent pathway and non-canonical β-catenin independent pathway (Angers and Moon 2009; Wang et al. 2014; Yuan et al. 2016). Further studies uncovered that the activation of β-catenin depended on the receptors to which Wnt ligands bind (Mikels and Nusse 2006; van Amerongen et al. 2012). For instance, Wnt ligands which bind to the Frizzled family of receptors along with LRP5/6 can trigger canonical pathway, while the ligands which bind to the Frizzled family of receptors along with ROR1 and ROR2 will trigger non-canonical pathway. Both canonical and non-canonical Wnt signaling pathways have been implicated in the mesenchymal stem cells (MSCs) mediated osteogenesis in mechanic loading bone (Arnsdorf et al. 2009; Baksh et al. 2007; Shi et al. 2012). At the TS during OTM, a similar stretched microenvironment was established, we hypothesized that Wnt signaling might also exert an important role in regulating osteogenesis in this place.
In the present study, we explored the expression pattern of canonical and non-canonical Wnt signals in a tooth movement model developed in mice to elucidate whether Wnt signaling pathway is involved in bone remodeling at the TS during OTM. The study will aid us to gain further insight into the molecular mechanism involved in the bone remodeling during OTM, which may provide us with a new therapeutic target to improve orthodontic treatment.
Materials and methods
Chemicals and regents
The primary antibodies against mice were anti-Wnt3a (Cell Signaling, #2391, 1:100), anti-Wnt5a (Cell Signaling, #2530, 1:100), anti-OCN (Abclonal, A1530, 1:200), anti-OSX (Chem Cruz, sc-22536-R, 1:200), anti-β-catenin (Abclonal, A2064, 1:200), anti-FZD4 (Abclonal, A8161, 1:200), anti-LRP5 (Abclonal, A5620, 1:200), anti-ROR2 (Abclonal, A0130, 1:200). The second antibodies used in double labeling immunofulorence staining were #A22220, cy3, goat anti-rabbit IgG (Abbkine, 1:200) and dylight 488, goat anti-rabbit IgG (Abbkine, 1:200). DAPI (4′,6-diamidino-2-phenylindole) used to stain the nucleus was purchased from Zsgb-bio (ZLI-9557). UltraSensitive™ SP (Rabbit) IHC Kit (KIT-9706), pepsin induced antigen retrieval solution (dig-3009) and DAB chromogenic reagent kit (DAB-0031/1031) were purchased from Maixin Biotech (Wuhan Hubei). All chemicals and reagents used in this study were of analytical grade.
Experimental tooth movement
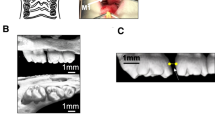
The study was approved by the Ethics Committee of the School of Stomatology, Wuhan University and in accordance with local laws and regulations. The animal experimental tooth movement model was established as we previously reported (Chang et al. 2015). The animals used were 8-week-old healthy male C57BL/6 mice, which were anesthetized by intraperitoneal injection with 0.4 mg/kg chloral hydrate (Sigma, St. Louis, MO, USA). The orthodontic appliance consisted of a nickel–titanium open-coil spring (Tomy, Tokyo, Japan), the distal end of which was directly bonded to the occlusal surface of the right first maxillary molar using a light-cured resin (Transbond, Unitek/3M). The other end was bonded to both upper incisors (Supplementary Fig. 1A). The amount of force produced by the activation of the coil was 0.35 N, measured by a tension gauge, which was adopted from the method proposed before (Taddei et al. 2012). There was no reactivation during the experimental period. The animals were divided into two groups: control groups (non-operated animals) and experimental groups. Mice were sacrificed at the following times: 0, 18 days (for micro-CT and histological analysis) or 0, 3, 6, 12 days (for immunohistochemistry and immunofluorescence analysis). For every set of experiments, five animals were used for each time-point.
Tissue processing
At the end of each experimental period, the animals were euthanized under deep anesthesia. The orthodontic appliance were removed and the whole maxillae including first, second, and third molars were dissected and further fixed in 4 % paraformaldehyde buffer (pH 7.4) for 24 h. After demineralisation in 10 % EDTA (pH 7.4) for 10 weeks and dehydration in increasing concentrations of methanol, the samples were embedded in paraffin. Sections of 5 μm, parallel to the long axis of the first upper molar, were obtained and mounted on glass slides. Sections were processed with hematoxylin and eosin (HE), and immunohistochemistry, immunofluorescence staining. Specific areas of the PDL were selected for morphological assessments and immunohistochemical and immunofluorescence studies. According to previous study, the center of rotation of the tooth was at the level of root apices, so we chose the middle and coronal thirds of distal periodontal site of the upper first molar distal root as our TS (Supplementary Fig. 1B) (Pavlin et al. 2000). The same areas in control teeth were chosen for analysis.
Micro-CT
Tooth movement was assessed using micro-CT scanning. The mice maxillary bone both in control group and experimental day 18 group were dissected after anesthesia and were scanned with a Scanco micro-CT (μCT50; Scanco Medical, Bassersdorf, Switzerland) at the same distance and orientation. Images were analyzed with Mimics 15.0 software to determine whether the first molar in our OTM model moved. Images were rotated and adjusted to ensure that we observed the narrowest gap between the first and second molars in both group.
