Abstract
The WNT/β-CATENIN signaling has been demonstrated to play critical roles in mouse tooth development, but little is known about the status of these molecules in human embryonic tooth. In this study, expression patterns of WNT/β-CATENIN signaling components, including WNT ligands (WNT3, WNT5A), receptors (FZD4, FZD6, LRP5), transducers (β-CATENIN), transcription factors (TCF4, LEF1) and antagonists (DKK1, SOSTDC1) were investigated in human tooth germ at the bud, cap and bell stages by in situ hybridization. All these genes exhibited similar but slightly distinct expression patterns in human tooth germ in comparison with mouse. Furthermore the mRNA expression of these genes in incisors and molars at the bell stage was also examined by real-time PCR. Our results reveal the status of active WNT/β-CATENIN signaling in the human tooth germ and suggest these components may also play an essential role in the regulation of human tooth development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mammalian tooth development depends on the reciprocal and sequential interactions between the ectoderm-derived dental epithelium and cranial neural crest-derived mesenchymal cells. While molecular and cellular mechanisms are being investigated in mice (Du et al. 2012; Feng et al. 2012; Tang et al. 2013; Yang et al. 2013; Lei et al. 2014), there is much less understood in humans. In developing human embryo, the dental placode develops into dental epithelium which thickens at 6 W (weeks of gestation). At 7–8 W, the dental epithelium proliferates and invaginates into the subjacent mesenchyme, forming the epithelial bud. At the cap stage (10–13 W), the epithelial bud undergoes specific folding and forms a transient epithelial cluster, called the enamel knot, in the central region of the bud, which is the signaling center involved in regulating tooth shape. At bell stage (14–18 W), ameloblasts and odontoblasts begin to differentiate (Gary et al. 2008).
Multiple signaling networks composed of numerous amounts of molecules participate in the regulation of tooth development. The Wingless-type MMTV integration site (WNT) signaling is one of the critical regulatory pathways. It has at least three branches, of which the WNT/β-CATENIN branch is the best studied and considered as the canonical WNT pathway. In the absence of WNT ligand, cytoplasmic β-CATENIN is associated with the scaffold proteins AXIN and adenomatous polyposis coli (APC), constitutively phosphorylated by glycogen synthase kinase 3β (GSK3β) and targeted for proteasome-mediated degradation. Binding of WNT ligand to frizzled (FZD) receptor and low-density lipoprotein receptor related proteins (LRP) 5/6 co-receptor inactivates the AXIN/APC/GSK3β/CKI complex and stabilizes cytoplasmic β-CATENIN. Subsequently, β-CATENIN translocates into nucleus and induces transcription of downstream genes through interactions with T cell-specific transcription factor (TCF) and lymphoid enhancer binding factor (LEF) (Huelsken and Behrens 2002).
The WNT/β-CATENIN signaling regulates tooth development at many levels (Liu and Millar 2010; Liu et al. 2008; Jarvinen et al. 2006). For instance, ligand Wnt3 shows specific expression in the enamel knot, and the overexpression of Wnt3 causes progressive loss of ameloblasts and reduction of enamel in postnatal mouse incisor teeth (Millar et al. 2003). In Lef1 knockout mice, tooth development arrests at the bud stage (van Genderen et al. 1994).Targeted epithelial overexpression of the secreted WNT antagonist Dickkopf 1(Dkk1) arrests tooth development at the lamina stage in mice (Liu et al. 2008), which is slightly earlier than the block to tooth development seen in Lef1 knockout mice. In embryonic mouse oral epithelium, constitutive activation of β-catenin results in multiple tooth buds (Pispa et al. 2004; Mustonen et al. 2003). In addition, mutations in Sostdc1 (Wise, Ectodin) encoding an inhibitor of Lrp5- and Lrp6-dependent WNT signaling leads to formation of supernumerary tooth in the diastemal region (Kassai et al. 2005).
Until now, most studies on the molecular mechanisms underlying tooth development were conducted in mice. The expression pattern and function of WNT signaling in human tooth development remain unknown. Different from mouse, human has two sets of teeth (the deciduous teeth and permanent teeth). The function of WNT signaling pathway in mice may not fully mimic that of human. Revealing the molecular mechanism during embryonic tooth development in normal human is essential for studying tooth regeneration or reconstitution of a bioengineered tooth organ. In this study, we examined the expression patterns of WNT/β-CATENIN signaling molecules during human incisor and molar development. Our data of the specific spatio-temporal patterns of WNT signaling in the developing human tooth germ demonstrate the WNT/β-CATENIN signaling cascade is crucial for early human odontogenesis.
