Abstract
Objectives
Recent studies indicate that high mobility group box protein 1 (HMGB1) can be released by necrotic and damaged cells and functions as an alarmin that is recognized by the innate immune system. Little is known about the role of HMGB1 within the periodontal ligament (PDL). Therefore, we examined HMGB1 expression by PDL cells in vitro and compared the findings to an in vivo model of orthodontically induced tooth root resorption. In addition, we addressed the question of whether a potentially anabolic intermittent administration of parathyroid hormone (iPTH) would modulate the expression of HMGB1.
Materials and methods
In confluent PDL cell cultures, HMGB1 messenger RNA (mRNA) expression was quantified by real-time polymerase chain reaction. In a rat model comprising 25 animals, mechanical loading for 5 days was followed by administration of either iPTH (1–34) systemically or sham injections for up to 56 days. HMGB1 expression was determined by means of immunohistochemistry and histomorphometry.
Results
The in vitro experiments revealed an inhibitory effect of iPTH on basal HMGB1 mRNA expression in confluent PDL cells. In vivo, the mechanical force-induced enhanced HMGB1 protein expression declined time dependently. Intermittent PTH further inhibited HMGB1 expression. The significantly higher basal HMGB1 protein expression in the former compression side was followed by a more pronounced time- and iPTH-dependent decline in the same area.
Conclusions
These data indicate a major role for HMGB1 in the regulation of PDL wound healing following mechanical load-induced tissue injury.
Clinical relevance
The findings point to the potential benefit of iPTH in the attempt to support these immune-associated reparative processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High mobility group box protein 1 (HMGB1) is a nuclear protein that binds DNA and regulates gene expression. It also serves as a multifunctional cytokine that mediates proinflammatory responses, but at the same time promotes wound healing [1, 2]. Outside the cell, HMGB1 can serve as an alarmin to activate the innate immune system and mediate a wide range of physiological and pathological responses. To function as an alarmin in cases of tissue damage or necrosis, HMGB1 translocates from the nucleus of the affected cells to the extracellular milieu or can be actively secreted by immune competent cells, binds to the receptor for advanced glycation end-products and the toll-like receptors 2 and 4 [3], and initiates tissue repair through a mechanism that imitates the necrotic process [4]. Like the RANK/receptor activator of nuclear factor kappa-B ligand (RANKL)/osteoprotegerin (OPG) axis, HMGB1 mediates the interaction of immune cells and alters chemotaxis, proliferation, and the expression of pro-inflammatory cytokines in target cells. Within the bone microenvironment, HMGB1 is chemotactic to osteoclasts and osteoblasts during enchondral ossification, as it is to monocytes and other immune and non-immune cells [5, 6].
Little is known about the role of HMGB1 in the regulation of the metabolism of the tooth supporting apparatus, including the periodontal ligament (PDL) and alveolar bone. Recently, the basal expression of HMGB1 was demonstrated in human PDL cells and a role for HMGB1 as a regulator of alveolar bone resorption was suggested based on the observation that this protein upregulates the expression of pro-inflammatory and osteoclastogenic cytokines such as interleukin (IL)-1ß, Il-6, IL-17, and RANKL [7]. It was reported that gingival crevicular fluid from patients with periodontitis contained HMGB1, whereas fluid from healthy patients did not, suggesting a potential role of HMGB1 protein in disease progression [8, 9]. In addition, human gingival fibroblasts (HGF) can be a source of HMGB1, by both active secretion and passive release, and HMGB1 from HGF may contribute to periodontal tissue destruction [10]. These findings provide evidence for a cross-talk of PDL cells and immune cells under inflammatory conditions with HMGB1 and RANKL representing key players in the control of alveolar bone resorption.
Tooth root resorption frequently occurs as a negative side effect of orthodontic tooth movement and also is a sequelae in the course of necrotic conditions as in periodontitis, after trauma or reimplantation of teeth [11–13]. While the origin of orthodontically induced root resorption has not been fully unraveled yet, several factors have been attributed a certain importance in its pathogenesis, including hereditary predisposition, individual susceptibility, and systemic, local, and anatomical factors associated with orthodontic mechanotherapy. In addition, chronological age, history of trauma, bone density, and humoral or immunological factors were identified as important co-factors [12]. Depending on the extent to which the tooth root is affected, its clinical integrity and, consequently, the prognosis of the tooth might be affected. Fortunately, the destructive process of root resorption is typically followed by a reparative activity mediated by PDL cells [14]. These cells represent a heterogeneous population that hosts several progenitor cell types which can be further induced to differentiate into cementoblasts, osteoblasts, and fibroblasts. A certain percentage of the PDL cells has been attributed stem cell-like properties such as self-renewal and multilineage differentiation potential suggesting an important role of those cells in the regeneration of periodontal tissues [15–21]. Recent experiments have demonstrated an osteoblast-like phenotype of a certain proportion of PDL cells as evidenced by the expression of bone-associated marker proteins and the respond to anabolic factors and hormones in an osteoblast-like manner [22].
