Abstract
The present study investigated the effect of salicylic acid (SA) on toxic symptoms, lipid peroxidation, reactive oxygen species generation and responses of antioxidative and glyoxalase systems in rice seedlings grown hydroponically under copper (Cu) stress for 48 h. Exposures of 75 and 150 μM Cu2+ caused toxicity symptoms (chlorosis, necrosis and rolling in leaves), sharp increases in malondialdehyde (MDA), hydrogen peroxide (H2O2) contents and lipoxygenase (LOX) activity with concomitant reductions of chlorophyll (Chl) and relative water content (RWC). Both levels of Cu decreased ascorbic acid (AsA), glutathione (GSH), non-protein thiol (NPT) and proline contents in roots but rather increased in leaves except that AsA decreased in leaves too. These results together with overaccumulation of superoxide (O •−2 ) and H2O2 in leaves revealed that Cu exposures induced oxidative stress. Contrary, SA-pretreatment (100 μM for 24 h) reduced toxicity symptoms and diminished Cu-induced increases in LOX activity, H2O2, MDA and proline contents while the levels of RWC, Chl, AsA and redox ratios were elevated. Higher levels of GSH and NPT were also observed in roots of SA-pretreated Cu-exposed seedlings. SA-pretreatment also exerted its beneficial role by inhibiting the Cu upward process. Studies on antioxidant enzymes showed that SA further enhanced the activities of superoxide dismutase, ascorbate peroxidase, glutathione reductase and glutathione peroxidase, and also elevated the depressed activities of catalase, dehydroascorbate reductase and glutathione S-transferase particularly at 150 μM Cu2+ stress. In addition, the activity of glyoxalase system (glyoxalase I and II) was further elevated by SA pretreatment in the Cu-exposed seedlings. These results concluded that SA-mediated retention of Cu in roots and enhanced capacity of both antioxidative and glyoxalase systems might be associated with the alleviation of Cu-toxicity in rice seedlings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Copper (Cu) is an essential structural and catalytic element of numerous proteins necessary for normal growth, development and stress tolerance of plants (Burkhead et al. 2009; Martínez-Peñalver et al. 2012). Cu is indeed required in small amounts by the plants; however, rapid industrialization and widespread uses of Cu-containing fertilizers cause elevated levels of Cu in soils (Burkhead et al. 2009). Due to inherent transitional property, Cu is readily uptaken by the plants, accumulates at high level and consequently disturbs major cellular processes like photosynthesis, respiration, nitrogen assimilation and cell wall metabolism (Cuypers et al. 2011). Excess Cu can become extremely toxic causing reduction of plant biomass, stunted growth, chlorosis and necrosis. Cu, even at mild excess, can disrupt protein structures, inactivate enzymes by binding to thiols and displace other essential metals manifesting their deficiencies in plants (Maksymiec 1997; Bouazizi et al. 2010). Another key feature of Cu toxicity is the induction of oxidative stress, as its redox-active nature can generate reactive oxygen species (ROS) like singlet oxygen (1O2), hydrogen peroxide (H2O2), superoxide (O •−2 ) and hydroxyl (OH•) radicals (Drazkiewicz et al. 2004; Contreras et al. 2009). Evidence from several plant species revealed that Cu also caused oxidative stress by mediating the activities of antioxidative enzymes (Drazkiewicz et al. 2004; Khatun et al. 2008). In addition to ROS, accumulation of methylglyoxal (MG), a cytotoxic compound, has been reported under abiotic stresses including heavy metals (Singla-Pareek et al. 2006; Hossain et al. 2009). At elevated level, MG can cause cell death by interacting directly with proteins, lipids, carbohydrates, DNA and also by inactivating antioxidant defense system (Yadav et al. 2005). Additionally, MG can produce ROS and stimulate oxidative stress generation unless it detoxified immediately (Saito et al. 2011; Hoque et al. 2012).
Plants adopted a variety of strategies to resist metal toxicity, including active efflux, sequestration and complexion inside the cells by strong ligands like cys-rich proteins, phytochelatins and GSH (Küpper et al. 2009). If these mechanisms are insufficient, intracellular metal concentration increases inducing oxidative stress. To combat oxidative stress, plant cells owned enzymatic and non-enzymatic antioxidants. Among the antioxidant enzymes, superoxide dismutase (SOD) dismutates O .−2 into H2O2 which is subsequently removed by catalases (CAT), glutathione peroxidases (GPX) and/or by ascorbate–glutathione cycle involving ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR) and glutathione reductase (GR). In addition, glutathione S-transferase (GST) is a key enzyme in the metabolisms of xenobiotics and secondary products (Lyubenova et al. 2007) Ascorbate (AsA) and glutathione (GSH) serve as the potent non-enzymatic antioxidants for direct scavenging of ROS and/or co-substrate of ascorbate–glutathione cycle (Foyer and Noctor 2011). Likewise, plant cells possess a MG detoxifying glyoxalase system comprises two enzymes; glyoxalase I (Gly I) and glyoxalase II (Gly II), which reduces MG to D-lactate by employing and regenerating GSH. Since GSH deals both the ascorbate–glutathione and glyoxalase systems, it may lead to a cross talk between these two important pathways. Numerous recent studies have claimed that efficient induction of ascorbate–glutathione and glyoxalase systems often correlated with elevated level of GSH and high GSH/GSSG ratio (El-Shabrawi et al. 2010; Upadhyaya et al. 2011; Hasanuzzaman et al. 2012).