Materials and methods
Sample preparation
We obtained 8–15 W (weeks of gestation) human embryonic tissue samples from aborted fetuses, which were provided by the Hospital for Woman and Children Health of Fujian Province. Written informed consents were obtained from participants for the use of their medically aborted embryos for scientific research. And the obtainment and application of human embryos in this study was permitted by the Ethical Committee of Fujian Normal University. The embryonic tissues were fixed in 4 % paraformaldehyde in phosphate-bufferedsaline (PBS, pH 7.4) at 4 °C overnight and then demineralized with 10 % ethylenediamine tetra-acetic acid (pH 7.4) at 4 °C from 2 days to 1 month depending on stages. Then the samples were processed for paraffin embedding and sectioned at 10 μm.
Probes and in situ mRNA hybridization
All the probes (WNT3, WNT5A, FZD4, FZD6, LRP5, CTNNB1, TCF4, LEF1, DKK1 and SOSTDC1) used for examining human gene expression in this study were purchased from Open Biosystems (Thermo Scientific Inc). cDNA fragments were subcloned into pBluesscript for riboprobe transcription.
Sections for in situ hybridization with DIG-labeled riboprobe were performed as previously described (Lin et al. 2007; Hu et al. 2013; Zhang et al. 2012). Sections were de-waxed in xylene, subsequently rehydrated through a graded series of alcohol and post-fixed in 4 % PFA. Digoxigenin (DIG) labeled RNA probes were prewarmed at 75 °C and hybridized to sections overnight at the temperature according to the TM value of the gene. Sense probes were used as negative control.
RNA isolation and real-time RT-PCR
Human embryonic tooth germs at 15 W (the bell stage) were isolated and two types of tooth germs (incisor and premolar) from one quadrant were respectively subjected to total RNA extraction for 3 times using RNeasy Mini Kit (QIAGEN) according to the manufacturer’s instructions. Template cDNAs were synthesized by reverse transcription of 1ug total RNAs using oligo (dT) primer and RevertAid™ M-MuLV Reverse Transcriptase (Fermentas, EU). Real-time PCR was carried out using 2×DyNAmo ColorFlash SYBR Green qPCR Kit (Thermo Scientific), and performed with ABI StepOnePlus Real-time PCR System (Applied Biosystems). Primers used in this assay were chosen from previous reports (Zhang et al. 2012). GAPDH was used as an internal control for correcting the expression levels of target genes. Relative mRNA expression levels of target genes in human molar germs were achieved using the ∆∆CT method in comparison with incisor germs. For each RNA sample, three replicates of real-time PCR were performed. Experimental data was shown as Mean and SD from at least three independent experiments. T test was analysis with ANOVA using the SigmaPlot 12.5 software. Differences with P < 0.05 or P < 0.01 were considered as statistically significant change.
Results
Human tooth morphogenesis and expression patterns of WNT3 and WNT5A ligands in the developing human tooth germ
Firstly, H&E staining was used to detect the human incisor and molar germ morphogenesis. At around 8 W (Weeks of gestation, bud stage), the dental epithelium invaginated into the subjacent mesenchyme, and dental mesenchymal cells condensed around the bud cells forming the epithelial bud of the primary teeth (Fig. 1a, d). At 12 W (cap stage), the epithelial bud folded and formed a transient epithelial cluster called the enamel knot in the central region of the bud (Fig. 1b, e). During the subsequent W bell stage, the epithelium and mesenchyme started to differentiate into ameloblasts and odontoblasts respectively, and the tooth germs assume bell-like structure (Fig. 1c, f). These data suggest the normal tooth morphogenesis in human tooth germ resembles that of mice.