Intermittently administered parathyroid hormone (iPTH) has been recognized as an anabolic treatment option in the healing process of bony defects which has led to approval by the US Federal Drug Administration for osteoporosis therapy [23, 24]. Experiments in healthy and estrogen-deficient rats proved its capacity to protect against periodontitis-associated bone loss and to modify the healing process [25, 26]. In a recent study, Bashutski et al. [27] demonstrated the effectiveness of iPTH on the healing process in alveolar and dental structures in humans after periodontal surgery. In a previous work, we demonstrated that PDL cells express the PTH-1-receptor and respond to an intermittent PTH (1–34) administration in an osteoblast-like manner. Changes in the production of osteoprotegerin after intermittent PTH treatment were demonstrated to modify the differentiation and resorptive activity of osteoclasts derived from immune cells [28–30]. These data and reports by others indicate that PDL cells are important regulators of PDL remodeling and within this process the interplay of HMGB1 and PTH regarding PDL metabolism remain to be elucidated.
In the present study, we addressed the regulatory role of HMGB1 in PDL tissue repair in a rat model of orthodontically induced sterile PDL tissue necrosis. We hypothesized that there would be a time-dependent change in HMGB1 expression after the discontinuation of the mechanical loading of the PDL tissue and that these alterations would be modified by an iPTH (1–34) administration. Furthermore, we compared both parameters in the former compression and tension zone of tooth movement in order to analyze the influence of the type of force applied.
Materials and methods
All experimental protocols were reviewed and approved by the ethics committee (reference number 029/08) and the local committee for animal care of the University of Bonn (Germany).
PDL cell collection and characterization
Human PDL cells were collected from the middle third of the roots of premolars of three different human donors aged between 12 and 14 years without pathological periodontal findings. The teeth had been extracted for orthodontic reasons and with written parental consent. Cells were cultured in DMEM containing 10 % fetal bovine serum and 0.5 % antibiotics (diluted from a stock solution containing 5,000 U/ml penicillin and 5,000 U/ml streptomycin; Biochrom AG, Berlin, Germany) and cultured at 37 °C in an atmosphere of 100 % humidity, 5 % CO2, and 95 % air. Prior to experimental use, PDL cells were characterized as described previously and shown to express several mesenchymal marker genes that indicating an osteoblast-like phenotype (data not shown) [31].
In vitro experiments, PTH administration, and real-time PCR
Fifth passage PDL cells were cultured in 24-well plates at a seeding density of 10,000 cells/well. At confluence, cells were exposed to 10−12 M PTH (1–34; Sigma Aldrich, Germany) for 1 h within a 48-h incubation cycle. For the remaining time, experimental media were replaced by regular culture media without PTH (1–34). These cycles were carried out three times resulting in a total experimental period of 6 days to mimic the anabolic effects of iPTH. Vehicle (water)-treated cultures served as controls. At harvest, the PTH (1–34) effect on HMGB1 messenger RNA (mRNA) expression was quantified by real-time polymerase chain reaction (PCR) as described previously [32]. The primer sequences used were as follows: HMGB1 sense 5′ATG GGC AAA GGA GAT CCT AAG AA-3′, antisense 5′ ATC TGC AGC AGT GTT ATT CCA CA-3′. Beta-actin served as the endogenous reference gene. To normalize the content of cDNA samples, the comparative threshold (Ct) cycle method, consisting in the normalization of the number of target gene copies versus the endogenous reference gene GAPDH, was used. For quantification and comparison, the ΔΔCt method was used [33].
Animals
Twenty five 3-month-old male Wistar rats (strain: Rattus norvegicus) with an average body weight of 300 g, obtained from Charles River Laboratories (Sulzfeld, Germany) were stabilized at the animal research facility of the University of Bonn. Rats were housed under specific pathogen-free conditions, in a room with continuously filtered air, maintained between 21 and 22 °C, with 40–60 % humidity on 12 h light and dark cycles and given free access to soft diet and water. Animal body weights were recorded before the onset and at the end of the experiment.