Histology and immunohistochemistry
Tissue sections were deparaffinized with xylene and rehydrated with decreasing concentrations of ethanol. After rehydration in deionized water, the tissues were stained with HE for morphology analysis. For immunohistochemistry staining, after rehydration the tissue section was incubated with 3 % hydrogen peroxide for 20 min to block inactivated endogenous peroxidase, and incubated with pepsin induced antigen retrieval solution for 20 min for antigen retrieval. The tissues were washed in phosphate-buffered saline (PBS) for three times after each step and then further blocked with 3 % goat serum for 30 min. Incubation with the primary antibody was performed at 4 °C overnight. Negative controls were incubated with PBS instead of the primary antibody. The next day, the tissues were washed in PBS for three times and incubated with biotin-conjugated second antibody and streptomyces avidin peroxidase for 20 min in turn and then washed in PBS for three times. DAB chromogenic reagent kit was used to visualize immunoreactivity in accordance with instructions, and hematoxylin was used to counterstained the sections. The images were acquired on OLYMPUS BX51 (Japan).
Double labeling immunofluorescence staining
Operations before blocking antigen followed as the mentioned above. The sections were incubated with the first primary antibody (Wnt5a) overnight, washed with PBS in the next day and incubated with cy3, goat anti-rabbit IgG (red) for 1 h. After blocked with 3 % goat serum for 30 min again, the sections were incubated with the second primary antibody (β-catenin) overnight, and on the third day incubated with dylight 488, goat anti-rabbit IgG (green) for 1 h. Nucleus were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). The fluorescent images were captured under a Leica DM4000B fluorescence microscope (Leica, Nussloch, Germany).
Statistical analyses
All experiments were performed at least three times and similar results were obtained. For quantitative analysis, five representative high-power fields (400× magnification) from each specimen were selected randomly and counted by two blinded researchers. The expression levels of each protein were calculated as percentage of positive cells per total cells. Statistical analysis of the data was performed by the Student’s t test and one-way ANOVA with Bonferroni post test. P < 0.05 was considered to be significant.
Results
Tooth movement and bone formation at TS in the OTM mice model
The OTM mice model was established according to our previous study (Chang et al. 2015) and the schema has been illustrated in Supplementary Fig. 1A, B. By a nickel–titanium open-coil spring, the tension force was exerted between the upper anterior teeth and right upper first (M1) molar. Following 18 days after mechanic loading, the distance between right upper first (M1) and second (M2) molar was gauged by micro-CT and HE staining. As the white arrow indicated in the 3-dimensional reconstruction of the micro-CT image in Supplementary Fig. 1C, the space between the two molars in experimental group (Exp 18 d) was wider than that in control group (Control). Measuring the distance between cemento-enamel junction (CEJ) of M1 and M2, a significant increase was exhibited in the experimental group relative to the control group (Supplementary Fig. 1D), consistent with the micro-CT analysis. The higher bone tissue volume between the M1 and M2 was also detected in experimental group compared to control group. Taken together, both the micro-CT and HE staining results indicated that the orthodontic tension successfully induced the tooth movement of right upper first molar (M1) in the established OTM mice model.
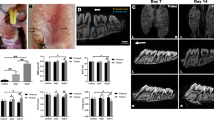
The achievement of OTM is triggered by bone remodeling, resulting in bone resorption at compression side (CS) and bone deposition at TS in the periodontium. Since the novel bone formation is essential for the long-term stability of OTM, we then analyzed the osteogenesis occurring at TS in the OTM mice model to further verify the reliability of our model. OCN and OSX, the markers of osteoblast differentiation, were used as indexes for bone formation (Baek et al. 2009). After mechanical loading for 0, 3, 6, 12 days, the expression of OCN and OSX at TS were detected in the OTM mice model. Our results revealed an increased expression of OSX and OCN in periodontium at TS compared to control group, which peaked on the 6th and the 12th day after mechanical loading and occurred in PDL cells of the perialveolar zone (Fig. 1a–p and quantitative analysis in Fig. 5a), where new bone formation took place. Interestingly, the expression of OSX is gradually confined to the osteoblasts near the surface of alveolar bone on day 12 after force application, indicating the osteogenesis was due to the mature of osteoblasts. The results were consistent with a previous study that demonstrated higher OCN levels at TS (Taddei et al. 2012). Thus, these results suggested the successful tooth movement and bone formation at TS in our established model.
