Abstract
The mitigating effects of melatonin (MT) treatment on salt-stressed seed germination capacity and of MT pretreatment (including the whole period of seed germination and seedling cultivation) on the salt tolerance of two rice (Oryza sativa L. ssp. japonica) cultivars, Liaojing 4 (LJ4, salt tolerant) and Nipponbare (Nipp, salt sensitive), and the related physiological and molecular events were investigated in this study. The results showed that when additional MT solutions (10–500 μM) were added, the NaCl-decreased seed germination potential (GP), germination index (GI) and vigor index (VI) of LJ4 and Nipp were clearly restored. When MT pretreatment occurred during the period of seed germination and seedling cultivation prior to NaCl stress, relative electrolytic leakage in roots and leaves clearly decreased and thus restored the root vigor and growth of both plants. This could be fulfilled by multiple physiological mechanisms. For example, improving seed germination ability (GP, GI and VI), strengthening root vigor, reducing Na+ and Cl− contents in roots and leaves (especially for Cl− in roots and Na+ in leaves), and enhancing the activities of antioxidant enzymes (such as catalase and superoxide dismutase) in roots and leaves resulted in a decrease in H2O2 level. Moreover, the reduced contents of Na+ and Cl− in the roots and leaves of both salt-stressed rice plants under MT pretreatment displayed clear relations with the enhanced transcription of OsSOS1 in roots and of OsCLC1 and OsCLC2 in roots and leaves. These results could indicate that soaking with MT during seed germination and/or root application of MT at the seedling stage are very simple operations that require small doses and can effectively solve the problems of low germination rate and poor seedling establishment in saline soils. Therefore, these results provide a theoretical basis and technical support for the chemical regulation of salt tolerance and cultivation practices of rice and other crops in saline areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Melatonin (N-acetyl-5-methoxy tryptamine) is an indoleamine-type hormone that is produced by the pineal gland in animals and regulates sleep-wake cycles (Hardeland et al. 2012). Melatonin was first discovered in plants in 1995 and shares a common biosynthetic route and similar physiological functions as the first discovered plant hormone (IAA); melatonin has been documented to be a potential growth regulator that enhances plant tolerance to many kinds of adverse environmental stresses, including salt (Li et al. 2012; Jiang et al. 2016; Zhang et al. 2014), drought (Wang et al. 2013; Meng et al. 2014; Ye et al. 2016), cold (Li et al. 2017), heavy metal (Li et al. 2016a; Hasan et al. 2015), and oxidative stresses (Szafránska et al. 2016), and delays leaf senescence (Liang et al. 2015) under both exogenous application and endogenous induction (Liang et al. 2017). In recent years, research on melatonin effects on plant growth, development and stress adaptation has received much attention, especially regarding the physiological and molecular mechanisms in plants exposed to many kinds of abiotic and biotic stress factors; tremendous and exciting progress has been obtained. It has been demonstrated that melatonin as a potent free radical scavenger and a broad-spectrum antioxidant (Zhang and Zhang 2014). Melatonin can effectively scavenge or reduce endogenous H2O2 (Tan et al. 2000) and regulate gene transcription levels related to antioxidant systems, thus alleviating salt injury to crops (Shi et al. 2015; Zhang et al. 2014). The specific physiological mechanisms mainly include improving seed germination ability under salt stress (Zhang et al. 2014); suppressing chlorophyll degradation, which enhances the photosynthetic rate (Wang et al. 2013; Li et al. 2012; Liang et al. 2015); strengthening the activities of antioxidant enzymes (e.g., CAT, SOD, peroxidase [POD], ascorbate peroxidase [APX], glutathione reductase [GR]) to achieve reactive oxygen species (ROS) detoxification; and, most importantly, regulating the uptake and transport of Na+, Cl− and K+ for ion homeostasis (Zhang et al. 2014; Jiang et al. 2016).
Soil salinity is one of the most important abiotic stresses threatening sustainable food production. Soil salinity poses a major constraint for many crop plants, and the vast majority of crop plants are glycophytes or are salt sensitive; therefore, increasing the salt tolerance of crops for biological improvement and comprehensive exploitation of saline soils is becoming the most crucial approaches for scientific research and agricultural practices (Munns and Tester 2008; Zhang et al. 2011; Himabindu et al. 2016). It is well documented that growth inhibition, development reduction, and yield decline of salt-affected crop plants mainly result from osmotic stress, ionic (including Na+ and Cl−) toxicity, nutritional imbalance, and oxidative damage. Certainly, plants have evolved different strategies for protection against salinity, including the synthesis of osmolytes or osmoprotectants; ion compartmentation at the intracellular, tissue or organic levels; the enhancement of enzymatic or nonenzymatic antioxidant systems; and changes in hormone levels and hormone-guided communications (Munns and Tester 2008; Kim et al. 2014; Muchate et al. 2016; Park et al. 2016). Ion transport is the fundamental factor determining salt tolerance in plants, and the ability to both regulate Na+ and Cl− uptake in roots and transport these ions to shoots is crucial for plant adaptations to saline environments (Munns and Tester 2008). As is known, Na+ efflux across the plasma membrane is attributed to the Salt Overly Sensitive1 (SOS1) Na+/H+ antiporter (Zhao et al. 2017), and Cl− uptake, transport and compartmentation are mediated by chloride channels (CLCs), which are widely distributed in cell membrane systems (mainly in the endomembranes) of prokaryotic and eukaryotic organisms (Wei et al. 2016). The pathways for improving plant stress tolerance may include stress hardening, chemical regulation and genetic engineering/breeding; however, plant breeding is time consuming and labor intensive (Tian et al. 2014; Hanin et al. 2016). In addition, the improvement of crop salt tolerance through chemical regulation measures has the advantages of shorter cycles, lower costs, and faster effects compared to the breeding of salt-tolerant cultivars. Many studies have shown that exogenous application of various plant growth regulators (such as glutathione, polyamines, γ-aminobutyric acid, and methanol), compatible solutes or osmoprotectants (such as proline, betaines, trehalose, and polyamines) can clearly improve the salt tolerance of plants or crops; this improvement is mainly fulfilled by increased contents of both osmolytes and antioxidants and enhanced activity of antioxidant enzymes (Li et al. 2012; Teh et al. 2015; Nahar et al. 2016; Wei et al. 2015a, b; Jia et al. 2017).