Experimental protocol in vivo
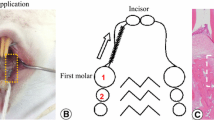
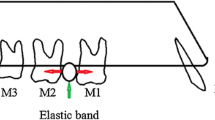
Animals were randomly subdivided into three experimental groups. The first group contained five rats and served as a control for the basal HMGB1 protein expression after the discontinuation of the force. The second and third group comprised 10 rats each and served as experimental groups to analyze the effect of time and iPTH on HMGB1 after 8 and 56 days. As described previously [34], tooth movement was realized by separation of the first and second maxillary molars in both quadrants according to the method described by Waldo and Rothblatt [35], resulting in a mesial movement of the first molar (for illustration, see Scheme 1). This treatment regimen was carried out for 5 days prior to discontinuation of the force. Thereafter, the appliance was removed and five animals of each experimental group received intermittent subcutaneous injections of 5 μg/kg body weight PTH [36] every second day for 8 or 56 days (for illustration, see Scheme 2). The other five animals of each group received sham injections of an equivalent dose of vehicle (water). The observation period up to day 8 after discontinuation of the force images the early phase of repair, whereas day 56 represents the late phase of repair. After the experimental period on days 0, 8, and 56, the animals were anesthetized with isoflurane before euthanasia via cervical dislocation and perfused with phosphate-buffered saline supplemented with 4 % paraformaldehyde for fixation purposes. Afterwards, the maxilla of each animal was dissected, divided in two halves, and prepared for light microscopical examination as recently described [37].
Histology
Before processing specimens for paraffin histology, they were decalcified in neutral 10 % ethylene diamine tetra-acetic acid. For orientation purposes, 5–7 μm serial sagittal sections were prepared and selected sections were stained with hematoxylin and eosin.
Immunohistochemistry
Tissue sections were processed for immunohistochemical detection of HMGB1 protein expression according to previously established protocols [34]. Sections were incubated with a polyclonal primary antibody of rabbit origin raised against a peptide mapping at the carboxy terminus of the protein (anti-HMGB1 s-2399, Epitomics, USA) in a 1:200 working solution of tris buffered saline with bovine serum albumin (TBS/BSA) at 4 °C overnight in a humidified chamber. A 1:100 dilution of a goat antirabbit immunoglobulin (Dako A/S, Denmark) was incubated as secondary antibody for 30 min. Following further rinsing, a PAP complex (1:150 in TBS/BSA; Dako A/S Denmark) was administered for 30 min prior to the visualization of antibody binding with 3,3′-diaminobenzidine (Sigma Chemicals, USA) solution for about 5 min. Thereafter, specimens were counterstained with Mayer’s hematoxylin, dehydrated, and cover-slipped for light microscopical analysis.
In order to prove the specificity of the immunoreactions, negative controls were carried out by (a) omitting the primary antibody or using non-immune IgG instead and (b) omitting both the primary and secondary antibody and using TBS/BSA instead. In addition, pre-adsorption controls, where the antibody was combined with a twofold excess of blocking peptide, were run in order to exclude unspecific binding of the antibodies to unrelated antigens.
Positive controls were carried out with sections of selected tissues carrying significant amounts of the HMGB1 antigen (rat bone) which were treated in the same way as the periodontal sections.
Histomorphometrical analysis
Randomly chosen light microscopical images of defined size (750 × 375 μm) were captured per specimen at the bifurcation of the maxillary first molars (magnification, ×200) in former compression and tension sides (microscope: Axioskope 2 Microscope; camera: Axio-Cam MRC; Carl Zeiss AG, Germany). In each image, the areas resembling PDL were analyzed. The number of immunoreactive cells was counted and calculated as a function of the total cell number with the help of the analyzing software axiovison (Zeiss, Jena, Germany). Extracellular immunoreactivity was not analyzed due to difficulties with quantification of such staining results. All counts were performed by the same investigator who has long experience in the interpretation of histological sections of the PDL and in the application of the computer software. Cells counts were performed automatically by the software after defining the respective thresholds by the researcher. To avoid bias, the investigator was blinded.
Statistical analysis
Reproducibility of the histomorphometrical readouts was ensured by analyzing 15 selected specimens in triplicate. The intraobserver error was demonstrated to be less than 4 %. For any given experiment, each data point represents the mean ± SEM. Each value is the mean ± SEM of five animals per group and three specimens per animal resulting in a total of 15 values per group. Variance and statistical significance of data were analyzed using Bonferroni’s modification of Student’s t test (SigmaStat 3.1, Systat Software, Germany). P values <0.05 were accepted to be significant.
