Abstract
Epithelial rests of Malassez (ERM), the only odontogenic epithelial structures in periodontal tissue, are proposed to correlate with root resorption, but the detailed mechanism remains unclear. Osteoprotegerin (OPG), the main inhibitor of osteoclastogenesis, plays a pivotal role in inhibiting root resorption, and ERM cells express OPG mRNA in vitro. Thus, in this study, we aimed to clarify OPG expression in ERM in vivo and to explore the role of OPG in ERM to determine whether ERM are associated with root resorption via OPG. We established Opg-knockout (Opg-KO) mice and detected the OPG expression in ERM by immunohistochemical staining in 4-, 6-, 10-, 26- and 52-week-old mice. The ERM of wild-type (WT) mice and Opg-KO mice were evaluated histologically at 4, 10 and 26 weeks of age. Orthodontic root resorption models were established, maxillae were collected after 4 weeks, and ERM were analysed by histomorphometric analysis. In our study, OPG displayed sustained expression in ERM, and OPG deficiency caused the destruction of ERM, characterized by irregular morphology and reduced numbers. Moreover, after orthodontic treatment, the loss of OPG severely damaged ERM, aggravating root resorption. Together, our results demonstrated that ERM expressed the OPG protein in vivo and that OPG deficiency resulted in morphological and quantitative damage to ERM. Furthermore, ERM may be associated with root resorption via OPG, thus helping to explain the mechanism underlying root resorption.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epithelial rests of Malassez (ERM) are the only odontogenic epithelial structures in the periodontium throughout adult life. During the development of the dental root, Hertwig’s epithelial root sheath (HERS) disintegrates into clusters and remains in the periodontium as ERM (Huang et al. 2009; Yamamoto et al. 2015). ERM are located near the cementum mainly in the furcation and cervical region of the periodontium with a net-like distribution around the entire root (Rincon et al. 2006). ERM are not useless clusters, but are involved in the maintenance of periodontium homeostasis, including maintaining the periodontal ligament space and preventing ankylosis (Xiong et al. 2013). In addition, as specific structures around the dental root, ERM are vital elements that help maintain root integrity and are correlated with the prevention of root resorption and cementum repair (Cordeiro et al. 2011; Kat et al. 2003; Bille et al. 2009). Fewer ERM are present in primary teeth than in permanent teeth, a phenomenon that might be associated with a higher risk of root resorption in primary teeth (Cordeiro et al. 2011). Nevertheless, the precise mechanism by which ERM protect against root resorption remains to be elucidated.
Osteoprotegerin (OPG), a secreted glycoprotein belonging to the tumour necrosis factor receptor (TNFR) superfamily, is widely distributed in oral tissue and is involved in the development of tooth germ, hard tissue mineralization, tooth eruption, and specifically protection against root resorption (Darcey and Qualtrough 2013; Ohazama et al. 2004; Wise 2009). OPG is generally thought to inhibit osteoclastogenesis during root resorption via the OPG/receptor activator of nuclear factor kappa-B ligand (RANKL)/receptor activator of nuclear factor kappa-B (RANK) pathway, damaging the sealing zone of the osteoclasts or inducing the apoptosis of osteoclasts and osteoclast precursor cells (Walsh and Choi 2014; Song et al. 2014; Liu et al. 2015). Recently, it was proposed that there are powerful protective factors around the dental root, including the cementum, periodontal ligament and epithelial rests. The special structure and remodelling ability exert significant protective effects on the root (Xiong et al. 2013; Arzate et al. 2015; Sokos et al. 2015). Many studies have focused on the protective role of OPG in the periodontal ligament and cementum against root resorption. Periodontal ligament cells isolated from non-resorbing deciduous teeth express abundant OPG but not RANKL, while cells from resorbing deciduous teeth predominantly express RANKL but decreased OPG, supporting the inhibitory effects of OPG in physiological root resorption (Fukushima et al. 2003). Moreover, our recent work demonstrated that Opg-knockout (Opg-KO) mice developed early-onset root resorption due to a reduction in cementum mineralization (Liu et al. 2016). However, little is known about how OPG affects ERM, the only odontogenic epithelial structure around the root in the periodontium.
