Abstract
The study was conducted at the grain-filling stage to elucidate the physiological and molecular mechanisms of the root to enhance yield under alternate wetting and drying (AWD) compared with conventional irrigation. Measurements of root dry weight (RDW), seed setting rate, total kernel weight, and grain yield were determined along with 2D electrophoresis to detect altered protein expression in response to moderate soil drying (MD) and the subsequent recovery phase as moderate wetting (MW) under AWD compared with continuous wetting under CI. We found significant enhancement in RDW as well as 14.30 % increase in inferior spikelets, seed setting and 10.32 g m−2 increase in final yield. Among the total 55 differentially expressed proteins, 26 proteins were differentially expressed under both MD treatment and MW treatment, whereas 14 proteins under MD and 15 proteins under MW showed distinct expression. Differentially expressed proteins were involved in redox homeostasis, signaling, defense, energy, photoassimilate remobilization and included 14-3-3 proteins, cysteine-rich receptor-like protein kinase, monodehydroascorbate reductase, ascorbate peroxidase, glutathione S-transferases, translationally controlled tumor protein, remorin C-terminal domain containing protein, protein disulfide isomerase, DnaK family protein, cysteine synthase, aminotransferase, phosphoglycerate mutase, pyruvate phosphate dikinase, ATP synthase, and abscisic acid stress ripening (ASR1). The differential expression ratio of the signaling, redox, and defense group proteins was almost the same under MD and MW. ABA signaling, amino acid synthesis, and N remobilization were upregulated under MD, and the enzymes involved in carbohydrate, energy, and transportation metabolism were upregulated under MW. In conclusion, at the rice grain-filling stage, AWD is a potential technique to trigger signaling and the enzymatic protein network for systematic senescence initiation, root enlargement for maximum nutrient uptake, and maximize photoassimilate remobilization for yield enhancement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice is one of the most important food crops in the world, and a primary source of food for more than half the world’s population (Khush 2005). At present, this population figure is estimated to be 7.1 billion which is expected to cross 9 billion in 2050 (Godfray and others 2010). Water shortage and land scarcity are constraints to producing enough food for this rapid population growth (Von Braun 2007). Asia is the most populated area of the world, where the traditional rice production system (CI) has been practiced through irrigated puddled soil for several centuries (Cassman and Pingali 1995). To grow rice through CI, water demand is two to three times more than the other important cereals such as wheat and maize. According to one estimate, up to 3000 L of water are required to produce 1 kg rice (Bouman and others 2007).
However, the scarcity of fresh water has resulted in a serious threat to the sustainability of the irrigated rice system in Asia (Carruthers and others 1997). Due to a looming water crisis, we must look for alternative water saving methodologies for sustainable rice production. A small savings of water due to a change in the current practice can result in a significant reduction in the total water consumption for rice farming (Bouman 2002). In the Philippines and China, alternative wet and moderate drying (AWD) trials at the grain-filling stage reduced water consumption from 13 to 30 % of the total water required under CI, with no yield decline (Cabangon and others 2001; Belder and others 2002).
Along with water savings, AWD during the mid- and late-grain-filling stages could enhance grain filling. Conventionally, it was thought that poor grain filling is the consequence of carbon source limitation. But, recent studies have shown that at the initial grain-filling stage, plants have adequate sucrose so carbohydrate supply should not be the major problem. The low activities of key enzymes in carbon metabolism can be the major reason for poor grain filling. Proper field practices, such as AWD can activate key enzymes to enhance systematic whole-plant senescence and accelerate the grain-filling rate (Yang and Zhang 2006). Further studies are needed using molecular approaches to investigate the AWD pathway including signaling, hormonal, defense, senescence, remobilization of specific gene expression, and the biochemical processes.
The root being the soil water status sensor (SWS) has a dramatic role at the grain-filling stage to enhance yield. The root functions as the primary SWS, and directly triggers a network regulating the stress response of the whole plant including reduced photosynthesis in the canopy and increased water and nutrient uptake through the root (Yang and others 2004a). So, the root is an important player to enhance rice yield under AWM (Patel and others 1984; Osaki and others 1997; Yang and others 2004b; MingMing and others 2010).
To explore the underlying mechanism of rice root system responses to AWD treatment as compared with CI at the grain-filling stage, a comparative proteomics approach was adopted based on a differential protein expression pattern along with monitoring the physiological responses of roots. AWD induced protein expression changes to trigger root growth, systematic senescence, remobilization of reserves from sheath-stem to grain pools and thus enhanced grain yield.
Materials and Methods
Research Material
Pot experiments were carried out in the experimental field of Agricultural Ecological key laboratory, Fujian Agriculture and Forestry University, Fuzhou, Fujian, China, during April to October, 2013. A large-panicle rice cultivar (Oryza sativa l. SSP. Indica) “Jin Hui 809” was used as research material. Plastic buckets 0.3 m length and 0.23 m bottom diameter were used. The soil was sandy loam with available nitrogen, phosphorus, and potassium at 190.60, 126.60, and 201.60 mg kg−1, respectively. Fertilizer application throughout the growth period was according to the dosage of 225 kg hm−2 converted into a barrel, the fertilizer proportion of N:P:K = 1:0.5:0.8, including 6:4 nitrogen ratio of basal dressing for tillers to top dressing for spike grains. The conventional irrigation method was used throughout the growing period until anthesis. At 6 days after anthesis, two different water irrigation treatments were adopted: alternate wetting and drying irrigation (AWD) and conventional irrigation (CI). Under AWD, the pots were not irrigated until the soil water potential reached –25 kPa at 15–20 cm depth, and then pot soil was irrigated up to 2–3 cm, this irrigation pattern was repeated until 1 week before the rice was harvested. Under CI, the soil was kept flooded with 2–3 cm water depth in the pots until 1 week before the rice was harvested. Each sampling, either at harvesting or before harvesting, was done in three replicates both from AWD and CI pots based on a randomized design and means were tested by the least significant difference at P < 0.05 (LSD0.05) following analysis of variance (ANOVA) using SPSS.
