Abstract
Periostin is essential for the integrity and function of the periodontal ligament (PDL), and periostin knockout is related to an enhanced inflammatory status in PDL. High mobility group box 1 (HMGB1), a late inflammatory cytokine, is up-regulated in PDL cells in response to mechanical stress. This study aimed to investigate the effect of periostin deficiency (Pn-/-) on HMGB1 expression in PDL during orthodontic tooth movement. We used 8-week-old male mice homozygous for the disrupted periostin gene and their wild-type (WT) littermates. Tooth movement was performed according to Waldo’s method, in which 0.5-mm-thick elastic bands were inserted between the first and second upper molars of anesthetized mice. After 3 days of mechanical loading, mice were fixed by transcardial perfusion of 4 % paraformaldehyde in phosphate buffer, and the maxilla was extracted for histochemical analyses. Compared with the WT group, Pn-/- mice showed higher basal expression of HMGB1 in the absence of mechanical loading. Following 3 days of orthodontic tooth movement, the PDL in the compression side of both groups was almost replaced by cell-free hyaline zones, and Pn-/- mice showed a much wider residual PDL than WT mice. In the tension side, the number of HMGB1-positive cells in PDL in both Pn-/- and WT groups increased remarkably without a significant difference between the two groups. Our findings suggest an inhibitory effect of periostin on HMGB1 production by PDL and confirmed the critical role of periostin in integrity of PDL collagen fibrils during orthodontic tooth movement, although mechanical loading is the predominant stimulant of HMGB1 expression relative to periostin deficiency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many molecular mediators for adaptive responses to stress are involved in the orthodontic force-induced PDL remodeling process. Periostin, a disulfide-linked 90 kDa secreted matricellular protein first identified by Takeshita et al. (Norris et al. 2008; Takeshita et al. 1993), is primarily expressed in the periosteum and PDL, which are subjected to constant mechanical stress, supporting effective transmission and distribution of force (Yamada et al. 2014) and maintaining the integrity of the PDL during occlusal function and inflammation (Choi et al. 2011). The up-regulation of periostin mRNA was found in PDL compression sites compared with tension sites in an experimental tooth movement model (Wilde et al. 2003). Periostin-null mice showed a dramatic loss of periodontal support and a compromised attachment apparatus that lead to pathologic tooth migration in a mechanically challenging environment (Rios et al. 2008). In addition, a drop in periostin levels and degradation of the PDL fiber system were observed in rats in the absence of mechanical stress (Choi et al. 2011). Based on the above evidence, it is suggested that periostin protein is involved in PDL maintenance and remodeling in response to mechanical stress.
High mobility group box protein 1 (HMGB1), a nonchromosomal nuclear protein that is constitutively expressed in the nucleus of eukaryotic cells, functions as a regulator for nucleosome structure maintenance and gene transcription regulation (Klune et al. 2008). HMGB1 also acts as an alarmin that can be actively secreted by activated immune cells or passively released by injured/dead cells to participate in the regulation of the immune response (Andersson et al. 2002; Yang et al. 2002). Recently, HMGB1 has been found basally expressed in periodontal tissue, e.g., in PDL and alveolar bone, and extracellularly released by human PDL cells. It can thus upregulate the expression of various pro-inflammatory and osteoclastogenic cytokines such as IL-1β, IL-6, IL-17, and RANKL by which HMGB1 could modulate alveolar bone resorption and remodeling (Kim et al. 2010). In addition, by using a rat experimental tooth movement model, Wolf et al. (2013c) showed HMGB1 expression was modulated in a time-dependent manner in both compression and tension sides. Our previous study also confirmed the orthodontic force-triggered up-regulation of HMGB1 in mice PDL (Lv et al. 2014a). Considering the altered expression of periostin and HMGB1 in PDL cells under stress condition, it is possible that there is a correlation, direct or indirect, between both proteins, which may be involved in the modulation of stress-induced PDL remodeling.
In this study, we examine the effect of periostin deficiency on the expression of HMGB1 in PDL in response to mechanical stimulus using periostin gene knockout mice.