ERM cells express OPG mRNA in vitro (Mizuno et al. 2005). Thus, it is worth determining the effects of OPG on ERM and further discussing their potential correlation with root resorption. Therefore, our present study aimed to investigate the expression and role of OPG in ERM and determine whether ERM have a relationship with root resorption via OPG. We clarified OPG protein expression in ERM in vivo and found that the loss of OPG damaged ERM morphology and quantity, accelerating root resorption. These results suggested that OPG deficiency destroyed ERM and that this destruction was related to root resorption.
Materials and methods
Animals
Wild-type (WT) (C57BL/6J background) and Opg-KO mice were provided by the Shanghai Research Center for Biomodel Organisms (Shanghai, China). They were maintained under standard conditions with a 12-h light and dark cycle at 25 °C and were provided with sufficient food and water. Opg-KO mice were genotyped by polymerase chain reaction (PCR) (Zhang et al. 2016). The animals were sacrificed at 4, 6, 10, 26 and 52 weeks, and the mandibles were separated. Six animals were provided for each phenotype at each age. All experimental procedures were approved by the Animal Use and Care Committee of Tongji University, and the animal experiment approval number was TJmed-013-31.
Orthodontic tooth movement
Eight-week-old WT mice and Opg-KO mice were used for orthodontic treatment. Orthodontic forces were delivered by coiled springs (wire size 0.1 mm; diameter 1 mm) with a force of approximately 20 g. Springs were tied between the left maxillary first molar and incisors with a ligature wire (diameter 0.2 mm). The animals were sacrificed after 4 weeks. The maxillae were separated—the left served as experimental groups, while the right served as internal controls. Nine animals were provided per phenotype, including six animals for histological evaluation and three animals for micro-computed tomography (μCT) analysis.
Tissue processing and H&E staining
The samples were fixed in 4% paraformaldehyde (PFA) (pH 7.4; Sangon Biotech, Shanghai, China) at 4 °C for 24 h. After decalcification in 10% ethylene diamine tetraacetic acid solution (EDTA) (pH 7.4; Sangon Biotech, Shanghai, China), samples were embedded in paraffin (Sigma, USA) and sectioned sagittally and transversely. Each section was sliced to a thickness of 4 μm and prepared for staining. After deparaffinization and rehydration, the sections were stained with haematoxylin and eosin (H&E) (Biotech Well, Shanghai, China) according to standard protocols.
Immunohistochemistry and immunofluorescence
Immunohistochemistry was performed using an UltraSitive S-P detection kit (MXB, Fuzhou, China). After deparaffinization and rehydration, the sections were incubated in hyaluronidase (Sigma, USA) at 37 °C for 1 h and with peroxidase blockers at room temperature for 10 min. After washing with PBS (Biotech Well, Shanghai, China), the sections were incubated with goat serum to prevent non-specific antibody binding. Next, the sections were incubated with primary antibody at 4 °C overnight. After washing in PBS, the sections were treated with biotinylated secondary antibodies for 10 min and washed in PBS. Colour reactions were then visualized using the DAB system (MXB, Fuzhou, China). For immunofluorescence, the sections were treated with goat serum after antigen retrieval. Immunolabelling was then performed at 4 °C overnight. After washing with PBS, the sections were incubated with secondary antibodies labelled with fluorescence for 1 h. After staining with 4′,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich, St. Louis, MO), the specimens were observed under a fluorescence microscope (Nikon Eclipse 80i, Japan). ERM in the furcation and cervical regions were compared. The antibodies used included rabbit anti-cytokeratin 19 (CK19) (dilution 1:500; Proteintech, USA), rabbit anti-OPG (dilution 1:200; Abcam, USA), mouse anti-cytokeratin 14 (CK14) (dilution 1:300; Abcam, USA), rabbit anti-desmoplakin (DSP) (dilution 1:300; GeneTex, USA), rabbit anti-PCNA (dilution 1:400; Abcam, USA), rabbit anti-caspase 9 (dilution 1:200; CST, USA), goat anti-mouse IgG (dilution 1:1000; Alexa Fluor 488; Abcam, USA) and goat anti-rabbit IgG (dilution 1:1000; Alexa Fluor 594; Abcam, USA).