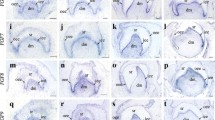
Human tooth morphogenesis and expression patterns of WNT signaling ligands during the developing human tooth germs. Morphogenesis of the early tooth germ development at 8-week bud stage (a), 12-week cap stage (b) and 15-week bell stage (c) in human primary incisor, and molar germ (d–f). WNT3 expression was rarely detected at 8.5-week bud stage and 12-week cap stage in the human primary incisor germ (g, h), and molar germ (j, k), but at the bell stage from a 15-week-old embryo exhibited WNT3 expression in the inner enamel epithelium in the human primary incisor (i) and molar germ (l). WNT5A strongly expression was detected at the 8.5-week bud stage, 12-week cap stage and 15-week bell stage in the human primary incisor and molar germ (m–r). DE dental epithelium, DP dental papilla, EK enamel knot, IEE inner enamel epithelium. Scale bar 50 μm (a, b, d, g, h, j, m, n, p), 100 μm (c, e, f, i, k, l, o, q, r)
Then in situ hybridization was performed to investigate the expression patterns of WNT signaling ligands, WNT3 and WNT5A in human embryonic tooth development (8–15 W). Ligand Wnt5a used to be considered as noncanonical WNT family members. But recent studies showed that Wnt5a could also regulate canonical WNT signaling via Ror2 or Frizzled4/Lrp5 receptors, suggesting a dual role for Wnt5a signaling in a receptor dependent manner (Mikels and Nusse 2006a; Lin et al. 2011). In the developing human incisor and molar germs, WNT3 was rarely expressed throughout the tooth germ at 8 and 12 W (Fig. 1g–h, j–k), and its expression was restricted in the internal enamel epithelium at the 15 W bell stage (Fig. 1i, l). However, WNT5A transcripts showed strong expression in oral epithelium and dental epithelium, and weaker expression throughout the remaining dental mesenchyme at the bud stage. At the 12 W cap stage, WNT5A highly expressed all around the tooth germ, including oral epithelium, dental epithelium, dental papilla mesenchyme and enamel knot. At the 15 W bell stage, expression of WNT5A transcripts was continually dense in outer enamel epithelium, inner enamel epithelium and dental mesenchyme (Fig. 1m–r).
Expression patterns of FZD4, FZD6 and LRP5 receptors in the developing human tooth germ
In situ hybridization was also used to determine the expression patterns of WNT signaling receptors FZD4, FZD6 and LRP5. Receptor FZD4 was not detected in dental epithelium and mesenchyme during 8 and 12 W in human incisor and molar germ (Fig. 2a–b, d–e). But at the bell stage (15 W), FZD4 was not detected in the incisors, while the expression was well localized in the internal enamel epithelium of molars (Fig. 2c, f). Another receptor FZD6 showed exclusively weak localization at dental epithelium and enamel knot of incisor and molar at 8 and 12 W, but was clearly observed in the internal and outer enamel epithelium and the surrounding mesenchymal cells during 15 W (Fig. 2g–l). In addition, the FZD6 signaling in incisor was stronger than that of molar. Low density lipoprotein receptor LRP5 was weakly detected in the dental epithelium and dental mesenchyme during the 8 W bud stage and the 12 W cap stage, but was obviously expressed in the internal enamel epithelium at the 15 W bell stage in both the human incisor and molar (Fig. 2m–r).
Expression patterns of WNT/β-CATENIN signaling receptors during the developing human tooth germs. FZD4 expression was not detected at 8.5-week bud stage and 12-week cap stage in the human primary incisor (a, b), and molar germ (d, e). FZD4 expression was weekly detected at 15-week bell stage in the incisor germ (c), but obviously detected in the inner enamel epithelium at 15-week-old embryo in the human primary molar germ (f). FZD6 expression was weekly detected in epithelium at 8-week (g), 12-week (h) in human primary incisor germ, and molar germ (j, k). FZD6 expression was well located at 15-week human primary incisor (i) and molar germ (l). LRP5 expression was weekly detected at the 8-week bud stage, and 12-week cap stage in human primary incisor (m, n) and molar germ (p, q), but at the bell stage from a 15-week-old embryo exhibited LRP5 expression in the inner enamel epithelium in the human primary incisor (o) and molar germ (r). DE dental epithelium, DP dental papilla, IEE inner enamel epithelium. Scale bar 100 μm (a–l), 50 μm (m–r)
Expression patterns of β-CATENIN, TCF4 and LEF1 in the developing human tooth germ
Transducer β-CATENIN, also known as CTNNB1, is a key molecule in canonical WNT pathway. The mRNA expression pattern of CTNNB1 during human tooth germ development was also examined by in situ hybridization. CTNNB1 was distinctively present in dental epithelial cells from the bud to bell stage. At 8 W, strong CTNNB1 expression was detected at the tip of the budding epithelium (Fig. 3a, d). At 12 W, the expression was observed in the dental epithelium, enamel knots and the mesenchyme surrounding epithelium (Fig. 3b, e). But at 15 W bell stage, CTNNB1 expression was limited in the inner enamel epithelium (Fig. 3c, f). The transcription factors TCF4 showed no expression during the bud and cap stages (8 and 12 W) (Fig. 3g, h, j, k), but positive TCF4 staining predominantly localized in internal enamel epithelium during the 15 W bell stage (Fig. 3i, l). Another transcription factor LEF1 was not detected in dental epithelium and mesentchyme at 8 W in both incisor and molar (Fig. 3m, p). However, LEF1 was expressed throughout the dental epithelium and mesenchyme of the incisor and molar at the cap stage (Fig. 3n, q), and had high expression in the internal and outer enamel epithelium at the bell stage in the developing human tooth germ (Fig. 3o, r).