Materials and methods
Animal and tissue preparation
Eight-week-old male mice homozygous for the disrupted periostin gene and their wild-type (WT) littermates were used in these experiments. Pn-/- mice were generated as described previously (Lv et al. 2014a). In brief, the targeted disruption of the periostin gene was performed in mouse embryonic stem cells using a homologous recombination, and the inserted Neo gene was deleted in deficient mice by crossing with CAG-Gre mice to excise the neo cassette; no periostin expression was observed in Pn-/- mice. All animal experiments in this study were conducted according to the Guidelines for Animal Experimentation of Shandong University, Hokkaido University, and Tokyo Institute of Technology.
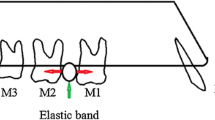
Waldo’s model was used to induce orthodontic tooth movement. In brief, an elastic band of 0.5 mm thickness and 2 mm width was inserted into the space between the right first and second maxillary molars under anesthesia. The untreated sides were used as controls. Mice were housed under specific pathogen-free conditions, in a room with continuously filtered air, maintained between 21 and 22 °C, with 40–60 % humidity on 12 h light and dark cycles and given free access to soft diet and water.
At 3 days after orthodontic force application, all animals were subjected to transcardial perfusion with 4 % paraformaldehyde in a 0.1 M phosphate buffer (pH = 7.4) after anesthesia with an intraperitoneal injection of 8 % chloral hydrate (400 mg/100 g body weight). Afterwards, the maxilla of each animal was dissected, divided in half, and immersed in the same fixative for an additional 24 h prior to decalcification with 10 % EDTA-2Na solution for 2 weeks at 4 °C. After that, specimens were dehydrated with ethanol solutions in ascending concentrations prior to paraffin embedding. Five-micrometer thick serial sections were prepared for histological analysis. Six animals were sacrificed for each group at each time point. Schematically illustrated in Fig. 1.
Immunohistochemistry for HMGB1 and periostin
After xylene treatment, dewaxed paraffin sections were pretreated with 0.3 % hydrogen peroxide for 30 min at room temperature. Then, sections were pre-incubated with 1 % bovine serum albumin in phosphate-buffered saline for 20 min to reduce non-specific staining. The treated sections were incubated for 2 h at room temperature with a rabbit anti-HMGB1 antibody (Epitomics, Burlingame, CA, USA) at a dilution of 1:200, or a rabbit anti-Periostin antibody (Santa Cruz, Dallas, TX, USA) at a dilution of 1:100. They were then immersed in horseradish peroxidase-conjugated secondary antibodies (Dako, Glostrup, Denmark) at a dilution of 1:100 for 1 h at room temperature. The immunoreaction was visualized with diaminobenzidine (Sigma-Aldrich, St. Louis, MO, USA). Staining results were assessed under light microscopy (BX53, Olympus Corp., Tokyo, Japan), and all sections were faintly counterstained with methyl green.
Immunostaining intensity was analyzed using Imagepro Plus 6.2 software (Media Cybernetics, Silver Spring, MD, USA). Positive reaction areas of periostin in the regions of PDL were manually selected in a color cube-based manner. At least six sections from each group were analyzed. All values are presented as mean ± standard deviation. Differences among the groups were assessed by the Student’s t test, and considered statistically significant at P < 0.01.
Counting of HMGB1-positive cells
Stained sections were observed and microscopical images were taken with a light microscope. Randomly chosen, equally-sized images of PDL tissue at the compression and tension sides of the maxillary first molars were captured for each specimen. The number of immunoreactive cells was counted and calculated with the aid of the cell analyzing software Imagepro Plus (Media Cybernetics). Extracellular immunoreactivity was not analyzed because of difficulties with quantification of such staining results. All operations were performed by the same person experienced in interpretation of histological sections. To avoid bias, the cell counting was performed automatically by software after definition of the corresponding thresholds.
