Abstract
Nitric oxide (NO), hydrogen peroxide (H2O2), and salicylic acid (SA) are well-known signaling molecules that play multifaceted roles in the stress tolerance of plants; however, their interactions during stress alleviation have not been well studied. We investigated the possible regulatory role of NO in H2O2- and SA-induced reduction of oxidative damage in salt-exposed rice seedlings. For this purpose, hydroponically grown 14-day-old seedlings were pretreated with 100 μM H2O2 or 100 μM SA in the presence or absence of 100 μM hemoglobin (Hb, a potent NO scavenger) for 24 h followed by salt stress (200 mM NaCl) for 72 h. Salt stress significantly increased the levels of H2O2, malondialdehyde, and proline whereas H2O2 and SA pretreatment reduced the values of these parameters. H2O2 and SA pretreatment also inhibited salt-induced loss of total chlorophyll and relative water content. Histochemical detection of reactive oxygen species [ROS: superoxide (O ·−2 ) and H2O2] indicated evident oxidative burst in the seedlings stressed with salt alone. Salt stress modulated the non-enzymatic and enzymatic antioxidants differentially; however, H2O2 and SA treatment prior to salt stress enhanced these antioxidants compared with the salt-stressed seedlings alone. H2O2 and SA pretreated salt-stressed seedlings also showed higher induction of the methylglyoxal (MG) detoxification system. Endogenous NO content was elevated following H2O2 and SA pretreatment over the experimental period. Adding Hb reduced the level of NO and subsequently abolished the beneficial effects of H2O2 and SA. Our results, therefore, suggest that NO might be involved in H2O2- and SA-induced reduction of oxidative damage through the upregulation of the antioxidant defense and MG detoxification systems to confer salt tolerance in rice seedlings. The data are of considerable value in elucidating the biochemical mechanisms of salt-stress tolerance and will augment the goal of developing appropriate and efficient methods for crop protection in saline environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Abiotic stress, being a natural part of every ecosystem, represents a major environmental constraint that confines plant biodiversity and accounts for more than 50 % yield loss of most major crops (Wang et al. 2007). Among various abiotic stresses, salinity is considered one of the brutal stress factors because of its effect on the growth, development, and productivity of plants (Iqbal et al. 2014). High soil salinity imposes various deleterious effects on plants such as ion toxicity, osmotic stress, nutritional imbalances, and oxidative stress (Türkan and Demiral 2009; Han et al. 2014). Salinity, like most abiotic stresses, elicits excessive production of reactive oxygen species (ROS) such as hydrogen peroxide (H2O2), hydroxyl radical (HO·), superoxide radical (O ·2 ) and singlet oxygen (1O2). Excess ROS are highly toxic to cellular components causing oxidative stress and thereby exacerbating cellular damage (Apel and Hirt 2004).
To minimize oxidative stress, plants have developed various stress tolerance mechanisms; however, the most important is the antioxidative system that encompasses both the enzymatic and non-enzymatic arsenals. The enzymatic mechanism underlies the interplay between various enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), glutathione S-transferase (GST), and the ascorbate–glutathione cycle enzymes: ascorbate peroxidase (APX), dehydroascorbate reductase (DHAR), monodehydroascorbate reductase (MDHAR) and glutathione reductase (GR). Non-enzymatic antioxidants such as ascorbic acid (AsA) and glutathione (GSH) can quench all kinds of ROS directly and also assist the enzymatic system to efficiently detoxify ROS (Gill and Tuteja 2010).
Methylglyoxal (MG) is a cytotoxic compound generally formed during amino acid, carbohydrate, and lipid metabolisms (Kaur et al. 2014). At the onset of abiotic stresses, cellular metabolism upturns and MG production reaches a lethal level (Yadav et al. 2005; Hossain et al. 2009). To protect cells from MG toxicity, plants possess an efficient glyoxalase system which detoxifies MG with the help of glyoxalase I (Gly I), glyoxalase II (Gly II), and GSH. Under stress conditions, glyoxalases are differentially regulated and their overexpression in transgenic plants confers significant tolerance to multiple abiotic stresses (Singla-Pareek et al. 2008; Álvarez Viveros et al. 2013 Mustafiz et al. 2014). A wealth of studies revealed that antioxidative and glyoxalase systems are intertwined, and their simultaneous induction offer increased resistance to abiotic stresses (El-Shabrawi et al. 2010; Upadhyaya et al. 2011; Mostofa and Fujita 2013).
In a saline environment, plants must respond and adapt their metabolism to survive in the harsh conditions. Acclimation under stress conditions depends on the plant’s ability to perceive the stimulus, transmit signals, and instigate biochemical changes that ultimately secures plant survival and reproduction (Hasegawa et al. 2000). Adaptation to oxidative stress is often correlated with enhanced antioxidant capacity. However, under severe stress conditions such as high salinity, antioxidant capacity may not be sufficient to withstand the damaging effect of oxidative stress (Gill and Tuteja 2010). Therefore, it is worth investigating the role of signal molecules that mediate the stress tolerance through enhancing antioxidant capacity. Among diverse signal molecules, hydrogen peroxide (H2O2), salicylic acid (SA), and nitric oxide (NO) have drawn significant attention in current research into plant stress biology.
H2O2, SA, and NO are involved in regulating a number of physiological processes and plant responses to several environmental stresses (Esim and Atici 2014; Miura and Tada 2014; Qiao et al. 2014). Numerous studies evinced that exogenous H2O2, SA, and NO exerted beneficial effects in alleviating salt-induced oxidative damage in various plant species (Xu et al. 2008; Li et al. 2011; Khan et al. 2014). In most cases, the protective mechanisms include the enhancement of antioxidant capacity against oxidative stress. They are also known to interact with each other and also with other signal molecules such as ABA, JA, Ca2+, and ethylene to instigate signaling cascades associated with the expression of ‘anti-stress’ genes (Saito et al. 2009; Petrov and Van Breusegem 2012; Qiao et al. 2014). It is well reported that SA induces the production of H2O2, and H2O2 can also modulate the biosynthesis of SA during various biotic and abiotic stresses (Jayakannan et al. 2015). Hence, whether H2O2 acts upstream or downstream of SA is still a matter of debate. On the other hand, H2O2 induces NO synthesis and accumulation, and NO may modulate H2O2 levels (Zhang et al. 2007; Neill et al. 2008). It is also hypothesized that NO signaling is based on interactions with plant hormones like SA (Song and Goodman 2001). SA may mediate or potentiate NO’s effects by altering the activity of various NO-regulated enzymes such as APX and CAT (Klessig et al. 2000). Therefore, a relationship/network must exist between these signal molecules and searching for the relationship between abiotic stress, H2O2, SA, and NO is challenging.