Expression patterns of WNT/β-CATENIN transducers and transcription factors during the developing human tooth germs. CTNNB1 (β-CATENIN) expression was detected in the epithelium at 8-week human primary incisor (a) and molar germ (d). CTNNB1 expression was seen in both the epithelium and the surrounding mesenchyme at 12-week human primary incisor (b) and molar germ (e). CTNNB1 expression was seen in the inner epithelium at 15-week human primary incisor (c) and molar germ (f). TCF4 showed no expression during the 8-week bud stage and 12-week cap stage in human primary incisor (g, h) and molar germ (j, k), but positive TCF4 staining was predominantly localized in internal enamel epithelium during the 15-week bell stage (i, l). LEF1 expression was not detected in the human tooth germ at the 8-week human primary incisor (m) and molar germ (p). LEF1 was expressed throughout the dental epithelium and mesenchyme of the incisor and molar at the 12-week cap stage (n, q), and had high localized expression in the internal and outer enamel epithelium at the 15-week bell stage in the developing human tooth germ (o–r). DE dental epithelium, DP dental papilla, EK enamel knot, IEE inner enamel epithelium. Scale bar 100 μm
Expression patterns of DKK1 and SOSTDC1 inhibitor in the developing human primary dentition
Dkk1 inhibits the WNT signaling pathway by binding to the WNT receptor complex. As in situ hybridization results showed, DKK1 was expressed throughout the epithelium at 8 W in the human incisor and molar (Fig. 4a, d). And the expression expanded to all of the tooth germ, including dental epithelium, dental mesenchyme and enamel knot at 12 W (Fig. 4b, e). At 15 W, DKK1 was observed in the inner enamel epithelium, outer enamel epithelium and dental mesenchyme (Fig. 4c, f). Another antagonist SOSTDC1 was also detected by in situ hybridization at the bud, cap and bell stages. From 8 to 15 W, the expression of SOSTDC1 was strongly detected in the dental epithelium and mesenchyme of incisor and molar germ (Fig. 4g–l). Negative controls for in situ hybridization in the developing human incisor and molar were shown in Fig. 4m–r.
Expression patterns of WNT/β-CATENIN inhibitors during the developing human tooth germs. DKK1 expression was mainly detected in the epithelium at the bud stage in human primary incisor (a) and molar germ (d). DKK1 expression was detected in the epithelium, mesenchyme and enamel knot at 12-week human primary incisor (b) and molar germ (e). DKK1 was observed in the inner enamel epithelium, outer enamel epithelium and dental mesenchyme at 15-week human primary incisor (c), and molar germ (f). SOSTDC1 expression was detected in dental epithelium and mesenchyme at the bud (g, j), cap (h, k) and bell (i, l) stages of the primary incisor and molar germ. Negative control for in situ hybridization in the developing human tooth germ (m–r). DE dental epithelium, DP dental papilla, IEE inner enamel epithelium. Scale bar 100 μm
Comparison of the mRNA expression of WNT/β-CATENIN signaling component in the developing human incisors and molars
To further confirm the mRNA expression levels of WNT/β-CATENIN signaling components in the developing human incisors and molars, we pooled these two types of tooth germs at the 15 W bell stage from all the available embryonic mandibular quadrant and isolated total RNAs from each pool. We then determined the expression levels of WNT signaling genes by Real-time RT-PCR. As shown in Fig. 5, the expression levels of FZD4 and LEF1 in molars were markedly higher than that of incisors, but FZD6 in molars was obviously lower than that of incisors. And there were no significant changes in WNT5A, LRP5, β-CATENIN, TCF4, DKK1 and SOSTDC1 of molars when compared with incisors. The changes in expression levels of these genes at the bell stage were basically matched with that detected by in situ hybridization.