Rice (Oryza sativa L.) is an important staple food crop of more than half of the world population and is well known to be a salt-sensitive crop (Kumar et al. 2013; Teh et al. 2015; Li et al. 2016b). Seed germination and seedling establishment are the most crucial stages in the life cycle of crop plants and are also the most fragile and vulnerable stages to harsh environments. High salinity during seed germination and early seedling growth can directly affect crop establishment, even resulting in entire crop failure or severe loss in yield (Zhang et al. 2014; Basnet et al. 2015; Chen et al. 2012). Liang et al. (2015) reported that melatonin treatment resulted in enhanced antioxidant protection and significantly enhanced he salt stress tolerance of rice seedlings by reducing chlorophyll degradation and suppressing the transcripts of senescence-associated genes. Generally, high salinity will result in serious Na+ and Cl− accumulations, marked K+ deficit, and disturbed K+/Na+ ratios in plant cells (Munns and Tester 2008). Proton pumps, ion channels and transporters play a vital role in the regulatory process of ion homeostasis in plants under salt stress (Sun et al. 2009; Yamaguchi et al. 2013). SOS1 is responsible for encoding a plasma membrane-localized Na+/H+ antiporter that is important in sodium extrusion, controlling long-distance Na+ transport from roots to shoots, oxidative stress responses, and intracellular pH homeostasis and is considered a superior salt tolerance determinant (Shi et al. 2002; Nie et al. 2015; Zhao et al. 2017). Rice OsSOS1 demonstrated the ability for Na+/H+ exchange in plasma membrane vesicles of yeast cells by reducing the net cellular Na+ content of those cells and suppressing the salt sensitivity of a sos1-1 mutant of Arabidopsis (Martínez-Atienza et al. 2007).
At present, OsCLC1 and OsCLC2 are the most reported CLC members in rice (Oryza sativa L. ssp. japonica cv. Nipponbare). OsCLC1 is expressed in most tissues, and promoted by salt treatment and shows more positive relation to salt tolerance due to transporting less Cl− to leaves, while OsCLC2 is expressed only in roots, nodes, internodes and leaf sheaths; however, both genes could rescue the growth phenotype of the yeast CLC gene mutant (ρgef1) under various ion stresses. The growth of loss-of-function rice mutants of both genes produced by the insertion of a retrotransposon (Tos17) was inhibited at all life stages (Nakamura et al. 2006; Diédhiou and Golldack 2006). However, to date, the related physiological and molecular bases of the effects of exogenously applied melatonin on rice seed germination under salt stress, seedling quality and salt tolerance and the maintenance of ion homeostasis in salt-stressed rice plants have not been reported. In this work, two rice (Oryza sativa L. ssp. japonica) cultivars, Liaojing 4 (LJ4) and Nipponbare (Nipp), that have different salt tolerances were chosen as the experimental materials. The effects of seed soaking with different concentrations of MT solutions on seed germination and seedling quality under salt stress were investigated. Changes in plant growth characteristics (including the damage rate); H2O2 content; antioxidant enzyme activity; the contents of Na+, K+ and Cl−; and the transcription patterns of the OsSOS1, OsCLC1, and OsCLC2 genes in the roots and leaves of rice plants pretreated with MT solutions during the whole period of seed germination and seedling culture were also analyzed. The objectives of this work were to uncover the physiological and molecular mechanisms of the homeostasis maintenance of salt ions and ROS during the process of exogenous melatonin application for enhancing rice seed germination and seedling growth under salt stress and to provide both a theoretical basis and technical support for effectively solving the problems of low germination rate and poor seedling vigor of rice in saline soils and of the chemical regulation and planting practices of rice and other crops in saline areas.
Materials and methods
Plant material and culture
Rice (Oryza sativa L. ssp. japonica) materials included cultivars Liaojing 4 (LJ4) and Nipponbare (Nipp). Seeds of similar size were surface-sterilized with 75% ethanol (W/V) for 1 min, after which they were sterilized with 0.1% HgCl2 (W/V) for 5 min followed by washing three times with distilled water for subsequent experiments during the seed germination and young seedling stages.
Experimental design 1: comparison of the salt tolerance of two rice cultivars
This experiment was conducted during the seed germination and young seedling stages. For seed germination, two salt treatments (150 and 200 mM NaCl) were established, and distilled water was used as a control. Sterilized rice seeds (cultivars LJ4 and Nipp) were soaked in distilled water for 8 h, after which they were transferred to Petri dishes (each with 15 seeds and 3 replicates) containing water-saturated filter papers and either 10 mL of treatment solution or distilled water to germinate in a constant-temperature incubator (28 ± 1) °C in dark conditions. The treatment solution was renewed every 2 days. Seed germination potential (GP) was measured at 4 days, and shoot length, germination index (GI), and vigor index (VI) were measured at 7 days. For the seedling stage, the sterilized and soaked rice seeds were sown in plastic containers containing double gauze and then germinated in a constant-temperature incubator (28 ± 1 °C) in dark conditions. The germinated seeds were transferred to a foam plate (each with 25 holes for uniform seedlings) covering a plastic cup containing 1/2 Kimura B nutrition solution (Liang et al. 2015) and maintained in a greenhouse. The greenhouse temperature was maintained at 26 ± 2/20 ± 2 °C (day/night), and the photoperiod was approximately 12/12 h (day/night). When rice seedlings grew to the 2-leaf/1-heart stage, they were randomly separated into 3 groups. The first group was grown continuously in 1/2 Kimura solution (control), the second was treated with 1/2 Kimura solution plus 100 mM NaCl, and the last was treated with 1/2 Kimura solution plus 120 mM NaCl. After 7 days of treatment, the morphological characteristics of rice plants were observed, imaged, and sampled for measurements of both fresh weight per plant and relative electrolyte leakage (REL) in the roots and leaves.
Experimental design 2: ameliorative effects of MT on salt injury to rice
This experiment was also conducted during the seed germination and young seedling stages. For seed germination, treatments including 120 mM NaCl plus 0, 10, 50, 100, 200, or 500 μM MT solutions were established together with distilled water as a control, and the seeds of both cultivars were germinated as described above. GP was measured at 4 days or 7 days later; the seedlings were then imaged and assayed for GI and VI. For the seedling stage, both rice seeds were soaked, germinated and cultivated at the 2-leaf/1-heart stage with different concentrations (0, 25, 50, 75 or 100 μM) of MT solutions. The seedlings were then stressed with 120 mM NaCl at 7 days and imaged. The plants pretreated with 75 μM MT were used as representatives to further analyze fresh weight; root vigor; REL of roots and leaves; H2O2 content; CAT and SOD activities; and Na+, K+ and Cl− contents. In addition, the transcriptional patterns of OsSLC1, OsCLC1 and OsCLC2 genes were compared in the roots and leaves of LJ4 and Nipp seedlings (including no MT pretreatment prior to NaCl stress, denoted as “NaCl”, and MT pretreatment prior to NaCl stress, denoted as “MT + NaCl”) within the 0, 3, 6, 12 and 24 h salt treatments.
Seed germination assays
Seeds were considered germinated when their shoot length was half of the seed length. The percentage of germinated seeds at 4 days was referred to as GP, and the germinated seeds were calculated at 7 days for GI and VI according to the method of Li et al. (2015) as follows: GI = ∑Gt/t, where Gt is the number of germinated seeds on Day t. VI was calculated as follows: VI = GI × S, where S is the shoot length of germinated seeds. Shoot length was directly measured using a ruler.