In case of severe or prolonged stress conditions, antioxidant and glyoxalase systems might be overwhelmed to withstand the adverse effects of ROS and MG. Therefore, treatments of plants with signaling molecules might be a well approach for acclimation to adverse environmental conditions. Salicylic acid (SA) acts as an endogenous hormone like growth regulator in plants, which has broad but divergent roles on damage development and stress acclimation (Metwally et al. 2003). Besides regulating numerous physiological and biochemical processes, SA appears to be an effective therapeutic agent in plant responses to biotic and abiotic stresses (Vicente and Plasencia 2011). Recent studies have evinced that SA alleviates a number of abiotic stresses including salinity (He and Zhu 2008), drought (Habibi 2012), hypoxia (Bai et al. 2009) and herbicide (Radwan 2012). It was also reported that SA induces adaptability to the toxicity of Cd, Hg, Ni, Pb and Mn (Guo et al. 2009; Zhou et al. 2009; Wang et al. 2009; Wang et al. 2011; Shi and Zhu 2008). In most of the above cases, SA prevented oxidative stress by enhancing antioxidant capacity. For instance, SA-induced H2O2 set up the rice plants to respond to Cd toxicity effectively by elevating non-enzymatic and enzymatic antioxidants (Guo et al. 2009). Recently, Wang et al. (2011) demonstrated that SA-mediated Pd-uptake, nutrient balance and alteration of CAT, POD and APX activities alleviates Pd-induced damages in vallisneria natants. Further, studies on SA-deficient transgenic rice and Thlaspi hyperaccumulators revealed that SA-regulated GSH homeostasis was implicated in the protection of oxidative stress (Kusumi et al. 2006, Freeman et al. 2005). The above findings provide a clue that SA-regulated GSH pool might have influence on the induction of antioxidative and glyoxalase systems during oxidative stress. No information, however, is available about the influence of SA on the glyoxalase system under heavy metal toxicity. Moreover, there is no study about the effect of SA on responses of the enzymatic and non-enzymatic defense systems to Cu-induced oxidative stress in plants. Our previous studies showed that simultaneous induction of antioxidative and glyoxalase systems conferred tolerance to Cd stress (Hossain et al. 2010; Hasanuzzaman et al. 2012). Further, the role of SA on antioxidant system under heavy metal stress was investigated in roots and leaves of rice plants separately (Panda and Patra 2007; Guo et al. 2007). We, therefore, aimed to investigate the influence of SA on Cu uptake, oxidative damages, responses of antioxidative and glyoxalase systems in both roots and leaves of rice seedlings. This study also focuses on the importance of simultaneous induction of antioxidative and glyoxalase systems in alleviating Cu-induced damages in rice seedlings.
Materials and methods
Plant materials, growth condition and treatments
Rice (Oryza sativa L. cv.BR 11) seeds were surface-sterilized with 1 % (v/v) sodium hypochlorite for 20 min, washed thoroughly with distilled water and then imbibed for 24 h in the dark. The seeds were sown on plastic nets floating on distilled water in 250 mL plastic beakers and kept in dark at 28 ± 2 °C for germination. After 48 h, uniformly germinated seeds were transferred and cultivated in plastic beakers with 7,500 times diluted nutrient solution (Hyponex, Japan) under the conditions of photon density: 100 μmol m−2 s−1, temperature: 25 ± 2 °C and relative humidity: 65–70 % in a growth chamber. Each plastic beaker contained ~50 rice seedlings. The nutrient solution (pH 5.5) renewed at every 3 days interval. Fourteen-day-old healthy rice seedlings were pretreated with 100 μM SA in the nutrient solution for 24 h and then exposed to 75 or 150 μM CuSO4 for 48 h. Cu doses were chosen appropriately to expose the seedlings from moderate to high toxicity by a preliminary experiment. Control seedlings were grown in nutrient solution only. This experiment includes total six treatments; control (Con), 100 μM salicylic acid (SA), 75 μM CuSO4 (Cu1), 100 μM salicylic acid + 75 μM CuSO4 (SA + Cu1), 150 μM CuSO4 (Cu2) and 100 μM salicylic acid + 150 μM CuSO4 (SA + Cu2). After 48 h of Cu stress treatment, the roots and second leaves were harvested from the seedlings for estimations of various physiological and biochemical parameters. Each treatment was replicated three times under the same conditions.
Determination of Cu content
Harvested root and leaf samples were oven dried at 80 °C for 48 h. Dried samples (0.1 g) were ground and acid digested with HNO3:HCLO4 (5:1 v/v) mixture for 24 h at 80 °C followed by Cu estimation using an atomic absorption spectrophotometer (Hitachi Z-5000, Japan).
Determination of total chlorophyll, proline and relative water contents
For estimation of total chlorophyll (Chl) content, leaves (0.5 g) were extracted in 80 % chilled acetone and Chl estimation was conducted according to the method of Arnon (1949). Proline (Pro) content was determined according to the method of Bates et al. (1973) using acid-ninhydrin solution. For estimation of relative water content (RWC), twenty leaf and root segments (4–5 cm) were weighed separately to determine fresh weights (FW). Leaf and root segments were then placed through two layers of filter paper and immersed in deionized water. When the leaf and root segments become fully turgid, the segments were gently dried with tissue paper and turgid weights (TW) were measured. Dry weights (DW) of the segments were measured after oven drying at 80 °C for 48 h. RWC was calculated using the following formula: RWC (%) = 100 × (FW − DW) / (TW − DW).
Determination of lipid peroxidation and hydrogen peroxide contents
Lipid peroxidation of roots and leaves was measured by estimating malondialdehyde (MDA) according to Heath and Packer (1968). The samples (0.5 g) were homogenized with 5 % (w/v) trichloroacetic acid (TCA) and centrifuged at 11,500×g for 12 min. The supernatant was mixed with 20 % TCA containing 0.5 % of TBA and heated at 95 °C for 30 min. MDA content was calculated from the difference in absorbance at 532 and 600 nm using extinction coefficient of 155 mM−1 cm−1. Hydrogen peroxide (H2O2) was extracted by homogenizing 0.5 g of samples with 50 mM K-phosphate buffer pH (6.5) and determined after reaction with 0.1 % TiCl4 in 20 % H2SO4 following the method of Hossain et al. (2010).
Histochemical detections of superoxide and H2O2
Superoxide (O •−2 ) and H2O2 were visualized in rice leaves according to the method of Wang et al. (2011) with modifications. Briefly, the second leaves were stained in 0.1 % nitroblue tetrazolium (NBT) or 1 % 3, 3-diaminobenzidine (DAB) solution for 8 h under dark and light, respectively. Incubated leaves were then decolorized by immersing in boiling ethanol which allowed visualization of blue insoluble formazan (for O •−2 ) or deep brown polymerization product (for H2O2). After cooling, glycerol was used to open the leaves and photographs were taken by placing the leaves between two glass plates.
Estimation of non-enzymatic antioxidants and non-protein thiols
Fresh roots and leaves (0.5 g) were homogenized separately in 3 mL of ice-cold 5 % meta-phosphoric acid containing 1 mM EDTA and centrifuged at 11,500×g for 12 min. Reduced and total AsA content were determined following the method of Dutilleul et al. (2003) with minor modifications. For estimating total AsA, the oxidized fraction was reduced by 0.1 M dithiothreitol. Reduced and total AsA content were assayed at 265 nm in 100 mM K-phosphate buffer (pH 7.0) with 1.0 U of ascorbate oxidase (AO). Oxidized ascorbate (DHA) = total AsA − reduced AsA. Based on enzymatic recycling, reduced glutathione (GSH), oxidized glutathione (GSSG) and total glutathione (GSH + GSSG) were determined according to Griffiths (1980). GSSG was determined after removal of GSH by 2-vinylpyridine derivatization. GSH was measured after subtracting the value of GSSG from total GSH. Non-protein thiols (NPT) were extracted in 3 % (w/v) sulfosalicylic acid and the content was measured at 412 nm according to the method of Ellman (1959) using Ellman’s reaction mixture (5 mM EDTA and 0.6 mM 5,5 o-dithiobis (2-nitrobenzoic acid) in 120 mM phosphate buffer, pH 7.5).