Real-time RT-PCR was used to detect the mRNA expression levels (mean ± SEM) of WNT/β-CATENIN signaling components, including WNT ligands (WNT5A), WNT receptors (FZD4, FZD6, LRP5), WNT transducers (β-CATENIN), WNT transcription factors (TCF4, LEF1) and WNT antagonists (DKK1, SOSTDC1) in the 15-week primary incisor and molar. Data were normalized to the average mRNA level of GAPDH. Statistical difference is indicated by asterisks (*P < 0.05, **P < 0.01)
Discussion
In mice, WNT signaling pathway has previously been shown to play important roles at various periods during tooth development, from the initiation to the bud, cap, and differentiation stages (Liu and Millar 2010). In the current study, the spatial and temporal expression patterns of WNT signaling components including the ligands (WNT3 and WNT5A), receptors (FZD4, FZD6 and LRP5), transducers and transcription factors (CTNNB1, TCF4 and LEF1), and antagonists (DKK1 and SOSTDC1) were examined in the developing human tooth germ during the bud, cap and bell stages. The expression of WNT3, FZD4, FZD6 and LRP5 in the developing human tooth germ was distinctly restricted in the dental epithelium at the bell stage. These observations indicate similar expression patterns of these genes in the developing human and mouse tooth germs. However, WNT5A showed strong localization not only around dental mesenchyme, but also in oral epithelium, dental epithelium during all of the stages in our study (Fig. 2m–r). And our immunohistochemistry data further confirmed the expression pattern of the WNT5A in human primary incisor and molar germ (data not shown), which is identical with our data of in situ hybridization and consistent with previous publciation that WNT5A protein was expressed in enamel epithelium cells, enamel knot and and stellate reticulum in developing human tooth germ (Peng et al. 2010a). In mice, Wnt5a mRNA is only expressed in mesenchymal cells with no overlying expression in the dental or oral epithelium (Kettunen et al. 2005; Sarkar and Sharpe 1999). These data suggests that WNT5A gene may be one of the key genes differ between human and mice in teeth. It was reported that Wnt5a plays dual role in noncanonical and canonical WNT pathway depending on the receptor (Mikels and Nusse 2006a; Lin et al. 2011). It was recently reported that WNT5A promoted differentiation and inhibited migration and proliferation in human dental papilla cells.(Peng et al. 2010b; Wang et al. 2013). The essential role of Wnt5A in the developing human tooth germ and human dental papilla might suggest its potential in the research of human regenerative dentistry (Peng et al. 2010b). Hence, more attention should be paid to noncanonical WNT pathway in the future to find the differences between human and mice in tooth germ.
In the WNT/β-CATENIN pathway, binding of the WNT ligands to the FZD receptors complex with LRP5/6 stabilizes β-CATENIN, leading to the transcription of its target genes through interaction with the transcription factors, TCF and LEF (Liu and Millar 2010). In mouse, β-catenin and Lef1 expression in the dental buds epithelium overlaps with expression of Pitx2, an essential transcription factor in the developing tooth (Sarkar and Sharpe 1999; Hjalt et al. 2000). Strong Lef1 expression is also detected in the dental mesenchyme in developing mouse tooth bud (Sarkar and Sharpe 1999). Similar expression patterns of β-CATENIN were found in dental epithelium of the embryonic human. However, LEF1 did not appear at the bud stage, instead was highly expressed in the dental epithelium and mesenchyme including stellate reticulum in human tooth germ at 12 weeks of gestation (cap stage), which had the same expression patterns with that of the bud stage in mice. This implies that LEF1 plays important role from the cap stages in the regulation of human tooth development, which is later than that in mice.
In mice, antagonist Dkk1 expression persists in the dental epithelium during the tooth initiation and bud stages, and then transfer to the dental mesenchyme from the cap stage to the differentiate stage (Liu et al. 2008; Fjeld et al. 2005). Another antagonist Sostdc1, also known as Ectodin, Wise or Usag-1, was identified as a gene encoding a conserved secreted protein capable of regulating canonical WNT signaling (Itasaki et al. 2003). But a recent study showed that it also binds to a subset of BMPs in vitro and influences Bmp signaling (Laurikkala et al. 2003). In mice, Sostdc1 is expressed in the dental epithelium and dental mesenchyme, except for the forming enamel knot at the tip of the upper tooth bud. The expression is fully negative from the enamel knot as well as the epithelium and mesenchyme surrounding at the cap stage (Laurikkala et al. 2003). In human, the expression patterns of DKK1 were strongly detected in the dental epithelium as that of mice at the bud stage, but the signaling expanded to the dental epithelium and mesenchyme at the cap and bell stages, which was slightly different from that of mice. SOSTDC1 transcripts were strongly detected in the dental epithelium and mesenchyme, and enamel knot from the 12th to 15th weeks of human tooth germs (Fig. 5h, i, k, l), which also was slightly different from that in mice. Hence, the WNT signals, and inhibitors probably act in concert to maintain a homeostasis for the regulation of tooth development.
Taken together, these results suggest that, the canonical WNT signaling pathway components exhibited similar but slightly distinct expression patterns in human tooth germ in comparison with mouse, suggesting that these components may also play an essential role in the regulation of human tooth development.