Seedling assays
LJ4 and Nipp seedlings (ten each) were selected randomly and fully rinsed in distilled water, after which they were dried with blotting paper for measurements of fresh weight per plant. REL in the roots and leaves of LJ4 and Nipp plants was assayed according to the method described by Hu et al. (2016). Root vigor was determined according to the triphenyltetrazolium chloride (TTC) method (Wang et al. 2012). H2O2 content was measured following the method of Cao et al. (2016). Specifically, root or leaf tissues (0.2 g) were homogenized with 2 mL of 0.1% (w/v) trichloroacetic acid (TCA) in an ice bath and then centrifuged at 12,000×g for 15 min at 4 °C. Then, 0.5 mL of the supernatant was added to 0.5 mL of 10 mM phosphate buffer (pH 7.0) and 1 mL of 1 M KI. The absorbance of the mixture was then read at 390 nm. Finally, the content of H2O2 was calculated using a standard curve. For assays of CAT and SOD activities, fresh rice roots or leaves (0.2 g) were homogenized using a mortar and pestle with 2 mL of 50 mM ice-cold phosphate buffer (pH 7.0) containing both 1 mM EDTA·Na2 and 0.5% (W/V) PVP. The homogenate was centrifuged at 15,000×g for 15 min at 4 °C. The supernatant was used as an enzyme extract for enzymatic activity, which was measured at 4 °C according to the methods of our previous study (Cao et al. 2016). Briefly, CAT activity was assayed spectrophotometrically at 240 nm in a 3-mL reaction mixture containing 0.1 mL of enzyme extract, 100 mM phosphate buffer (pH 7.0), 0.1 μM EDTA, and 0.1% H2O2. The decomposition of H2O2 was measured by following the decrease in absorbance at 240 nm for 3 min and quantified by its molar extinction coefficient (39.4 mmol L−1 cm−1). One unit of CAT activity was defined as a change in absorbance of 0.1 units min−1 caused by the addition of the enzyme extract (Qiu et al. 2014). SOD activity was assayed using the photochemical nitroblue tetrazolium (NBT) method (Li et al. 2011). The reaction mixture contained 100 mM phosphate buffer (pH 7.8), 130 mM methionine, 750 μM NBT, 20 μM riboflavin, 0.1 mM EDTA·Na2, 505 μL of deionized water, and 80 μL of enzyme extract in a 3-mL volume. One unit of SOD activity was defined as the amount of enzyme required to cause 50% inhibition of NBT reduction monitored at 560 nm. Extractions and assays of Na+, K+ and Cl− were performed according to our previous methods (Wei et al. 2015a, b; Zhou and Yu 2009).
RNA extraction and analysis of OsCLC1, OsCLC2 and OsSOS1 gene expression
Gene-specific primers for OsCLC1, OsCLC2 and OsSOS1 were designed using Primer Premier software (ver. 5.0) as follows: OsCLC1-F: 5′-CTACGTACGGGCGCATAGTT-3′; OsCLC1-R: 5′-ACGAGCATGCCAAAGGGAG-3′; OsCLC2-F: 5′-ATCGAGAGCCTCGACTACGA-3′; OsCLC2-R: 5′-ACCTGCACTTTCCCTTTAGCA-3′; OsSOS1-F: 5′-CATTCGTATCTGGGCTAA-3′; and OsSOS1-R: 5′-CATTTCTTGATTTGGTGTA-3′. Total RNAs were isolated from the frozen roots or leaves of LJ4 and Nipp plants using a TRIzol reagent kit and were used for the synthesis of cDNA based on the Hieff™ First Strand cDNA synthesis Super Mix for RT-qPCR (YEASEN, Shanghai, China). The rice actin gene (AK100267) with the forward and reverse primers 5′-AGTGTCTGGATTGGAGGAT-3′ and 5′-TCTTGGCTTAGCATTCTTG-3′, respectively, was used as an internal reference. qRT-PCR was performed with 384-well plates using the QuantStudio 5 Real-Time PCR System (ThermoFisher Scientific China, Inc., Shanghai), where 0.4 µL of primers (1 μM each), 50 ng of prepared cDNA (2 μL), 7.2 μL of ddH2O, and 10 μL of SYBR mix were combined and brought to a final volume of 20 μL per well. PCR cycles were set up as follows: 95 °C for 5 min, 95 °C for 10 s, and 58 °C for 20 s, with a total of 40 amplification cycles. The gene relative expression levels were normalized and calibrated according to the 2−ΔΔCT method (Schmittgen and Livak 2008), with the rice actin gene as the internal reference, and the results were presented as the means ± SDs of three replicates.
Statistical analysis
All data were analyzed and presented as the means ± SDs for each treatment (n = 3; n = 10 for plant fresh weight) using SPSS software (ver. 20.0). The data were subjected to the one-way analysis of variance (ANOVA), and the mean differences were compared using Duncan’s test (P < 0.05).
Results
Comparison of salt tolerance in rice cultivars LJ4 and Nipp
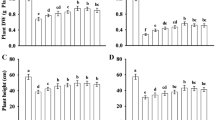
When treated with 150 and 200 mM NaCl for 4 days or 7 days, respectively, the seed germination potential (GP) and shoot length of rice cultivars LJ4 and Nipp were significantly less than those of the untreated controls; moreover, the reduction ranges of GP under the 200 mM NaCl treatment or the shoot length of Nipp under the 150 mM NaCl treatment were clearly greater than those of LJ4 (P < 0.05) (Fig. 1A, B). When two rice seedlings (at the 2-leaf/1-heart stage) were treated with 100 mM NaCl for 7 days, only shorter plant height but no visible yellow leaves were displayed compared to the control; however, after treatment with 120 mM NaCl for 7 days, leaves clearly became visibly yellow, especially for Nipp. At the same time, the fresh weight per plant of LJ4 and Nipp seedlings and REL in roots and leaves were significantly lower and higher than those of the control, respectively, especially under 120 mM NaCl stress; the reduction range of fresh weight per plant or the increase range of REL the in roots or leaves of Nipp showed significant (P < 0.05) differences compared with those of LJ4 (Fig. 1C, D). Thus, salt tolerance of LJ4 is stronger than that of Nipp at both the seed germination and young seedling stages; these plants were respectively used as salt-tolerant and the salt-sensitive plants for the subsequent studies.