Root Dry Weight Measurement
The roots were sampled every 5 days from flowering stage through maturity (5, 10, 15, 20, 25, 30, and 35 day) in four replications from AWD and CI. For sampling, plants were taken out from plastic buckets. Each bucket contained three plants and was considered as one replication. After carefully removing soil, roots were washed with water, paper dried, and blanched in oven at 105 °C for 30 min, then, dried at 80 °C for 48 h until constant weight and RDW was calculated.
Yield Measurement
The first and second kernels of each panicle were designated as superior and the third and fourth as inferior kernels. Four replications were performed for each parameter and each replication was the average of four measurements. Panicles having at least five solid grains were denoted as an effective panicle. At harvesting, the number of effective panicles (panicles m−2), grain number per panicle, seed setting percentage of superior and inferior spikelets were measured from both AWD and CI rice plants. Then, grains per sampled plant were dried at 70°C up to a constant weight, dehulled and the thousand-kernel weight (TKW) (g) and yield (g m−2) were measured.
Protein Extraction
Sampling was done at two stages with three biological replicates for protein extraction; 1st at moderate soil drying (MD), when the soil water potential reached −25 kPa at 15–20 cm depth and the 2nd at moderate soil wetting (MW), when the soil water potential reached 0 kPa at 15–20 cm depth (almost 48 h after irrigation). Sampling was also done from CI as control at the same time on both stages. For sampling, plants were taken out from plastic buckets. Soil was removed and roots were washed thoroughly with water, paper dried, cleaned roots were cut into equal pieces, homogenized well into 5.0 g samples, frozen immediately in liquid nitrogen, and stored at −80 °C prior to protein extraction. The protein extraction protocol was followed with some modifications from Wang (2006). Briefly, 5 g of freeze-dried roots mixed with a little polyvinyl pyrrolidone (PVP) and liquid nitrogen, were ground into fine powder. The sample was resuspended in 10 mL 100 % acetone followed by centrifugation at 16,000×g for 30 min. This step was repeated thrice until the supernatant was achromatic. Then, the protein pellet was lyophilized in a vacuum centrifuge. The lyophilized powder was again resuspended in 10 mL precooled buffer containing 30 % sucrose, 1.5 % SDS, 4 % β-mercaptoethanol using 1.5 M Tris–Hcl (pH 8.8) as solvent, and 10 mL of Tris (dimethylaminomethyl) phenol, then, sonicated with occasional vortexing for 1 h, subsequently centrifuged at 16,000×g for 30 min at 4 °C. The upper phenol phase was dissolved in five times the volume of precooled 0.1 M ammonium acetate methanol-solution using 100 % methanol as solvent and kept at −20 °C overnight. The thawed sample was centrifuged at 16,000×g for 30 min at 4 °C. After supernatant decanting, the precipitant was washed by the precooled, 100 % acetone containing 0.07 % β-mercaptoethanol. This step was repeated 2–3 times until the supernatant became transparent. Finally, the protein pellet was vacuum-dried and this powdered protein was dissolved in a lysis buffer (pH 8.0) containing 8 M urea, 4 % CHAPS, 40 mM Tris, and 65 mM DTT. The mixture was homogenized for 1 h by ultra-sonification and centrifuged at 16,000×g for 30 min under 4 °C. The supernatant fluid was collected and stored at −80 °C for proteomic analysis. Protein concentration was measured through Bradford method using the BSA (bovine serum albumin) as a standard (Bradford 1976).
2-D Electrophoresis and Protein Spots Selection
The extracted root proteins were separated by 2D-PAGE using isoelectric focusing (IEF) gel strips (linear, 24 cm long, immobiline dry, pH 4–7) for the first dimension and SDS-PAGE (26 cm × 20 cm) for the second dimension. The 2D electrophoresis process was carried out in a 2D-Electrophoresis Apparatus (GE Healthcare). Protein (1.3 mg) was loaded in each IEF strip. A series of electrophoreses were performed such as gradient to 500 V for 1 h; gradient to 1000 V for 2 h; gradient to 8000 V for 3 h; hold at 8000 V for 3 h; and gradient to 1000 V protection voltage for 24 h. The strips were equilibrated in an equilibration buffer (0.1 M Tris–HCl pH 8.8, 6 M urea, 30 % (v/v) glycerol, and 2 % (w/v) SDS) on a shaking table two times. At the first time, the strips were equilibrated in equilibration buffer I (65 mM DTT) and kept shaking for 15 min. At the second time, they were treated in equilibration buffer II (2.5 % (w/v) IAA) and kept shaking for 15 min. The second dimension electrophoresis was performed on SDS-PAGE comprising 12 % (v/v) polyacrylamide gels at 15 mA current per gel until the end of electrophoresis. The gels were stained with Colloidal Coomassie Blue G-250 for at least 12 h. Protein gels were scanned with the GE Image scanner III, and reproducible differential protein spots were detected using Imagemaster 5.0 software.
In-Gel Protein Digestion
Differential protein spots were transferred into 1.5 mL Eppendorf tubes. Each protein sample was washed twice with deionized water for 10 min, destained twice with 100 μL of acetonitrile (ACN) (50 %)/100 mM NH4HCO3 (50 %) for 10 min, and dehydrated with 100 % ACN. Finally, samples were digested with 20 μL of trypsin (12.5 μg/mL) using 50 mM NH4HCO3 as solvent for 30 min on ice and then, incubated at 37 °C overnight. The termination reaction was carried out with 0.2 % v/v formic acid and after centrifugation the supernatant was used for LC–ESI–MS/MS analysis.