Despite numerous reports detailing the mechanism of NO in stress tolerance, the involvement of NO in H2O2- and SA-induced oxidative stress reduction is not properly demonstrated. Moreover, to the best of our knowledge, there is little information available on the role of NO induced by H2O2 and SA pretreatment in the modulation of the antioxidative and glyoxalase systems in rice seedlings under salt stress. In this study, we aimed to examine the role of NO in exogenous H2O2- and SA-mediated salt tolerance in rice seedlings. Our results provide strong evidence that H2O2 and SA pretreatment induces the production of NO, which then plays an important role in reducing salt-induced oxidative damage through modulating the antioxidant defense and glyoxalase systems.
Materials and methods
Plant materials, growth conditions and treatments
Rice (Oryza sativa L. cv. BR11) seeds were surface sterilized with 1 % (v/v) sodium hypochlorite solution for 20 min, washed with distilled water and imbibed for 24 h. The seeds were sown on plastic nets floating on distilled water in 250 mL plastic beakers and kept in the dark at 28 ± 2 °C for germination. After 48 h, uniformly germinated seeds were transferred to a growth chamber and grown in a commercial hydroponics solution (Hyponex, Japan). The hyponex consisted of 8 % N, 6.43 % P, 20.94 % K, 8 % S, 11.8 % Ca, 3.08 % Mg, 0.07 % B, 0.24 % Fe, 0.03 % Mn, 0.0014 % Mo, 0.008 % Zn, and 0.003 % Cu. The hyponex was diluted to 5000-fold and the pH was adjusted to 5.5. The nutrient solution was renewed every 3 days. Each plastic beaker contained about 60 rice seedlings. The seedlings were grown under controlled conditions (photon density: 100 µmol m−2 s−1, temperature: 26 ± 2 °C, RH: 65–70 %). Fourteen-day-old seedlings were pretreated with hyponex solution containing 100 μM SA or 100 μM hydrogen peroxide (H2O2) in the presence or absence of 100 μM hemoglobin (Hb, a potent NO scavenger) for 24 h. The roots of the pretreated and non-pretreated seedlings were then washed with distilled water and exposed to salt stress by applying 200 mM sodium chloride (NaCl) in the nutrient solution for 72 h. This salt concentration was selected based on a preliminary experiment where 200 mM NaCl application resulted in visible toxicity symptoms such as chlorosis and leaf rolling. This experiment consisted of nine treatments as follows: (1) control (Con), (2) 100 μM hydrogen peroxide (H2O2), (3) 100 μM SA, (4) 100 μM Hb, (5) 200 mM sodium chloride (NaCl), (6) 100 μM H2O2 + 200 mM NaCl (H2O2 + NaCl), (7) 100 μM H2O2 + 100 μM Hb + 200 mM NaCl (H2O2 + Hb + NaCl), (8) 100 μM SA + 200 mM NaCl (SA + NaCl), and (9) 100 μM SA + 100 μM Hb + 200 mM NaCl (SA + Hb + NaCl) (Supplemental Figure and Table). After 72 h of growth under the above conditions, the second leaf of rice seedlings was harvested to determine various physiological and biochemical parameters. Each treatment was replicated three times under the same experimental conditions.
Determining lipid peroxidation, hydrogen peroxide and proline content
Lipid peroxidation of the second leaves was measured by estimating malondialdehyde (MDA) according to the method of Heath and Packer (1968). Fresh leaf samples (0.5 g) were homogenized with 5 % (w/v) trichloroacetic acid (TCA) and centrifuged at 11,500g for 12 min. The supernatant was mixed with 20 % TCA containing 0.5 % TBA and heated at 95 °C for 30 min. MDA content was calculated by the difference in absorbance at 532 and 600 nm using an extinction coefficient of 155 mM−1 cm−1. Hydrogen peroxide (H2O2) was extracted by homogenizing 0.5 g of fresh leaf samples with K-phosphate buffer (pH 6.5) and determined after reaction with 0.1 % TiCl4 in 20 % H2SO4 following the method of Mostofa and Fujita (2013). Proline (Pro) content was determined according to the method of Bates et al. (1973).
Determining relative water content and total chlorophyll content
Relative water content (RWC) was determined as described by Mostofa et al. 2014a. To determine total chlorophyll (Chl) content, leaves (0.5 g) were homogenized in 10 mL 80 % (v/v) chilled acetone and centrifuged at 10,000g for 10 min. The absorbance of the supernatant was recorded at 645 and 663 nm against 80 % (v/v) acetone blank. Total Chl content was calculated according to the formula of Arnon (1949).
where V = volume of 80 % (v/v) acetone (mL), W = weight of sample (g).
Histochemical detection of reactive oxygen species (O ·−2 and H2O2)
Superoxide (O ·−2 ) and H2O2 were detected in rice leaves according to the method of Mostofa and Fujita (2013). Briefly, the second leaves were stained in 0.1 % nitroblue tetrazolium (NBT) or 1 % 3, 3-diaminobenzidine (DAB) solution for 12 h under dark and light, respectively. Incubated leaves were then decolorized by immersing in boiling ethanol to detect blue insoluble formazan (for O ·−2 ) or deep brown polymerization product (for H2O2). After cooling, photographs were taken by placing the leaves between two glass plates.
Estimating non-enzymatic antioxidants
Fresh leaves (0.5 g) were homogenized in 3 mL of ice-cold 5 % meta-phosphoric acid containing 1 mM EDTA and centrifuged at 11,500g for 12 min. Reduced and total AsA content were determined following the method of Dutilleul et al. (2003) with minor modifications. To estimate total AsA, the oxidized fraction was reduced by 0.1 M dithiothreitol. Reduced and total AsA content were assayed at 265 nm in 100 mM K-phosphate buffer (pH 7.0) with 1.0 U of ascorbate oxidase (AO). Oxidized ascorbate (DHA) = total AsA − reduced AsA. Based on enzymatic recycling, reduced glutathione (GSH), oxidized glutathione (GSSG), and total glutathione (GSH + GSSG) were determined according to the method of Griffiths (1980). GSSG was determined after removing GSH by 2-vinylpyridine derivatization. GSH was measured after subtracting the value of GSSG from total GSH.
Extracting and assaying enzymes
To extract enzymes, fresh leaf samples (0.5 g) were homogenized separately with a reaction mixture containing 50 mM K-phosphate buffer (pH 7.0), 100 mM KCl, 1 mM AsA, 5 mM β-mercaptoethanol, and 10 % (w/v) glycerol in pre-chilled mortars and pestles. The homogenate was centrifuged at 11,500g for 15 min and the resultant supernatants were collected to analyse enzyme activities and protein content. All procedures were performed at 0–4 °C.