Expression of OCN and OSX at tension site (TS) of upper first molar of OTM mice model. Histological section of the periodontium surrounding distobuccal root of M1 was analyzed by immunohistochemistry for OCN (a–d) and OSX (i–l) at TS of perialveolar zone in control group (Control) and experiment group following mechanical loading for 3 days (Exp 3d), 6 days (Exp 6d), 12 days (Exp 12d). The red boxed regions in a–d and i–l are shown at a higher magnification in e–h, m–p, respectively. r Root, ab alveolar bone, p periodontium, black arrow direction of force. (Color figure online)
Wnt/β-catenin signaling pathway might participate in bone formation at TS during OTM mediated by Wnt5a not Wnt3a
Canonical Wnt/β-catenin signaling pathway has been reported to promote bone formation by positively regulating osteogenic differentiation through activating the expression of osteogenestic related target genes, such as Runx2, OSX and OPN (osteoprotegerin) (Qiao et al. 2011; Xu et al. 2015). We therefore detected the activation of canonical Wnt/β-catenin signaling pathway at the TS of PDL during OTM in our established mice model. Our results revealed the positive immunoreactivity of β-catenin appearing at nucleus of cells adjacent to the alveolar bone at day 6 after mechanical loading (Fig. 2a–h), which was consistent with a previous in vitro study that mechanical loading activated β-catenin in periodontal ligament cells (PDLCs). And the nucleus expression of β-catenin was enhanced and peaked at day 12 after mechanical loading (Fig. 5b), and gradually extended from the surface alveolar bone to nearly the whole periodontium. It is well known that the nuclear expression of β-catenin symbols the activation of canonical signaling pathway since β-catenin can enter the nucleus in the target cells and interact with Lef1/Tcf transcription factors to induce the expression of specific target genes (Jho et al. 2002; Kim et al. 2015). Therefore, the results indicated us that β-catenin signaling pathway was activated at the TS of PDL during OTM, which may contribute to novel osteogenesis observed in the Fig. 1 and Supplementary Fig. 1. We next explored the expression of Wnt3a, a typical canonical Wnt ligand, which was found no detectable difference between experimental and control group after mechanical loading for 3, 6, 12 days (Figs. 2i–p, 5b). Since the Wnt3a expression was not detected in our mice model, it is suggested that the activation of β-catenin signaling pathway was not mediated by Wnt3a. Recently, there were studies shown that representative non-canonical Wnt5a ligand could directly activate the β-catenin signaling pathway in vitro and in vivo (Mikels and Nusse 2006; van Amerongen et al. 2012). We further analyzed the expression of Wnt5a at the TS of PDL during OTM. As shown in the Figs. 2q–x, 5b, the Wnt5a expression was increased from day 3 to day 6 after mechanical loading and the expression was distributed throughout the periodontium, consistent with a previous study in which OTM model was developed in rats (Isogai et al. 2015). On day 12, the Wnt5a expression became weaker compared to that on day 3 and day 6. The high expression of Wnt5a and low expression of Wnt3a at the TS of PDL after mechanical loading indicated us that tension force exerted on the cells residing in PDL might induce Wnt5a but not Wnt3a, which may further activate Wnt/β-catenin signaling pathway, resulting in osteogenesis at TS.
Immunohistochemistry for β-catenin, Wnt3a and Wnt5a in periodontium in response to tension force during OTM. Immunohistochemistrical stainings of β-catenin (a–d), Wnt3a (i–l) and Wnt5a (q–t) at the tension site (TS) of the periodontium around the distobuccal root of right upper first molar in control (Control) and experimental groups 3 days (Exp 3d), 6 days (Exp 6d) and 12 days (Exp 12d) after mechanical loading. The boxed regions in a–d, i–l, q–t are shown at a higher magnification in e–h, m–p, u–x, respectively. r Root, ab alveolar bone, p periodontium, black arrow direction of force
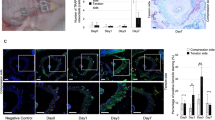
To further determine the relationship between the increased Wnt5a expression and the activation of β-catenin, we explored the co-localization of Wnt5a and β-catenin at the TS of PDL in the mice of control and experimental group using double labeling immunofluorescence staining. The results in Fig. 3 shows that spatially, Wnt5a expression was mainly localized in the cytoplasm of cells residing at the TS of PDL while the β-catenin expression in the nucleus. Temporally, the expression pattern of Wnt5a is prior to the activation of β-catenin. Wnt5a was induced on day 3 after mechanical loading and peaked on day 6, then gradually decreased till day 12. And the activation of β-catenin occurred on day 6 after mechanical loading and lasted until day 12. The sequential expression of Wnt5a and β-catenin activation suggested us that non-canonical Wnt5a may be implicated in the activation of β-catenin in the tension force-induced bone formation during OTM.