It is well known that the incisors and molars possess several differences in shape and size in the human tooth germ. For instance, molar is larger and has several cusps, while incisors only has one cusp. Therefore, the molecular mechanisms underlying the development of molar and incisors may be different. In order to investigate whether the WNT signaling molecules are responsible for the differential histomorphogenesis of the incisors and molars, we performed Real-time RT-PCR to determine the gene expression levels of WNT signaling in the human incisors and molars at 15 W (bell stage). We found that the gene expression levels of receptors FZD4 and FZD6, transcription factors LEF1 in molar were markedly different that of incisors, suggesting FZD4, FZD6 and LEF1 of WNT/β-CATENIN signaling components may be the key molecules make difference between incisors and molars. More studies will be needed to study the functional roles of FZD4 and FZD6 during the development of molars and incisor, which may provide in-depth understanding of the developmental processes of the tooth germs.
In addition to its role in tooth development and morphogenesis, the WNT/β-CATENIN signaling pathway is also associated with the pathogenesis of human oral diseases. For example, the role of WNT pathway in various types of oral tumor is well established. Differential expression of several WNT components including WNT3, WNT5A, WNT10A, LRP5, β-CATENIN, AXIN2, APC and DKK1, have been reported in normal tooth tissues and oral cancers (Adaimy et al. 2007; Blanton et al. 2004; Bohring et al. 2009; Foulkes 1995; Niemann et al. 2004; Rickels et al. 2005; Roessler et al. 2000; Sekine et al. 2002, 2003). Nuclear localized β-CATENIN is detected in follicular and plexiform-type ameloblastomas, and these tumors are occasionally linked to gain of function mutation of β-CATENIN or loss of function mutation of APC (Kawabata et al. 2005; Mikels and Nusse 2006b; Siriwardena et al. 2009). WNT signaling components are overexpressed in adenoid cystic carcinoma (ACC), another common salivary gland tumor subtype, and nuclear localized β-CATENIN and mutations in WNT signaling genes including β-CATENIN, APC and AXIN1 are detected in some cases (Daa et al. 2004, 2005; Frierson et al. 2002). This finding revealed that Wnt/β-CATENIN signaling is critical in formation of some oral tissue tumors, as well as in embryonic development of the various structures of the oral cavity (Liu and Millar 2010).
In conclusion, the specific spatio-temporal patterns showed that canonical WNT signaling components including WNT3, WNT5A, FZD4, FZD6, LRP5, β-CATENIN, LEF1, DKK1 and SOSTDC1 exhibit similar expression patterns in the developing human and mouse tooth germs. Among them, WNT5A, DKK1 and SOSTDC1 genes showed wider spatial expression in the developing human tooth germ as compared to that in mice. And FZD4, FZD6 and LEF1 showed difference in the expression levels of incisors and molars at the bell stage. All of our data revealed that WNT/β-CATENIN signaling cascade is crucial for early human tooth patterning during morphogenesis.
References
Adaimy L, Chouery E, Megarbane H, Mroueh S, Delague V, Nicolas E, Belguith H, de Mazancourt P, Megarbane A (2007) Mutation in WNT10A is associated with an autosomal recessive ectodermal dysplasia: the odonto-onycho-dermal dysplasia. Am J Hum Genet 81(4):821–828
Blanton SH, Bertin T, Serna ME, Stal S, Mulliken JB, Hecht JT (2004) Association of chromosomal regions 3p21.2, 10p13, and 16p13.3 with nonsyndromic cleft lip and palate. Am J Med Genet A 125A(1):23–27
Bohring A, Stamm T, Spaich C, Haase C, Spree K, Hehr U, Hoffmann M, Ledig S, Sel S, Wieacker P, Ropke A (2009) WNT10A mutations are a frequent cause of a broad spectrum of ectodermal dysplasias with sex-biased manifestation pattern in heterozygotes. Am J Hum Genet 85(1):97–105
Daa T, Kashima K, Kaku N, Suzuki M, Yokoyama S (2004) Mutations in components of the Wnt signaling pathway in adenoid cystic carcinoma. Mod Pathol 17(12):1475–1482