Comparison of salt tolerance in rice cultivars LJ4 and Nipp at the stages of seed germination (A GP, B shoot length) and young seedlings (C fresh weight per plant, D relative electrolytic leakage-REL). Means in bars followed by different letters show significant difference (P < 0.05), and asterisk represents significant level (P < 0.05) between the parameters of two rice cultivars under the treatment of same NaCl concentration
Effects of MT treatment on seed germination of LJ4 and Nipp under salt stress
Under 120 mM NaCl stress, the seed germination of LJ4 and Nipp was markedly inhibited; GP, GI and VI significantly decreased compared with those of the non-treated controls, and effects on Nipp were generally greater than on LJ4. When treated with 120 mM NaCl plus additional 10–500 μM MT, the reduced seed GP, GI and VI of Nipp and LJ4 clearly recovered, and GP and GI were almost restored to control levels (P > 0.05). Further analysis showed that the recovery effect of MT treatments with different concentrations (10–500 μM) on aforementioned seed germination parameters did not work in a concentration-dependent manner (Fig. 2). Therefore, in order to save costs, we may consider selecting lower concentrations of MT for research on the alleviation mechanisms of MT regarding rice salt damage or the practical application of MT. It is just for this reason that in following seedling trials we adopted lower concentrations (25–100 μM) of MT and observed morphological effects on salt-stressed rice plants, and we focused on the analysis of physiological and molecular mechanisms using the 75 μM MT treatment.
Effects of MT treatment on seed germination of (A germination appearance at 7 days, B seed germination potential-GP, C seed germination index-GI, D seed vigor index-VI) of rice cultivars LJ4 and Nipp under salt stress. Means in bars followed by different letters show significant difference (P < 0.05)
Effects of MT pretreatment on growth, root vigor and REL in the roots and leaves of salt-stressed rice seedlings
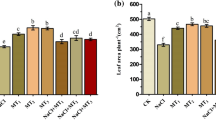
Regarding treatment with MT at different concentrations (0–100 μM), rice seeds of cultivars LJ4 and Nipp were soaked, germinated and cultivated to seedlings at the 2-leaf/1-heart stage. Then, following stress with 120 mM NaCl for 7 days, the growth of both rice seedlings treated with no MT (0 μM NaCl) was clearly suppressed. The leaves partially became yellow along with significantly reduced plant fresh weight and root vigor. REL in the roots and leaves increased (P < 0.05) compared with those of the control, while seedlings cultivated with 25, 50, 75 and 100 μM MT were clearly mitigated from salt injury (Fig. 3A). Further analysis of salt-stressed plants pretreated with 75 μM MT showed that fresh weight per plant and root vigor evidently increased and that REL values in the roots and leaves significantly decreased, although there was still an obvious gap with the control values (Fig. 3B–D). These results are also very consistent with the mitigating effects of MT application on salt damage symptoms in rice plants.
Effects of MT pretreatment on growth phenotype (A), fresh weight per plant (B), root vigor (C) and relative electrolytic leakage (REL) in roots and leaves (D) of rice cultivars LJ4 and Nipp seedlings under salt stress. Means in bars followed by different letters show significant difference (P < 0.05)
Effects of MT pretreatment on H2O2 content, CAT and SOD activities in the roots and leaves of salt-stressed rice seedlings
When LJ4 and Nipp seedlings with no MT pretreatment were stressed with 120 mM NaCl for 7 days, the H2O2 contents in roots and leaves significantly increased (P < 0.05) compared with those of the control, and the increase in leaves was more obvious. However, when LJ4 and Nipp seedlings pretreated with 75 μM MT were exposed to salt stress, H2O2 contents in roots and leaves clearly decreased compared to those of seedlings without MT pretreatment; however, under 120 mM NaCl for 7 days, the decrease in leaves was more apparent (Fig. 4A). We also investigated the changes in the activity of two representative antioxidant enzymes, CAT and SOD, in both salt-stressed rice seedlings with or without MT pretreatment. We found that NaCl treatment could result in different degrees of enhancement effects on CAT and SOD activities in the roots and leaves of both rice seedlings. Also, MT pretreatment could cause subsequent positive synergistic effects, and the activity of these enzymes in the leaves of LJ4 was more distinct (Fig. 4B, C).
Effects of MT pretreatment on the contents of Na+, K+ and Cl− in the roots and leaves of salt-stressed rice seedlings
The contents of Na+ and Cl− in the roots and leaves of LJ4 and Nipp seedlings with no MT pretreatment under 120 mM NaCl for 7 days were significantly increased in comparison with those of the control (P < 0.05), while K+ contents decreased markedly. However, there was no change in the leaves of Nipp, and correspondingly, Na+/K+ ratios in the roots and leaves of both rice seedlings largely increased. When pretreated with 75 μM MT before NaCl stress, the salt-induced increases in Na+ and Cl− contents and Na+/K+ ratios in the roots and leaves clearly decreased, especially in the roots of LJ4. However, no recovery effects of MT pretreatment on the decreased K+ level resulting from salt stress were found in the roots and leaves of the rice seedlings of either cultivar (Fig. 5).
Effects of MT pretreatment on the transcriptional patterns of OsCLC1, OsCLC2 and OsSOS1 in the roots and leaves of salt-stressed rice seedlings
The relative expressions of OsCLC1 and OsCLC2 in the roots and leaves of LJ4 and Nipp seedlings without or with MT pretreatment first showed an increasing tendency but then a decreasing one under 120 mM NaCl stress for 0, 3, 6, 12 and 24 h, most of them reached the maximum when stressed for 6 h. MT pretreatment displayed synergistic enhancement effects, in which the relative expressions of OsCLC1 in the roots of LJ4 (MT pretreatment increased the relative expression level from 5.61 fold to 10.62 fold at 6 h of salt stress) and the relative expression of OsCLC1 and OsCLC2 in the roots of Nipp (MT pretreatment increased the relative expression of OsCLC1 from 18.82 fold to 27.27 fold and that of OsCLC2 from 1.60 fold to 2.41 fold) were more prominent (Fig. 6A, B). During the process of 24 h of salt stress, the highest relative expressions of OsSOS1 in the roots and leaves of both rice seedlings with or without MT pretreatment mostly occurred in the NaCl treatment for 6 h, aside from the continual increase in the roots of Nipp. Moreover, MT pretreatment also showed further enhancement effects on OsSOS1 expression together with NaCl stress, and this expression in roots of both rice cultivars was more prominent than in the leaves (Fig. 6C).