Extraction and assay of enzymes
For extraction of enzymes, fresh root and leaf samples (0.5 g) were homogenized separately with a reaction mixture containing 50 mM K-phosphate buffer (pH 7.0), 100 mM KCl, 1 mM AsA, 5 mM β-mercaptoethanol and 10 % (w/v) glycerol in pre-chilled mortars and pestles. The homogenate was centrifuged at 12,500×g for 15 min and the resultant supernatants were collected for analysis of enzyme activities and protein content. All procedures were performed at 0–4 °C.
Lipoxygenase (LOX, EC 1.13.11.12) activity was estimated according to the method of Doderer et al. (1992) by monitoring the increase in absorbance at 234 nm using linoleic acid as a substrate. Superoxide dismutase (SOD, EC 1.15.1.1) activity was estimated according to El-Shabrawi et al. (2010) which based on xanthine–xanthine oxidase system. The reaction mixture contained K-phosphate buffer (50 mM), NBT (2.24 mM), catalase (0.1 units), xanthine oxidase (0.1 units), xanthine (2.36 mM) and enzyme extract. Catalase was added to avoid the H2O2-mediated possible inactivation of CuZn-SOD. SOD activity was expressed as units (i.e., amount of enzyme required to inhibit NBT reduction by 50 %) min−1 mg−1 protein. Catalase (CAT, EC 1.11.1.6) activity was measured according to Hossain et al. (2010). Ascorbate peroxidase (APX, EC 1.11.1.11) activity was determined by monitoring the decrease in absorbance at 290 nm as AsA was oxidized, according to Nakano and Asada (1981). Monodehydroascorbate reductase (MDHAR, EC 1.6.5.4) activity was measured by using 1 U of AO and the oxidation rate of NADPH was followed at 340 nm (Hossain et al. 1984). Dehydroascorbate reductase (DHAR, EC 1.8.5.1) activity was measured by following the formation of AsA from DHA at 265 nm using GSH (Nakano and Asada 1981). Glutathione reductase (GR, EC 1.6.4.2) activity was measured by following the decrease in the absorbance of NADPH at 340 nm for GSSG-dependent oxidation of NADPH, as described by Foyer and Halliwell (1976). Glutathione S-transferase (GST, EC 2.5.1.18) activity was determined by the method of Hossain et al. (2010). Glutathione peroxidase (GPX, EC: 1.11.1.9) activity was measured as described by Elia et al. (2003) using H2O2 as a substrate. Glyoxalase I (Gly I, EC 4.4.1.5) assay was carried out according to Hossain et al. (2009) using MG as a substrate. Glyoxalase II (Gly II, EC 3.1.2.6) activity was determined according to Hossain et al. (2010) by monitoring the formation of GSH at 412 nm.
The protein content was determined following the method of Bradford (1976) using bovine serum albumin (BSA) as a standard.
Statistical analysis
The data were subjected to a one-way analysis of variance (ANOVA) and the mean differences were compared by Duncan’s multiple range test (DMRT) using IRRISTAT version 3. Different letters indicate significant differences between treatments at P < 0.05. Data represented in the table and figures are as means ± standard deviations (SD) of three replicates for each treatment.
Results
Effects of SA pretreatment on visual appearances in Cu-treated rice seedlings
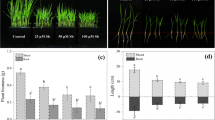
After 48 h of Cu exposures, rice seedlings showed severe morphological disturbances, especially at Cu2 stress. Visible toxicity symptoms like chlorosis, necrosis and rolling of the leaves were noticed upon Cu impositions (Fig. 1). The roots of Cu stressed seedlings become reddish and health was also deteriorated compared to that of the control seedlings. However, SA pretreatment prior to Cu stresses decreased the toxic effects and retained the seedlings growth similar to the control seedlings.
Visual aspects of Cu-toxicity on rice seedlings with and without SA pretreatment (a). Necrotic lesions in Cu2 treated rice leaves (b) and necrotic lesion not detected in SA + Cu2 treated rice leaves (c). Con, SA, Cu1, SA + Cu1, Cu2 and SA + Cu2 correspond to control (only hyponex), 100 μM salicylic acid, 75 μM CuSO4, 100 μM salicylic acid + 75 μM CuSO4, 150 μM CuSO4 and 100 μM salicylic acid + 150 μM CuSO4, respectively. Photographs were taken after 48 h of Cu treatments
Effects of SA pretreatment on MDA, H2O2 and Pro contents
MDA and H2O2 are frequently used as index of oxidative stress. MDA and H2O2 contents showed significant rises in roots and leaves of both Cu1 and Cu2 stresses compared to that of control (Table 1). The induction of MDA and H2O2 was more pronounced in roots than that of leaves in Cu-stressed seedlings. On the other hand, SA pretreatment significantly reduced MDA and H2O2 contents in roots and leaves of both groups of Cu-stressed seedlings. In comparison with control, H2O2 content also increased by 26 % in roots and 27 % in leaves of SA-pretreated non-stressed seedlings. In roots, Pro content was decreased by 27 and 48 % at Cu1 and Cu2 stresses, respectively. On the other hand, Pro content in rice leaves remarkably increased in response to both levels of Cu and it was particularly significant (825 %) at Cu2 stress (Table 1). SA pretreatment did not increase Pro contents in roots, but in leaves it returned to the control level upon Cu exposures.
Effects of SA pretreatment on Cu-induced overproduction of ROS
ROS like O •−2 and H2O2 were overproduced as indicated by scattered dark blue spots and brown polymerization products, respectively in the second leaves of Cu-stressed seedlings (Fig. 2a, b). However, SA pretreatment prior to Cu stresses considerably diminished the ROS accumulations.