Masson staining and reconstruction
Serial sections were stained with Masson staining (KeyGEN, Shanghai, China) according to standard protocols. The sections were consecutively stained with haematoxylin for 5 min, Ponceau 2R for 5 min, phosphomolybdic acid for 1 min and light green for 5 min. Images of serial sections were obtained under a microscope and reconstructed using VGstudio.
Tartrate-resistant acid phosphatase (TRAP) staining
A TRAP staining kit (Sigma-Aldrich, St Louis, MO) was used with the following detailed protocol. After deparaffinization and rehydration, sections were incubated in a substrate solution (naphthol AS-BI phosphoric acid solution, fast garnet GBC base solution, tartrate solution, acetate solution, sodium nitrite solution and citrate solution) at 37 °C for 30 min. After washing in PBS, sections were stained with methyl green (Sigma-Aldrich, St. Louis, MO) for 5 min.
Microcomputed tomography
The maxillae of mice after orthodontic tooth movement were fixed in 4% PFA for 24 h and digested in 0.4% collagenase I (Sigma-Aldrich, St. Louis, MO) for 48 h. Next, the first molars were separated carefully with a stereoscopic microscope (Stemi 508; Carl Zeiss, Jena, Germany) and analysed using a microcomputed tomography-50 system (Scanco Medical, Bassersdorf, Switzerland). The scanning accuracy was 10 μm.
Statistical analysis
Eight sections were selected from each specimen for use in statistical analysis, which was performed using SPSS 20.0 software. The results were presented as the mean ± SD, determined using Student’s t test. Significance was achieved at p < 0.05.
Results
Expression of OPG in ERM in WT mice
To explore the effects of OPG on ERM, we first clarified the expression of OPG in ERM in WT mice. Using serial sections and immunohistochemistry, ERM were observed to be stained with CK19, one of the cytoskeletal proteins characteristic of epithelial cells, and OPG displayed a sustained expression pattern in CK19-positive ERM in WT mice. In 4- and 6-week-old mice, ERM exhibited small clusters that only contained several cells, and the OPG expression was strong. With increasing age, although the ERM clusters grew larger with more cells, OPG expression was weaker at 10, 26 and 52 weeks (Fig. 1).
Irregular morphology of ERM in Opg-KO mice
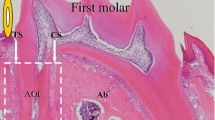
To investigate the role of OPG in ERM, we observed changes in ERM in Opg-KO mice. In WT mice, CK19-positive clusters were present around the entire root at 4 weeks (Fig. 2). However, with increasing age, the ERM were mainly embedded in the furcation and cervical regions of the periodontium, and the distribution of ERM in Opg-KO mice was similar to that in WT mice (Fig. 2, Supplementary Fig. 1). However, the morphology was different. In WT mice, the ERM always showed tight cell connections at 4, 10 and 26 weeks (Fig. 3a1–c1). Although the clusters exhibited no obvious differences in Opg-KO mice and WT mice at 4 and 10 weeks (Fig. 3a1, b1, d1, e1), apparent loose epithelial cells were observed in Opg-KO mice but not in WT mice at 26 weeks (Fig. 3c1, f1). In addition, according to ERM reconstruction visualized by Masson staining, the observed cells presented a cobblestone-like appearance in WT mice at 26 weeks (Fig. 3a3). However, in Opg-KO mice, the cells showed an elongated shape, and the cell connections were no longer compact (Fig. 3b3). The epithelial cell connections in ERM mainly consisted of desmosomes (Suzuki et al. 2006). DSP, an important component of the desmosome, was no longer clearly visualized in Opg-KO mice compared with WT mice at 26 weeks (Fig. 3b2, e2). In addition, the cell shape became elongated and not polygonal, as observed by detecting the immunofluorescence of CK14, a type of epithelial cytoskeletal protein (Fig. 3a2, d2).