Discussion
In the process of rice planting or production, soil salinity is one of the major constraints to seed germination, seedling establishment, plant growth and yield. Among them, the early stages of seed germination and seedling establishment are more susceptible to salt stress and constitute the primary link for deciding whether rice can be planted in saline fields and determining rice output (Zhang et al. 2014; Basnet et al. 2015; Chen et al. 2012). Rice seedlings pretreated with 10 and 20 µM MT for 2 weeks could delay dark- and salt-induced leaf senescence by directly or indirectly counteracting the cellular accumulation of H2O2, revealing enhanced antioxidant protection of exogenous MT application as a potent free radical scavenger (Liang et al. 2015). In this study, at the rice seed germination and/or young seedling stages, the enhancement effects of MT treatment or pretreatment were studied in two rice cultivars (LJ4 and Nipp) that have different salt tolerances (Fig. 1). The results demonstrated that these measures could clearly improve seed germination ability (shown as GP), strengthen seedling quality (GI and VI) under salt stress or alleviate salt damage to plants (biomass, root activity, REL, etc.) (Figs. 2, 3); however, no obvious correlations between the ameliorative roles in salt injury and the salt tolerance of cultivars LJ4 and Nipp were observed (Fig. 1). This differs from the results of our previous studies on salt-stressed soybean using foliar sprays of methanol (Wei et al. 2015a, b) or on seed soaking with soybean isoflavones (Tian et al. 2014), which indicated better alleviating effects on relatively salt-sensitive soybean cultivars. The differential effects may be attributed to MT as a kind of endogenous plant growth regulator acting in trace amounts (Zhang et al. 2015). In addition, in this study, the alleviating effects of MT on rice seed germination (10–500 µM) or seedling establishment (25–100 µM) under salt stress all showed no clear MT concentration or dose-dependent effects (Figs. 2, 3A). However, Dawood and El-Awadi (2015) reported that seed treatments involving seed priming or pre-sowing with MT could alleviate salinity stress on Vicia faba L. plants irrigated with diluted seawater, and MT at 500 mM had a more pronounced effect than did 100 mM MT. Conversely, Wei et al. (2015a, b) reported that coating seeds with MT significantly promoted soybean growth and seed production, but like other plant hormones, MT displayed weak effects at higher concentrations; in field tests, 50 μM MT-treated seedlings seemed to be healthier than were the control or 100 μM-treated seedlings.
With respect to the physiological and molecular events of MT treatment or pretreatment on improving seed germination ability or seedling growth status under many kinds of adverse environments, many studies have mainly demonstrated the oxidative protection of MT as an antioxidant by its ability to enhance antioxidant enzyme and non-enzyme systems or via its ability to regulate gene expression, and through its secondary effects on plant water relations, growth, photosynthesis, ion homeostasis, and leaf senescence (Meng et al. 2014; Liang et al. 2015; Jiang et al. 2016; Ye et al. 2016). Furthermore, Zhang et al. (2014) showed that the enhanced seed germination of cucumber under NaCl stress after pretreatment with exogenous MT contributed to a rapid decrease in ABA content and a significant increase in GA (especially GA4) levels during the early stages of germination, which suggests regulation of MT occurs during the biosynthesis and catabolism of ABA and GA4. Li et al. (2017) demonstrated that melatonin, a potent long-distance signal, may be translocated from the treated leaves or roots of Citrullus lanatus L. to distant untreated tissues via vascular bundles, leading to systemic induction of cold tolerance. These findings also suggest MT effects have characteristics of transitivity and persistence. In addition, according to many studies, large differences occur in the methods, including pretreatment (seed priming, seedling culture) (Zhang et al. 2014; Dawood and El-Awadi 2015), foliar spray (Ye et al. 2016), root or rhizospheric application (Jiang et al. 2016), or mixing together with other antioxidants (e.g., ascorbic acid) (Kostopoulou et al. 2015). Also, the concentration of MT used varies substantially in terms of researchers, plant species, and stress factors, among other things. Ye et al. (2016) reported that foliar-sprayed MT (100 µM) could increase maize seedling drought tolerance by alleviating drought-induced photosynthetic inhibition and oxidative damage (both enzymatic and nonenzymatic antioxidant activity). Jiang et al. (2016) suggested that root- or rhizospheric-applied MT (1 µM) could enhance maize salt tolerance by significantly increasing K+ contents and K+/Na+ ratios in the shoots and decreasing the Na+ content in the leaves of maize plants under 100 mM NaCl stress. Similarly, cucumber seeds pretreated/soaked with 1 µM MT showed enhanced germination rates under 150 mM NaCl stress (Zhang et al. 2014). Even lower concentrations (<200 nM) of exogenously applied MT to the roots of Vitis vinifera cuttings prior to 10% PEG-induced drought treatment could improve the resistance by its antioxidant and osmoprotectant effects (Meng et al. 2014). This can fully demonstrate that the enhancement effects of MT on plants or the adaptation of crops to many kinds of stressful environmental factors are related not only to internal causes, such as species, growth and development stage, but also to external causes, such as individual stress factors, application method, dosage, etc. Indeed, regarding the high commercial cost of MT and for reducing or minimizing the cost of practical applications as much as possible, lower MT concentrations should be promoted.
With respect to the mechanisms of MT alleviation effects on plant stress damage, the feature of MT being a good antioxidant has often been the focus and is well analyzed (Zhang et al. 2015; Meng et al. 2014; Liang et al. 2015; Jiang et al. 2016). In this study, clear decreases in H2O2 contents were displayed in the roots and leaves of both salt-stressed LJ4 and Nipp plants pretreated with MT at the seed germination and young seedling stages, and the activities of relevant antioxidant enzymes (such as CAT and SOD) were also enhanced (Fig. 4). Oxidative stress is secondarily derived from various biotic and abiotic stresses (except for direct treatments, such as methyl viologen or H2O2) that affect plants or crops (Hossain et al. 2015). Different rice cultivars and genotypes often exhibit clear differences in salt tolerance. Kabir et al. (2016) suggested that the salt-tolerant rice genotype Pokkali displays greater induction of OsNAS1 (coding for nicotianamine synthase), OsPCS1 (for phytochelatin synthase), and DREB1A (a transcription factor) transcripts under salt stress and significant increases in CAT, GR, and SOD activity in roots compared to the salt-sensitive genotype BRRI 3. Jiang et al. (2016) demonstrated the strengthening activities of antioxidant enzymes, e.g., CAT, SOD, APX, and GR, by MT application to achieve ROS detoxification or homeostasis in salt-stressed maize seedlings. This may indicate a possible relationship between MT treatment and antioxidant defense in rice under salt stress.
Among the variety of stressful injuries to germinating seeds or young seedlings under salty environments, ionic toxicity is the primary and most severe type. Thus, employing effective solutions for ion homeostasis is a key strategy for plants to improve salt tolerance (Zhao et al. 2017). In this study, when two rice seedlings without MT pretreatment were subjected to 120 mM NaCl for 7 days, the contents of Na+ and Cl− in roots and leaves significantly increased compared with those of the control, and K+ content clearly decreased; these were results occur in many other glycophytes. Accordingly, Na+/K+ ratios in roots and leaves significantly increased when both rice plants pretreated with 75 µM MT at the seed germination and young seedling stages were exposed to NaCl treatment, although no recovery effect on the reduced K+ content was observed. The contents of Na+ and Cl− and the Na+/K+ ratio in the roots and leaves all decreased to varying degrees, of which the decreases in Na+ content and the Na+/K+ ratio in leaves and that of the Cl− content in the roots all reached significant levels (P > 0.05) (Fig. 5). This may be the direct physiological cause for the enhancement of rice salt tolerance by MT pretreatment. In addition, there is evidence indicating that environmental stress can increase the level of endogenous MT in plants (Zhang et al. 2015).