Double labeling immunofluorescence staining of Wnt5a (red) and β-catenin (green) at the tension site of the periodontium around the distobuccal root of right upper first molar during OTM. Nucleus was counterstained with DAPI (blue). Significantly high expression of Wnt5a and activation of β-catenin were observed in the same cells on experimental groups 3 days (Exp 3d) and 6 days (Exp 6d) after mechanical loading. (Color figure online)
Wnt5a mediated β-catenin activation may be dependent on the complex receptors of FZD4 and LRP5 but not ROR2
Since some studies have shown that the role of Wnt5a in activating or inhibiting β-catenin signaling pathway was determined by the different receptors (Sakisaka et al. 2015; van Amerongen et al. 2012), we further detected the expression of distinct receptors at TS in our OTM mice model. It has been demonstrated that Wnt5a can directly activate Wnt/β-catenin signaling pathway by binding to FZD4 and LRP5 receptor (Mikels and Nusse 2006) while antagonizing the β-catenin signaling through Wnt/Ca2+ pathway and ROR2 co-receptor (Ishitani et al. 2003; Mikels and Nusse 2006). Therefore, we detected the expression of receptor FZD4, LRP5 and ROR2 in the following experiments. At the TS of PDL in the OTM mice model as shown in Figs. 4a–h, 5c, the expression of FZD4 was upregulated from day 3 to day 6 after mechanical loading, while downregulated on day 12, which was expressed in the similar manner compared with Wnt5a. Meanwhile, the expression of LRP5 was increasing from day 3 to day 12 after mechanical loading (Figs. 4i–p, 5c). And both the positive staining of FZD4 and LRP5 are present in the periodontium near alveolar bone. In contrast, ROR2 was weakly expressed in all the groups with no obvious difference between control and experimental groups (Figs. 4q–x, 5c). Taken together, the results indicated that the highly expressed Wnt5a might activate the β-catenin signaling pathway through FZD4 and LRP5 receptors.
Immunohistochemistry for FZD4, LRP5 and ROR2 at the tension site (TS) of the periodontium during OTM. Immunoreactivity of FZD4 (a–d), LRP5 (i–l) and ROR2 (q–t) at the TS of the periodontium around the distobuccal root of right upper first molar in control (Control) and experimental groups after mechanical loading for 3 days (Exp 3d), 6 days (Exp 6d) and 12 days (Exp 12d). The boxed regions in a–d, i–l, q–t are shown at a higher magnification in e–h, m–p, u–x, respectively. r Root, ab alveolar bone, p periodontium, black arrow direction of force
Discussion
In our present study, we preliminarily explored the expression pattern of canonical and non-canonical Wnt signaling pathway related molecules in the established OTM mice model in order to uncover the mechanism of bone remodeling at the TS of PDL during OTM. And our study indicated the positive expression of β-catenin in the PDLCs near the alveolar bone, which might contribute to the osteogenesis, since the critical factors of osteogenesis, OSX and OCN, are the downstream target genes of β-catenin. We found a transient induction of Wnt5a, LRP5, FZD4 and OSX peaking on 6th day after orthodontic force loading and gradually decreasing on 12th day. And the phenomenon is concomitant with many OTM studies (Salomão et al. 2014; Xu et al. 2015) in which long observation period is adopted. Meanwhile, the expression levels of β-catenin and OCN, the downstream proteins, was increased continuously during the observation time window, suggesting the activation of osteogenesis and Wnt signaling pathway occur in a strict chronological order. Moreover, the calcein and alizarin red double staining assays as we mentioned in a previous study suggested the abundant mineral deposition after 6 days (Chang et al. 2015). These results corroborated the sustaining bone remodeling during orthodontic treatment. As we mentioned before, Wnt signaling pathways could be divided into two categories, the canonical and non-canonical pathway based on the activation of β-catenin. It was reported that Wnt3a, which was considered as the most common activator of canonical Wnt pathway, could induce bone formation. However, our immunochemistry results showed Wnt5a but not Wnt3a was highly expressed at the TSs of PDL during OTM. Along with increased expression of LRP5 and FZD4, negligible expression of ROR2, our results indicate that the activation of β-catenin signaling pathway might be signaled by Wnt5a, which further induces osteogenesis.
During OTM, PDL cells under mechanical stimulus release various molecules including neurotransmitters, cytokines, growth factors, colony-stimulating factors (cytokines that involved in maturing of various leucocyte, macrophage, and monocyte line), and arachidonic acid metabolites, to provide a favorable microenvironment for periodontal tissue remodeling (Zainal Ariffin et al. 2011). In the case of TSs, the setting-up microenvironment tips the bone remodeling balance towards bone formation rather than bone resorption, indicated by the upregulation of osteogenic related transcription factors and inhibitors of osteoclast formation via different signaling pathways (d’Apuzzo et al. 2013; Di Domenico et al. 2012; Lv et al. 2014). Wnt signaling pathway has been considered as one of the main mechanism affecting bone development and bone mass (Leucht et al. 2008; Minear et al. 2010). Since mutations in the LRP5 gene was discovered to be related with alternations in human bone mass (Kobayashi et al. 2016), the crucial role of canonical Wnt/β-catenin pathway in promoting bone formation while repressing bone resorption has been established by a great number of studies (Kobayashi et al. 2016; Okamoto et al. 2014). The activated Wnt/β-catenin signaling was found to function through regulating osteoblast differentiation by inducing expression of OSX (Heo et al. 2010; Nemoto et al. 2016), a transcription factor promoting osteoblast differentiation, and through preventing osteoclast differentiation by inducing osteoprotegerin (Baron and Kneissel 2013), an osteoclast inhibitory factor. Moreover, Wnt/β-catenin signaling determined stromal cells fate shifting from adipocytes to osteoblasts through the suppression of Cebp/a and Ppar-γ expression (Bennett et al. 2005; Ross et al. 2000). In the case of alveolar bone and periodontal tissue remodeling, Lim et al. (2015) demonstrated the role of Wnt signaling in promoting osteogenesis using gain- and loss-of-function approaches by Lrp5 ACT mice and Ad-Dkk1 treated mice.