Results
In vitro experiments
Real-time PCR analysis revealed a basal HMGB1 mRNA expression in confluent PDL cell cultures that was down regulated significantly by the chosen iPTH (1–34) regimen (PTH, 0.31 ± 0.16 vs. control; 1.09 ± 0.19; Fig. 1).
Effect of an intermittent 10−12 M PTH (1–34) administration on the regulation of HMGB1 specific mRNA expression in fifth passage, confluent human PDL cells. Vehicle-treated cultures served as controls (vehicle). HMGB1 mRNA expression was determined by realtime PCR. Data were acquired from one of two separate experiments, both yielding comparable results. Each value is the mean + SEM for six independent cultures. *P < 0.05, experimental group vs. vehicle control
In vivo experiments
Effect of mechanical force on the rat periodontium
Directly after discontinuation of the mechanical force, resorption sites could be observed primarily at the mesial surfaces of the alveolar bone. Hyaline zones indicated tissue degradation caused by sterile necrosis. Attachment loss and tooth root resorption occurred predominantly in areas of acellular root cementum. With proceeding time (day 56), hyaline zones were mostly restructured as tissue repair occurred as described before (Figs. 2a and 3a) [34].
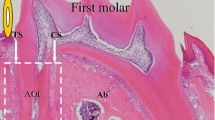
a Upper left panel bifurcation area of the first rat molar with the former compression (CS) and tension side (TS) of orthodontic tooth movement; HE magnification, ×50; other panels: HMGB1 immunohistochemistry at former compression side after the discontinuation of the force at day 0 (d0) for baseline expression and at days 8 and 56 following vehicle treatment (d8v, d56v) or iPTH (1–34) administration (d8PTH, d56PTH); DAB, magnification, ×200; periodontal ligament (PDL), dentin (D), alveolar bone (B). b Histomorphometrical semiquantitative assessment of iPTH (1–34)-induced changes in HMGB1 protein expression in the former compression side after the discontinuation of orthodontic force. Each value is the mean ± SEM for five animals per group and three specimens per animal resulting in a total of 15 values per group. *P < 0.05, ***P < 0.001, experimental group vs. control (day 0); ## P < 0.01, PTH (1–34)-treated group vs. sham-injected animals at the same time point
a Upper left panel: section of the PDL showing the result of a negative control experiment using non-immune IgG instead of the primary antibody; magnification, ×200; other panels: HMGB1 immunohistochemistry at former tension side after the discontinuation of the force at day 0 (d0) for baseline expression and at days 8 and 56 following vehicle treatment (d8v, d56v) or iPTH (1–34) administration (d8PTH, d56PTH); DAB, magnification, ×200; periodontal ligament (PDL), dentin (D), alveolar bone (B). b Histomorphometrical semiquantitative assessment of iPTH (1–34)-induced changes in HMGB1 protein expression in the tension side after the discontinuation of the orthodontic force. Each value is the mean ± SEM for five animals per group and three specimens per animal resulting in a total of 15 values per group. ***P < 0.001, experimental group vs. control at day 0; # P < 0.05, ## P < 0.01, PTH-treated group vs. sham-injected group after the same observation period
HMGB1 protein expression and effects of iPTH (1–34)
In the former compression side, distinct HMGB1 protein expression by PDL cells was observed directly after discontinuation of the orthodontic force, but decreased significantly over time. The majority of the immunoreactive cells could be clearly qualified as fibroblasts by their characteristic spindle-shaped morphology, which is unequivocally distinguishable from cells of the monocyte/macrophage lineage or from epithelial remnants of Malassez (ERM). Vimentin staining of selected tissue sections confirmed this classification (data not shown). ERM were only visible in very few specimens, but those were immunopositive for HMGB1. In the early phase of repair on day 8, HMGB1 immunoreactivity was reduced by 20.54 %. This decrease continued until the late phase of repair on day 56, where only 38 % of the PDL cells were immunopositive (day 0, 51.7 ± 2.06; day 8, 41.08 ± 2.83, day 56, 19.74 ± 2.21).
An iPTH (1–34) administration inhibited HMGB1 protein expression significantly in both, the early and late phase of repair at days 8 and 56 in both, the early phase of periodontal repair at day 8 (vehicle 41.08 ± 2.83 vs. PTH 26.75 ± 3.13) and the late phase at day 56 (vehicle 19.74 ± 2.21 vs. PTH 6.70 ± 1.60) compared to the sham-injected animals at the same time point (Fig. 2a and b).