Morphological damage to ERM in Opg-KO mice. a1–f1 The morphology of ERM in Opg-KO mice was the same as that in WT mice at 4 and 10 weeks, but the cell connection became loose at 26 weeks. a2–f2 CK14, a type of epithelial cytoskeletal protein, and DSP, an important protein of the desmosome, were expressed more disorganized in Opg-KO mice than in WT mice at 26 weeks. a3–b3 Masson staining and reconstruction revealed cobblestone-like cells and impacted cell connections in WT mice at 26 weeks, but the cell shape became elongated in Opg-KO mice. D dentin, PDL periodontal ligament, P pulp
Decreased number of ERM in Opg-KO mice
In addition to the irregular morphology of ERM, we found that the number of ERM was reduced in Opg-KO mice at 10 and 26 weeks, and there was a significant difference between the mean number of clusters in the two types of mice (p < 0.05) (Fig. 4a). To account for the decreased number of the clusters, we detected the proliferation and apoptosis of epithelial cells. The number of PCNA-positive cells contained in the clusters was decreased in Opg-KO mice at 10 and 26 weeks (Fig. 4b), while the expression of caspase-9 in ERM was stronger (Fig. 4c). OPG has been proposed to play a protective role against root resorption (Darcey and Qualtrough 2013). Given these findings, we sought to investigate the correlation between ERM and root resorption after the loss of OPG.
Quantitative changes in ERM in Opg-KO mice. a ERM numbers (red arrow) were reduced in Opg-KO mice at 10 and 26 weeks compared with those in WT mice. b PCNA-positive epithelial cells in ERM (red circle) were decreased in Opg-KO mice at 10 and 26 weeks. c Caspase-9 expression in ERM (red circle) was weaker in Opg-KO mice at 10 and 26 weeks. The data were analysed as the mean ± SD, *p < 0.05. D dentin, PDL periodontal ligament, P pulp. (Color figure online)
Notable root resorption associated with dramatic destruction of ERM in Opg-KO mice after orthodontic treatment
We found that OPG deficiency caused damage to ERM morphology and quantity. To further investigate whether this destruction was related to root resorption via OPG, orthodontic root resorption models in WT and Opg-KO mice were established because orthodontic force is one of the common clinical causes of root resorption (Wishney 2017). The OPG expression was detected in the experiment groups both in WT and Opg-KO mice (Fig. 5a). Using μCT and H&E staining, abundant absorptive lesions were observed on the root surface in Opg-KO mice 4 weeks after orthodontic tooth movement, and the defect was much more severe than that in WT mice (Fig. 5b, c). In addition, increased numbers of TRAP-positive cells were found in the resorption lacuna on the surface of the cementum in Opg-KO mice compared to those in WT mice (Fig. 5d). Interestingly, a loose connection between epithelial cells was observed in Opg-KO mice after treatment, but there was no visible morphological change in the ERM of WT mice (Fig. 6a). Moreover, the number of ERM was decreased in both WT mice and Opg-KO mice after treatment, but ERM were specifically reduced in Opg-KO mice compared with WT mice after treatment (Fig. 6b, c).
Root resorption with destroyed ERM 4 weeks after orthodontic treatment. a The OPG protein was expressed in CK19-positive clusters in WT mice but not in Opg-KO mice after treatment. b μCT revealed abundant pits (yellow arrow) on the surface of the dental root in Opg-KO mice compared with that in WT mice after orthodontic tooth movement. c The root exhibited much more defects (black arrow) with H&E staining in Opg-KO mice than in WT mice after orthodontic tooth movement. d TRAP-positive cells (black arrow) were increased after orthodontic treatment, specifically in Opg-KO mice. D dentin, PDL periodontal ligament, P pulp. (Color figure online)
Destroyed ERM in Opg-KO mice 4 weeks after orthodontic treatment. a ERM cell connections were loose in Opg-KO mice but not in WT mice after treatment. b, c ERM numbers (red arrow) were reduced after treatment and the numbers reduced much more in Opg-KO mice than that in WT mice. The data were analysed as the mean ± SD, *p < 0.05. D dentin, PDL periodontal ligament. (Color figure online)
Discussion
OPG, an inhibitor of osteoclastogenesis, plays an important role in protecting the dental root (Tyrovola et al. 2008). Many scholars have focused on the role of OPG in the inhibition of odontoclasts or its protective functions against root resorption in the periodontal ligament and cementum (Iglesias-Linares and Hartsfield 2017). However, the effects of OPG on ERM, the only epithelial structure present in the periodontium throughout adult life, remain unclear. Therefore, it is necessary to investigate the potential role of OPG in ERM. In our study, OPG deficiency destroyed ERM, enhancing orthodontic root resorption. Our data indicated that OPG loss resulted in morphological and quantitative damage to ERM and that ERM might be relevant to root resorption via OPG.