Results
Periostin expression in periodontal tissue of WT mice after orthodontic tooth movement
To investigate the periostin expression profiles in periodontal tissue during orthodontic tooth movement, we performed immunohistochemistry and found that the expression of periostin was predominantly observed in the PDL of control specimens without experimental tooth movement. There was no apparent difference in intensity of immunostaining between the mesial and distal PDL surrounding the root in control specimens (Fig. 2a, b, e). The cells expressing periostin protein were mainly fibroblasts in PDL; a region of osteoblasts on the surface of alveolar bone was also positively stained. However, osteocytes located in alveolar bone were negative for periostin (Fig. 2a, b). After 3 days of experimental tooth movement, the expression of periostin was slightly reduced at the tension side compared with the control group (Fig. 2a, c, e), although without significant difference. At the compression side, the immunostaining for periostin was dramatically lowered to an undetectable level along with the formation of hyaline zones (Fig. 2b, d, e).
Immunohistochemistry for periostin and statistical analysis for periostin immunochemical intensity. At 0 day, periostin was predominantly localized in PDL and uniformly distributed in the mesial and distal PDL surrounding the root (a, b). There is no significant difference between the mesial and distal PDL surrounding the root (e). Following orthodontic tooth movement, periostin expression in PDL was weakened slightly compared with 0 day group at tension sides (c, e). At the compression side, due to the formation of cell free hyaline zones, the immunostaining for periostin was dramatically lowered even to an undetectable level (d). P < 0.01. PDL periodontal ligament; D dentin; Ab alveolar bone; TS tension side; CS compression side. a–d, ×400
HMGB1 expression in PDL of the compression side in both WT and Pn-/- groups after orthodontic tooth movement
In untreated WT mice, PDL at the compression side showed a basal expression of HMGB1 and the faint immunostaining for this protein was mainly located at the cell nucleus of fibroblasts (Fig. 3a). Compared with WT mice, PDL at the compression side in Pn-/- mice exhibited a higher basal expression of HMGB1 with a larger number of HMGB1-positive cells (Fig. 3a, b, e). After 3 days of orthodontic tooth movement, the PDL of both groups was almost replaced by cell-free hyaline zones filled with disorganized collagen fibers, thus no visible HMGB1-positive cells were observed in these areas (Fig. 3c, d). However, the residual PDL of the compression side was much wider in Pn-/- mice than in their WT littermates (Fig. 3c, d, f).
The HMGB1 expression level and the width of PDL at compression side. In Pn-/- group, the number of HMGB1 positive cells was significantly increased compared with WT group (a–d, i). Following 3 days of orthodontic tooth movement, there were apparent hyaline zones formed in both group (the red dotted line) (e–h). There were only a few HMGB1 positive cells in the compression sides. Statistical analysis for width of PDL (j). The width of PDL in both groups showed narrowed, however, in the Pn-/- group, the width of compressed periodontal ligament was wider than that of WT group at 3 days after orthodontic tooth movement. P < 0.01. PDL periodontal ligament; D dentin; Ab alveolar bone; CS compression side. a–d, ×400. (Color figure online)
HMGB1 expression in PDL of the tension side in both WT and Pn-/- groups after orthodontic tooth movement
For the untreated WT mice, PDL at this side showed similar levels of basal expression of HMGB1 compared with the compression side (Figs. 3a, 4a). Similar to the manifestation of the compression side, Pn-/- mice also displayed a higher level of basal expression of HMGB1 in PDL cells than in WT mice (Fig. 4a, b, e). After 3 days of orthodontic force application, PDL in both groups exhibited different extents of stretch (Fig. 4c, d). This increased PDL width in Pn-/- mice was about 25 % less than that of WT mice (Fig. 4f). More importantly, the numbers of HMGB1-positive cells in PDL of this side from WT and Pn-/- mice were both increased compared with their respective unloaded counterparts (Fig. 4a–d), but there was no significant difference between the two groups (Fig. 4c–e).