Wnt5a has been demonstrated to has a dual role in regulating bone homeostasis via signaling different receptors and then activating canonical and non-canonical pathway (Mikels and Nusse 2006; van Amerongen et al. 2012). Several lines of evidences have shown that Wnt5a is involved in the osteoblast differentiation. Wnt5a heterozygous mice exhibited an osteopenic phenotype, with a low bone mass and high bone marrow adipogenesis, which is also consistent with the phenotypes of osteoblast-lineage cell-specific Wnt5a-deficient mice (Wnt5a cKO) (Maeda et al. 2012). And in Wnt5a-deficient osteoblasts, the expression of osteoblast differentiation marker: Alp, OSX and OCN, was declined whereas that of adipocyte marker: aP2, was increased. These findings suggest us that the mechanism by which Wnt5a regulates osteogenesis is due to inhibiting adipogenesis by suppressing Ppar-γ transcriptional activity through CAMKII-TAK1-TAB 2-NLK-dependent noncanonical Wnt signaling and promoting osteoblastogenesis by inducing expression of Runx2 (Takada et al. 2007). And Wnt5a can also promote bone formation in aid of canonical Wnt/β-catenin pathway. In osteoblast-lineage cells, Okamoto et al. (2014), showed that Wnt5a-mediated non-canonical signaling pathway could increase sensitivity of canonical Wnt/β-catenin pathway for Wnt3a, a typical canonical Wnt ligands, by up-regulating the expression of LPR5/6. Moreover, Wnt5a also has the capability to directly activate Wnt/β-catenin signaling following binding to a receptor complex of LRP5 and FZD4 in HEK293 cells as previously reported (Mikels and Nusse 2006). In our experiments, the co-expression of Wnt5a and β-catenin in combination with high expression of FZD4 and LRP5 suggests us that the osteogenesis occurring at TS of PDL during OTM may be attributed to the Wnt5a mediating canonical Wnt/β-catenin pathway activation. However, some studies also highlighted the important role of Wnt5a in osteoclast formation. It has been shown by Maeda et al. (2012) that Wnt5a/ROR2/JNK crosstalk signaling between osteoblasts and osteoclast precursors enhances osteoclast differentiation and bone resorption in mice, indicating that Wnt5a/ROR2 signaling could promote osteoclastogenesis (Maeda et al. 2012). ROR2 was extremely important in both osteoclast precursor differentiation and regulation of bone-resorbing activity of mature osteoclasts. Thus, we next explored the expression of ROR2 at the TS of PDL in the mice model. The results showed that few ROR2 was expressed at the TS, indicating that non-canonical Wnt5a/ROR2 signaling pathway might not be activated. Thus, taken all these together, we proposed that Wnt5a that was expressed at the TS of PDL during OTM, might play an important role in osteogenesis but not osteoclast differentiation.
In addition, several controversies exist in the literature regarding the role of Wnt5a in activating or inhibiting canonical Wnt/β-catenin signaling pathway. On one hand, Wnt5a is reported to antagonize the β-catenin signaling by inhibiting downstream of β-catenin stabilization through Wnt/Ca2+ pathway (Ishitani et al. 2003) and ROR2 co-receptor (Mikels and Nusse 2006) or by inducing β-catenin degradation through seven in absentia homolog 2 (SIAH2) (Topol et al. 2003). On the other hand, it has been demonstrated that Wnt5a can positively regulate Wnt/β-catenin signaling pathway either by directly activating via co-expression of FZD4 and LRP5 receptors (Mikels and Nusse 2006) or by indirectly enhancing via increasing the expression of LRP5/6, a well-known co-receptor of canonical Wnt3a (Okamoto et al. 2014). And the dual and opposite role of Wnt5a played in regulating canonical Wnt/β-catenin signaling has also been demonstrated by Nusse using purified Wnt5a in vitro and tetO-Wnt5a; Rosa26rtTA-double inducible transgenic mice in vivo (van Amerongen et al. 2012). The discrete signaling pathways initiated by the same molecular have been attributed to activation of two distinct receptors, thus carrying out their diverse physiological response. In addition, a great number of studies have enlightened us that the Wnt signaling outputs are dictated by receptors but not Wnt ligands, that are receptor availability expressed by receiving cells determines the multiple signaling activities in the complex physiological context (He et al. 1997; Umbhauer et al. 2000).