Our recent study showed that OPG played a vital role in preventing root resorption and cementum destruction (Liu et al. 2016). Interestingly, in this study, OPG displayed a sustained expression pattern in ERM, which was consistent with mRNA levels (Mizuno et al. 2005). OPG is widely expressed in some epithelial cells, such as mammary epithelial cells in breast cancer, and plays a role in cell survival, proliferation, migration and apoptosis (Weichhaus et al. 2015; Goswami and Sharma-Walia 2016). We suggest that OPG may exert an effect on ERM. Our study showed that OPG loss caused morphological and quantitative damage to ERM. In terms of morphological changes, ERM were no longer regular in Opg-KO mice. The expression of CK14, a type of cytoskeletal protein in epithelial cells, was altered in ERM in Opg-KO mice. The cytoskeleton of most animal cells consists of a meshwork of three types of filaments—microfilaments, microtubules and intermediate filaments (IFs)—and contain cytokeratins (Geisler and Leube 2016; Pierozan and Pessoa-Pureur 2017). Previous studies have shown that OPG affects the sealing zone, which is the cytoskeletal structure unique to mature osteoclasts, as well as actin fibres and microtubules of endothelial cells. Thus, cell morphologies were changed, and the skeletal changes are associated with the activation of Src, FAK and ERK1/2 (Liu et al. 2015; Kobayashi-Sakamoto et al. 2010). However, the mechanism by which OPG affects cytokeratin must to be further discussed.
In addition, we observed reduced numbers of ERM clusters in Opg-KO mice, characterized by decreased numbers of proliferative cells but stronger expression of apoptosis-related proteins. As a soluble decoy receptor for TNF-related apoptosis-inducing ligand (TRAIL), OPG inhibits the binding of TRAIL homotrimers to TRAIL-R1 and TRAIL-R2 on the surface of target cells, preventing TRAIL-induced apoptosis (Bernardi et al. 2016). Moreover, a previous study showed that intra-tibial tumours from MCF-7 cells overexpressing OPG demonstrated increased numbers of Ki67-positive cells, and the proliferation of human mammary epithelial cells was significantly increased in the presence of recombinant human OPG (Weichhaus et al. 2015; Goswami and Sharma-Walia 2015). Overall, our study found that OPG affected ERM, which might further help to explain the correlation between ERM and root resorption.
Root resorption of permanent teeth is the pathological loss of dentin or cementum and may lead to tooth mobility or even tooth loss (Ahangari et al. 2010). Protecting against root resorption and illuminating the underlying mechanisms are essential. In addition to the periodontal ligament and cementum, ERM might be associated with protection against root resorption (Xiong et al. 2013). In this study, absorptive lesions and TRAP-positive cells on the surface of the cementum were accompanied by decreased ERM after orthodontic treatment. Similarly, another study showed that physiological root resorption was associated with a loss of continuity in the ERM network and the incursion of blood vessels (Kat et al. 2003). In addition, the number of osteoclasts on both the root and alveolar bone surface significantly increased with the disappearance of ERM after denervation of the inferior alveolar nerve, suggesting a potential inhibitory role for ERM osteoclast activation (Fujiyama et al. 2004). In the current study, we also found that OPG loss destroyed ERM, and more defects and TRAP-positive cells appeared on the root surface after orthodontic treatment. Thus, ERM might have a relationship with root resorption via OPG.