In the former tension side, similar results were obtained as described for the compression side. Likewise, the initially obvious HMGB1 expression declined significantly over time and iPTH (1–34) further enhanced this effect (day 0, 55.73 ± 0.93; day 8, vehicle 51.33 ± 3.642 vs. PTH 40.79 ± 1.52; day 56, vehicle 24.57 ± 10.21 vs. PTH 11.24 ± 1.87) (Fig. 3a and b).
Comparison of HMGB1 protein expression and the iPTH effect in the former compression vs. tension side
The comparison of the former compression and tension side revealed a higher HMGB1 immunoreactivity in the compression side at all experimental time points in both, the sham-injected and iPTH (1–34)-treated group. In addition, the time- and iPTH-dependent reduction of HMGB1 expression was more pronounced in the former compression side displaying statistical significance only after 8 days as opposed to just a trend after 56 days (Fig. 4a and b).
Comparison of the relative changes in HMGB1 protein expression in the former tension vs. compression side in sham-injected (a) and iPTH (1–34)-treated animals (b). After the discontinuation of the 5-day orthodontic force (day 0), the respective injections were carried out for 8 (day 8) and 56 (day 56) days, respectively. Each value represents the mean ± SEM for five animals per group and three specimens per animal resulting in a total of 15 values per group. *P < 0.05, **P<0.01, tension side vs. compression side at a particular time point
Discussion
The present study demonstrated a basal HMGB1 expression in PDL cells both at the transcriptional and translational level in vitro and in vivo. Furthermore, the in vivo results showed a time-dependent reduction of HMGB1 immunoreactivity in a rat model of orthodontically induced sterile necrosis and subsequent repair and iPTH (1–34) further enhanced this decline.
In a first approach, we proved HMGB1 mRNA expression in PDL cell cultures and observed a significant inhibitory effect of iPTH on the expression level. While the former is in line with previous reports [7], comparable data for the latter only exists for the osteoblastic secretion of HMGB1 that was reported to be influenced by PTH [5].
Thereafter, we wanted to transfer the in vitro findings to an in vivo model in order to examine the physiological significance. The animal model used in this study was first described by Waldo and Rothblatt [35] in order to analyze necrosis and tissue repair processes within the PDL of rats. The insertion of an elastomeric ring between the molars creates zones of compressive and tensile forces within the PDL. Following the discontinuation of the force after 5 days, the typical formation of cell-free hyaline zones can be observed in former compression sides indicating sterile necrosis. Subsequent repair processes have been described at the histological level in detail [34, 37, 38]. Altogether, this model is well-characterized and suitable for the investigation of tissue damage and repair in the PDL. Nevertheless, as discussed previously [34], one might argue that the discontinuation of the mechanical force after 5 days might actually result in a reversal of the force, which means that cells that were exposed to a compressive force initially then would be subjected to a slight tensile force and vice versa. Since the force-induced necrosis on the former compression side results in the absence of cells, this interference might only be of importance for the first days after discontinuation of the force at the tension side, but not for the long-term remodeling and reparative processes.
Our immunohistochemical findings demonstrated a high HMGB1 protein expression by PDL fibroblasts in vivo in response to mechanical loading which corroborates a possible role of HMGB1 as an alarmin indicating tissue damage and as a mediator to enhance acute repair processes [4, 39]. The time-dependent decline of HMGB1 we observed during the experimental period of up to 56 days underlines the importance of the protein primarily for early rather than late repair processes. This interpretation is further substantiated by cell culture experiments with osteoblasts showing the translocation of HMGB1 from the nucleus to the cytoplasm to the extracellular matrix to initiate tissue repair [4, 39, 40]. The significantly stronger decline of HMGB1 protein expression on the former compression side as compared to the tension side within the first 8 days of the repair period might be explained by a principally different reaction of PDL cells to tensional or compressive forces or by differences in the microenvironment at the tissue level as a consequence of widening or narrowing of the periodontal ligament. On the other hand, in the light of the above considerations with the reversal of the force in the former tension side, a longer exposure of those cells to the mechanical force has to be discussed. Consequently, the PDL cells in this area might have released HMGB1 for a prolonged period to initiate the subsequent reparative response. Besides PDL fibroblasts, ERM could be detected sporadically in the specimens investigated and those very few were positive for HMGB1. ERM are discrete clusters of pale cells with an epithelial phenotype interconnected by desmosomes and ensheathed by a basal lamina that arise from fragmentation of the Hertwig’s root sheath [41]. Although the exact function of ERM remains to be elucidated, they might very well contribute to the regulation of bone remodeling during orthodontic tooth movement as suggested by their cytokine, growth factor, and extracellular matrix-degrading proteinase expression profile [42–46]. However, the small number of ERMs did not allow for a quantitative analysis and comparison between control and iPTH-treated specimens. Unpublished data from our lab indicates that ERMs do not express the PTH-1-receptor, which is in line with a lack of evidence in the literature, indicating that iPTH most probably cannot affect HMGB1 expression in ERM.