LC–ESI–MS/MS Analysis and Protein Identification
The parameters of equipment were performed by the protocol of Zhang and others (2012). Briefly, high-performance liquid chromatography: Thermo Scientific Accera System; Chromatographic Column: BioBasic C18 Column (100 × 0.18 mm, the particle size: 5 μm); Loading quantity of sample: 10 μL; Mobile phase: Solvent A was 0.1 % HCO2H (formic acid) mixed in water, and Solvent B was 0.1 % HCO2H mixed in ACN; Gradient: held at 2 % Solvent B for 2 min, and increased linearly up to 90 % Solvent B over the course of 60 min. The peptides were eluted from a C18 column at a flow rate of 160 μL/min and then electro-sprayed directly into an LTQ mass spectrometer using a spray voltage of 3.5 kV and a constant capillary temperature of 275 °C.
Data Analysis
Data acquisition was performed under data-dependent MS/MS scanning mode. Mass spectrometry analysis of the raw data obtained in Proteome Discoverer1.2 relative quantitative analysis software and database retrieval was performed through UNIPROT database (http://www.uniprot.org/ download the Oryza sativa. Fasta protein libraries 2.6 Software Analysis). For functional analysis and characterization of proteins, Mapman software Version 3.6.0RC1 was used. For graphical analysis, Origi 8.0 was used.
Results
AWD Effect on RDW and Yield
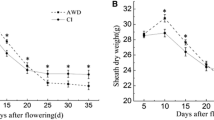
Figure 1 showed that under AWD and CI, dry matter of rice roots declined as the grain-filling process proceeded toward the harvest stage due to progressive metabolite mobilization from root to grain. The RDW under AWD was significantly higher than that of CI from 10 DAF up to 35 DAF. Consistent with more RDW, Table 1 showed that the rice seed setting rate in inferior spikelets was also enhanced up to 14.30 %, the thousand-kernel weight was increased up to 2.98 %, and ultimately the yield was increased up to 10.32 % under AWD as compared with CI. This increasing yield trend indicated a promising role of AWD at the grain-filling stage. Yet, we did not find any significant difference in some measurements like the effective panicle (panicles m−2), grain number per panicles, and seed setting rate in superior spikelets (Table 1).
Graphical demonstration of root dry weight (g) trend from anthesis to grain-filling stage under the alternative wetting and drying (AWD) in comparison with conventional irrigation (CI). Each point represents the mean ± SE of three independent experiments. Analysis of variance showed a significant increase in RDW under AWD (P < 0.005)
AWD Effect on Root Proteins Expression
To further understand the underlying mechanism on a molecular basis, we subsequently carried out a comprehensive proteomic analysis. Root proteins extracted from two stages, moderate drought (MD at −25 kPa), and 48 h after moderate wetting (MW at 0 kPa) along with well-watered pots (CI) as control, were separated by 2-DE technique. Gels are presented in Fig. 2. Through Image master 5.0 software; 72 reproducible, differential protein spots (Fig. 3) were screened based on ≧1.5 as upregulation and ≦0.5 as downregulation parameters and finally LC–ESI–MS/MS identified 71 proteins (Table 2). Some proteins were detected as fragments of the same proteins (Table 3; SD). Finally, 55 screened differentially expressed root proteins were analyzed through Venn graph (Fig. 4). Proteins (47.3 %, 26/55) were differentially expressed under both MD and MW stages, whereas 25.5 % (14/55) and 27.3 % (15/55) of proteins were distinctly expressed under MD and MW, respectively. Among 26 reproducible proteins, 10 proteins were upregulated and 16 were downregulated under MD, whereas 14 proteins were upregulated and 12 were downregulated under MW (Fig. 4). Up- and downregulation trends among distinctly expressed proteins were almost the same under MD and WD (Fig. 4).
Representative 2-DE gel electrophoresis images of the rice root proteome sampled at 10D, 20D, and 30D after anthesis at moderate drying (MD), moderate wetting (MW) stages under the alternative wetting, and drying (AWD) in comparison with conventional irrigation (CI). Extracted proteins were separated by 2D-SDS-PAGE and stained using Coomassie brilliant blue. The MW (in kiloDalton) and pI (isoelectric point) of the proteins are shown on the left and at the top, respectively
A total of 55 screened differentially expressed root proteins were analyzed by MapMan Software Version 3.6.0RC1 using Loc IDs (Table 4; SD) for their functional annotation. On the basis of MapMan ontology, differential proteins were categorized into twelve groups (Fig. 5). All groups including stress/redox defense response (21.82 %), signaling proteins (9.09 %), hormone (3.64 %), carbohydrate metabolism (20 %), energy metabolism (9.09 %), transportation (5.4 %), and alcoholic fermentation (5.45 %) were important in regulation of grain filling. Some proteins functionally uncharacterized by MapMan such as prefoldin subunit 4 (loc_os03g43020.1) were found to be involved in root structural and enzymatic protein stability based on previous reports (Fig. 6).