The HMGB1 expression level and the width of PDL at tension side. There was a basal expression of HMGB1 in both untreated groups (a–d), and in Pn-/- group, the number of HMGB1 positive cells exhibited a significant increase. At 3 day after orthodontic tooth movement, HMGB1 expression was increased significantly in tension side of both groups, but the increased value of PDL width in Pn-/- mice was less than that of WT mice (e–i). Statistical analysis for HMGB1 positive cells (i) and width of PDL (j). Following orthodontic tooth movement, both groups showed a wider tensed PDL, and WT mice showed a wider tensed PDL than Pn-/- mice. P < 0.01. PDL periodontal ligament; D dentin; Ab alveolar bone; TS tension side. a–d, ×400
Discussion
In this study, we examined the expression of HMGB1 by using immunohistochemistry analysis in WT and Pn-/- mice periodontal tissue during experimental tooth movement. In 1954, Waldo et al. introduced a method for inducing tooth movement through inserting an elastic band into the space between maxillary first molar and second molar. Compared with the Ni–Ti coil spring method now in wide use, Waldo’s method is characterized by its ease of application and good short-term stability (1–5 days). Therefore, it is suitable for evaluating the effect of short-term orthodontic force on the local periodontal tissue. Specially, Wolf et al. (2013c) reported the peak expression of HMGB1 in periodontal ligament cells following 3 days of mechanical loading. Furthermore, our previous study showed the fully formed cell free hyaline zones in PDL of the compression side at day 3 after the application of mechanical stress using Waldo’s method (Lv et al. 2014b). Based on the above-mentioned evidence, we choose Waldo’s method to induce orthodontic tooth movement and day 3 as our targeted time point in this experiment.
Our previous study demonstrated that periostin plays an essential role in the function of collagenolytic enzymes such as cathepsin K, MMP1 and MMP2 in the compressed PDL after orthodontic force application. This aided the degradation of collagen fibers and facilitating orthodontic force-induced tooth movement, while deficiency of periostin inhibited the elimination of collagen fibers and obstructed the corresponding tooth movement (Lv et al. 2014b). In this study, we obtained similar results showing that both groups exhibited extensive formation of cell-free hyaline zones at the PDL of the compression side and the PDL at this side was less compressed in Pn-/- mice than in WT mice. Although the critical roles of periostin in collagen fibrillogenesis and connective tissue integrity have been highlighted in the literature (Choi et al. 2011; Norris et al. 2007; Rios et al. 2008), it appears that the tendency of excessive tooth migration caused by periostin deficiency-induced defects in biomechanical properties of the periodontium is counter-balanced by the impairment of degradation of collagen fibers resulting from periostin deficiency.
We have revealed, for the first time to our knowledge, that PDL of Pn-/- mice exhibited a higher level of basal HMGB1 expression than that in WT mice in the absence of mechanical force. It is demonstrated that periostin-null mice develop with a highly inflamed background in their tooth-supporting tissue including PDL and alveolar bone (Rios et al. 2005), and the inflammatory response is though to be associated with a high level of HMGB1 expression (Yang et al. 2002). Hence, these findings suggest that it might be the periostin deficiency-induced systemic inflammatory response that upregulated HMGB1 expression in PDL. Additionally, other than the indirect regulation, it is also possible that periostin directly inhibited HMGB1 expression by interacting with specific receptors expressed on periodontal ligament fibroblasts. However, the exact regulatory role of periostin on HMGB1 expression of PDL cells and the underline mechanism still needs further in vitro confirmation.