Histochemical detection of O •−2 (a) and H2O2 (b) with nitroblue tetrazolium (NBT) and 3, 3-diaminobenzidine (DAB), respectively in rice leaves. Other details were as in the Fig. 1
Effects of SA pretreatment on RWC, Chl and Cu contents
RWC decreased by 18 and 39 % in roots and by 15 and 30 % in leaves of Cu1 and Cu2 stresses, respectively compared to control values (Table 2). However, SA pretreatment significantly prevented the reduction of RWC in rice seedlings under Cu stresses. Total Chl content in second leaves of Cu-treated seedlings declined sharply at both levels of Cu stresses (10 and 29 % at Cu1 and Cu2 stresses, respectively). However, SA pretreatment prior to Cu stresses significantly increased Chl contents (Table 2). In non-stress condition, SA pretreatment also decreased Chl content significantly compared to that of control.
The Cu content in roots and leaves increased with increasing Cu concentrations in the nutrient solution (Table 2). However, Cu accumulation was always higher in the roots than that of leaves. On the other hand, SA pretreatment had little effects on Cu accumulation in roots but significantly inhibited Cu translocation from roots to leaves.
Effects of SA pretreatment on AsA content
Cu-exposures drastically decreased AsA content in roots and leaves of both Cu1 and Cu2 stresses whereas DHA content significantly decreased in roots but increased in leaves compared to control value (Fig. 3a–d). AsA/DHA ratio in leaves at both doses of Cu significantly decreased, whereas in roots, it was decreased only at Cu1 stress (Fig. 3e, f). However, SA-pretreated Cu-exposed seedlings showed prevention in the declines of AsA and maintained the level 33 and 52 % higher in roots and 17 and 139 % higher in leaves compared to that of Cu1 and Cu2 stresses, respectively. SA pretreatment also maintained AsA/DHA ratio similar to the control value in Cu-stressed seedlings.
Effects of SA pretreatment on reduced (a, b) oxidized (c, d) and ratio of reduced to oxidized (e, f) AsA contents in roots and leaves of Cu-treated rice seedlings. Data represented as mean ± SD. Different letters on vertical bars indicate significant differences at P < 0.05 applying DMRT. Other details were as in the Fig. 1
Effects of SA pretreatment on GSH and NPT contents
The changes in GSH content followed an opposite pattern in roots and leaves of Cu-stressed seedlings. As shown in Fig. 4a, GSH contents significantly declined in roots whereas increased in leaves at both level of Cu-exposures. GSSG contents increased by 63 and 198 % in roots but 151 and 668 % in leaves of Cu1 and Cu2 stresses, respectively. However, SA pretreatment prevented the declines in GSH contents in roots and maintained the level 116 and 423 % above compared to that of the seedlings exposed to Cu1 and Cu2 stresses, respectively. In leaves, SA pretreatment reduced Cu induced increase of GSH; however, it was significantly higher than the control value. Further, SA-pretreatment significantly increased GSH/GSSG ratio at both level of Cu-exposures compared to that of Cu stresses only. In roots, NPT content significantly decreased at both levels of Cu but the reduction was drastic (54 %) at Cu2 stress (Fig. 4g, h). NPT contents in leaves increased by 35 and 134 % at Cu1 and Cu2 stresses, respectively. However, SA pretreated Cu-stressed seedlings showed rises in NPT contents in roots but reduction in leaves compared to their respective stresses.
Effects of SA pretreatment on reduced (a, b), oxidized (c, d), ratio of reduced to oxidized (e, f) GSH and NPT (g, h) contents in roots and leaves of Cu-treated rice seedlings. Data represents as mean ± SD. Different letters on vertical bars indicate significant differences at P < 0.05 applying DMRT. Other details were as in the Fig. 1
Effects of SA pretreatment on LOX and ROS detoxifying enzymes
At both level of Cu, LOX activity significantly increased in roots however, the increase was notable (472 %) in Cu2 stress whereas in leaves, LOX activity only significantly increased at Cu2 stress (Fig. 5a, b). In SA-pretreated Cu-stressed seedlings, LOX activity significantly reduced in both roots and leaves compared to their respective stresses. In roots, SOD activities significantly induced upon Cu exposures and the inductions were 156 % at Cu1 and 436 % at Cu2 compared to control value. In leaves, SOD activity remained unchanged at Cu1 but significantly increased at Cu2 (Fig. 5c, d). Upon SA pretreatment, SOD activity further increased at Cu1 but decreased at Cu2 in roots. However, in leaves, SA pretreatment further increased SOD activity at both level of Cu stresses. CAT activity remained unchanged in roots but significantly increased in leaves of Cu1 stress. At Cu2 stress, CAT activities were decreased by 34 % in roots and 24 % in leaves (Fig. 5e, f). But SA pretreatment further increased CAT activity in leaves at Cu1 stress but significantly alleviated the decline of CAT activity at Cu2 stress in both roots and leaves. In non-stress condition, SA pretreatment also showed significant reductions in CAT activities in both roots and leaves.
Effects of SA pretreatment on activities of LOX (a, b), SOD (c, d) and CAT (e, f) in roots and leaves of Cu-treated rice seedlings. Data represents as mean ± SD. Different letters on vertical bars indicate significant differences at P < 0.05 applying DMRT. Other details were as in the Fig. 1
APX activity increased by 43 % at Cu1 and 65 % at Cu2 in roots, whereas in leaves it was significantly increased only at Cu2 over the control value (Fig. 6a, b). SA pretreatment further increased APX activity at Cu1 in both roots and leaves. In SA-pretreated Cu2-stressed seedlings, APX activity was unchanged in roots but further increased in leaves. MDHAR activity increased by 42 % at Cu1 but remained as control value at Cu2 stress in roots (Fig. 6c, d). In leaves, MDHAR activity significantly increased only at Cu2 compared to the control value. SA pretreatment had no positive effects on MDHAR activities in roots but slightly increased the activity in leaves only at Cu1.
Effects of SA pretreatment on activities of APX (a, b), MDHAR (c, d), DHAR (e, f) and GR (g, h) in roots and leaves of Cu-treated rice seedlings. Data represents as mean ± SD. Different letters on vertical bars indicate significant differences at P < 0.05 applying DMRT. Other details were as in the Fig. 1
DHAR activity drastically reduced in response to both level of Cu stresses in roots (37 % at Cu1 and 48 % at Cu2) (Fig. 6e, f). In leaves, DHAR activity slightly increased at Cu1 but remained as control level at Cu2. However, pretreatment with SA did not increase DHAR activity at Cu1 but it was significantly increased in both roots and leaves of Cu2-stressed seedlings. GR activity increased by 30 % at Cu1 and 73 % at Cu2 in roots whereas it remained unchanged at Cu1 but significantly increased at Cu2 in leaves (Fig. 6g, h). In SA-pretreated Cu-exposed seedlings, GR activity further boosted in roots at both levels of Cu but declined only at Cu2 in leaves. However, GR activities were significantly up-regulated in SA-pretreated Cu-exposed seedlings compared to the control value.