Comparative description of the functional proteins groups expressed under the moderate wetting (MD) and the moderate drying (MW) through pie and bar graphs. The pie diagram shows the differential proteins classification based on biological function between MD and WD. The bar graph shows functional classification of differential proteins based on up- and downregulated protein groups
Alternative cycles of MW and MD highly upregulated redox, defense, and signaling group proteins. Among the signaling group, cysteine-rich receptor-like protein kinase (loc_os04g56430.1) and 14-3-3 protein (loc_os02g36974.1) were upregulated, whereas kinase pfkB family (loc_os02g41590.1) and WD repeat-containing protein (loc_os01g49290.1) were downregulated. Among the redox and stress defense group, two isoforms of glutathione S-transferase (loc_os03g04240.1, loc_os03g04240.1) were only upregulated under MD, no differential expression was found under MW. Two isoforms of ascorbate peroxidase (loc_os03g17690.1, loc_os07g49400.2) were only upregulated in MW but not under MD. Of the two isoforms of monodehydroascorbate reductase, one (loc_os08g44340.1) was upregulated under MD and other (loc_os09g39380.1) was upregulated under MW. Disulfide isomerase was upregulated in both stages, salt stress root protein (loc_os01g13210.1) was upregulated in MD but downregulated in MW. DnaK family protein (loc_os02g53420.1) was upregulated in both, whereas dirigent (loc_os10g18820.1, loc_os10g18870.1) was only upregulated in MD.
MD stress upregulated proteins involved in protein synthesis network stability including remorin C-terminal domain protein (loc_os04g45070.1, loc_os10g36000.1) involved in RNA regulation and aminotransferase (loc_os10g25130.1), ketol-acid reductoisomerase (loc_os01g46380.1), cysteine synthase (loc_os01g74650.3), involved in amino acid metabolism. Among hormone metabolism-related proteins, abscisic stress-ripening protein (loc_os11g06720.1) and kelch repeat protein (loc_os09g07460.1) were upregulated under MD. Glutamine synthetase (loc_os03g12290.1) involved in N-metabolism was also upregulated.
Following the MD cycle (protein synthesis network), the MW cycle resulted in upregulation of important enzymatic proteins involved in carbohydrate, energy, and transportation metabolism. Carbohydrate metabolism-related proteins included phosphoglycerate kinase (loc_os06g45710.1), phosphoglycerate mutase (loc_os01g60190.1), and jacalin-like lectin (loc_os01g24710.4); energy and transportation metabolism proteins included ATP synthase (loc_os09g08910.1, loc_os06g37180.1), succinyl-CoA ligase (loc_os02g40830.2), lactate/malate dehydrogenase (loc_os05g49880.1, loc_os10g33800.1), transketolase (loc_os06g04270.1), and transaldolase (loc_os01g70170.1).
Discussion
AWD treatment (Fig. 1) significantly enhanced RDW, consistent with a previous report that low water potential induces downstream signals through enhanced root growth for maximum water and nutrient uptake (Saab and others 1990). We also detected that AWD treatment at the grain-filling stage increased the yield up to 10.32 % with a significantly enhanced seed setting rate in inferior spikelets up to 14.30 % and TKW up to 2.98 g (Table 1), consistent with the report that moderate drought stress at the grain-filling stage enhances reallocation of prestored carbon to the grains and accelerates the grain-filling rate especially in inferior spikelets as compared to the well-watered condition (Yang and others 2001b). Water deficit during grain filling also induces systematic senescence to maximize the remobilization of carbon into grains (Yang and others 2001a). In this paper, we have investigated the molecular mechanism for the observed effects of AWD. Our proposed model based on the involvement of significantly altered protein expression profile is shown in Fig. 7 and discussed below.
AWD treatment activated important signaling molecules in root cells including 14-3-3 protein (loc_os02g36974.1), cysteine-rich receptor-like protein kinase (RLKs; loc_os04g56430.1), and abscisic stress-ripening protein (ABA; loc_os11g06720.1). RLKs (loc_os04g56430.1) transmit stress signals into cells interior machinery through coordination of a membrane spanning segment and an extracellular cytoplasmic domain (Walker 1994). In addition, 14-3-3 proteins (loc_os02g36974.1) generally act as activators, repressors, adapters, or chaperones which interact physically with target (client) proteins to execute signal transduction (Chung and others 1999; Sehnke and others 2002). ABA (loc_os11g06720.1) hormonal signaling in root triggers root cell growth to maximize water and nutrient uptake (Saab and others 1990; Schoonheim and others 2007). Through long-distance signaling, ABA regulates stomatal conductance, decreases transpirational water loss (Zhang and Davies 1991; Schroeder and others 2001), and represses expression of certain photosynthesis-related gene families to inhibit photosynthesis and initiates programmed cell death (Bartholomew and others 1991).
Along with signaling activation, root cell longevity was also upregulated through redox homeostasis and the defense network. Key antioxidant defense enzymes involved in the plant cell detoxification system such as the cytosolic/mitochondrial glutathione S-transferases (GSTs; EC 2.5.1.18; loc_os10g38489.1, loc_os03g04240.1) and monodehydroascorbate reductase (MDAR; loc_os08g44340.1) were upregulated under moderate drying. This upregulated antioxidant defense network detoxifies toxic products of lipid oxidation and S-glutathiolated proteins generated by oxidative stress (Awasthi and others 2005; Dixon and others 2002). In addition to redox homeostasis, these enzymes have also been involved in signal transduction pathways by interacting with important signaling proteins in a non-enzymatic way (Dixon and others 2002; Laborde 2010).
Under MW, upregulation of monodehydroascorbate reductase (MDAR; loc_os09g39380.1) and ascorbate peroxidase (APx; EC, 1.11.1.11; loc_os07g49400.2, loc_os03g17690.1) enzymes may regulate the glutathione-ascorbate cycle (GAC) to detoxify ROS (H2O2 into H2O) in root cells. The coupling of APx and MDA reductase can scavenge H2O2 using ascorbate as a specific electron donor (Bloom and others 2004; Teixeira and others 2006; Leterrier and others 2005).