As well known, orthodontic force applied to teeth generates mechanical loading, which in turn elicits diverse and complex biological responses in periodontal tissues that further caused bone formation at the tension side and bone resorption at the CS (Lv et al. 2014). Cytoskeleton reorganization which is the consequent effects of mechanics induced deformation of the receipt cells induces the activation of YAP/TAZ by transmitting the co-transcription factor into nuclei, which further arises complex biological responses dependent on cell types (Zhao et al. 2012). It has been reported that in MSCs, activation of YAP/TAZ under mechanical stimulation up-regulated the osteogenic related genes by enhancing the transcription activity of Runx2 (Yang et al. 2014). A recent study demonstrated the complex interaction between YAP/TAZ and canonical and alternative Wnt signaling pathways, indicating the crucial role of Wnt signaling pathway in the mechanical transduction (Park et al. 2015). Indeed, various works have already indicated the close relationship between Wnt signaling pathway and mechanical transduction (Kang and Robling 2014). Robinson et al. (2006) reported that mechanical loading resulted in the increased expression of Wnt pathway and Wnt/β-catenin target genes including Wnt10B, SFRP1, and others. Moreover, another study by Sawakami et al. (2006) revealed that the loss of LRP5 signaling in mice yielded a significant decrease in bone-mineral density and bone strength, especially under the mechanical loading, similar to the human patients with loss-of-function mutations in LRP5. LRP5 was also associated with the increased cell responsiveness to mechanical loading in bone and bone formation cells (Kang and Robling 2014). Here, in our present study, we reported the activation of Wnt signaling pathway at the TS of PDL under orthodontic force, but not the CS, indicating the mechanical transduction in OTM might also be closely associated with Wnt signaling pathway. However, the precise role and underlying mechanism of the Wnt pathway activation in orthodontic mechanical loading are still needed to be well studied in the future.
Collectively, the present study demonstrated that the activation of β-catenin occurring through Wnt5a was associated with the tension force-induced bone formation during OTM. However, the role of Wnt5a in bone remodeling at the TS of the PDL during OTM is not sufficient, and the underlying mechanism of action affecting this event remains elusive and controversial. Besides, the cooperation that exists between the two Wnt signaling pathways during osteoblastogenesis remains to be elucidated and need to be further investigated.
Abbreviations
- OTM:
-
Orthodontic tooth movement
- OCN:
-
Osteocalcin
- OSX:
-
Osterix
- PDLC:
-
Periodontal ligament cells
- TS:
-
Tension site
- CS:
-
Compression site
- CEJ:
-
Cemento-enamel junction
- FZD4:
-
Frizzled 4
- LRP5:
-
Low-density lipoprotein receptor-related protein 5
- ROR2:
-
Receptor tyrosine kinase-like orphan receptor 2
References
Angers S, Moon RT (2009) Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol 10:468–477. doi:10.1038/nrm2717
Arnsdorf EJ, Tummala P, Jacobs CR (2009) Non-canonical Wnt signaling and N-cadherin related beta-catenin signaling play a role in mechanically induced osteogenic cell fate. PLoS One 4:e5388. doi:10.1371/journal.pone.0005388
Baek WY, Lee MA, Jung JW, Kim SY, Akiyama H, de Crombrugghe B, Kim JE (2009) Positive regulation of adult bone formation by osteoblast-specific transcription factor osterix. J Bone Miner Res 24:1055–1065. doi:10.1359/jbmr.081248
Baksh D, Boland GM, Tuan RS (2007) Cross-talk between Wnt signaling pathways in human mesenchymal stem cells leads to functional antagonism during osteogenic differentiation. J Cell Biochem 101:1109–1124. doi:10.1002/jcb.21097
Baron R, Kneissel M (2013) WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 19:179–192. doi:10.1038/nm.3074
Bennett CN, Longo KA, Wright WS, Suva LJ, Lane TF, Hankenson KD, MacDougald OA (2005) Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci USA 102:3324–3329. doi:10.1073/pnas.0408742102
Chang M, Lin H, Luo M, Wang J, Han G (2015) Integrated miRNA and mRNA expression profiling of tension force-induced bone formation in periodontal ligament cells. In Vitro Cell Dev Biol Anim 51:797–807. doi:10.1007/s11626-015-9892-0
Cui J et al (2016) The effect of calcitriol on high mobility group box 1 expression in periodontal ligament cells during orthodontic tooth movement in rats. J Mol Histol 47:221–228. doi:10.1007/s10735-016-9669-0
d’Apuzzo F, Cappabianca S, Ciavarella D, Monsurro A, Silvestrini-Biavati A, Perillo L (2013) Biomarkers of periodontal tissue remodeling during orthodontic tooth movement in mice and men: overview and clinical relevance. Sci World J 2013:105873. doi:10.1155/2013/105873
Di Domenico M et al (2012) Cytokines and VEGF induction in orthodontic movement in animal models. J Biomed Biotechnol 2012:201689. doi:10.1155/2012/201689