Superoxide dismutase (EC 1.15.1.1) activity was estimated according to the method of El-Shabrawi et al. (2010), which is based on a xanthine–xanthine oxidase system. The reaction mixture contained K-phosphate buffer (50 mM), NBT (2.24 mM), CAT (0.1 units), xanthine oxidase (0.1 units), xanthine (2.36 mM), and enzyme extract. SOD activity was expressed as units (that is, amount of enzyme required to inhibit NBT reduction by 50 %) min−1 mg−1 protein. CAT (EC 1.11.1.6) activity was measured according to the method of Mostofa and Fujita (2013). APX (EC 1.11.1.11) activity was determined by monitoring the decrease in absorbance at 290 nm as AsA was oxidized, according to the method of Nakano and Asada (1981). MDHAR (EC 1.6.5.4) activity was measured by using 1 U of AO and the oxidation rate of NADPH was determined at 340 nm (Hossain et al. 1984). DHAR (EC 1.8.5.1) activity was measured by monitoring the formation of AsA from DHA at 265 nm using GSH (Nakano and Asada 1981). GR (EC 1.6.4.2) activity was measured by monitoring the decrease in the absorbance of NADPH at 340 nm for GSSG-dependent oxidation of NADPH, as described by Foyer and Halliwell (1976). GST (EC 2.5.1.18) activity was determined according to the method of Hossain et al. (2013b). GPX (EC: 1.11.1.9) activity was measured as described by Elia et al. (2003) using H2O2 as a substrate. Gly I (EC 4.4.1.5) assay was carried out according to the method of Hossain et al. (2009) using MG as a substrate. Gly II (EC 3.1.2.6) activity was determined according to the method of Hossain et al. (2013a) by monitoring the formation of GSH at 412 nm. Protein content was determined following the method of Bradford (1976) using bovine serum albumin (BSA) as a standard.
Estimating nitric oxide content
Nitric oxide (NO) generation was determined by estimating nitrite (NO2 −) content following the method of Zhou et al. (2005). Leaves (0.5 g) were homogenized in a mortar and pestle with 3 mL of 50 mM ice-cold acetic acid buffer (pH 3.6, containing 4 % zinc diacetate). The homogenates were centrifuged at 11,500g for 15 min at 4 °C. The supernatant was collected. The pellet was washed with 1 mL of extraction buffer and centrifuged as before. The two supernatants were combined and 0.1 g of charcoal was added for neutralization. After vortex and filtration, the filtrate was leached and collected. The mixture of 1 mL of filtrate and 1 mL of the Greiss reagent (1 % sulfanilamide and 0.1 % N-1-napthylethylenediamine dihydrochloride in 5 % phosphoric acid solution) was incubated at room temperature for 30 min. Absorbance of the reaction mixture was read at 540 nm and the content of NO was determined from a calibration curve developed with sodium nitrite (NaNO2) as standard.
Statistical analysis
The data were subjected to one-way analysis of variance (ANOVA) and different letters indicate significant differences between treatments at p < 0.05, according to Duncan’s multiple range test (DMRT) using IRRISTAT version 3. Data represented in the table and figures are means ± standard deviations of three replicates for each treatment.
Results
Oxidative stress
Hydrogen peroxide (H2O2) content and lipid peroxidation
The degree of oxidative injury of tissues is related to the level of endogenous H2O2. In this experiment, salt exposure (200 mM, 72 h) increased the level of H2O2 by 99 % compared with control (Table 1). On the other hand, 100 μM SA and 100 μM H2O2 treatment prior to salt stress significantly reduced the level of H2O2 compared with the seedlings stressed with salt alone. To ascertain the role of NO in H2O2- and SA-induced salt tolerance, we used Hb as a NO scavenger. Applying 100 μM Hb with SA and H2O2 increased the level of H2O2 by 70 and 78 %, respectively in salt-exposed seedlings (Table 1) compared with control. H2O2 pretreatment also increased the level of H2O2 by 16 % compared with control whereas in the case of SA and Hb, the level remained at the control level.
Malondialdehyde content was measured as the indicator of the extent of lipid peroxidation in the rice leaves. Salt stress increased the level of MDA by 157 % compared with control (Table 1). However, pretreatment with SA and H2O2 significantly decreased the salt-induced MDA level. In comparison to control, applying Hb with SA and H2O2 resulted in a significantly higher level of MDA in salt-exposed rice seedlings. The level of H2O2 in H2O2 + Hb + NaCl and SA + Hb + NaCl groups was even 8 and 14 % higher than that of the salt-stressed only seedlings, respectively (Table 1). SA, H2O2, and Hb pretreatment did not affect the level of MDA compared with control in the non-stressed condition.
Reactive oxygen species (ROS: O ·−2 and H2O2)
Reactive oxygen species (O ·−2 and H2O2) were overproduced in the second leaves of the salt-stressed rice seedlings (Fig. 1a, b). NBT staining was used to confirm salt-induced overproduction of O ·−2 . Compared with the control group, an increased amount of O ·−2 was observed as scattered dark blue spots in the leaf plates of the salt-stressed only seedlings (Fig. 1a). Similarly, DAB staining of the leaves was performed to show H2O2 accumulation. A marked increase in brown polymerization products, which indicates the over-accumulation of H2O2, was observed in the salt-stressed seedlings compared with the control group (Fig. 1b). However, SA and H2O2 treatment prior to salt stress considerably diminished O ·−2 and H2O2 accumulation in the leaves of the salt-stressed seedlings. On the other hand, applying Hb abolished the capacity of SA and H2O2 to reduce the accumulation of O ·−2 and H2O2 in the salt-stressed seedlings (Fig. 1a, b).
Effect of exogenous H2O2 and SA on O ·−2 (a) and H2O2 (b) production in rice seedlings with or without NaCl stress. Con, NaCl, SA, H2O2, SA + NaCl, SA + Hb + NaCl, H2O2 + NaCl, and H2O2 + Hb + NaCl correspond to control, 200 mM NaCl, 100 μM SA, 100 μM H2O2, 100 μM SA + 200 mM NaCl, 100 μM SA + 100 μM Hb + 200 mM NaCl, 100 μM H2O2 + 200 mM NaCl, and 100 μM H2O2 + 100 μM Hb + 200 mM NaCl, respectively
Proline (Pro) content
Salt treatment increased the level of Pro by 669 % compared with control. On the other hand, SA and H2O2 treatment prior to salt stress significantly reduced the level of Pro compared with the seedlings stressed with salt alone (Table 1). Applying Hb with SA and H2O2 resulted in a noticeable increase in Pro level and it was 1169 and 1508 % higher than the control level. The combination of H2O2, Hb, and NaCl (H2O2 + Hb + NaCl) resulted in the highest level of Pro among the treatments. SA, H2O2, and Hb pretreatment did not affect the level of Pro compared with control in the non-stressed condition (Table 1).