Using modern molecular biological methods, such as transcriptome and RNA-sequence analysis, it can be shown that the MT alleviation of plant stress injury or the enhancement of stress adaptation is related to the regulation of gene expression. Wei et al. (2015a, b) found that coating soybean seed with MT could up-regulate the expression of salt stress-inhibited genes involved in cell division, photosynthesis, carbohydrate metabolism, fatty acid biosynthesis, and ascorbate metabolism, hence conferring salt and drought stress tolerance to soybean plants. Li et al. (2017) revealed that an abundance of cold defense-related genes involved in signal sensing and transduction, transcriptional regulation, protection and detoxification, and hormone signaling could mediate MT-induced cold tolerance. With respect to the molecular bases of MT pretreatment on saline ion absorption or redistribution in the roots and leaves of salt-stressed rice plants, we mainly focused on and analyzed the transcriptional differences of CLCs (OsCLC1, OsCLC2) and OsSOS1 genes related to cellular absorption, transport and exclusion as well as intracellular compartmentation of Na+ and Cl− in rice plants under salt stress. The results showed that, besides the continuous enhancement of OsSOS1 expression in the roots of Nipp, the transcriptional patterns of OsCLC1, OsCLC2 and OsSOS1 in the roots and leaves of LJ4 and Nipp seedlings without MT pretreatment first showed an increasing tendency but then a decreasing one under 120 mM NaCl stress for 0, 3, 6, 12 and 24 h; the majority of these genes reached the maximum when stressed for 6 h, and MT pretreatment displayed synergistic enhancement effects, in which the relative expression of OsCLC1 and OsSOS1 in the roots of LJ4 and Nipp and the relative expression of OsCLC1 and OsCLC2 in the roots and leaves of LJ4 and Nipp were more prominent (Fig. 6). The synergistic enhancement of OsSOS1 expression in the salt-treated roots of both rice cultivars by MT pretreatment may directly contribute to Na+ export from roots and retention in stems, thus preventing Na+ from reaching photosynthetic leaf tissues (Olías et al. 2009). This phenomenon could directly result in decreases in Na+ content and Na+/K+ ratios in roots and leaves. The Cl− content in plants is mainly controlled by an anion transporter protein family represented by CLCs (Wei et al. 2016). Under salt stress, the accumulated Cl− in plants of the salt-sensitive rice cultivar IR29, especially in the leaves, was significantly higher than that in the salt-tolerant cultivar Pokkali, which may be due to the inhibition and enhancement of OsCLC1 gene transcription in IR29 and Pokkali, respectively (Diédhiou and Golldack 2006). In our study, the transcription of OsCLC1 and OsCLC2 in the roots and leaves of cultivars LJ4 and Nipp were enhanced under salt stress, and MT pretreatment prior to salt stress could also further strengthen their expression (Fig. 6A, B). This finding suggests that MT pretreatment would be beneficial to further reduce the Cl− content in salt-stressed rice roots and leaves, to synergistically improve Cl− and Na+ homeostasis together with the OsSOS1-encoded protein-mediated decrease in Na+ content, and to ultimately achieve the salt tolerance improvement of the tested rice plants. However, the mechanism of the participation of OsCLC1 and OsCLC2 in MT pretreatment to regulate Cl− homeostasis in salt-stressed rice plants as well as determining whether other OsCLC members participate in this process is worth further discovery.
Conclusion
Melatonin application at seed germination and/or young seedling stages could improve the germination ability and seedling quality of both rice cultivars LJ4 and Nipp under salt stress, thus clearly enhancing their salt tolerance. This phenomenon might be simultaneously fulfilled by multiple physiological processes. For example, improving seed germination quality (GP, GI and VI), strengthening root vigor, reducing the contents of Na+ and Cl− in roots and leaves (especially for Cl− contents in roots and Na+ levels in leaves), and enhancing the activities of antioxidant enzymes (such as CAT and SOD) in roots and leaves to reduce the content of reactive H2O2. Therefore, the reduced contents of Na+ and Cl− in the roots and leaves of both salt-stressed rice plants under MT pretreatment were positively related to the enhanced transcription of OsSOS1 in roots and of OsCLC1 and OsCLC2 in roots and leaves. In addition, these results indicate that soaking during seed germination and/or root application at the seedling stage with MT, a non-toxic and harmless indoleamine-type plant growth regulator, is a very simple operation that requires small doses and can markedly improve seed germination capacity and seedling quality under salt stress in the short-term. This process effectively solves the problems of low germination rate and poor seedling establishment in saline soils and provides a theoretical basis and technical support for the chemical regulation of salt tolerance and cultivation practices of rice and other crops in saline areas.
Abbreviations
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- CLCs:
-
Chloride channels
- GI:
-
Germination index
- GP:
-
Germination potential
- GR:
-
Glutathione reductase
- MT:
-
Melatonin
- ROS:
-
Reactive oxygen species
- REL:
-
Relative electrolytic leakage
- SOS1:
-
Salt-overly-sensitive1
- SOD:
-
Superoxide dismutase
- TTC:
-
Triphenyltetrazolium chloride
- VI:
-
Vigor index
References
Basnet RK, Duwal A, Tiwari DN, Xiao D, Monakhos S, Bucher J, Visser RGF, Groot SPC, Bonnema G, Maliepaard C (2015) Quantitative trait locus analysis of seed germination and seedling vigor in Brassica rapa reveals QTL hotspots and epistatic interactions. Front Plant Sci 6:1032. doi:10.3389/fpls.2015.01032
Cao C, Yu B, Zhao X, Wei P, Song J, Chen L, Wang M (2016) Ameliorative effects of foliar 2,3-dihydroporphyrin iron(III) spray on seedling growth and seed traits of salt-stressed rapeseed plants. Agron J 108(4): 1455–1462. doi:10.2134/agronj2015.0600