Effects of SA pretreatment on glutathione metabolizing enzymes
GPX activity was found to increase by 70 and 138 % in roots, and by 13 and 56 % in leaves at Cu1 and Cu2 stresses, respectively (Fig. 7a, b). On the other hand, GPX activities were further enhanced by SA pretreatment at Cu1 in both roots and leaves. But at Cu2 stress, SA pretreatment did not increase GPX activity in roots but boosted the activity in leaves significantly.
Effects of SA pretreatment on activities of GPX (a, b) and GST (c, d) in roots and leaves of Cu-treated rice seedlings. Data represents as mean ± SD. Different letters on vertical bars indicate significant differences at P < 0.05 applying DMRT. Other details were as in the Fig. 1
As shown in Fig. 7, GST activity significantly increased at Cu1 stress while it was drastically inhibited at Cu2 (20 % in roots and 48 % in leaves) compared to the control value. On the other hand, SA pretreatment further increased GST activity only at Cu1, but alleviated the inhibition of GST activity at Cu2.
Effects of SA pretreatment on glyoxalase enzymes
Gly I activity was significantly enhanced at both level of Cu stresses in roots whereas it was significantly increased only at Cu2 in leaves over the control value (Fig. 8a, b). SA-pretreated Cu-exposed seedlings also showed further enhancement of Gly I activities in roots compared to the seedlings subjected to Cu stresses only. In leaves, SA pretreatment also increased Gly I activity only at Cu2 compared to that of Cu2 stress only. Activities of Gly II were enhanced by 68 % at Cu1 and 30 % at Cu2 in roots whereas in leaves, it was elevated by 24 % only at Cu2 over the control level (Fig. 8c, d). SA-pretreated Cu-exposed seedlings also showed further enhancements of Gly II activities (17 % at Cu1 and 50 % at Cu2) in roots whereas in leaves, it was further increased only at Cu1 in comparison with their respective stresses.
Effects of SA pretreatment on activities of Gly I (a, b) and Gly II (c, d) in roots and leaves of Cu-treated rice seedlings. Data represents as mean ± SD. Different letters on vertical bars indicate significant differences at P < 0.05 applying DMRT. Other details were as in the Fig. 1
Discussion
Despite being an essential micronutrient, excess Cu can exhibit detrimental effects on plant growth, development and productivity. Cu induced toxicity symptoms and ROS generations leading to cell death are well documented in different plant species (Liu et al. 2012; Thounaojam et al. 2012). In this study, we observed progressive toxicity symptoms in leaves with increasing doses of Cu (Fig. 1) which were strongly correlated with H2O2 and MDA contents (Table 1). Increased H2O2, MDA levels and over-accumulation of O •−2 and H2O2 (Fig. 2) indicated that Cu induced oxidative stress. This study also provided evidence that Cu induced oxidative stress was effectively mitigated by pretreating rice seedlings with SA. We showed that SA pretreatment followed by Cu stresses displayed attenuated toxicity symptoms coupled with lower H2O2 and MDA contents and also less accumulation of O •−2 and H2O2. In the current findings, roots showed higher accumulation of Cu than the leaves which is consistent with the report of Thounaojam et al. (2012). Higher induction of H2O2 and MDA in roots also demonstrated that roots are the primary target of Cu toxicity. However, SA mediated inhibition of Cu upward process might be regarded as one of the potential physiological effect of SA in rice seedlings. Similarly, SA application inhibiting the uptake of Cd and Pd had been reported in other plants (Shi and Zhu 2008; Wang et al. 2011). Chlorosis and disturbances in plant water-balance are general consequences of metal toxicity. In this experiment, Cu-induced marked declines in Chl and RWC (Table 2) indicated that Cu exerted its toxicity by inhibiting Chl biosynthesis and generating osmotic stress. These effects were reverted by SA pretreatment, suggesting its protecting role against Cu induced toxicity which also corroborated with previous reports (Metwally et al. 2003; Chen et al. 2007). Pro accumulation appeared to be a suitable indicator of heavy metal stress experienced by the plants. Interestingly, we observed an opposite trend of Pro accumulation in roots and leaves of Cu-stressed seedlings (Table 1) which was in accordance with Chen et al. (2004). Reduced Pro levels in roots indicated that the direct contact of roots with Cu caused a severe damage of root structures which ultimately led to a destruction of Pro. Contrary, a remarkable increase in Pro contents in leaves of Cu-stressed seedlings suggested its functional significance in the osmoregulation. SA-pretreatment caused a little protection of Pro in roots and reduction of Cu-induced Pro content in leaves which indicated that the seedlings were partially relieved from Cu stresses. Metwally et al. (2003) also observed a reduction of this stress metabolite after pretreatment with SA in Cd-stressed barley plants. LOX is an iron containing oxidative enzyme associated with higher lipolytic activity in membranes (Molassiotis et al. 2006) during stress conditions. In our experiment, increased LOX activity contributed to the enhanced lipid peroxidation as supported by higher MDA levels in Cu-stressed seedlings (Fig. 5). However, reduction in LOX activity and MDA content by SA reflecting its beneficial role against Cu-mediated membrane lipid peroxidation. Our findings are in agreement with those of Wang et al. (2009) who reported that SA lowered the LOX activity in Ni-stressed maize plants.