Protein disulfide isomerase (PDI; loc_os11g09280.1) was upregulated under both MD and MW plays an important role in nascent protein disulfide formation, the rearrangement of incorrect disulfides, and thiol-dependent redox reactions (Lundstrom and Holmgren 1990; Freedman and others 1998). It also works as an essential folding catalyst and chaperone (Laboissiere and others 1995). Upregulation of PDI and DnaK family/70 kDa hsp stress-specific proteins (loc_os02g53420.1, loc_os11g47760.1) may assist maintenance of root cell proteins in their functional conformations and avoid the aggregation of non-native proteins (Wang and others 2004). Being a 70 kDa hsp distinct multidomain structure (ATP-binding site, the peptide recognition and binding site), it also plays a regulatory role in energy metabolism (Kiang and Tsokos 1998) through protein translocation, signal transduction, and transcriptional activation (Vierling 1991; Miemyk 1997; Bukau and Horwich 1998). As a molecular chaperone, prefoldin protein (loc_os03g43020.1) upregulation may further promote protein network stability (Smith and others 2000).
Increased expression of enzymatic and non-enzymatic antioxidant defense pathways including MDAR, APx, GSTs, and PDI as discussed above, may scavenge excessive ROS and maintain redox homeostasis. ROS at controlled levels serve as second messengers in signal transduction cascades during sensing of environmental change. This results in appropriate adjustments to gene expression, metabolism, and physiology (Foyer and Noctor 2005) as part of root cell defense.
Amino acid concentration is positively correlated with the rate of N uptake and remobilization (Lalonde and others 2004), AWD treatment upregulated mitochondrial cysteine synthase (loc_os01g74650.3), and ketol-acid reductoisomerase (loc_os01g46380.1) enzymes involved in important amino acid biosynthesis cysteine, valine, leucine, isoleucine etc. (Lithgow and others 2004; Tyagi and others 2005). Glutamine synthetase (loc_os03g12290.1) upregulation enhances synthesis of glutamine (Gln), the predominant free amino acid in phloem for N remobilization (Simpson and Dalling 1981). This shift in amino acid balance has the potential to trigger programmed N uptake and the remobilization network (Good and others 2004; Herrera-Rodriguez and others 2006). Aminotransferase (loc_os10g25130.1) further catalyzed transamination reactions and triggered amino acids remobilization (Beatty and others 2009). The cysteine-containing molecules also contribute to enhance root antioxidant defense properties (Carmel-Harel and Storz 2000; Bulaj and others 1998).
Induction of MW after MD stress induced upregulation of phosphoglycerate mutase (PGM; loc_os01g60190.1), phosphoglycerate kinase (loc_os06g45710.1), and pyruvate phosphate dikinase (PPDK; loc_os03g31750.1) involved in glycolysis and gluconeogenesis, respectively. In addition to the glycolysis and gluconeogenic pathway protein upregulation, we also detected jacalin-like lectin (JRLs; loc_os01g24710.4) upregulation under the MW cycle. JRLs have a carbohydrate binding site and are located in the cytoplasm and vacuole (Van Damme and others 2002).
The β-sheet of JRLs is non-covalently bound to the barrel which enlarges the sugar-binding pocket, thus facilitating the entrance of larger sugar moieties (Van Damme and others 2002; Arockia Jeyaprakash and others 2005), promoting remobilization. Lectins have been proposed as major storage proteins, however, many lectins in plant defense are well-documented as important components of plant innate immunity (Fernandez-del-Carmen and others 2013). Under MW, C remobilization triggered through coordinative expression of PGM, PPDK, and JRLs, might be partly responsible for greater grain size and higher yield.
Under AWD, a feedback on Calvin cycle activity due to inhibited photosynthesis causes soluble carbohydrate accumulation (Mirzaei and others 2013). ATP provides the energy for remobilization of these assimilates to the grain. In our studies, we found upregulation of ATP synthase (EC 3.6.3.14; loc_os09g08910.1, loc_os06g37180.1), as well as TCA cycle enzymes such as lactate/malate dehydrogenase (loc_os05g49880.1, loc_os10g33800.1) and succinyl-CoA ligase (loc_os02g40830.2).
The ATPase activity has a significant positive correlation with the accumulation of grain photoassimilates in the form of starch and total sugar contents in wheat (Zhou and others 2009), and rice grain filling (Qiyu and Zhiqiang 1989). In root, H+-ATPase abundance in epidermal, endodermis, and phloem cells (Parets-Soler and others 1990; Jahn and others 1998) establishes the proton gradient for the membrane energization used for transport processes including root nutrient uptake and photoassimilate remobilization. During grain filling, upregulation of the TCA cycle may lead to improved uptake and transport of metabolites to grains.
The TCA cycle can also provide energy for the activated H+-ATPase detected in our results. This enzyme extrudes protons and decreases the apoplastic pH activating enzymes involved in cell wall loosening (Hager 2003). Further, ABA (abscisic stress-ripening protein) also triggers auxin transport in the root tip which activates proton secretion in the root tip to maintain root elongation and root hair development under moderate water stress (Xu and others 2013). The transported auxin also activates the plasma membrane H+-ATPase to release more protons along the root tip and trigger cell growth (Xu and others 2013).
Dirigent (78 kD native protein) upregulation triggers the stereoselective bimolecular phenoxy radical coupling, especially in lignin and lignan biosynthesis (Davin and Lewis 1995; Davin and others 1997). Lignin strengthens the cell wall structure along with secondary cell wall formation to maintain functional stability under stress and assists long-distance water conductance (Denness and others 2011). Secretory protein (loc_os10g34920.1) also supports the synthesis of many complex cell wall components (Bassham and others 2008). The TCA cycle and root hormonal activation as discussed above through MD and MW alternation might improve root ATP energy level, respiratory network, secondary cell wall synthesis, and root dry weight accumulation (Fig. 1), along with uptake of metabolites.