After the application of mechanical force in WT mice, the expression of periostin in PDL of the tension side was not significantly different from the unloaded counterpart, which does not conform with the reports that periostin mRNA expression was decreased in tension sites during experimental tooth movement (Wilde et al. 2003). Meanwhile, the number of HMGB1-positive cells was increased in the PDL of the tension side. This finding is consistent with the well-recognized stimulatory effect of mechanical stress on HMGB1 production (Wolf et al. 2013a, b, c). For Pn-/- mice, the number of HMGB1 positive cells in PDL at the tension side was also increased, with no significant differences compared with WT mice. It is seemed that the promotive effect of periostin deficiency on HMGB1 expression was so inconsiderable compared with that of mechanical loading that lead to the similar extent of increase in the number of HMGB1-positive cells between two groups. Nevertheless, it has been reported that periostin is not only essential for the integrity and function of the PDL during mechanical loading, but it also promotes cellular tolerance against stress and inhibits cell death (Rios et al. 2008). Deficiency of periostin is associated with defects in cell–cell and cell–matrix connections (Norris et al. 2007). Thus, under mechanical stress, the PDL tissue of Pn-/- mice is vulnerable to damage and tearing. Therefore, theoretically, with the same extent of tension, PDL of Pn-/- mice should be easier to be stretched. However, the periostin deficiency-induced impairment of collagen fiber degradation impeded tooth movement and reduced the value of tension force applied to PDL at the tension side indicated by the less expanded width of PDL at the tension side in Pn-/- mice compared with their WT littermates. Primary, tension of the PDL alters blood vessel morphology and the vascular response in the alveolar socket (Kuitert et al. 1988; Noda et al. 2009), leading to a reduction in blood volume (Packman et al. 1977), which can eventually induce local hypoxia and apoptosis in PDL cells (Rygh 1973). Both hypoxia-caused cell injury and apoptosis of cells are well recognized as stimulators for HMGB1 expression and release (Andersson et al. 2002; Bianchi 2007; Wang et al. 1999). Therefore, presumably, less tension force means less morphologic change of blood vessel and less hypoxia-induced injury to PDL cells, which further reduces HMGB1 expression. Moreover, stress is an independent activator for HMGB1 expression. Experiments in vivo and vitro demonstrated that stress could induce HMGB1 release and translocation from the nucleus to the cytoplasm in an intensity-dependent manner (Wolf et al. 2013c). Thus, the attenuated tension force in the tension side contributed to less enhanced HMGB1 expression by PDL cells in Pn-/- mice, which might generate a similar increase in the number of HMGB1-positive cells to that of WT mice combined with the additional promotive effect of periostin deficiency on HMGB1 expression.
In conclusion, our study reveals that periostin deficiency increases basal expression of HMGB1 in mice, suggesting a direct or indirect inhibitory effect of periostin on HMGB1 production. In the process of orthodontic tooth movement, mechanical force might act as a common mediator, regulating the expression of HMGB1 and periostin in experimental tooth movement, which further contributes to maintenance of periodontal integrity and remodeling of periodontal tissue (Fig. 5)
Schematic diagram for HMGB1 expression profiles in PDL of Pn-/- mice during experimental tooth movement. Normal PDL cells exhibit low level of basal expression of HMGB1. Both periostin deficiency and mechanical stress can promote HMGB1 expression by PDL cells independently. At the compression side, the PDL was almost replaced by cell free hyaline zones due to hypoxia, but because of the impeded collagen fiber degradation and the resulting obstruction of tooth movement, it showed a wider residual compressed PDL than their WT littermates. At the condition of mechanical stress, periostin deficiency causes collagen fibers degradation impairment of PDL at compression side, which obstructs orthodontic tooth movement and further results in less stretch of PDL at the tension side. Less stretch is reasonably associated with less tensile force in PDL of tension side and less increase of HMGB1 expression compared with WT mice. However, the additional promotive effect of periostin deficiency on HMGB1 production makes up for it, leading to a comparable HMGB1 expression level to WT mice
.
References
Andersson U, Erlandsson-Harris H, Yang H, Tracey KJ (2002) HMGB1 as a DNA-binding cytokine. J Leukoc Biol 72:1084–1091
Bianchi ME (2007) DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 81:1–5
Choi JW, Arai C, Ishikawa M, Shimoda S, Nakamura Y (2011) Fiber system degradation, and periostin and connective tissue growth factor level reduction, in the periodontal ligament of teeth in the absence of masticatory load. J Periodontal Res 46:513–521