Root resorption is a common complication of orthodontic tooth movement, and orthodontic force is the main cause (Feller et al. 2016). Tooth movement is achieved by bone remodeling when an appropriate orthodontic force is applied (Sun et al. 2017; Wang et al. 2017; Gu et al. 2017), but heavy orthodontic forces lead to increased numbers of odontoclasts (Krishnan 2017). The force was found to affect ERM cells in vitro (Koshihara et al. 2010), which may help to explain the association of root resorption with reduced numbers of ERM after orthodontic treatment in WT mice in our study, supporting the correlation between root resorption and ERM. Additionally, we observed notable root resorption with damaged ERM in Opg-KO mice after treatment, suggesting a role for OPG in root resorption. Furthermore, other cells such as periodontal ligament cells express OPG in the oral cavity (Wada et al. 2001). Periodontal ligament remodeling is essential during orthodontic tooth movement (Cui et al. 2016; Fu et al. 2016), and orthodontic root resorption is closely related to injury and necrosis of the periodontal ligament (Krishnan 2017). OPG expression has been shown to decrease in periodontal ligament cells with severe orthodontically induced root resorption (Yamaguchi et al. 2006). Although our study focused on the correlation between ERM and root resorption, orthodontic root resorption is not only associated with ERM. However, the precise relationship between ERM and root resorption and the specific role of OPG requires further study.
In summary, we clarified the expression of OPG in ERM in vivo and found that OPG deficiency resulted in morphological and quantitative damage in ERM. ERM might be associated with root resorption via OPG. These data may help to elucidate the mechanisms of root resorption and provide new insights into the protection of the dental root.
References
Ahangari Z, Nasser M, Mahdian M, Fedorowicz Z, Marchesan MA (2010) Interventions for the management of external root resorption. Cochrane Database Syst Rev 16:CD008003. https://doi.org/10.1002/14651858.CD008003.pub2
Arzate H, Zeichner-David M, Mercado-Celis G (2015) Cementum proteins: role in cementogenesis, biomineralization, periodontium formation and regeneration. Periodontol 2000 67:211–233. https://doi.org/10.1111/prd.12062
Bernardi S, Bossi F, Toffoli B, Fabris B (2016) Roles and clinical applications of OPG and TRAIL as biomarkers in cardiovascular disease. Biomed Res Int. https://doi.org/10.1155/2016/1752854
Bille ML, Nolting D, Kjær I (2009) Immunohistochemical studies of the periodontal membrane in primary teeth. Acta Odontol Scand 67:382–387. https://doi.org/10.1080/00016350903160589
Cordeiro MM, Santos BZ, Reyes-Carmona JF, Figueiredo CP (2011) Primary teeth show less protecting factors against root resorption. Int J Paediatr Dent 21:361–368. https://doi.org/10.1111/j.1365-263X.2011.01132.x
Cui J, Li J, Wang W, Han X, Du J, Sun J, Feng W, Liu B, Liu H, Amizuka N, Li M (2016) The effect of calcitriol on high mobility group box 1 expression in periodontal ligament cells during orthodontic tooth movement in rats. J Mol Histol 47:221–228. https://doi.org/10.1007/s10735-016-9669-0
Darcey J, Qualtrough A (2013) Resorption: part 1. Pathology, classification and aetiology. Br Dent J 214:439–451. https://doi.org/10.1038/sj.bdj.2013.431
Feller L, Khammissa RA, Thomadakis G, Fourie J, Lemmer J (2016) Apical external root resorption and repair in orthodontic tooth movement: biological events. Biomed Res Int. https://doi.org/10.1155/2016/4864195
Fu HD, Wang BK, Wan ZQ, Lin H, Chang ML, Han GL (2016) Wnt5a mediated canonical Wnt signaling pathway activation in orthodontic tooth movement: possible role in the tension force-induced bone formation. J Mol Histol 47:455–466. https://doi.org/10.1007/s10735-016-9687-y