Relative water content (RWC)
Relative water content decreased by 17 % in salt-stressed seedlings compared with control (Table 1). However, SA and H2O2 pretreatment inhibited the loss of RWC in the salt-stressed seedlings and the loss was only 7 and 5 %, respectively compared with control. Applying Hb with SA and H2O2 decreased RWC by 25 and 23 %, respectively in the salt-stressed seedlings compared with control (Table 1). SA, H2O2, and Hb pretreatment did not affect RWC compared with control in the non-stressed condition.
Total chlorophyll (Chl) content
Total Chl content decreased by 16 % in the salt-stressed seedlings compared with control (Table 1). However, SA and H2O2 treatment prior to salt exposure significantly rescued the level of total Chl compared with the salt-stressed only seedlings. Applying Hb with SA and H2O2 decreased total Chl content by 25 and 15 %, respectively in the salt-stressed seedlings compared with control. The loss of total Chl was pronounced in the combination of SA, Hb, and NaCl (SA + Hb + NaCl). SA, H2O2, and Hb pretreatment did not affect total Chl content in the non-stressed condition (Table 1).
Non-enzymatic antioxidants
Ascorbic acid (AsA) and AsA/DHA ratio
Salt exposure decreased the level of AsA by 49 % compared with control (Table 2). SA and H2O2 treatment prior to salt stress showed 11 and 18 % loss of AsA level compared with the salt-stressed only seedlings, respectively (Table 2). Therefore, SA pretreatment maintained higher AsA level than that of H2O2 pretreatment. Applying Hb with SA and H2O2 was not effective in rescuing the loss of the total AsA level in the salt-stressed seedlings. Moreover, the level of AsA in H2O2 + Hb + NaCl group was 18 % lower than that of salt-stressed only seedlings. SA pretreatment did not affect the level of AsA whereas H2O2 pretreatment increased AsA level by 5 % compared with control in the non-stress condition. The changes in the ratio of reduced to oxidized ascorbic acid (AsA/DHA) showed a significant decrease in the salt-stressed only seedlings compared with control (Table 2). Although the AsA/DHA ratios in the H2O2- and SA-pretreated seedlings were lower than control, it was significantly higher than the salt-stressed only seedlings. Applying Hb with H2O2 and SA also resulted in a lower AsA/DHA ratio compared with control and the H2O2- and SA-pretreated salt-stressed seedlings.
Glutathione (GSH) and GSH/GSSG ratio
GSH content increased by 84 % in response to salt stress compared with control (Table 2). H2O2 and SA pretreatment did not affect the level of GSH in the non-stressed seedlings. However, in stress conditions the GSH level significantly decreased with H2O2 and SA pretreatment but the level was 30 and 58 % higher than that of control (Table 2). Applying Hb with H2O2 and SA pretreatment significantly increased the level of GSH and the level was even 10 and 19 % higher than in the salt-stressed only seedlings, respectively. The ratio of reduced to oxidized GSH (GSH/GSSG) showed a significant decrease upon salt stress compared with control (Table 2). On the other hand, H2O2 and SA pretreatment maintained significantly higher GSH/GSSG ratios upon exposure to salt stress. Applying Hb with H2O2 and SA pretreatment resulted in a significant lower GSH/GSSG ratios compared with control, and the SA- and H2O2-pretreated salt-stressed seedlings. H2O2 pretreatment also significantly reduced the GSH/GSSG ratio compared with control in the non-stressed condition (Table 2).
Enzymatic antioxidants
Activities of ascorbate–glutathione cycle enzymes (APX, MDHAR, DHAR, GR)
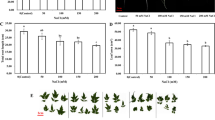
Ascorbate peroxidase activity increased by 36 % in response to salt stress compared with control (Fig. 2a). H2O2 and SA treatment prior to salt stress further intensified the activity of APX and it was 24 and 9 % higher than in the salt-stressed seedlings. On the other hand, applying Hb with H2O2 and SA pretreatment markedly diminished the activity of APX in the salt-stressed seedlings, where the decrease was more prominent with SA pretreatment. Notably, APX activity also increased by 19 % in the H2O2-pretreated non-stressed seedlings compared with control.
Effect of exogenous H2O2 and SA on the ascorbate–glutathione cycle enzyme (APX, MDHAR, DHAR and GR) activities in rice seedlings with or without NaCl stress. Vertical bars represent standard deviation of the mean (n = 3). Different letters indicate significant differences between treatments at p < 0.05, according to DMRT
Monodehydroascorbate reductase activity increased by 59 % in response to salt stress compared with control (Fig. 2b). On the other hand, H2O2 and SA pretreatment prior to salt stress was not effective in increasing MDHAR activity in the salt-stressed seedlings. However, applying Hb with H2O2 and SA pretreatment markedly decreased (26 and 38 %, respectively) the activity of MDHAR in the salt-stressed seedlings compared with the seedlings stressed with salt alone.
Dehydroascorbate reductase activity also increased by 65 % in response to salt stress compared with control (Fig. 2c). H2O2 pretreatment did not increase DHAR activity, whereas SA pretreatment further increased the activity in the salt-stressed seedlings. Applying Hb with H2O2 increased DHAR activity even higher than in the salt-stressed only seedlings, but it decreased significantly when Hb was applied with SA. Notably, DHAR activity increased by 39 and 50 % in the seedlings pretreated with SA and H2O2 under non-stress conditions.
Glutathione reductase activity increased by 45 % in response to salt stress compared with control (Fig. 2d). H2O2 and SA treatment prior to salt stress did not alter GR activity compared with the salt-stressed only seedlings; however, the activity was 42 and 30 % higher than that of control. On the other hand, GR activity remained the same as with the salt-stressed seedlings following application of Hb with H2O2 and SA prior to salt stress.
Activities of SOD, CAT, GPX, GST enzymes
Superoxide dismutase activity increased by 29 % in response to salt stress compared with control (Fig. 3a). On the other hand, H2O2 and SA treatment prior to salt stress significantly decreased the activity of SOD compared with the salt-stressed only seedlings; however, the activity was 13 and 17 % higher than that of control. Applying Hb with H2O2 and SA pretreatment prior to salt stress did not increase SOD activity in the salt-stressed seedlings. Under non-stress condition, SOD activity slightly decreased upon H2O2 and SA pretreatment (5 and 9 %, respectively) compared with control.
Catalase activity decreased by 17 % in the salt-stressed seedlings compared with control (Fig. 3b). In contrast, SA and H2O2 treatment prior to salt stress significantly restored the activity of CAT compared with the salt-stressed only seedlings. Applying Hb with H2O2 and SA pretreatment markedly decreased the activity of CAT (20 and 12 %, respectively) in the salt-stressed seedlings. Under non-stress condition, H2O2 pretreatment did not affect CAT activity, whereas SA pretreatment increased the activity by 11 % compared with control.