Feller L, Khammissa RA, Schechter I, Thomadakis G, Fourie J, Lemmer J (2015) Biological events in periodontal ligament and alveolar bone associated with application of orthodontic forces. Sci World J 2015:876509. doi:10.1155/2015/876509
Garlet TP, Coelho U, Silva JS, Garlet GP (2007) Cytokine expression pattern in compression and tension sides of the periodontal ligament during orthodontic tooth movement in humans. Eur J Oral Sci 115:355–362. doi:10.1111/j.1600-0722.2007.00469.x
He X, Saint-Jeannet JP, Wang Y, Nathans J, Dawid I, Varmus H (1997) A member of the frizzled protein family mediating axis induction by Wnt-5A. Science 275:1652–1654
Heo JS, Lee SY, Lee JC (2010) Wnt/beta-catenin signaling enhances osteoblastogenic differentiation from human periodontal ligament fibroblasts. Mol Cells 30:449–454. doi:10.1007/s10059-010-0139-3
Huang C, Ogawa R (2010) Mechanotransduction in bone repair and regeneration. FASEB J 24:3625–3632. doi:10.1096/fj.10-157370
Ishitani T et al (2003) The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca2+ pathway to antagonize Wnt/beta-catenin signaling. Mol Cell Biol 23:131–139
Isogai N et al (2015) Wnt5a stimulates the bone formation in tension side during orthodontic tooth movement. Int J Oral Med Sci 13:8. doi:10.5466/ijoms.13.120
Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F (2002) Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol 22:1172–1183
Kang KS, Robling AG (2014) New insights into Wnt-Lrp5/6-beta-catenin signaling in mechanotransduction. Front Endocrinol 5:246. doi:10.3389/fendo.2014.00246
Kim JY, Kim BI, Jue SS, Park JH, Shin JW (2012) Localization of osteopontin and osterix in periodontal tissue during orthodontic tooth movement in rats. Angle Orthod 82:107–114. doi:10.2319/030911-173.1
Kim I, Hur J, Jeong S (2015) HuR represses Wnt/beta-catenin-mediated transcriptional activity by promoting cytoplasmic localization of beta-catenin. Biochem Biophys Res Commun 457:65–70. doi:10.1016/j.bbrc.2014.12.052
Kobayashi Y, Uehara S, Udagawa N, Takahashi N (2016) Regulation of bone metabolism by Wnt signals. J Biochem 159:387–392. doi:10.1093/jb/mvv124
Krishnan V, Davidovitch Z (2006) Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofac Orthop 129:469 e1–e32. doi:10.1016/j.ajodo.2005.10.007
Lee SY, Yoo HI, Kim SH (2015) CCR5-CCL Axis in PDL during orthodontic biophysical force application. J Dent Res 94:1715–1723. doi:10.1177/0022034515603926
Leucht P, Minear S, Ten Berge D, Nusse R, Helms JA (2008) Translating insights from development into regenerative medicine: the function of Wnts in bone biology. Semin Cell Dev Biol 19:434–443. doi:10.1016/j.semcdb.2008.09.002
Li J et al (2015) Altered distribution of HMGB1 in the periodontal ligament of periostin-deficient mice subjected to Waldo’s orthodontic tooth movement. J Mol Histol 46:303–311. doi:10.1007/s10735-015-9619-2
Lim WH, Liu B, Mah SJ, Yin X, Helms JA (2015) Alveolar bone turnover and periodontal ligament width are controlled by Wnt. J Periodontol 86:319–326. doi:10.1902/jop.2014.140286
Lv S et al (2014) Histochemical examination of cathepsin K, MMP1 and MMP2 in compressed periodontal ligament during orthodontic tooth movement in periostin deficient mice. J Mol Histol 45:303–309. doi:10.1007/s10735-013-9548-x
Lv S et al (2015) Expression of HMGB1 in the periodontal tissue subjected to orthodontic force application by Waldo’s method in mice. J Mol Histol 46:107–114. doi:10.1007/s10735-014-9606-z
Mabuchi R, Matsuzaka K, Shimono M (2002) Cell proliferation and cell death in periodontal ligaments during orthodontic tooth movement. J Periodontal Res 37:118–124
Maeda K et al (2012) Wnt5a-Ror2 signaling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis. Nat Med 18:405–412. doi:10.1038/nm.2653
Masella RS, Meister M (2006) Current concepts in the biology of orthodontic tooth movement. Am J Orthod Dentofac Orthop 129:458–468. doi:10.1016/j.ajodo.2005.12.013
Mikels AJ, Nusse R (2006) Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol 4:e115. doi:10.1371/journal.pbio.0040115
Minear S et al (2010) Wnt proteins promote bone regeneration. Sci Transl Med 2:29ra30. doi:10.1126/scitranslmed.3000231
Nemoto E et al (2016) Wnt3a signaling induces murine dental follicle cells to differentiate into cementoblastic/osteoblastic cells via an osterix-dependent pathway. J Periodontal Res 51:164–174. doi:10.1111/jre.12294
Novackova S, Marek I, Kaminek M (2011) Orthodontic tooth movement: bone formation and its stability over time. Am J Orthod Dentofac Orthop 139:37–43. doi:10.1016/j.ajodo.2009.11.011
Okamoto M et al (2014) Noncanonical Wnt5a enhances Wnt/beta-catenin signaling during osteoblastogenesis. Sci Rep 4:4493. doi:10.1038/srep04493
Park HW et al (2015) Alternative Wnt signaling activates YAP/TAZ. Cell 162:780–794. doi:10.1016/j.cell.2015.07.013