Daa T, Kaku N, Kashima K, Nakayama I, Yokoyama S (2005) Expression of beta-catenin, E-cadherin and cyclin D1 in adenoid cystic carcinoma of the salivary gland. J Exp Clin Cancer Res 24(1):83–87
Du J, Wang Q, Wang L, Wang X, Yang P (2012) The expression pattern of FHL2 during mouse molar development. J Mol Histol 43(3):289–295
Feng J, McDaniel JS, Chuang HH, Huang O, Rakian A, Xu X, Steffensen B, Donly KJ, MacDougall M, Chen S (2012) Binding of amelogenin to MMP-9 and their co-expression in developing mouse teeth. J Mol Histol 43(5):473–485
Fjeld K, Kettunen P, Furmanek T, Kvinnsland IH, Luukko K (2005) Dynamic expression of Wnt signaling-related Dickkopf1, -2, and -3 mRNAs in the developing mouse tooth. Dev Dyn 233(1):161–166
Foulkes WD (1995) A tale of four syndromes: familial adenomatous polyposis, Gardner syndrome, attenuated APC and Turcot syndrome. QJM 88(12):853–863
Frierson HF Jr, El-Naggar AK, Welsh JB, Sapinoso LM, Su AI, Cheng J, Saku T, Moskaluk CA, Hampton GM (2002) Large scale molecular analysis identifies genes with altered expression in salivary adenoid cystic carcinoma. Am J Pathol 161(4):1315–1323
Gary C, Schoenwolf SBB, Brauer PR, Francis-West PH (2008) Larsen’s Human Embryology, 4th edn. Churchill Livingstone, London
Hjalt TA, Semina EV, Amendt BA, Murray JC (2000) The Pitx2 protein in mouse development. Dev Dyn 218(1):195–200
Hu X, Zhang S, Chen G, Lin C, Huang Z, Chen Y, Zhang Y (2013) Expression of SHH signaling molecules in the developing human primary dentition. BMC Dev Biol 13:11
Huelsken J, Behrens J (2002) The Wnt signalling pathway. J Cell Sci 115(Pt 21):3977–3978
Itasaki N, Jones CM, Mercurio S, Rowe A, Domingos PM, Smith JC, Krumlauf R (2003) Wise, a context-dependent activator and inhibitor of Wnt signalling. Development 130(18):4295–4305
Jarvinen E, Salazar-Ciudad I, Birchmeier W, Taketo MM, Jernvall J, Thesleff I (2006) Continuous tooth generation in mouse is induced by activated epithelial Wnt/beta-catenin signaling. Proc Natl Acad Sci USA 103(49):18627–18632
Kassai Y, Munne P, Hotta Y, Penttila E, Kavanagh K, Ohbayashi N, Takada S, Thesleff I, Jernvall J, Itoh N (2005) Regulation of mammalian tooth cusp patterning by ectodin. Science 309(5743):2067–2070
Kawabata T, Takahashi K, Sugai M, Murashima-Suginami A, Ando S, Shimizu A, Kosugi S, Sato T, Nishida M, Murakami K, Iizuka T (2005) Polymorphisms in PTCH1 affect the risk of ameloblastoma. J Dent Res 84(9):812–816
Kettunen P, Loes S, Furmanek T, Fjeld K, Kvinnsland IH, Behar O, Yagi T, Fujisawa H, Vainio S, Taniguchi M, Luukko K (2005) Coordination of trigeminal axon navigation and patterning with tooth organ formation: epithelial-mesenchymal interactions, and epithelial Wnt4 and Tgfbeta1 regulate semaphorin 3a expression in the dental mesenchyme. Development 132(2):323–334
Laurikkala J, Kassai Y, Pakkasjarvi L, Thesleff I, Itoh N (2003) Identification of a secreted BMP antagonist, ectodin, integrating BMP, FGF, and SHH signals from the tooth enamel knot. Dev Biol 264(1):91–105
Lei H, Liu H, Ding Y, Ge L (2014) Immunohistochemical localization of Pax6 in the developing tooth germ of mice. J Mol Histol. doi:10.1007/s10735-014-9564-5
Lin D, Huang Y, He F, Gu S, Zhang G, Chen Y, Zhang Y (2007) Expression survey of genes critical for tooth development in the human embryonic tooth germ. Dev Dyn 236(5):1307–1312
Lin M, Li L, Liu C, Liu H, He F, Yan F, Zhang Y, Chen Y (2011) Wnt5a regulates growth, patterning, and odontoblast differentiation of developing mouse tooth. Dev Dyn 240(2):432–440
Liu F, Millar SE (2010) Wnt/beta-catenin signaling in oral tissue development and disease. J Dent Res 89(4):318–330
Liu F, Chu EY, Watt B, Zhang Y, Gallant NM, Andl T, Yang SH, Lu MM, Piccolo S, Schmidt-Ullrich R, Taketo MM, Morrisey EE, Atit R, Dlugosz AA, Millar SE (2008) Wnt/beta-catenin signaling directs multiple stages of tooth morphogenesis. Dev Biol 313(1):210–224
Mikels AJ, Nusse R (2006a) Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol 4(4):e115
Mikels AJ, Nusse R (2006b) Wnts as ligands: processing, secretion and reception. Oncogene 25(57):7461–7468