Given the stimulatory role of HMGB1 on the resorbing activity of osteoclasts, as evidenced by an inhibition of bone resorption and enhanced bone mineral density in RAGE knockout mice [6], the increased expression of the protein after the discontinuation of the force in our experiments indicates the HMGB1-mediated initiation of the removal of the necrotic tissue. In support of this interpretation, the principled involvement of cells of the monocyte/macrophage lineage in the clearance of cellular debris in the early phase of repair following orthodontic tooth movement was shown previously, e.g., by ED-1, ED-2, and TRAP immunostaining [38, 47]. In the present investigation, cells of the monocyte/macrophage lineage stained positively for CD68 sporadically (data not shown), but the small size and number of the immunoreactive cells and the potential influence of the sectional plane did not allow for further quantification regarding differences between the control and iPTH specimens. Apart from its action as a proinflammatory cytokine, HMGB1 was found to exert chemotactic effects on skin fibroblasts and keratinocytes in vitro and accelerated wound healing in diabetic mice [2]. In another study, HMGB1 also stimulated wound healing by enhancing 3 T3 fibroblast proliferation and migration [1]. Thus, in our model of orthodontically induced tissue injury, HMGB1 might deliver dual benefit by enhancing the activity of cells of the monocyte/macrophage lineage to clear the cellular debris in the hyalinized zones on the one hand and at the same time act as a chemoattractant and proliferative signal for PDL cells to promote wound healing processes.
The necessity of HMGB1 for the RANKL induced differentiation of osteoclast precursors was demonstrated both in vitro and in vivo [6]. Kim et al. reported on HMGB1-mediated periodontal bone resorption through the modulation of osteoclastogenic cytokine levels, including RANKL, in human PDL cells [7]. In another study, Yang et al. linked HMGB1 to an enhanced RANKL/OPG steady state mRNA ratio in osteoblastic bone marrow stroma cells [6]. Those findings provide a reasonable basis for the connection of the present findings to our recent data on the role of RANKL and OPG in the regulation of PDL repair processes which were obtained in the same animal model [34]. In those experiments, an increase of RANKL in the early phase of repair, which was interpreted to serve the activation of osteoclasts and, subsequently, the removal of necrotic tissue, was followed by a shift of the OPG/RANKL balance in favor of tissue formation in the late phase of repair. These events were positively influenced by iPTH (1–34). The apparent interplay of these regulatory proteins allows for the conclusion that, in our experiments, HMGB-1 itself and the HMGB1-induced stimulation of RANKL expression in the early phase of repair enhance the removal of cellular debris. With increasing time, the decline in HMGB1 expression is linked to a downregulation of RANKL and a simultaneous increase of the OPG/RANKL ratio indicating the onset of the reparative phase which could be demonstrated histologically. Both events, the inhibition of HMGB1 and the increase of the OPG/RANKL ratio were enhanced by iPTH (1–34). Consequently, the iPTH (1–34) induced inhibition of HMGB1 immunoreactivity in our model, which could appear disadvantageous at first sight, might be counterbalanced by iPTH (1–34) stimulation of the OPG/RANKL ratio in support of wound healing events. Support for this conclusion comes from the field of osteoimmunology, where a tight link of regulatory processes between stroma and immune cells (particularly T cells) has been established as crucial prerequisite for the mediation of the effect of PTH. This link apparently involves components of the RANK/RANKL/OPG system as well as HMGB1 as key regulators [48, 49].
In summary, our results indicate a regulatory role for HMGB1 in the response of PDL cells to tissue damage induced by mechanical loading and in the initiation of the subsequent repair processes. The mapping of intercellular cytokine signaling networks that functionally couple immune and osseous tissues provides potential clinical targets for immune associated regenerative periodontal treatment regimens. The intermittent administration of PTH (1–34) seems to be a promising strategy to support these approaches.