A switch from central carbon metabolism to alcoholic fermentation may be important for starch synthesis and accumulation during grain development (Xu and others 2008). Upregulation of dehydrogenase (loc_os11g10510.1) and thiamine pyrophosphate enzyme (loc_os05g39310.1; loc_os05g39320.1) transitioned from cell growth and differentiation to starch synthesis.
Abscisic acid stress ripening (ASR1; loc_os11g06720.1) is also proposed to have a role in re-routing the metabolites from source to sink leading to the senescence of the source organs as it can serve as a transcription factor of the gene encoding a hexose transporter during ripening (Fillion and others 1999). MD upregulated grain setting defect 1 (GSD1), encodes a putative remorin protein (loc_os10g36000, loc_os04g45070). The expression level of GSD1 may serve as a means by which the distribution of photoassimilates among different tissues is regulated, having important implications for improving rice yield (Gui and others 2014). These proteins serve as the traffic control centers of the phloem in directing the transport of photoassimilates (Oparka and Turgeon 1999).
In conclusion, adoption of the AWD treatment at the grain-filling stage resulted in improved grain yield. We hypothesis that the underlying molecular mechanism of yield enhancement is based on upregulation of GF14-3-3, ABA, and RLKs signaling molecules which directly or indirectly induced root growth through downstream signaling and triggered systematic senescence through long-distance signaling. Dirigent and secretory protein activation maintained structural defense of root cells through secondary cell wall synthesis. Redox homeostasis triggered by the antioxidant defense proteins may protect root cell membrane lipids, proteins, and DNA/RNA. Protein disulfide isomerase and 70 kDa heat shock protein may stabilize root protein functional conformation. A feedback on the Calvin cycle activity due to inhibited photosynthesis may upregulate glycolysis and gluconeogenesis to enhance soluble carbohydrates accumulation and remobilization through jacalin-like lectin upregulation. In addition, cysteine synthase, ketol-acid reductoisomerase, glutamine synthetase, and aminotransferase activation may shift the amino acid balance and exploit N uptake. ATP synthase, lactate/malate dehydrogenase, and succinyl-CoA ligase activation may increase the energy available in root cells for these C, N assimilates remobilization. Abscisic acid stress ripening, grain setting defect 1 (GSD1), and dirigents may serve as the traffic control centers of the phloem in directing photoassimilate transport from source to sink, especially toward inferior spikelet grain filling, which might have helped to increase rice yield up to 10.23 g m−2 (Table 1) under AWD as compared to CI.
References
Arockia Jeyaprakash A, Jayashree G, Mahanta S, Swaminathan C, Sekar K, Surolia A, Vijayan M (2005) Structural basis for the energetics of jacalin-sugar interactions: promiscuity versus specificity. J Mol Biol 347(1):181–188
Awasthi YC, Ansari G, Awasthi S (2005) Regulation of 4-hydroxynonenal mediated signaling by glutathione S-transferases. Methods Enzymol 401:379–407
Bartholomew DM, Bartley GE, Scolnik PA (1991) Abscisic acid control of rbcS and cab transcription in tomato leaves. Plant Physiol 96(1):291–296
Bassham DC, Brandizzi F, Otegui MS, Sanderfoot AA (2008) The secretory system of Arabidopsis. The Arabidopsis Book/American Society of Plant Biologists 6
Beatty PH, Shrawat AK, Carroll RT, Zhu T, Good AG (2009) Transcriptome analysis of nitrogen efficient rice overexpressing alanine aminotransferase. Plant Biotechnol J 7(6):562–576
Belder P, Bouman B, Spiertz J, Lu G, Quilang E (2002) Water use of alternately submerged and nonsubmerged irrigated lowland rice. Water wise rice production IRRI-Plant Research International:51–61
Bloom A, Zwieniecki M, Passioura J, Randall L, Holbrook N, St Clair D (2004) Water relations under root chilling in a sensitive and tolerant tomato species. Plant Cell Environ 27(8):971–979
Bouman B (2002) Water-wise rice production, vol 1. International Rice Research Institute
Bouman B, Humphreys E, Tuong T, Barker R (2007) Rice and water. Adv Agron 92:187–237
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bukau B, Horwich AL (1998) The Hsp70 and Hsp60 chaperone machines. Cell 92(3):351–366
Bulaj G, Kortemme T, Goldenberg DP (1998) Ionization-reactivity relationships for cysteine thiols in polypeptides. Biochemistry 37(25):8965–8972
Cabangon R, Castillo E, Bao L, Lu G, Wang G, Cui Y, Tuong T, Bouman B, Li Y, Chen C (2001) Impact of alternate wetting and drying irrigation on rice growth and resource-use efficiency. Barker R, Loeve R, Li YH, Tuong TP (eds), 55–80
Carmel-Harel O, Storz G (2000) Roles of the glutathione-and thioredoxin-dependent reduction systems in the Escherichia coli and Saccharomyces cerevisiae responses to oxidative stress. Ann Rev Microbiol 54(1):439–461
Carruthers I, Rosegrant MW, Seckler D (1997) Irrigation and food security in the 21st century. Irrigat Drain Syst 11(2):83–101
Cassman KG, Pingali PL (1995) Intensification of irrigated rice systems: learning from the past to meet future challenges. Geo J 35(3):299–305