Chen F, Liu L, Chen F, Jia G (2012) The ecological characteristics of seed germination and seedling establishment of Manglietia patungensis: implication for species conservation. Am J Plant Sci 3:1455–1461. doi:10.4236/ajps.2012.310175
Dawood MG, El-Awadi ME (2015) Alleviation of salinity stress on Vicia faba L. plants via seed priming with melatonin. Acta Biol Colomb 20(2):223–235. doi: 10.15446/abc.v20n2.43291
Diédhiou CJ, Golldack D (2006) Salt-dependent regulation of chloride channel transcripts in rice. Plant Sci 170:793–800
Hanin M, Ebel C, Ngom M, Laplaze L, Masmoudi K (2016) New insights on plant salt tolerance mechanisms and their potential use for breeding. Front Plant Sci 7:1787. doi:10.3389/fpls.01787
Hardeland R, Madrid AJ, Tan DX, Reiter RJ (2012) Melatonin, the circadian multioscillator system and health: the need for detailed analyses of peripheral melatonin signaling. J Pineal Res 52:139–166
Hasan MK, Ahammed GJ, Yin L, Shi K, Xia X, Zhou Y, Yu J, Zhou J (2015) Melatonin mitigates cadmium phytotoxicity through modulation of phytochelatins biosynthesis, vacuolar sequestration, and antioxidant potential in Solanum lycopersicum L. Front Plant Sci 6:601. doi:10.3389/fpls.00601
Himabindu Y, Chakradhar T, Reddy MC, Kanygin A, Redding KE, Chandrasekhar T (2016) Salt-tolerant genes from halophytes are potential key players of salt tolerance in glycophytes. Environ Exp Bot 124:39–63
Hossain MA, Bhattacharjee S, Armin S-M, Qian P, Xin W, Li H-Y, Burritt DJ, Fujita M, Tran L-SP (2015) Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: insights from ROS detoxification and scavenging. Front Plant Sci 6:420. doi:10.3389/fpls.2015.00420
Hu SB, Zhou Q, An J, Yu BJ (2016) Cloning of PIP genes in drought-tolerant vetiver grass and responses of transgenic VzPIP2;1 soybean plants to water-deficit stress. Biol Plant 60(4):655–666. doi:10.1007/s10535-016-0631-5 doi
Jia Y, Zou D, Wang J, Sha H, Liu H, Inayat MA, Sun J, Zheng H, Xia N, Zhao H (2017) Effects of c-aminobutyric acid, glutamic acid, and calcium chloride on rice (Oryza sativa L.) under cold stress during the early vegetative stage. J Plant Growth Regul 36:240–253. doi:10.1007/s00344-016-9634-x
Jiang C, Cui Q, Feng K, Xu D, Li C, Zheng Q (2016) Melatonin improves antioxidant capacity and ion homeostasis and enhances salt tolerance in maize seedlings. Acta Physiol Plant 38:82. doi:10.1007/s11738-016-2101-2
Kabir AH, Zaman R, Begum MC, Haque A, Swaraz AM, Noor IM, Alam MZ, Haider SA (2016) Upregulation of OsNAS1, OsPCS1, and DREB1A transcripts along with antioxidative defense confers salt tolerance in rice (Oryza sativa L. cv Pokkali). Arch Agron Soil Sci 62:1381–1395. doi:10.1080/03650340.2016.1149817
Kim YH, Khan AL, Waqas M, Shim JK, Kim DH, Lee KY, Lee IJ (2014) Silicon application to rice root zone influenced the phytohormonal and antioxidant responses under salinity stress. J Plant Growth Regul 33:137–149. doi:10.1007/s00344-013-9356-2
Kostopoulou Z, Therios I, Roumeliotis E, Kanellis AK, Molassiotis A (2015) Melatonin combined with ascorbic acid provides salt adaptation in Citrus aurantium L. seedlings. Plant Physiol Biochem 86:155–165. doi:10.1016/j.plaphy.2014.11.021
Kumar K, Kumar M, Kim S-R, Ryu H, Cho Y-G (2013) Insights into genomics of salt stress response in rice. Rice 6:27. doi:10.1186/1939-8433-6-27
Li JT, Qiu ZB, Zhang XW, Wang LS (2011) Exogenous hydrogen peroxide can enhance tolerance of wheat seedlings to salt stress. Acta Physiol Plant 33:835–842. doi:10.1007/s11738-010-0608-5
Li C, Wang P, Wei ZW, Liang D, Liu CH, Yin LH, Jia DF, Fu MY, Ma FW (2012) The mitigation effects of exogenous melatonin on salinity-induced stress in Malus hupehensis. J Pineal Res 53(3):298–306. doi:10.1111/j.1600-079X.2012.00999.x
Li L, Li J, Shen M, Zhang C, Dong Y (2015) Cold plasma treatment enhances oilseed rape seed germination under drought stress. Sci Rep 5:13033. doi:10.1038/srep13033
Li MQ, Hasan KM, Li CX, Ahammed GJ, Xia XJ, Shi K, Zhou YH, Reiter RJ, Yu JQ, Xu MX, Zhou J (2016a) Melatonin mediates selenium-induced tolerance to cadmium stress in tomato plants. J Pineal Res. doi:10.1111/jpi.12346
Li Z, Azeem S, Zhang Z, Li Z, Zhao H, Lin W (2016b) Promising role of moderate soil drying and subsequent recovery through moderate wetting at grain-filling stage for rice yield enhancement. J Plant Growth Regul 35:838–850. doi:10.1007/s00344-016-9587-0
Li H, Chang J, Zheng J, Dong Y, Liu Q, Yang X, Wei C, Zhang Y, Ma J, Zhang X (2017) Local melatonin application induces cold tolerance in distant organs of Citrullus lanatus L. via long distance transport. Sci Rep 7:40858. doi:10.1038/srep40858
Liang C, Zheng G, Li W, Wang Y, Hu B, Wang H, Wu H, Qian Y, Zhu XG, Tan DX, Chen SY, Chu C (2015) Melatonin delays leaf senescence and enhances salt stress tolerance in rice. J Pineal Res 59:91–101. doi:10.1111/jpi.12243
Liang C, Li A, Yu H, Li W, Liang C, Guo S, Zhang R, Chu C (2017) Melatonin regulates root architecture by modulating auxin response in rice. Front Plant Sci 8:134. doi:10.3389/fpls.00134
Martínez-Atienza J, Jiang X, Garciadeblas B, Mendoza I, Zhu JK, Pardo JM, Quintero FJ (2007) Conservation of the salt overly sensitive pathway in rice. Plant Physiol 143:1001–1012
Meng J-F, Xu T-F, Wang Z-Z, Fang YL, Xi Z-M, Zhang Z-W (2014) The ameliorative effects of exogenous melatonin on grape cuttings under water-deficient stress: antioxidant metabolites, leaf anatomy, and chloroplast morphology. J Pineal Res 57:200–212. doi:10.1111/jpi.12159
Muchate NS, Nikalje GC, Rajurkar NS, Suprasanna P, Nikam TD (2016) Plant salt stress: adaptive responses, tolerance mechanism and bioengineering for salt tolerance. Bot Rev. doi:10.1007/s12229-016-9173-y
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Nahar K, Hasanuzzaman M, Rahman A, Alam MM, Mahmud JA, Suzuki T, Fujita M (2016) Polyamines confer salt tolerance in mung bean (Vigna radiata L.) by reducing sodium uptake, improving nutrient homeostasis, antioxidant defense, and methylglyoxal detoxification systems. Front Plant Sci. doi:10.3389/fpls.2016.01104