Kim YS, Lee YM, Park JS, Lee SK, Kim EC (2010) SIRT1 modulates high-mobility group box 1-induced osteoclastogenic cytokines in human periodontal ligament cells. J Cell Biochem 111:1310–1320
Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A (2008) HMGB1: endogenous danger signaling. Mol Med 14:476–484
Kuitert RB, van de Velde JP, Hoeksma JB, Prahl-Andersen B (1988) Tissue changes in the rabbit periodontal ligament during orthodontic tooth movement. Acta Morphol Neerl-Scand 26:191–206
Lv S et al (2014a) Expression of HMGB1 in the periodontal tissue subjected to orthodontic force application by Waldo’s method in mice. J Mol Histol. doi:10.1007/s10735-014-9606-z
Lv S et al (2014b) Histochemical examination of cathepsin K, MMP1 and MMP2 in compressed periodontal ligament during orthodontic tooth movement in periostin deficient mice. J Mol Histol 45:303–309
Noda K, Nakamura Y, Kogure K, Nomura Y (2009) Morphological changes in the rat periodontal ligament and its vascularity after experimental tooth movement using superelastic forces. Eur J Orthod 31:37–45
Norris RA et al (2007) Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J Cell Biochem 101:695–711
Norris RA, Borg TK, Butcher JT, Baudino TA, Banerjee I, Markwald RR (2008) Neonatal and adult cardiovascular pathophysiological remodeling and repair: developmental role of periostin. Ann N Y Acad Sci 1123:30–40
Packman H, Shoher I, Stein RS (1977) Vascular responses in the human periodontal ligament and alveolar bone detected by photoelectric plethysmography: the effect of force application to the tooth. J Periodontol 48:194–200
Rios H et al (2005) Periostin null mice exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease-like phenotype. Mol Cell Biol 25:11131–11144
Rios HF, Ma D, Xie Y, Giannobile WV, Bonewald LF, Conway SJ, Feng JQ (2008) Periostin is essential for the integrity and function of the periodontal ligament during occlusal loading in mice. J Periodontol 79:1480–1490
Rygh P (1973) Ultrastructural changes in pressure zones of human periodontium incident to orthodontic tooth movement. Acta Odontol Scand 31:109–122
Takeshita S, Kikuno R, Tezuka K, Amann E (1993) Osteoblast-specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem J 294((Pt 1)):271–278
Wang H et al (1999) HMG-1 as a late mediator of endotoxin lethality in mice. Science 285:248–251
Wilde J, Yokozeki M, Terai K, Kudo A, Moriyama K (2003) The divergent expression of periostin mRNA in the periodontal ligament during experimental tooth movement. Cell Tissue Res 312:345–351
Wolf M, Lossdorfer S, Abuduwali N, Jager A (2013a) Potential role of high mobility group box protein 1 and intermittent PTH (1–34) in periodontal tissue repair following orthodontic tooth movement in rats. Clin Oral Invest 17:989–997
Wolf M, Lossdorfer S, Craveiro R, Gotz W, Jager A (2013b) Regulation of macrophage migration and activity by high-mobility group box 1 protein released from periodontal ligament cells during orthodontically induced periodontal repair: an in vitro and in vivo experimental study. J Orofac Orthop = Fortschr der Kieferorthop: Organ/Off J Deutsche Ges fur Kieferorthop 74:420–434
Wolf M, Lossdorfer S, Kupper K, Jager A (2013c) Regulation of high mobility group box protein 1 expression following mechanical loading by orthodontic forces in vitro and in vivo. Eur J Orthod. doi:10.1093/ejo/cjt037
Yamada S, Tauchi T, Awata T, Maeda K, Kajikawa T, Yanagita M, Murakami S (2014) Characterization of a novel periodontal ligament-specific periostin isoform. J Dent Res 93:891–897
Yang H, Wang H, Czura CJ, Tracey KJ (2002) HMGB1 as a cytokine and therapeutic target. J Endotoxin Res 8:469–472
Acknowledgments
This study was partially supported by the National Nature Science Foundation of China (Nos. 81271965; 81470719; 81311140173) and Specialized Research Fund for the Doctoral Program of Higher Education (No. 20120131110073) to Li M. The Shandong Province Science and Technique Foundation, China (No. 2014GSF118093) to Guo J.
Ethical Standards
The animals were maintained and all experimental procedures were approved by the Committee on the Ethics of Animal Experiments of Shandong University, Hokkaido University, and Tokyo Institute of Technology. All surgery was performed under chloral hydrate anesthesia, and all efforts were made to minimize suffering.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Juan Li and Wei Feng have equally contributed to this article.
Rights and permissions
About this article
Cite this article
Li, J., Feng, W., Liu, B. et al. Altered distribution of HMGB1 in the periodontal ligament of periostin-deficient mice subjected to Waldo’s orthodontic tooth movement. J Mol Hist 46, 303–311 (2015). https://doi.org/10.1007/s10735-015-9619-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-015-9619-2