Fujiyama K, Yamashiro T, Fukunaga T, Balam TA, Zheng L, Takano-Yamamoto T (2004) Denervation resulting in dento-alveolar ankylosis associated with decreased Malassez epithelium. J Dent Res 83:625–629
Fukushima H, Kajiya H, Takada K, Okamoto F, Okabe K (2003) Expression and role of RANKL in periodontal ligament cells during physiological root-resorption in human deciduous teeth. Eur J Oral Sci 111:346–352
Geisler F, Leube RE (2016) Epithelial intermediate filaments: guardians against microbial infection? Cells. https://doi.org/10.3390/cells5030029
Goswami S, Sharma-Walia N (2015) Osteoprotegerin secreted by inflammatory and invasive breast cancer cells induces aneuploidy, cell proliferation and angiogenesis. BMC Cancer 15:935. https://doi.org/10.1186/s12885-015-1837-1
Goswami S, Sharma-Walia N (2016) Osteoprotegerin rich tumor microenvironment: implications in breast cancer. Oncotarget 7:42777–42791. https://doi.org/10.18632/oncotarget.8658
Gu Q, Guo S, Wang D, Zhou T, Wang L, Wang Z, Ma J (2017) Effect of corticision on orthodontic tooth movement in a rat model as assessed by RNA sequencing. J Mol Histol 48:199–208. https://doi.org/10.1007/s10735-017-9718-3
Huang X, Bringas P Jr, Slavkin HC, Chai Y (2009) Fate of HERS during tooth root development. Dev Biol 334:22–30. https://doi.org/10.1016/j.ydbio.2009.06.034
Iglesias-Linares A, Hartsfield JK Jr (2017) Cellular and molecular pathways leading to external root resorption. J Dent Res 96:145–152. https://doi.org/10.1177/0022034516677539
Kat PS, Sampson WJ, Wilson DF, Wiebkin OW (2003) Distribution of the epithelial rests of Malassez and their relationship to blood vessels of the periodontal ligament during rat tooth development. Aust Orthod J 19:77–86
Kobayashi-Sakamoto M, Isogai E, Holen I (2010) Osteoprotegerin induces cytoskeletal reorganization and activates FAK, Src, and ERK signaling in endothelial cells. Eur J Haematol 85:26–35. https://doi.org/10.1111/j.1600-0609.2010.01446.x
Koshihara T, Matsuzaka K, Sato T, Inoue T (2010) Effect of stretching force on the cells of epithelial rests of malassez in vitro. Int J Dent. https://doi.org/10.1155/2010/458408
Krishnan V (2017) Root resorption with orthodontic mechanics: pertinent areas revisited. Aust Dent J 62 Suppl 1:71–77. https://doi.org/10.1111/adj.12483
Liu W, Xu C, Zhao H, Xia P, Song R, Gu J, Liu X, Bian J, Yuan Y, Liu Z (2015) Osteoprotegerin induces apoptosis of osteoclasts and osteoclast precursor cells via the Fas/Fas ligand pathway. PLoS ONE 10:e0142519. https://doi.org/10.1371/journal.pone.0142519
Liu Y, Du H, Wang Y, Liu M, Deng S, Fan L, Zhang L, Sun Y, Zhang Q (2016) Osteoprotegerin-knockout mice developed early onset root resorption. J Endod 42:1516–1522. https://doi.org/10.1016/j.joen.2016.07.008
Mizuno N, Shiba H, Mouri Y, Xu W, Kudoh S, Kawaguchi H, Kurihara H (2005) Characterization of epithelial cells derived from periodontal ligament by gene expression patterns of bone-related and enamel proteins. Cell Biol Int 29:111–117
Ohazama A, Courtney JM, Sharpe PT (2004) Opg, rank, and rankl in tooth development: co-ordination of odontogenesis and osteogenesis. J Dent Res 83:241–244
Pierozan P, Pessoa-Pureur R (2017) Cytoskeleton as a target of quinolinic acid neurotoxicity: insight from animal models. Mol Neurobiol. https://doi.org/10.1007/s12035-017-0654-8
Rincon JC, Young WG, Bartold PM (2006) The epithelial cell rests of Malassez—a role in periodontal regeneration? J Periodontal Res 41:245–252
Sokos D, Everts V, de Vries TJ (2015) Role of periodontal ligament fibroblasts in osteoclastogenesis: a review. J Periodontal Res 50:152–159. https://doi.org/10.1111/jre.12197