Chung HJ, Sehnke PC, Ferl RJ (1999) The 14-3-3 proteins: cellular regulators of plant metabolism. Trends Plant Sci 4:367–371
Davin LB, Lewis NG (1995) Lignin and lignan biochemical pathways in plants: an unprecedented discovery in phenolic coupling. An Acad Bras Cienc Suppl 3(67):363–378
Davin LB, Wang HB, Crowell AL, Bedgar DL, Martin DM, Sarkanen S, Lewis NG (1997) Stereoselective bimolecular phenoxy radical coupling by an auxiliary (dirigent) protein without an active center. Science 275:362–366
Denness L, McKenna JF, Segonzac C, Wormit A, Madhou P, Bennett M, Mansfield J, Zipfel C, Hamann T (2011) Cell wall damage-induced lignin biosynthesis is regulated by a reactive oxygen species-and jasmonic acid-dependent process in Arabidopsis. Plant Physiol 156(3):1364–1374
Dixon DP, Davis BG, Edwards R (2002) Functional divergence in the glutathione transferase superfamily in plants identification of two classes with putative functions in redox homeostasis in Arabidopsis thaliana. J Biol Chem 277(34):30859–30869
Fernandez-del-Carmen A, Juarez P, Presa S, Granell A, Orzaez D (2013) Recombinant jacalin-like plant lectins are produced at high levels in Nicotiana benthamiana and retain agglutination activity and sugar specificity. J Biotechnol 163(4):391–400
Fillion L, Ageorges A, Picaud S, Coutos-Thévenot P, Lemoine R, Romieu C, Delrot S (1999) Cloning and expression of a hexose transporter gene expressed during the ripening of grape berry. Plant Physiol 120(4):1083–1094
Foyer CH, Noctor G (2005) Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ 28(8):1056–1071
Freedman RB, Dunn AD, Ruddock LW (1998) Protein folding: a missing redox link in the endoplasmic reticulum. Curr Biol 8(13):468–470
Godfray HCJ (2010) Food security: the challenge of feeding 9 billion people. Science 327(5967):812–818
Good AG, Shrawat AK, Muench DG (2004) Can less yield more? Is reducing nutrient input into the environment compatible with maintaining crop production? Trends Plant Sci 9(12):597–605
Gui J, Liu C, Shen J, Li L (2014) Grain setting defect1, encoding a remorin protein, affects the grain setting in rice through regulating plasmodesmatal conductance. Plant Physiol 166(3):1463–1478
Hager A (2003) Role of the plasma membrane H+-ATPase in auxin-induced elongation growth: historical and new aspects. J Plant Res 116(6):483–505
Herrera-Rodriguez MB, Maldonado JM, Perez-Vicente R (2006) Role of asparagine and asparagine synthetase genes in sunflower (Helianthus annuus) germination and natural senescence. J Plant Physiol 163(10):1061–1070
Jahn T, Baluska F, Michalke W, Harper JF, Volkmann D (1998) Plasma membrane H + ATPase in the root apex: evidence for strong expression in xylem parenchyma and asymmetric localization within cortical and epidermal cells. Physiol Plant 104(3):311–316
Khush GS (2005) What it will take to feed 5.0 billion rice consumers in 2030. Plant Mol Biol 59(1):1–6
Kiang JG, Tsokos GC (1998) Heat shock protein 70 kDa: molecular biology, biochemistry, and physiology. Pharmacol Ther 80(2):183–201
Laboissiere MC, Sturley SL, Raines RT (1995) The essential function of protein-disulfide isomerase is to unscramble non-native disulfide bonds. J Biol Chem 270(47):28006–28009
Laborde E (2010) Glutathione transferases as mediators of signaling pathways involved in cell proliferation and cell death. Cell Death Differ 17(9):1373–1380
Lalonde S, Wipf D, Frommer WB (2004) Transport mechanisms for organic forms of carbon and nitrogen between source and sink. Annu Rev Plant Biol 55:341–372
Leterrier M, Corpas FJ, Barroso JB, Sandalio LM, Luis A (2005) Peroxisomal monodehydroascorbate reductase. Genomic clone characterization and functional analysis under environmental stress conditions. Plant Physiol 138(4):2111–2123
Lithgow JK, Hayhurst EJ, Cohen G, Aharonowitz Y, Foster SJ (2004) Role of a cysteine synthase in Staphylococcus aureus. J Bacteriol 186:1579–1590
Lundstrom J, Holmgren A (1990) Protein disulfide-isomerase is a substrate for thioredoxin reductase and has thioredoxin-like activity. J Biol Chem 265(16):9114–9120
Miemyk J (1997) The 70 kDa stress-related proteins as molecular chaperones. Trends Plant Sci 2(5):180–187
MingMing H, Fan H, Kai W, FengYun Z (2010) Effects of different kinds of exogenous auxin on the growth of rice roots under cadmium stress. Agric Sci Technol-Hunan 11(7):45–48
Mirzaei M, Soltani N, Sarhadi E, George IS, Neilson KA, Pascovici D, Shahbazian S, Haynes PA, Atwell BJ, Salekdeh GH (2013) Manipulating root water supply elicits major shifts in the shoot proteome. J Proteome Res 13(2):517–526
Oparka KJ, Turgeon R (1999) Sieve elements and companion cells-traffic control centers of the phloem. Plant Cell 11:739–750
Osaki M, Shinano T, Matsumoto M, Zheng T, Tadano T (1997) A root-shoot interaction hypothesis for high productivity of field crops. In: Plant nutrition for sustainable food production and environment pp 669–674
Parets-Soler A, Pardo JM, Serrano R (1990) Immunocytolocalization of plasma membrane H + -ATPase. Plant Physiol 93(4):1654–1658