Maintenance and regeneration of high levels of non-enzymatic antioxidants are crucial for reducing deleterious effects of ROS during stress conditions. AsA plays a key role as primary antioxidant by scavenging ROS directly and also represents a cellular reservoir to regenerate α-tocopherol, a lipophilic antioxidant (Foyer and Noctor 2011). Our investigation showed that Cu treatment led to substantial declines in AsA and AsA/DHA ratio (Fig. 3), which might be one of the ways Cu induces its toxicity. This is probably due to increased oxidation for scavenging overproduced ROS and inefficient recycling by decreased DHAR activity. This alteration shifted the redox status toward the oxidized state which aggravated oxidative stress by reducing antioxidant capacity. However, amendment of this trend by SA pretreatment might restrict the membrane damage and prevent oxidative injury, and ultimately shepherd to a better antioxidant capacity against Cu-phytotoxicity. SA-pretreatment also enhanced AsA content in rice and cucumber under Cd and Mn stresses, respectively (Guo et al. 2007; Shi and Zhu 2008). GSH, besides scavenging free radicals directly, is regarded as an integral part of antioxidative system to keep ROS under control during stress conditions. Our findings suggested that higher load of Cu and ROS in roots might contribute to the oxidation of GSH to GSSG and concomitant decrease of GSH and GSH/GSSG ratio (Fig. 4). On the other hand, higher induction of GSH in leaves might be due to compensation of AsA loss or increased activities of GR and/or decreased activities of DHAR and GST. Similar pattern of GSH homeostasis under Cd and Cu stresses also reported in rice plants previously (Guo et al. 2007; Thounaojam et al. 2012). Corresponding with GR activity, SA-pretreatment maintained higher GSH in roots and effectively counteracted the Cu induced changes of GSH in leaves while maintained a high GSH/GSSG ratio. Enhanced GSH contents at least in roots and high GSH/GSSG ratio, in both roots and leaves under SA pretreatment may partly account for the higher capacity for oxidative defense during Cu-exposures. NPT comprises of several acid-soluble thiol-components, such as cysteine, GSH and phytochelatins which may involve in detoxification of heavy metals (De Vos et al. 1992). In this study (Fig. 3), reduced level of GSH and higher level of NPT in roots of SA-pretreated Cu-stressed seedlings suggested that GSH might be incorporated into phytochelatins which restricts Cu in roots and thus represented another defensive mechanism against Cu stress. In parallel to our study, SA increased NPT content in rice under cadmium stress (Guo et al. 2007) while SA decreased NPT content in alfalfa exposed to mercury (Zhou et al. 2009).
To explore the possible role of SA against Cu-mediated damages, we focused on the enzymes of ascorbate–glutathione and glyoxalase cycles supplementing SOD, CAT, GPX and GST. By dismutating O •−2 into H2O2, SOD serves the first line of enzymatic defense against oxidative stress. Our results showed that enhancement of SOD activity and declines in CAT activity in Cu-stressed seedlings complied with the higher levels of H2O2 in roots and leaves (Table 1; Fig. 5). This imbalance also facilitated the overproduction of deadly OH• by Cu via Fenton-type reaction. However, a study by Jiang et al. (2012) on Cu-tolerant species P. fortunei indicated that the enhanced activities of SOD and CAT were crucial for coping with the oxidative stress of Cu. In our study, further enhancement of SOD activity and recovery of CAT activity following SA-pretreatment in Cu-stressed seedlings maintained a balance between O •−2 and H2O2 which contributed to the tolerance to Cu-mediated oxidative stress. The similar results have been demonstrated in rice and cucumber following Cd and Mn stresses, respectively (Panda and Patra 2007; Shi and Zhu 2008). Coinciding with H2O2 levels, slight inhibition of CAT and APX by SA might influence H2O2 signaling which played important role against oxidative stress induced by Cu (Guo et al. 2007; Chao et al. 2010; Shi and Zhu 2008).
In addition to CAT, removal of H2O2 largely dependant on the activities of GPX and the enzymes involved in ascorbate–glutathione cycle. Additionally, co-ordinated activities of H2O2 metabolizing enzymes are crucial for enhanced tolerance to oxidative stress. Wang et al. (2009) reported that SA-induced coordinated roles of SOD with APX, MDHAR, DHAR and GR counteracted oxidative stress in Ni-treated maize. However, in this study, the obtained results are varied in response to Cu stresses and/or SA pretreatment. Our result showed that increased GPX and APX activities in Cu-stressed seedlings might not be sufficient to deal with excess H2O2 under depressed activity of CAT. Further, lower activity of MDHAR in roots and decreased activity of DHAR in both roots and leaves interrupted the ascorbate–glutathione cycle and thus led to an accumulation of H2O2 (Table 1; Fig. 6). This is also correlated with higher DHA level which indicating inefficient recycling of AsA via ascorbate–glutathione cycle. In contrast, SA-pretreatment enhanced H2O2 detoxification by further elevating the activities of GPX, APX and DHAR as evident by lower level of H2O2. GR-mediated regeneration of GSH may contribute to the maintenance of AsA and thiol group, PC synthesis and substrates for GPX, GST and glyoxalase system in plant stress tolerance. Although SA did not stimulate GR activities in leaves, it was further increased in roots which were also correlated with the higher level of GSH, AsA and redox ratios (Fig. 6). Similarly, SA had no effect on GR activity in rice leaves (Panda and Patra 2007) but increased in alfalfa roots under Hg stress (Zhou et al. 2009). GST is another important antioxidative component often involved in nucleophilic conjugation of GSH to a wide variety of organic molecules. In this study, GST activity induced at low dose of Cu but significantly decreased at higher dose (Fig. 7) which might be the result of direct binding of Cu to the catalytic site of the enzyme (Lyubenova et al. 2007). On the other hand, SA-pretreated Cu-stressed seedlings maintained enhanced GST activities and thus contributed to the reduction of Cu-induced H2O2 and MDA levels, and subsequently affirmed higher tolerance to Cu toxicity. Our results are consistent with Popova et al. (2012).
MG is an inevitable product of abiotic stresses which can again aggravate ROS production. Therefore, detoxification of this compound is also a priority to register higher tolerance to oxidative stress. In addition to MG detoxification, glyoxalase system also contributes to the redox homeostasis by regenerating GSH. Since GSH is the integral component of both antioxidative and glyoxalase systems, harmonization of these systems by maintaining enhanced GSH and GSH/GSSG ratio might be a well strategy to augment abiotic stress tolerance. Several recent studies highlighted that co-ordinate induction of these systems conferred enhanced tolerance to abiotic stresses including heavy metals (Hossain et al. 2010; Upadhyaya et al. 2011; Hasanuzzaman et al. 2012). The present study clearly demonstrated that under reduced level of GSH in roots and depressed GSH/GSSG ratio in both roots and leaves, several antioxidant enzymes (e.g. GST, GPX, DHAR) and Gly I might not be getting sufficient GSH for their actions, which ultimately enhanced the levels of ROS and MG. Hoque et al. (2010) suggested that MG can cause toxicity to cells by depleting thiol, GSH and altering the redox homeostasis. It is also hypothesized that reduced activities of CAT and GST, and less stimulation of SOD and GPX in Cu-stressed seedlings might also be the result of MG toxicity (Choudhary et al. 1997; Hoque et al. 2010, 2012). Contrary, SA-pretreated Cu-stressed seedlings maintained a higher level of GSH and GSH/GSSG ratio which facilitated the further stimulation of both antioxidant and glyoxalase systems to minimize oxidative stress. In accordance with Upadhyaya et al. 2011, enhanced antioxidant capacity might also contribute to the improved glyoxalase activity with higher GSH level and GSH/GSSG ratio resulting in decreased MG/ROS toxicity. The results of this study and our previous findings (Hossain et al. 2010; Hasanuzzaman et al. 2012) affirmed that simultaneous induction of ROS and MG detoxification systems by SA could be an effective strategy to induce Cu-tolerance in rice seedlings.