Glutathione peroxidase activity increased by 46 % in the salt-stressed seedlings compared with control (Fig. 3c). H2O2 and SA pretreatment prior to salt stress further intensified the activity of GPX, and it was 17 and 18 % higher than that of the seedlings stressed with salt alone. On the other hand, applying Hb with H2O2 and SA pretreatment markedly decreased the activity of GPX in the salt-stressed seedlings; however, the decrease was more prominent with SA pretreatment. Under non-stress condition, H2O2 pretreatment did not affect CAT activity, whereas SA pretreatment increased the activity by 56 % compared with control.
Glutathione S-transferase activity decreased by 23 % in the salt-stressed seedlings compared with control (Fig. 3d). On the other hand, H2O2 and SA treatment prior to salt stress significantly increased the activity of GST compared with control and the salt-stressed only seedlings. Applying Hb with H2O2 and SA pretreatment drastically decreased (30 and 41 %, respectively) the activity of CAT in the salt-stressed seedlings. Under non-stressed conditions, H2O2 and SA pretreatment did not alter the activity of CAT.
Glyoxalase cycle enzymes (Gly I, Gly II)
Glyoxalase I activity increased by 24 % in the salt-stressed seedlings compared with control (Fig. 4a). H2O2 and SA pretreatment prior to salt stress did not alter this activity compared with the salt-stressed only seedlings. However, applying Hb with H2O2 and SA pretreatment prior to salt stress decreased the activity of Gly I by 20 and 18 %, respectively compared with the seedlings stressed with salt alone. Under non-stress condition, H2O2 and SA pretreatment did not alter the activity of Gly I.
Effect of exogenous H2O2 and SA on the activities of the glyoxalase cycle enzymes (Gly I and Gly II) in rice seedlings with or without NaCl stress. Vertical bars represent standard deviation of the mean (n = 3). Different letters indicate significant differences between treatments at p < 0.05, according to DMRT
Glyoxalase II activity increased by 15 % in the salt-stressed seedlings compared with control (Fig. 4b). H2O2 and SA pretreatment prior to salt stress further increased Gly II activity (20 and 23 %, respectively) compared with the salt-stressed only seedlings. However, applying Hb with SA and H2O2 pretreatment decreased the activity of Gly II in the salt-stressed seedlings. Under non-stress condition, H2O2 and SA pretreatment increased Gly II activity by 15 and 25 %, respectively compared with control.
Nitric oxide (NO) content
To explore whether H2O2 and SA pretreatment induces NO generation, we determined the endogenous nitrite (NO2 −) level before pretreatment, after pretreatment, and after salt treatment. After 24 h of H2O2 and SA pretreatment, the NO level increased by 85 and 96 %, respectively compared with control (Fig. 5). However, applying Hb with H2O2 and SA pretreatment reduced the level of NO, and the level was similar to that of control. Salt treatment for 72 h decreased the level of NO by 12 % compared with control. The NO level in the SA- and H2O2-pretreated salt-stressed seedlings was 148 and 118 % higher, respectively than that of the salt-stressed only seedlings. On the other hand, applying Hb with SA and H2O2 pretreatment sustained the decrease in NO content after 72 h of salt treatment (Fig. 5).
Effect of exogenous H2O2 and SA on endogenous level of NO in the presence and absence of NO scavenger (Hb) in rice leaves with or without salt stress. NO level was measured before pretreatment, after pretreatment, and after treatment with NaCl. Vertical bars represent standard deviation of the mean (n = 3). Different letters indicate significant differences between treatments at p < 0.05, according to DMRT
Discussion
High soil salinity induces ionic toxicity and osmotic stress which impair plant growth and development by affecting normal plant metabolism. Furthermore, oxidative stress is a common consequence of salinity leading to membrane damage and cell death (Türkan and Demiral 2009). Plant responses to oxidative stress are complicated and involve many metabolic processes that are thought to be regulated by signal molecules such as NO, H2O2, and SA (Iqbal et al. 2014; Qiao et al. 2014). The individual roles of NO, H2O2, and SA have been extensively studied, but their interactions in salt tolerance are not properly understood. We investigated the role of H2O2 and SA in alleviating salt-induced oxidative damage and considered NO as a part of this protective mechanism. Our results showed that salt stress resulted in a significant accumulation of ROS (O ·−2 and H2O2) (Table 1; Fig. 1), which can cause lipid peroxidation, protein oxidation, and DNA mutation (Tanou et al. 2012). Increased ROS accumulation has also been observed in the leaves of Brassica and rice upon NaCl stress (Kumar et al. 2013; Khare et al. 2014, Mostofa et al. 2015). As expected, the MDA level concomitantly increased with the level of ROS, suggesting that salt stress triggered evident oxidative burst through membrane damage in leaf tissues, which corroborates previous findings (Mishra et al. 2013). In contrast, H2O2 and SA pretreatment significantly addressed salt-induced oxidative stress by lowering the accumulation of ROS and the levels of H2O2 and MDA. This study has shown that H2O2 and SA pretreatment increased NO content, which suppressed oxidative damage induced by salt stress. It has been reported that an Arabidopsis mutant, Atnoa1, with a reduced endogenous NO level is more sensitive to salt stress than wild type (Zhao et al. 2007). We observed that salinity decreased the level of NO and the seedlings subsequently suffered from more oxidative stress. On the other hand, H2O2- and SA-induced NO accumulation has been reported in maize leaves and Arabidopsis (Zhang et al. 2007; Sun et al. 2010), which also occurred in our study. Therefore, our current study proposed that H2O2- and SA-induced alleviation of oxidative damage is attributed to NO accumulation under salt stress. The protective effect of NO against oxidative stress has been reported in rice, cucumber, and cotton seedlings under salinity stress (Uchida et al. 2002; Lin et al. 2012; Liu et al. 2014).
Pro has been considered an important metabolite for acclimation of plants, especially during water deficit conditions (Iqbal et al. 2014). In this study, a salt-induced increase in Pro level exhibited an inverse relationship with RWC (Table 1), suggesting that the level of Pro accumulation reflects stressed symptoms rather than stress resistance. Decreased RWC further concentrated the ions in the leaf tissues, which ultimately bleaches photosynthetic pigments and diminishes photosynthesis in stressed plants (Khan et al. 2014). Our results showed that H2O2 and SA pretreatment reduced salt-induced Pro accumulation, which might be attributed to the increased activity of proline dehydrogenase induced by NO (Lopez-carrion et al. 2008). NO is also capable of maintaining water relations and Chl biosynthesis under a saline condition (Khan et al. 2012). Similar to our results, exogenous H2O2 and SA improved leaf water status and photosynthetic efficiency in salt-exposed mungbean and wheat seedlings (Nazar et al. 2011; Ashfaque et al. 2014).