Song R, Gu J, Liu X, Zhu J, Wang Q, Gao Q, Zhang J, Cheng L, Tong X, Qi X, Yuan Y, Liu Z (2014) Inhibition of osteoclast bone resorption activity through osteoprotegerin-induced damage of the sealing zone. Int J Mol Med 34:856–862. https://doi.org/10.3892/ijmm.2014.1846
Sun J, Du J, Feng W, Lu B, Liu H, Guo J, Amizuka N, Li M (2017) Histological evidence that metformin reverses the adverse effects of diabetes on orthodontic tooth movement in rats. J Mol Histol 48:73–81. https://doi.org/10.1007/s10735-016-9707-y
Suzuki M, Matsuzaka K, Yamada S, Shimono M, Abiko Y, Inoue T (2006) Morphology of Malassez’s epithelial rest-like cells in the cementum: transmission electronmicroscopy, immunohistochemical, and TdT-mediated dUTP-biotin nick end labeling studies. J Periodontal Res 4:280–287
Tyrovola JB, Spyropoulos MN, Makou M, Perrea D (2008) Root resorption and the OPG/RANKL/RANK system: a mini review. J Oral Sci 50:367–376
Wada N, Maeda H, Tanabe K, Tsuda E, Yano K, Nakamuta H, Akamine A (2001) Periodontal ligament cells secrete the factor that inhibits osteoclastic differentiation and function: the factor is osteoprotegerin/osteoclastogenesis inhibitory factor. J Periodontal Res 36:56–63
Walsh MC, Choi Y (2014) Biology of the RANKL–RANK–OPG system in immunity, bone, and beyond. Front Immunol 5:511. https://doi.org/10.3389/fimmu.2014.00511
Wang C, Gu W, Sun B, Zhang Y, Ji Y, Xu X, Wen Y (2017) CTHRC1 promotes osteogenic differentiation of periodontal ligament stem cells by regulating TAZ. J Mol Histol 48:311–319. https://doi.org/10.1007/s10735-017-9729-0
Weichhaus M, Chung ST, Connelly L (2015) Osteoprotegerin in breast cancer: beyond bone remodeling. Mol Cancer 14:117. https://doi.org/10.1186/s12943-015-0390-5
Wise GE (2009) Cellular and molecular basis of tooth eruption. Orthod Craniofac Res 12:67–73. https://doi.org/10.1111/j.1601-6343.2009.01439.x
Wishney M (2017) Potential risks of orthodontic therapy: a critical review and conceptual framework. Aust Dent J 62(Suppl 1):86–96. https://doi.org/10.1111/adj.12486
Xiong J, Gronthos S, Bartold PM (2013) Role of the epithelial cell rests of Malassez in the development, maintenance and regeneration of periodontal ligament tissues. Periodontol 63:217–233. https://doi.org/10.1111/prd.12023
Yamaguchi M, Aihara N, Kojima T, Kasai K (2006) RANKL increase in compressed periodontal ligament cells from root resorption. J Dent Res 85:751–756
Yamamoto T, Yamada T, Yamamoto T, Hasegawa T, Hongo H, Oda K, Amizuka N (2015) Hertwig’s epithelial root sheath fate during initial cellular cementogenesis in rat molars. Acta Histochem Cytochem 48:95–101. https://doi.org/10.1267/ahc.15006
Zhang L, Liu M, Zhou X, Liu Y, Jing B, Wang X, Zhang Q, Sun Y (2016) Role of osteoprotegerin (OPG) in bone marrow adipogenesis. Cell Physiol Biochem 40:681–692
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (Grant Nos: 81570966, 81371141).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10735_2018_9771_MOESM1_ESM.tif
Supplementary material 1. Fig. 1. The magnification of the ERM distribution in WT and Opg-KO mice. a The distribution of ERM (yellow arrow) in the cervical region of the periodontium. b The distribution of ERM (yellow arrow) in the furcation region of the periodontium. D dentin, P pulp (TIF 70970 KB)
10735_2018_9771_MOESM2_ESM.tif
Supplementary material 2. Fig. 2. The IgG control for the expression of OPG protein in WT mice. D dentin, PDL periodontal ligament, D dentin, PDL periodontal ligament (TIF 46835 KB)
Rights and permissions
About this article
Cite this article
Wang, Y., Liu, M., Deng, S. et al. Osteoprotegerin deficiency causes morphological and quantitative damage in epithelial rests of Malassez. J Mol Hist 49, 329–338 (2018). https://doi.org/10.1007/s10735-018-9771-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-018-9771-6