In conclusion, although roots and leaves responded differentially, as a whole our findings revealed that poor induction of ascorbate–glutathione and glyoxalase systems together with reduced activities of CAT and GST are allied with the generation of oxidative stress in Cu-treated seedlings as evident by elevated levels of H2O2, MDA and declines in RWC, Chl, AsA and GSH contents. SA, on the other hand, exerted its protective effects at least by inhibiting Cu translocation, enhancing enzymatic and non-enzymatic antioxidants, and by simultaneous induction of antioxidative and glyoxalase systems which contributed to the cutback of oxidative burden close to the basal level.
References
Arnon DT (1949) Copper enzymes in isolated Chloroplast polyphenol oxidase in Beta vulgaris. Plant Physiol 24:1–15
Bai TH, Li CY, Ma FW, Shu HR, Han MY (2009) Exogenous salicylic acid alleviates growth inhibition and oxidative stress induced by hypoxia stress in Malus robusta Rehd. J Plant Growth Regul 28:358–366
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Bouazizi H, Jouili H, Geitmann A, Ferjani EE (2010) Copper toxicity in expanding leaves of Phaseolus vulgaris L.: antioxidant enzyme response and nutrient element uptake. Ecotoxicol Environ Saf 73:1304–1308
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Burkhead JL, Reynolds KAG, Abdel-Ghany SE, Cohu CM, Pilon M (2009) Copper homeostasis. New Phytol 182:799–816
Chao YY, Chen CY, Huang W, Kao CH (2010) Salicylic acid-mediated hydrogen peroxide accumulation and protection against Cd toxicity in rice leaves. Plant Soil 329:327–337
Chen CT, Chen TH, Lo KF, Chiu CY (2004) Effects of proline on copper transport in rice seedlings under excess copper stress. Plant Sci 166:103–111
Chen J, Zhu C, Li L, Sun Z, Pan X (2007) Effects of exogenous salicylic acid on growth and H2O2-metabolizing enzymes in rice seedlings under lead stress. J Environ Sci 19:44–49
Choudhary D, Chandra D, Kale RK (1997) Influence of methylglyoxal on antioxidant enzymes and oxidative damage. Toxicol Lett 93:141–152
Contreras L, Mella D, Moenne A, Correa JA (2009) Differential responses to copper-induced oxidative stress in the marine macroalgae Lessonia nigrescens and Scytosiphon lomentaria (Phaeophyceae). Aquat Toxicol 94:94–102
Cuypers A et al (2011) The cellular redox state as a modulator in cadmium and copper responses in Arabidopsis thaliana seedlings. J Plant Physiol 168:309–316
De Vos RCH, Vonk MJ, Vooijs R, Schat H (1992) Glutathione depletion due to copper-induced phytochelatin synthesis causes oxidative stress in Silene cucubalus. Plant Physiol 98:853–858
Doderer A, Kokkelink I, van der Veen S, Valk B, Schram A, Douma A (1992) Purification and characterization of two lipoxygenase isoenzymes from germinating barley. Biochim Biophys Acta 112:97–104
Drazkiewicz M, Skórzyńska-Polit E, Krupa Z (2004) Cu-induced oxidative stress and antioxidant defence in Arabidopsis thaliana. Biometals 17:379–387
Dutilleul C, Driscoll S, Cornic G, De Paepe R, Foyer CH, Noctor G (2003) Functional mitochondrial complex I is required by tobacco leaves for optimal photosynthetic performance in photorespiratory conditions and during transients. Plant Physiol 131:264–275
Elia AC, Galarini R, Taticchi MI, Dorr AJM, Mantilacci L (2003) Antioxidant responses and bioaccumulation in Ictalurus melas under mercury exposure. Ecotoxicol Environ Saf 55:162–167
Ellman G (1959) Tissue sulphydryl groups. Arch Biochem Biophys 32:70–77
El-Shabrawi H, Kumar B, Kaul T, Reddy MK, Singla-Pareek SL, Sopory SK (2010) Redox homeostasis, antioxidant defense, and methylglyoxal detoxification as markers for salt tolerance in Pokkali rice. Protoplasma 245:85–96
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155:12–18
Freeman JL, Garcia D, Kim D, Hopf A, Salt DE (2005) Constitutively elevated salicylic acid signals glutathione-mediated nickel tolerance in Thlaspi nickel hyperaccumulators. Plant Physiol 137:1082–1091
Griffiths OW (1980) Determination of glutathione and glutathione disulphide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106:207–212
Guo B, Liang YC, Zhu YG, Zhao FJ (2007) Role of salicylic acid in alleviating oxidative damage in rice roots (Oryza sativa) subjected to cadmium stress. Environ Pollut 147:743–749
Guo B, Liang Y, Zhu Y (2009) Does salicylic acid regulate antioxidant defense system, cell death, cadmium uptake and partitioning to acquire cadmium tolerance in rice? J Plant Physiol 166:20–31
Habibi G (2012) Exogenous salicylic acid alleviates oxidative damage of barley plants under drought stress. Acta Biol Szegediensis 56:57–63
Hasanuzzaman M, Hossain MA, Fujita M (2012) Exogenous selenium pretreatment protects rapeseed seedlings from cadmium-induced oxidative stress by upregulating antioxidant defense and methylglyoxal detoxification systems. Biol Trace Elem Res 149:248–261
He Y, Zhu ZJ (2008) Exogenous salicylic acid alleviates NaCl toxicity and increases antioxidative enzyme activity in Lycopersicon esculentum. Biol Plant 52:792–795
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stochiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hoque TS (2012) Methylglyoxal-induced stomatal closure accompanied by peroxidase-mediated ROS production in Arabidopsis. J Plant Physiol 169:979–986
Hoque MA, Uraji M, Banu MNA, Mori IC, Nakamura Y, Murata Y (2010) The effects of methylglyoxal on glutathione S-transferase from Nicotiana tabacum. Biosci Biotechnol Biochem 74:2124–2126
Hossain MA, Nakano Y, Asada K (1984) Monodehydroascorbate reductase in spinach chloroplasts and its participation in the regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol 25:385–395