Reactive oxygen species accumulation is an obvious consequence of stress reactions and plants possess an inherent antioxidant system to detoxify ROS. Interconversion of ROS and their subsequent elimination are mainly done by SOD, CAT, peroxidases, and ascorbate–glutathione cycle enzymes (Gill and Tuteja 2010). In the present study, higher SOD activity increased the conversion of O ·−2 to H2O2 under salt stress (Fig. 3a; Table 1), which corroborates the finding of Mishra et al. (2013) and indicated that SOD activity was prompted by an increased O ·−2 level. However, a profound decrease in CAT activity trapped H2O2 (Fig. 3b; Table 1) and exacerbated oxidative stress through the production of the deadly hydroxyl (OH·) radical in the presence of redox active metals (Apel and Hirt 2004). In contrast, H2O2 and SA pretreatment decreased SOD activity, which paralleled the level of O ·−2 (Figs. 1a, 3a) and suggested that H2O2- and SA-induced NO scavenged O ·−2 directly rather than modulated SOD activity. At the same time, elevating CAT activity by pretreating with H2O2 and SA prevented H2O2-mediated oxidative damage (Fig. 3b; Table 1). Our results, therefore, support the notion that NO itself is a ROS scavenger and modulator of the activities of antioxidant enzymes that help to overcome oxidative stress. GPX and GST are ubiquitous enzymes that play vital roles in cellular detoxification of H2O2, lipid hydroperoxides, and other endobiotic substrates by conjugating GSH (Roxas et al. 2000). We noted an increase in GPX activity and a decrease in GST activity (Fig. 3c, d), which corroborates previous report (Mostofa et al. 2015) and indicated that these enzymes are differentially regulated by salinity stress. Our recent reports (Mostofa et al. 2015; Hossain and Fujita 2013; Hossain et al. 2013b) and other literature (Roxas et al. 2000) indicate that higher GPX and GST activity correlates with enhanced salt tolerance. We observed a significant increase in GPX and GST activities following H2O2 and SA pretreatment, which indicated that both these enzymes increased GSH-dependent peroxide scavenging that leads to reduced oxidative damage.
It is well known that the ascorbate–glutathione cycle constitutes the key H2O2 detoxification pathway and its high efficiency is a requisite for oxidative stress tolerance. In this cycle, APX decomposes H2O2, and MDHAR, DHAR, and GR are involved in the regeneration of reduced AsA and GSH (Gill and Tuteja 2010). Several studies on salt-sensitive and salt-tolerant cultivars reported that the ascorbate–glutathione cycle responded differentially during salinity stress. Mishra et al. (2013) and Hossain et al. (2013a) reported that salt-tolerant species exhibited enhanced activity of this cycle in protecting against salt-induced oxidative stress. In contrast, Lee et al. (2013) observed increased enzyme activity in salt-sensitive rice cultivars, which is thought to be induced by oxidative-stress signaling associated with salt sensitivity rather than salt tolerance. In the current study, despite enhanced activity of the ascorbate–glutathione cycle enzymes, the H2O2 level remained elevated, indicating that inducing these enzymes might not be sufficient to reduce the higher oxidative load (Figs. 1, 2a–d; Table 1). On the other hand, a substantial decline in AsA content, the AsA/DHA and GSH/GSSG ratios, and a significant increase in GSH content (Table 2) suggested that salt stress perturbed the whole antioxidant system, which facilitated the accumulation of ROS. A decrease in AsA content and the AsA/DHA ratio was previously reported by Lee et al. (2013) and Mishra et al. (2013) in salt-sensitive rice cultivars. In line with our findings, a time- and dose-dependent progressive increase in GSH content was observed in Vicia faba seedlings (Younis et al. 2010) and rice cultivars (El-Shabrawi et al. 2010) under salt stress, which suggest that the cellular GSH level correlates with the intensity of stress. In contrast, H2O2 and SA pretreatment maintained a higher AsA level and AsA/DHA and GSH/GSSG ratios, further enhanced the activities of APX and DHAR, and reduced the activity of MDHAR, while it maintained GR activity as it was in the seedlings stressed with salt alone (Fig. 2a–d). Therefore, we assumed that H2O2 was reasonably detoxified by APX, and AsA regeneration was accomplished via DHAR activity instead of MDHAR. Reduced GR activity might have contributed to the decreased level of GSH in H2O2- and SA-pretreated salt-stressed seedlings (Fig. 2d; Table 2). However, we suggest that a coordinated and finely regulated action of all the enzymes of the ascorbate–glutathione cycle in conjunction with that of other ROS-processing enzymes are functional in H2O2- and SA-pretreated rice seedlings that helped to reduce the level of ROS and thereby alleviated salt-induced oxidative stress.
Previously, we have shown that applying H2O2 and SA enhanced the activities of glyoxalase enzymes in Brassica and rice under drought and copper stresses, respectively (Hossain and Fujita 2013; Mostofa and Fujita 2013). In this study, we found that pretreatment with H2O2 and SA maintained Gly I activity similar to salt stress but further enhanced Gly II activity, indicating their imperative roles in MG detoxification. We assumed that enhanced Gly II activity also contributed to maintaining the physiological level of GSH as well as a higher GSH/GSSG ratio, which facilitated the higher activities of APX, DHAR, GPX, and GST for scavenging ROS (Table 2; Figs. 2a, c, 3c, d). In line with our previous findings (Mostofa and Fujita 2013; Mostofa et al. 2014a, b, 2015), this result also suggests that H2O2 and SA pretreatment presumably assisted in cellular detoxification of ROS and MG and thereby renders the rice seedlings more tolerant to salt-induced oxidative stress.
The key feature of the current study is that when we used NO scavenger (Hb) together with H2O2 and SA during pretreatment, all the beneficial effects of SA and H2O2 were abolished. Reversal of activities of SOD, CAT, GPX, GST, APX, MDHAR and GR, and Gly I and Gly II under co-application of Hb with H2O2 and SA pretreatment coincided with the increased accumulation of ROS (O ·−2 , H2O2), MDA, and Pro, and decreased levels of RWC, AsA, and total Chl, which suggests the involvement of NO in modulating oxidative stress under salt stress. Enhanced activities of CAT, GPX, GST, APX, DHAR, and Gly II coupled with higher NO content in H2O2- and SA-pretreated salt-stressed seedlings clearly suggest a regulatory role for NO in the antioxidant defense and MG detoxification systems. It is also known that NO interacts with H2O2 and SA at the level of biosynthesis, and regulation of gene expression or protein activities (Klessig et al. 2000; Zottini et al. 2007). NO induced by H2O2 and SA might have an immense effect on salt tolerance as NO is known to maintain a diverse network with other growth regulators. For instance, the cellular NO level is affected by ABA, JA, SA, cytokinin, auxin, polyamine, and brassinosteroids, and interplay with these molecules modulate molecular, cellular, and whole-plant responses to various environmental stimuli (Xu et al. 2008; Saito et al. 2009; Khan et al. 2012; Qiao et al. 2014).