Millar SE, Koyama E, Reddy ST, Andl T, Gaddapara T, Piddington R, Gibson CW (2003) Over- and ectopic expression of Wnt3 causes progressive loss of ameloblasts in postnatal mouse incisor teeth. Connect Tissue Res 44(Suppl 1):124–129
Mustonen T, Pispa J, Mikkola ML, Pummila M, Kangas AT, Pakkasjarvi L, Jaatinen R, Thesleff I (2003) Stimulation of ectodermal organ development by Ectodysplasin-A1. Dev Biol 259(1):123–136
Niemann S, Zhao C, Pascu F, Stahl U, Aulepp U, Niswander L, Weber JL, Muller U (2004) Homozygous WNT3 mutation causes tetra-amelia in a large consanguineous family. Am J Hum Genet 74(3):558–563
Peng L, Dong G, Xu P, Ren LB, Wang CL, Aragon M, Zhou XD, Ye L (2010a) Expression of Wnt5a in tooth germs and the related signal transduction analysis. Arch Oral Biol 55(2):108–114
Peng L, Ren LB, Dong G, Wang CL, Xu P, Ye L, Zhou XD (2010b) Wnt5a promotes differentiation of human dental papilla cells. Int Endod J 43(5):404–412
Pispa J, Mustonen T, Mikkola ML, Kangas AT, Koppinen P, Lukinmaa PL, Jernvall J, Thesleff I (2004) Tooth patterning and enamel formation can be manipulated by misexpression of TNF receptor Edar. Dev Dyn 231(2):432–440
Rickels MR, Zhang X, Mumm S, Whyte MP (2005) Oropharyngeal skeletal disease accompanying high bone mass and novel LRP5 mutation. J Bone Miner Res 20(5):878–885
Roessler E, Du Y, Glinka A, Dutra A, Niehrs C, Muenke M (2000) The genomic structure, chromosome location, and analysis of the human DKK1 head inducer gene as a candidate for holoprosencephaly. Cytogenet Cell Genet 89(3–4):220–224
Sarkar L, Sharpe PT (1999) Expression of Wnt signalling pathway genes during tooth development. Mech Dev 85(1–2):197–200
Sekine S, Shibata T, Kokubu A, Morishita Y, Noguchi M, Nakanishi Y, Sakamoto M, Hirohashi S (2002) Craniopharyngiomas of adamantinomatous type harbor beta-catenin gene mutations. Am J Pathol 161(6):1997–2001
Sekine S, Sato S, Takata T, Fukuda Y, Ishida T, Kishino M, Shibata T, Kanai Y, Hirohashi S (2003) Beta-catenin mutations are frequent in calcifying odontogenic cysts, but rare in ameloblastomas. Am J Pathol 163(5):1707–1712
Siriwardena BS, Kudo Y, Ogawa I, Tilakaratne WM, Takata T (2009) Aberrant beta-catenin expression and adenomatous polyposis coli gene mutation in ameloblastoma and odontogenic carcinoma. Oral Oncol 45(2):103–108
Tang R, Wang Q, Du J, Yang P, Wang X (2013) Expression and localization of Nell-1 during murine molar development. J Mol Histol 44(2):175–181
van Genderen C, Okamura RM, Farinas I, Quo RG, Parslow TG, Bruhn L, Grosschedl R (1994) Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev 8(22):2691–2703
Wang C, Zhao Y, Su Y, Li R, Lin Y, Zhou X, Ye L (2013) C-Jun N-terminal kinase (JNK) mediates Wnt5a-induced cell motility dependent or independent of RhoA pathway in human dental papilla cells. PLoS One 8(7):e69440
Yang J, Wan C, Nie S, Jian S, Sun Z, Zhang L, Chen Z (2013) Localization of Beclin1 in mouse developing tooth germs: possible implication of the interrelation between autophagy and apoptosis. J Mol Histol 44(6):619–627
Zhang M, Shi J, Huang Y, Lai L (2012) Expression of canonical WNT/beta-CATENIN signaling components in the developing human lung. BMC Dev Biol 12:21
Acknowledgments
We would like to thank the Hospital for Women and Children of Fujian Province for providing aborted human embryonic tissues for this research. This study was supported by Grants from the Natural Science Foundation of China (NSFC) (No. 81200761), Natural Science Foundation of Fujian Province (2011J01146), Doctoral Program of Higher Education of China (20123503120005), Scientific Research Fund of Fujian Provincial Education Department (JA12081), and Excellent Young key Teachers Program of Fujian Normal University (fjsdjk2012077).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, B., Li, H., Liu, Y. et al. Expression patterns of WNT/β-CATENIN signaling molecules during human tooth development. J Mol Hist 45, 487–496 (2014). https://doi.org/10.1007/s10735-014-9572-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-014-9572-5