References
Ranzato E, Patrone M, Pedrazzi M et al (2010) Hmgb1 promotes wound healing of 3 T3 mouse fibroblasts via RAGE-dependent ERK1/2 activation. Cell Biochem Biophys 57:9–17
Straino S, Di Carlo A, Mangoni A et al (2008) High-mobility group box 1 protein in human and murine skin: involvement in wound healing. J Invest Dermatol 128:1545–1553
Dumitriu IE, Baruah P, Valentinis B et al (2005) Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J Immunol 174:7506–7515
Ulloa L, Messmer D (2006) High-mobility group box 1 (HMGB1) protein: friend and foe. Cytokine Growth Factor Rev 17:189–201
Charoonpatrapong K, Shah R, Robling AG et al (2006) HMGB1 expression and release by bone cells. J Cell Physiol 207:480–490
Yang J, Shah R, Robling AG et al (2008) HMGB1 is a bone-active cytokine. J Cell Physiol 214:730–739
Kim YS, Lee YM, Park JS et al (2010) SIRT1 modulates high-mobility group box 1-induced osteoclastogenic cytokines in human periodontal ligament cells. J Cell Biochem 111:1310–1320
Morimoto Y, Kawahara KI, Tancharoen S et al (2008) Tumor necrosis factor-alpha stimulates gingival epithelial cells to release high mobility-group box 1. J Periodontal Res 43:76–83
Ebe N, Hara-Yokoyama M, Iwasaki K et al (2011) Pocket epithelium in the pathological setting for HMGB1 release. J Dent Res 90:235–240
Feghali K, Iwasaki K, Tanaka K et al (2009) Human gingival fibroblasts release high-mobility group box-1 protein through active and passive pathways. Oral Microbiol Immunol 24:292–298
Brezniak N, Wasserstein A (2002) Orthodontically induced inflammatory root resorption. Part II: the clinical aspects. Angle Orthod 72:180–184
Krishnan V, Davidovitch Z (2006) Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofacial Orthop 129(469):e1–e32
Taithongchai R, Sookkorn K, Killiany D (1996) Facial and dentoalveolar structure and the prediction of apical root shortening. Am J Orthod Dentofacial Orthop 110:296–302
Owman-Moll P, Kurol J (1998) The early reparative process of orthodontically induced root resorption in adolescents—location and type of tissue. Eur J Orthod 20:727–732
Chen SC, Marino V, Gronthos S et al (2006) Location of putative stem cells in human periodontal ligament. J Periodontal Res 41:547–553
Chou AM, Sae-Lim V, Lim T et al (2002) Culturing and characterization of human periodontal ligament fibroblasts—a preliminary study. Mater Sci Eng 20:77–83
Nagatomo K, Komaki M, Sekiya I et al (2006) Stem cell properties of human periodontal ligament cells. J Periodontal Res 41:303–310
Seo BM, Miura M, Gronthos S et al (2004) Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364:149–155
Trubiani O, Di Primio R, Traini T et al (2005) Morphological and cytofluorimetric analysis of adult mesenchymal stem cells expanded ex vivo from periodontal ligament. Int J Immunopathol Pharmacol 18:213–221
Nohutcu RM, Somerman MJ, McCauley LK (1995) Dexamethasone enhances the effects of parathyroid hormone on human periodontal ligament cells in vitro. Calcif Tissue Int 56:571–577
Ouyang H, McCauley LK, Berry JE et al (2000) Response of immortalized murine cementoblasts/periodontal ligament cells to parathyroid hormone and parathyroid hormone-related protein in vitro. Arch Oral Biol 45:293–303
Lekic PC, Rajshankar D, Chen H et al (2001) Transplantation of labeled periodontal ligament cells promotes regeneration of alveolar bone. Anat Rec 262:193–202
Sone T, Fukunaga M, Ono S et al (1995) A small dose of human parathyroid hormone (1–34) increased bone mass in the lumbar vertebrae in patients with senile osteoporosis. Miner Electrolyte Metab 21:232–235
Verhaar HJ, Lems WF (2010) PTH analogues and osteoporotic fractures. Expert Opin Biol Ther 10:1387–1394
Barros SP, Silva MA, Somerman MJ et al (2003) Parathyroid hormone protects against periodontitis-associated bone loss. J Dent Res 82:791–795
Marques MR, da Silva MA, Manzi FR et al (2005) Effect of intermittent PTH administration in the periodontitis-associated bone loss in ovariectomized rats. Arch Oral Biol 50:421–429
Bashutski JD, Eber RM, Kinney JS et al (2010) Teriparatide and osseous regeneration in the oral cavity. N Engl J Med 363:2396–2405
Lossdorfer S, Gotz W, Jager A (2005) PTH (1–34) affects osteoprotegerin production in human PDL cells in vitro. J Dent Res 84:634–638