In conclusion, our results revealed that salt treatment imposed evident oxidative stress, whereas H2O2 and SA alleviated oxidative damage through modulating the antioxidant defense and glyoxalase systems. H2O2 and SA pretreatment elevated the level of endogenous NO that participated in reducing oxidative parameters like ROS and MDA through its antioxidant properties and signaling role. Removing NO by Hb subsequently damaged the rice seedlings in the presence of H2O2 and SA and confirmed that the beneficial role of H2O2 and SA, at least in part, depends on NO. It could be proposed that SA and H2O2 may use a common signaling mechanism where NO plays the most vital role in improving oxidative stress tolerance. Although our study did not emphasize osmotic and ionic stresses, the obtained results have enormous importance in elucidating the biochemical mechanism of salt tolerance in crop plants. The present findings established a NO-dependent role for H2O2 and SA; however, further research is required to find the direct targets and exact mechanisms of H2O2 and SA in NO metabolism that would augment the molecular understanding of NO-mediated salt-stress tolerance.
References
Álvarez Viveros MF, Inostroza-Blancheteau C, Timmermann T, González M, Arce-Johnson P (2013) Overexpression of Gly I and Gly II genes in transgenic tomato (Solanum lycopersicum Mill.) plants confers salt tolerance by decreasing oxidative stress. Mol Biol Rep 40:3281–3290
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Arnon DT (1949) Copper enzymes in isolated chloroplast polyphenol oxidase in Beta vulgaris. Plant Physiol 24:1–15
Ashfaque F, Khan MIR, Khan NA (2014) Exogenously applied H2O2 promotes proline accumulation, water relations, photosynthetic efficiency and growth of wheat (Triticum aestivum L.) under salt stress. Annu Res Rev Biol 4:105–120
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Dutilleul C, Driscoll S, Cornic G, De Paepe R, Foyer CH, Noctor G (2003) Functional mitochondrial complex I is required by tobacco leaves for optimal photosynthetic performance in photorespiratory conditions and during transients. Plant Physiol 131:264–275
Elia AC, Galarini R, Taticchi MI, Dorr AJM, Mantilacci L (2003) Antioxidant responses and bioaccumulation in Ictalurus melas under mercury exposure. Ecotoxicol Environ Saf 55:162–167
El-Shabrawi H, Kumar B, Kaul T, Reddy MK, Singla-Pareek SL, Sopory SK (2010) Redox homeostasis, antioxidant defense, and methylglyoxal detoxification as markers for salt tolerance in Pokkali rice. Protoplasma 245:85–96
Esim N, Atici O (2014) Nitric oxide improves chilling tolerance of maize by affecting apoplastic antioxidative enzymes in leaves. Plant Growth Regul 72:29–38
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Griffiths OW (1980) Determination of glutathione and glutathione disulphide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106:207–212
Han Y, Yin S, Huang L (2014) Towards plant salinity tolerance-implications from ion transporters and biochemical regulation. Plant Growth Regul. doi:10.1007/s10725-014-9997-6
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Ann Rev Plant Physiol Plant Mol Biol 51:463–499
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stochiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hossain MA, Fujita M (2013) Hydrogen Peroxide priming stimulates drought tolerance in mustard (Brassica juncea L.) seedlings. Plant Gene Trait 4:109–123
Hossain MA, Nakano Y, Asada K (1984) Monodehydroascorbate reductase in spinach chloroplasts and its participation in the regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol 25:385–395
Hossain MA, Hossain MZ, Fujita M (2009) Stress induced changes of methylglyoxal level and Glyoxalase I activity in pumpkin seedlings and cDNA cloning of glyoxalase I gene. Aust J Crop Sci 3:53–64
Hossain MA, Ismail MR, Uddin MK, Islam MZ, Ashrafuzzaman M (2013a) Efficacy of ascorbate–glutathione cycle for scavenging H2O2 in two contrasting rice genotypes during salinity stress. Aust J Crop Sci 7:1801–1808
Hossain MA, Mostofa MG, Fujita M (2013b) Cross protection by cold-shock to salinity and drought stress-induced oxidative stress in mustard (Brassica campestris L.) seedlings. Mol Plant Breed 4:50–70
Iqbal N, Umar S, Khan NA, Khan MIR (2014) A new perspective of phytohormones in salinity tolerance: regulation of proline metabolism. Environ Exp Bot 100:34–42
Jayakannan M, Bose J, Babourina O, Rengel Z, Shabala S (2015) Salicylic acid in plant salinity stress signalling and tolerance. Plant Growth Regul. doi:10.1007/s10725-015-0028-z
Kaur C, Ghosh A, Pareek A, Sopory SK, Singla-Pareek SL (2014) Glyoxalases and stress tolerance in plants. Biochem Soc Trans 42:485–490
Khan MN, Siddiqui MH, Mohammad F, Naeem M (2012) Interactive role of nitric oxide and calcium chloride in enhancing tolerance to salt stress. Nitric Oxide 27:210–218
Khan MIR, Asgher M, Khan NA (2014) Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean (Vigna radiata L.). Plant Physiol Biochem 80:67–74
Khare T, Kumar V, Kishor PBK (2014) Na+ and Cl− ions show additive effects under NaCl stress on induction of oxidative stress and the responsive antioxidative defense in rice. Protoplasma. doi:10.1007/s00709-014-0749-2
Klessig DF et al (2000) Nitric oxide and salicylic acid signaling in plant defense. PNAS 97:8849–8855
Kumar D, Yusuf MA, Singh P, Sardar M, Sarin NB (2013) Modulation of antioxidant machinery in α-tocopherol-enriched transgenic Brassica juncea plants tolerant to abiotic stress conditions. Protoplasma 250:1079–1089
Lee MH, Cho EJ, Wi SG, Bae H, Kim JE, Cho JY, Lee S, Kim JH, Chung BY (2013) Divergences in morphological changes and antioxidant responses in salt-tolerant and salt-sensitive rice seedlings after salt stress. Plant Physiol Biochem 70:325–335
Li J-T, Qiu Z-B, Zhang X-W, Wang L-S (2011) Exogenous hydrogen peroxide can enhance tolerance of wheat seedlings to salt stress. Acta Physiol Plant 33:835–842
Lin Y, Liu Z, Shi Q, Wang X, Wei M, Yang F (2012) Exogenous nitric oxide (NO) increased antioxidant capacity of cucumber hypocotyl and radicle under salt stress. Sci Hortic 142:118–127