Patel C, Ghilyal B, Tomar V (1984) Nutrient flow rates in rice roots under varying drainage conditions. Plant Soil 77(2):243–252
Qiyu H, Zhiqiang W (1989) The relationship between the ATPase activity of the rice spike and the grain filling. J Fujian Agric For Univ (Nat Sci Edit) 4:001
Saab IN, Sharp RE, Pritchard J, Voetberg GS (1990) Increased endogenous abscisic acid maintains primary root growth and inhibits shoot growth of maize seedlings at low water potentials. Plant Physiol 93(4):1329–1336
Schoonheim PJ, Sinnige MP, Casaretto JA, Veiga H, Bunney TD, Quatrano RS, de Boer AH (2007) 14-3-3 adaptor proteins are intermediates in ABA signal transduction during barley seed germination. Plant J 49(2):289–301
Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D (2001) Guard cell signal transduction. Annu Rev Plant Biol 52(1):627–658
Sehnke PC, DeLille JM, Ferl RJ (2002) Consummating signal transduction the role of 14-3-3 proteins in the completion of signal-induced transitions in protein activity. Plant Cell 14:S339–S354
Simpson RJ, Dalling MJ (1981) Nitrogen redistribution during grain growth in wheat (Triticum aestivum L.): III. Enzymology and transport of amino acids from senescing flag leaves. Planta pp 447–456
Smith A, Datta SP, Smith GH, Campbell PN, Bentley R, McKenzie H (2000) Oxford dictionary of biochemistry and molecular biology. Oxford University Press, Oxford
Teixeira FK, Menezes-Benavente L, Galvao VC, Margis R, Margis-Pinheiro M (2006) Rice ascorbate peroxidase gene family encodes functionally diverse isoforms localized in different subcellular compartments. Planta 224(2):300–314
Tyagi R, Lee YT, Guddat LW, Duggleby RG (2005) Probing the mechanism of the bifunctional enzyme ketol-acid reductoisomerase by site-directed mutagenesis of the active site. FEBS J 272:593–602
Van Damme EJ, Hause B, Hu J, Barre A, Rouge P, Proost P, Peumans WJ (2002) Two distinct jacalin-related lectins with a different specificity and subcellular location are major vegetative storage proteins in the bark of the black mulberry tree. Plant Physiol 130(2):757–769
Vierling E (1991) The roles of heat shock proteins in plants. Annu Rev Plant Biol 42(1):579–620
Von Braun J (2007) The world food situation: new driving forces and required actions. International Food Policy Research Institute, Washington
Walker JC (1994) Structure and function of the receptor-like protein kinases of higher plants. Plant Mol Biol 26(5):1599–1609
Wang W, Vinocur B, Shoseyov O, Altman A (2004) Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci 9(5):244–252
Wang W, Vignani R, Scali M, Cresti M (2006) A universal and rapid protocol for protein extraction from recalcitrant plant tissues for proteomic analysis. Electrophor–Int J 27(13):2782–2786
Xu SB, Li T, Deng ZY, Chong K, Xue Y, Wang T (2008) Dynamic proteomic analysis reveals a switch between central carbon metabolism and alcoholic fermentation in rice filling grains. Plant Physiol 148(2):908–925
Xu W, Jia L, Shi W, Liang J, Zhou F, Li Q, Zhang J (2013) Abscisic acid accumulation modulates auxin transport in the root tip to enhance proton secretion for maintaining root growth under moderate water stress. New Phytol 197(1):139–150
Yang J, Zhang J (2006) Grain filling of cereals under soil drying. New Phytol 169(2):223–236
Yang J, Zhang J, Wang Z, Zhu Q, Liu L (2001a) Water deficit induced senescence and its relationship to the remobilization of pre-stored carbon in wheat during grain filling. Agron J 93(1):196–206
Yang J, Zhang J, Wang Z, Zhu Q, Wang W (2001b) Hormonal changes in the grains of rice subjected to water stress during grain filling. Plant Physiol 127(1):315–323
Yang C, Yang L, Yang Y, Ouyang Z (2004a) Rice root growth and nutrient uptake as influenced by organic manure in continuously and alternately flooded paddy soils. Agric Water Manag 70(1):67–81
Yang J, Zhang J, Wang Z, Xu G, Zhu Q (2004b) Activities of key enzymes in sucrose-to-starch conversion in wheat grains subjected to water deficit during grain filling. Plant Physiol 135(3):1621–1629
Zhang J, Davies WJ (1991) Antitranspirant activity in xylem sap of maize plants. J Exp Bot 42(3):317–321
Zhang Z, Chen J, Li Z (2012) Differential proteomic expressions between superior and inferior spikelets of rice in response to varied nitrogen treatments. Aust J Crop Sci 6:316–325
Zhou ZQ, Li JW, Deng XY, Wang LK, Mei FZ, Zou LP (2009) The ATPase activity in phloem cells and its relation to the accumulation of photo-assimilates in developing caryopsis during wheat grain filling. Sci Agric Sinica 7:2314–2325
Acknowledgments
This work was supported by Fujian-Taiwan Joint Innovative Centre for Germplasm Resources and cultivation of crop (Fujian 2011 Program, [2015] 75).
Author information
Authors and Affiliations
Corresponding author
Additional information
Zhong Li, Saadia Azeem, and Zhixing Zhang have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Z., Azeem, S., Zhang, Z. et al. Promising Role of Moderate Soil Drying and Subsequent Recovery Through Moderate Wetting at Grain-Filling Stage for Rice Yield Enhancement. J Plant Growth Regul 35, 838–850 (2016). https://doi.org/10.1007/s00344-016-9587-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-016-9587-0