Lossdorfer S, Gotz W, Rath-Deschner B et al (2006) Parathyroid hormone (1–34) mediates proliferative and apoptotic signaling in human periodontal ligament cells in vitro via protein kinase C-dependent and protein kinase A-dependent pathways. Cell Tissue Res 325:469–479
Lossdorfer S, Gotz W, Jager A (2011) PTH (1–34)-induced changes in RANKL and OPG expression by human PDL cells modify osteoclast biology in a co-culture model with RAW 264.7 cells. Clin Oral Investig 15:941–952
Lossdörfer S, Kraus D, Abuduwali N et al (2011) Intermittent PTH(1–34) regulates the osteoblastic differentiation of human periodontal ligament cells via protein kinase C- and protein kinase A-dependent pathways in vitro. J Periodontal Res 46:318–326
Lossdorfer S, Kraus D, Jager A (2010) Aging affects the phenotypic characteristics of human periodontal ligament cells and the cellular response to hormonal stimulation in vitro. J Periodontal Res 45:764–771
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25:402–408
Lossdorfer S, Yildiz F, Gotz W et al (2010) Anabolic effect of intermittent PTH (1–34) on the local microenvironment during the late phase of periodontal repair in a rat model of tooth root resorption. Clin Oral Investig 14:89–98
Waldo CM, Rothblatt JM (1954) Histologic response to tooth movement in the laboratory rat; procedure and preliminary observations. J Dent Res 33:481–486
Sato M, Vahle J, Schmidt A et al (2002) Abnormal bone architecture and biomechanical properties with near-lifetime treatment of rats with PTH. Endocrinology 143:3230–3242
Gotz W, Kunert D, Zhang D et al (2006) Insulin-like growth factor system components in the periodontium during tooth root resorption and early repair processes in the rat. Eur J Oral Sci 114:318–327
Jager A, Kunert D, Friesen T et al (2008) Cellular and extracellular factors in early root resorption repair in the rat. Eur J Orthod 30:336–345
Bianchi ME (2007) DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 81:1–5
Savill J, Dransfield I, Gregory C et al (2002) A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol 2:965–975
Gotz W, Lossdorfer S, Kruger U et al (2003) Immunohistochemical localization of insulin-like growth factor-II and its binding protein-6 in human epithelial cells of Malassez. Eur J Oral Sci 111:26–33
Meghji S, Qureshi W, Henderson B et al (1996) The role of endotoxin and cytokines in the pathogenesis of odontogenic cysts. Arch Oral Biol 41:523–531
Liu F, Abiko Y, Nishimura M et al (2001) Expression of inflammatory cytokines and beta-defensin 1 mRNAs in porcine epithelial rests of Malassez in vitro. Med Electron Microsc 34:174–178
Ohshima M, Nishiyama T, Tokunaga K et al (2000) Profiles of cytokine expression in radicular cyst-lining epithelium examined by RT-PCR. J Oral Sci 42:239–246
Kale S, Kocadereli I, Atilla P et al (2004) Comparison of the effects of 1,25 dihydroxycholecalciferol and prostaglandin E2 on orthodontic tooth movement. Am J Orthod Dentofacial Orthop 125:607–614
Rincon JC, Young WG, Bartold PM (2006) The epithelial cell rests of Malassez—a role in periodontal regeneration? J Periodontal Res 41:245–252
Jager A, Radlanski RJ, Gotz W (1993) Demonstration of cells of the mononuclear phagocyte lineage in the periodontium following experimental tooth movement in the rat. An immunohistochemical study using monoclonal antibodies ED1 und ED2 on paraffin-embedded tissues. Histochemistry 100:161–166
Pacifici R (2010) T cells: critical bone regulators in health and disease. Bone 47:461–471
Pacifici R (2010) The immune system and bone. Arch Biochem Biophys 503:41–53
Acknowledgments
The authors thank Mrs. Schaffrath for expert technical assistance and the German Research Foundation (DFG) as well as the Medical Faculty of the University of Bonn for providing a research grant (KFO 208, TP8, LO-1181/2-2). The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
M. Wolf and S. Lossdörfer contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wolf, M., Lossdörfer, S., Abuduwali, N. et al. Potential role of high mobility group box protein 1 and intermittent PTH (1–34) in periodontal tissue repair following orthodontic tooth movement in rats. Clin Oral Invest 17, 989–997 (2013). https://doi.org/10.1007/s00784-012-0777-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-012-0777-2