Nakamura A, Fukuda A, Sakai S, Tanaka Y (2006) Molecular cloning, functional expression and subcellular localization of two putative vacuolar voltage-gated chloride channels in rice (Oryza sativa L.). Plant Cell Physiol 47:32–42
Nie W, Xu L, Yu B (2015) A putative soybean GmsSOS1 confers enhanced salt tolerance to transgenic Arabidopsis sos1-1 mutant. Protoplasma 252:127–134. doi:10.1007/s00709-014-0663-7
Olías R, Eljakaoui Z, Pardo JM, Belver A (2009) The Na+/H+ exchanger SOS1 controls extrusion and distribution of Na+ in tomato plants under salinity conditions. Plant Signal Behav 4(10):973–976
Park HJ, Kim WY, Yun DJ (2016) A new insight of salt stress signaling in plant. Mol Cells 39(6):447–459. doi:10.14348/molcells.2016.0083
Qiu ZB, Guo JL, Zhu AJ, Zhang L, Zhang MM (2014) Exogenous jasmonic acid can enhance tolerance of wheat seedlings to salt stress. Ecotox Environ Safe 104:202–208. doi:10.1016/j.ecoenv.2014.03.014
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR by comparative CT method. Nat Protoc 3:1101–1108
Shi HZ, Quintero J, Pardo JM, Zhu JK (2002) The putative plasma membrane Na+/H+ antiporter SOS1 controls long distance Na+ transport in plants. Plant Cell 14(2):465–477
Shi H, Jiang C, Ye T, Tan D, Reiter RJ, Zhang H, Liu R, Chen Z (2015) Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in bermudagrass [Cynodon dactylon (L). Pers.] by exogenous melatonin. J Exp Bot 66:681–694
Sun J, Chen S-L, Dai S-X, Wang R-G, Li N-Y, Shen X, Zhou X-Y, Lu C-F, Zheng X-J, Hu Z-M, Zhang Z-K, Song J, Xu Y (2009) Ion flux profiles and plant ion homeostasis control under salt stress. Plant Signal Behav 4:261–264
Szafránska K, Reiter RJ, Posmyk MM (2016) Melatonin application to Pisum sativum L. seeds positively influences the function of the photosynthetic apparatus in growing seedlings during paraquat-induced oxidative stress. Front Plant Sci 7:1663. doi:10.3389/fpls.2016.01663
Tan DX, Manchester LC, Reiter RJ, Plummer BF, Limson J, Weintraub ST, Qi W (2000) Melatonin directly scavenges hydrogen peroxide: a potentially new metabolic pathway of melatonin biotransformation. Free Radical Bio Med 29:1177–1185
Teh CY, Mahmood M, Shaharuddin NA, Ho CL (2015) In vitro rice shoot apices as simple model to study the effect of NaCl and the potential of exogenous proline and glutathione in mitigating salinity stress. Plant Growth Regul 75(3):771–781. doi:10.1007/s10725-014-9980-2
Tian F, Jia T, Yu B (2014) Physiological regulation of seed soaking with soybean isoflavones on drought tolerance of Glycine max and Glycine soja. Plant Growth Regul 74(3):229–237. doi:10.1007/s10725-014-9914-z
Wang CJ, Yang W, Wang C, Gu C, Niu DD, Liu HX, Wang YP, Guo JH (2012) Induction of drought tolerance in cucumber plants by a consortium of three plant growth-promoting rhizobacterium strains. PLoS ONE 7:e52565. doi:10.1371/journal.pone.0052565
Wang P, Sun X, Li C, Wei ZW, Liang D, Ma FW (2013) Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J Pineal Res 54:292–302. doi:10.1111/jpi.12017
Wei P, Chen D, Jing R, Zhao C, Yu B (2015a) Ameliorative effects of foliar methanol spraying on salt injury to soybean seedlings differing in salt tolerance. Plant Growth Regul 75:133–141. doi:10.1007/s10725-014-9938-4
Wei W, Li Q-T, Chu Y-N, Reiter RJ, Yu X-M, Zhu D-H, Zhang W-K, Ma B, Lin Q, Zhang J-S, Chen S-Y (2015b) Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J Exp Bot 66(3):695–707. doi:10.1093/jxb/eru392
Wei P, Wang L, Liu A, Yu B, Lam HM (2016) GmCLC1 confers enhanced salt tolerance through regulating chloride accumulation in soybean. Front Plant Sci. doi:10.3389/fpls.2016.01082
Yamaguchi T, Hamamoto S, Uozumi N (2013) Sodium transport system in plant cells. Front Plant Sci. doi:10.3389/fpls.2013.00410
Ye J, Wang S, Deng X, Yin L, Xiong B, Wang X (2016) Melatonin increased maize (Zea mays L.) seedling drought tolerance by alleviating drought-induced photosynthetic inhibition and oxidative damage. Acta Physiol Plant 38:48. doi:10.1007/s11738-015-2045-y
Zhang HM, Zhang YQ (2014) Melatonin: a well-documented antioxidant with conditional pro-oxidant actions. J Pineal Res 57:131–146. doi:10.1111/jpi.12162
Zhang XK, Zhou QH, Cao JH, Yu BJ (2011) Differential Cl−/salt tolerance and NaCl-induced alternations of tissue and cellular ion fluxes in Glycine max, Glycine soja and their hybrid seedlings. J Agron Crop Sci 197:329–339. doi:10.1111/j.1439-037X.2011.00467.x
Zhang H-J, Zhang N, Yang R-C, Wang L, Sun Q-Q, Li D-B, Cao Y-Y, Weeda S, Zhao B, Ren S, Guo Y-D (2014) Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA4 interaction in cucumber (Cucumis sativus L.). J Pineal Res 57:269–279. doi:10.1111/jpi.12167
Zhang N, Sun Q, Zhang H, Cao Y, Weeda S, Ren S, Guo Y-D (2015) Roles of melatonin in abiotic stress resistance in plants. J Exp Bot 66(3):647–656. doi:10.1093/jxb/eru336
Zhao X, Wei P, Liu Z, Yu B, Shi H (2017) Soybean Na+/H+ antiporter GmsSOS1 enhances antioxidant enzyme activity and reduces Na+ accumulation in Arabidopsis and yeast cells under salt stress. Acta Physiol Plant 39:19. doi:10.1007/s11738-016-2323-3
Zhou Q, Yu BJ (2009) Accumulation of inorganic and organic osmolytes and its role in osmotic adjustment in NaCl-stressed vetiver grass seedlings. Russ J Plant Physiol 56:678–685
Acknowledgements
We thank the National Science-Technology Support Plan Projects of China (2015BAD01B01), the National Natural Science Foundation of China (31671604, U1603111) for the financial support provided for this work.
Author information
Authors and Affiliations
Contributions
YBJ and LXJ designed the experiments. LXJ, CYQ and YYF performed the experiments, and YBJ and LXJ analyzed the data and wrote the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Li, X., Yu, B., Cui, Y. et al. Melatonin application confers enhanced salt tolerance by regulating Na+ and Cl− accumulation in rice. Plant Growth Regul 83, 441–454 (2017). https://doi.org/10.1007/s10725-017-0310-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-017-0310-3