Hossain MA, Hossain MZ, Fujita M (2009) Stress induced changes of methylglyoxal level and Glyoxalase I activity in pumpkin seedlings and cDNA cloning of glyoxalase I gene. Aust J Crop Sci 3:53–64
Hossain MA, Hasanuzzaman M, Fujita M (2010) Up-regulation of antioxidant and glyoxalase systems by exogenous glycinebetaine and proline in mung bean confer tolerance to cadmium stress. Physiol Mol Biol Plants 26:259–272
Jiang ZF, Huang SZ, Han YL, Zhao JZ, Fu JJ (2012) Physiological response of Cu mine tailing remediation of Paulownia fortunei (Seem) Hemsl. Ecotoxicology 21:759–767
Khatun S, Ali MB, Hahna E, Paeka K (2008) Copper toxicity in Withania somnifera: growth and antioxidant enzymes responses of in vitro grown plants. Environ Exp Bot 64:279–285
Küpper H, Götz B, Mijovilovich A, Küpper FC, Meyer-Klaucke W (2009) Complexation and toxicity of copper in higher plants. I. Characterization of copper accumulation, speciation, and toxicity in Crassula helmsii as a new copper accumulator. Plant Physiol 15:702–714
Kusumi K, Yaeno T, Kojo K, Hirayama M, Hirokawa D (2006) The role of salicylic acid in the glutathione-mediated protection against photo-oxidative stress in rice. Physiol Plant 128:651–666
Liu N, Lin Z, Mo H (2012) Metal (Pb, Cd, and Cu)-induced reactive oxygen species accumulations in aerial root cells of the Chinese banyan (Ficus microcarpa). Ecotoxicology 21:2004–2011
Lyubenova L, Götz C, Golan-Goldhirsh A, Schröder P (2007) Direct effect of Cd on GSH S-transferase and GSH reductase from Calystegia sepium. Int J Phytorem 9:465–473
Maksymiec W (1997) Effect of copper on cellular processes in higher plants. Photosynthetica 34:321–342
Martínez-Peñalver A, Graña E, Reigosa MJ, Sánchez-Moreiras AM (2012) The early response of Arabidopsis thaliana to cadmium- and copper-induced stress. Environ Exp Bot 78:1–9
Metwally A, Finkemeier I, Georgi M, Dietz KJ (2003) Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol 132:272–281
Molassiotis A, Sotiropoulos T, Tanou G, Diamantidis G, Therios I (2006) Boron induced oxidative damage and antioxidant and nucleolytic responses in shoot tips culture of the apple rootstock EM9 (Malus domestica Borkh). Environ Exp Bot 56:54–62
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Panda S, Patra H (2007) Effect of salicylic acid potentiates cadmium-induced oxidative damage in Oryza sativa L. leaves. Acta Physiol Plant 29:567–575
Popova LP, Maslenkova LT, Ivanova AP, Stoinova Z (2012) Role of Salicylic Acid in Alleviating Heavy Metal Stress. In: Ahmad P, Prasad MNV (eds) Environmental adaptations and stress tolerance of plants in the era of climate change. Springer, New York, Dordrecht, Heidelberg, London, pp 441–466
Radwan DEM (2012) Salicylic acid induced alleviation of oxidative stress caused by clethodim in maize (Zea mays L.) leaves. Pestic Biochem Physiol 102:182–188
Saito R, Yamamoto H, Makino A, Sugimoto T, Miyake C (2011) Methylglyoxal functions as Hill oxidant and stimulates the photoreduction of O2 at photosystem I: a symptom of plant diabetes. Plant, Cell Environ 34:1454–1464
Shi Q, Zhu Z (2008) Effects of exogenous salicylic acid on manganese toxicity, element contents and antioxidative system in cucumber. Environ Exp Bot 63:317–326
Singla-Pareek SL, Yadav SK, Pareek A, Reddy MK, Sopory SK (2006) Transgenic tobacco overexpressing Glyoxalase pathway enzymes grow and set viable seeds in zinc-spiked soils. Plant Physiol 140:613–623
Thounaojam TC, Panda P, Mazumdar P, Kumar D, Sharma GD, Sahoo L, Panda SK (2012) Excess copper induced oxidative stress and response of antioxidants in rice. Plant Physiol Biochem 53:33–39
Upadhyaya CP, Venkatesh J, Gururani MA, Asnin L, Sharma K, Ajappala H, Park SW (2011) Transgenic potato overproducing L-ascorbic acid resisted an increase in methylglyoxal under salinity stress via maintaining higher reduced glutathione level and glyoxalase enzyme activity. Biotechnol Lett 33:2297–2307
Vicente MR, Plasencia J (2011) Salicylic acid beyond defence: its role in plant growth and development. J Exp Bot 62:3321–3338
Wang H, Feng T, Peng X, Yan M, Tang X (2009) Up-regulation of chloroplastic antioxidant capacity is involved in alleviation of nickel toxicity of Zea mays L. by exogenous salicylic acid. Ecotoxicol Environ Saf 72:1354–1362
Wang C, Zhang S, Wang P, Hou J, Qian J, Ao Y, Lu J, Li L (2011) Salicylic acid involved in the regulation of nutrient elements uptake and oxidative stress in Vallisneria natans (Lour.) Hara under Pb stress. Chemosphere 84:136–142
Yadav SK, Singla-Pareek SL, Reddy MK, Sopory SK (2005) Methylglyoxal detoxification by glyoxalase system: a survival strategy during environmental stresses. Physiol Mol Biol Plants 11:1–11
Zhou ZS, Guo K, Abdou-Elbaz A, Yang ZM (2009) Salicylic acid alleviates mercury toxicity by preventing oxidative stress in roots of Medicago sativa. Environ Exp Bot 65:27–34
Acknowledgments
The first author is grateful to the Ministry of Education, Culture, Sports, Science and Technology, Japan for providing financial supports. We are thankful to Mr. Abidur Rahman, Mr. Mohammad Anwar Hossain, Mr. Md. Mesbah Uddin Ansary and two anonymous reviewers for their suggestions for the improvement of the manuscript. We also extend our thanks to Professor Tatsuhiro Matsuo and Mr Masaru Ochiai for assisting in determination of Cu content by atomic absorption spectrophotometer.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mostofa, M.G., Fujita, M. Salicylic acid alleviates copper toxicity in rice (Oryza sativa L.) seedlings by up-regulating antioxidative and glyoxalase systems. Ecotoxicology 22, 959–973 (2013). https://doi.org/10.1007/s10646-013-1073-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-013-1073-x