Liu S, Dong Y, Xu L, Kong J (2014) Effects of foliar applications of nitric oxide and salicylic acid on salt-induced changes in photosynthesis and antioxidative metabolism of cotton seedlings. Plant Growth Regul 73:67–78
Lopez-Carrion AI, Castellano R, Rosales MA, Ruiz JM, Romero L (2008) Role of nitric oxide under saline stress: implications on proline metabolism. Biol Plant 52:587–591
Mishra P, Bhoomika K, Dubey RS (2013) Differential responses of antioxidative defense system to prolonged salinity stress in salt-tolerant and salt-sensitive Indica rice (Oryza sativa L.) seedlings. Protoplasma 250:3–19
Miura K, Tada Y (2014) Regulation of water, salinity, and cold stress responses by salicylic acid. Front Plant Sci 5:4
Mostofa MG, Fujita M (2013) Salicylic acid alleviates copper toxicity in rice (Oryza sativa L.) seedlings by up-regulating antioxidative and glyoxalase systems. Ecotoxicology 22:959–973
Mostofa MG, Seraj ZI, Fujita M (2014a) Exogenous sodium nitroprusside and glutathione alleviate copper toxicity by reducing copper uptake and oxidative damage in rice (Oryza sativa L.) seedlings. Protoplasma 251:1373–1386
Mostofa MG, Yoshida N, Fujita M (2014b) Spermidine pretreatment enhances heat tolerance in rice seedlings through modulating antioxidative and glyoxalase systems. Plant Growth Regul 73:31–44
Mostofa MG, Hossain MA, Fujita M (2015) Trehalose pretreatment induces salt tolerance in rice (Oryza sativa L.) seedlings: oxidative damage and co-induction of antioxidant defense and glyoxalase systems. Protoplasma 252:461–475
Mustafiz A et al (2014) A unique Ni2+-dependent and methylglyoxal-inducible rice glyoxalase I possesses a single active site and functions in abiotic stress response. Plant J 78:951–963
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nazar R, Iqbal N, Syeed S, Khan NA (2011) Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mungbean cultivars. J Plant Physiol 168:807–815
Neill S, Barros R, Bright J, Desikan R, Hancock J, Harrison J, Morris P, Ribeiro D, Wilson I (2008) Nitric oxide, stomatal closure, and abiotic stress. J Exp Bot 59:65–176
Petrov VD, Van Breusegem F (2012) Hydrogen peroxide—a central hub for information flow in plant cells. AoB Plants. doi:10.1093/aobpla/pls014
Qiao WH, Li CN, Fan LM (2014) Cross-talk between nitric oxide and hydrogen peroxide in plant responses to abiotic stresses. Environ Exp Bot 100:84–93
Roxas VP, Lodhi SA, Garrett DK, Mahan JR, Allen RD (2000) Stress tolerance in transgenic tobacco seedlings that overexpress glutathione S-transferase/glutathione peroxidase. Plant Cell Physiol 41:1229–1234
Saito N, Nakamura Y, Mori IC, Murata Y (2009) Nitric oxide functions in both methyl jasmonate signaling and abscisic acid signaling in Arabidopsis guard cells. Plant Signal Behav 4:119–120
Singla-Pareek SL, Yadav SK, Pareek A, Reddy MK, Sopory SK (2008) Enhancing salt tolerance in a crop plant by overexpression of glyoxalase II. Transgenic Res 17:171–180
Song F, Goodman RM (2001) Activity of nitric oxide is dependent on, but is particularly required for function of salicylic acid in the signaling pathway in tobacco systemic acquired resistance. Mol Plant-Microbe Int 14:1458–1462
Sun LR, Hao FS, Lu BS, Ma LY (2010) AtNOA1 modulates nitric oxide accumulation and stomatal closure induced by salicylic acid in Arabidopsis. Plant Signal Behav 5:1022–1024
Tanou G, Filippou P, Belghazi M, Job D, Diamantidis G, Fotopoulos V, Molassiotis A (2012) Oxidative and nitrosative-based signaling and associated post-translational modifications orchestrate the acclimation of citrus plants to salinity stress. Plant J 72:585–599
Türkan I, Demiral T (2009) Recent developments in understanding salinity tolerance. Environ Exp Bot 67:2–9
Uchida A, Jagendorf AT, Hibino T, Takabe T (2002) Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice. Plant Sci 163:515–523
Upadhyaya CP, Venkatesh J, Gururani MA, Asnin L, Sharma K, Ajappala H, Park SW (2011) Transgenic potato overproducing L-ascorbic acid resisted an increase in methylglyoxal under salinity stress via maintaining higher reduced glutathione level and glyoxalase enzyme activity. Biotechnol Lett 33:2297–2307
Wang W, Vinocur B, Altman A (2007) Plant responses to drought, salinity and extreme temperatures towards genetic engineering for stress tolerance. Planta 218:1–14
Xu Q, Xu X, Zhao Y, Jiao K, Herbert SJ, Hao L (2008) Salicylic acid, hydrogen peroxide and calcium-induced saline tolerance associated with endogenous hydrogen peroxide homeostasis in naked oat seedlings. Plant Growth Regul 54:249–259
Yadav SK, Singla-Pareek SL, Ray M, Reddy MK, Sopory SK (2005) Transgenic tobacco plants overexpressing glyoxalase enzymes resist an increase in methylglyoxal and maintain higher reduced glutathione levels under salinity stress. Fed Eur Biochem Soc Lett 579:6265–6271
Younis ME, Hasaneen MNA, Kazamel AMS (2010) Exogenously applied ascorbic acid ameliorates detrimental effects of NaCl and mannitol stress in Vicia faba seedlings. Protoplasma 239:39–48
Zhang A, Jiang M, Zhang J, Ding H, Xu S, Hu X, Tan M (2007) Nitric oxide induced by hydrogen peroxide mediates abscisic acid-induced activation of the mitogen-activated protein kinase cascade involved in antioxidant defense in maize leaves. New Phytol 175:36–50
Zhao MG, Tian QY, Zhang WH (2007) Nitric oxide synthase-dependent nitric oxide production is associated with salt tolerance in Arabidopsis. Plant Physiol 144:206–217
Zhou B, Guo Z, Xing J, Huang B (2005) Nitric oxide is involved in abscisic acid-induced antioxidant activities in Stylosanthes guianensis. J Exp Bot 56:3223–3228
Zottini M, Costa A, Michele RD, Ruzzene M, Carimi F, Schiavo FL (2007) Salicylic acid activates nitric oxide synthesis in Arabidopsis. J Exp Bot 58:1397–1405
Acknowledgments
M. G. Mostofa gratefully acknowledges the funding from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mostofa, M.G., Fujita, M. & Tran, LS.P. Nitric oxide mediates hydrogen peroxide- and salicylic acid-induced salt tolerance in rice (Oryza sativa L.) seedlings. Plant Growth Regul 77, 265–277 (2015). https://doi.org/10.1007/s10725-015-0061-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-015-0061-y