Pavlin D, Gluhak-Heinrich J (2001) Effect of mechanical loading on periodontal cells. Crit Rev Oral Biol Med 12:414–424
Pavlin D, Goldman ES, Gluhak-Heinrich J, Magness M, Zadro R (2000) Orthodontically stressed periodontium of transgenic mouse as a model for studying mechanical response in bone: the effect on the number of osteoblasts. Clin Orthod Res 3:55–66
Qiao LJ, Kang KL, Heo JS (2011) Simvastatin promotes osteogenic differentiation of mouse embryonic stem cells via canonical Wnt/beta-catenin signaling. Mol Cells 32:437–444. doi:10.1007/s10059-011-0107-6
Robinson JA et al (2006) Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem 281:31720–31728. doi:10.1074/jbc.M602308200
Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA (2000) Inhibition of adipogenesis by Wnt signaling. Science 289:950–953
Sakisaka Y, Tsuchiya M, Nakamura T, Tamura M, Shimauchi H, Nemoto E (2015) Wnt5a attenuates Wnt3a-induced alkaline phosphatase expression in dental follicle cells. Exp Cell Res 336:85–93. doi:10.1016/j.yexcr.2015.06.013
Salomão MFL, Reis SRA, Vale VLC, Machado CV, Meyer R, Nascimento ILO (2014) Immunolocalization of FGF-2 and VEGF in rat periodontal ligament during experimental tooth movement. Dental Press J Orthod 19(3):67–74. doi:10.1590/2176-9451.19.3.067-074.oar
Sawakami K et al (2006) The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem 281:23698–23711. doi:10.1074/jbc.M601000200
Shi Y et al (2012) Uniaxial mechanical tension promoted osteogenic differentiation of rat tendon-derived stem cells (rTDSCs) via the Wnt5a-RhoA pathway. J Cell Biochem 113:3133–3142. doi:10.1002/jcb.24190
Sprogar S, Vaupotic T, Cor A, Drevensek M, Drevensek G (2008) The endothelin system mediates bone modeling in the late stage of orthodontic tooth movement in rats. Bone 43:740–747. doi:10.1016/j.bone.2008.06.012
Taddei SR, Moura AP, Andrade I Jr, Garlet GP, Garlet TP, Teixeira MM, da Silva TA (2012) Experimental model of tooth movement in mice: a standardized protocol for studying bone remodeling under compression and tensile strains. J Biomech 45:2729–2735. doi:10.1016/j.jbiomech.2012.09.006
Taddei SR et al (2013) The effect of CCL3 and CCR1 in bone remodeling induced by mechanical loading during orthodontic tooth movement in mice. Bone 52:259–267. doi:10.1016/j.bone.2012.09.036
Takada I et al (2007) A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-gamma transactivation. Nat Cell Biol 9:1273–1285. doi:10.1038/ncb1647
Takimoto A et al (2015) Scleraxis and osterix antagonistically regulate tensile force-responsive remodeling of the periodontal ligament and alveolar bone. Development 142:787–796. doi:10.1242/dev.116228
Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y (2003) Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J Cell Biol 162:899–908. doi:10.1083/jcb.200303158
Umbhauer M, Djiane A, Goisset C, Penzo-Mendez A, Riou JF, Boucaut JC, Shi DL (2000) The C-terminal cytoplasmic Lys-thr-X–X–X-Trp motif in frizzled receptors mediates Wnt/beta-catenin signalling. EMBO J 19:4944–4954. doi:10.1093/emboj/19.18.4944
van Amerongen R, Fuerer C, Mizutani M, Nusse R (2012) Wnt5a can both activate and repress Wnt/beta-catenin signaling during mouse embryonic development. Dev Biol 369:101–114. doi:10.1016/j.ydbio.2012.06.020
Wang B et al (2014) Expression patterns of WNT/beta-CATENIN signaling molecules during human tooth development. J Mol Histol 45:487–496. doi:10.1007/s10735-014-9572-5
Xu J, Li Z, Hou Y, Fang W (2015) Potential mechanisms underlying the Runx2 induced osteogenesis of bone marrow mesenchymal stem cells. Am J Transl Res 7:2527–2535
Yamashita Y, Sato M, Noguchi T (1987) Alkaline phosphatase in the periodontal ligament of the rabbit and macaque monkey. Arch Oral Biol 32:677–678
Yang C, Tibbitt MW, Basta L, Anseth KS (2014) Mechanical memory and dosing influence stem cell fate. Nat Mater 13:645–652. doi:10.1038/nmat3889
Yuan YP et al (2016) Canonical and non-canonical Wnt signaling control the regeneration of amputated rodent vibrissae follicles. J Mol Histol 47:1–8. doi:10.1007/s10735-015-9648-x
Zainal Ariffin SH, Yamamoto Z, Zainol Abidin IZ, Megat Abdul Wahab R, Zainal Ariffin Z (2011) Cellular and molecular changes in orthodontic tooth movement. Sci World J 11:1788–1803. doi:10.1100/2011/761768
Zhao B, Li L, Wang L, Wang CY, Yu J, Guan KL (2012) Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev 26:54–68. doi:10.1101/gad.173435.111
Acknowledgments
This study was supported by the National Natural Science Foundation of China (NSFC); NSFC Number: 81371169.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Hai-Di Fu and Bei-Ke Wang have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fu, HD., Wang, BK., Wan, ZQ. et al. Wnt5a mediated canonical Wnt signaling pathway activation in orthodontic tooth movement: possible role in the tension force-induced bone formation. J Mol Hist 47, 455–466 (2016). https://doi.org/10.1007/s10735-016-9687-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-016-9687-y