Abstract
To study the exogenous salicylic acid (SA) to alleviate the cadmium (Cd) toxicity in ryegrass (Lolium perenne L.), ryegrass plants subjected to 100 μM CdCl2 exposure were treated with different concentrations of SA, and Cd toxicity was evaluated by the decreases in plant growth and chlorophyll content. In Cd-treated plants, the activities of antioxidant enzymes, such as superoxide dismutase, peroxidase and catalase, decreased dramatically in both shoots and roots, whereas the accumulation of superoxide anion (O ·-2 ), hydrogen peroxide (H2O2) and malondialdehyde (MDA) increased significantly. Excess Cd also decreased soluble protein and ascorbic acid (AsA) contents, increased accumulation of Cd in both shoots and roots; furthermore, the absorption of micronutrients was inhibited. Addition of 200 μM SA had the most significant alleviating effect against Cd toxicity while the addition of 400 μM SA had no significant effect with Cd treatment. Addition of 100, 200, 300 μM SA considerably increased chlorophyll content and the activities of antioxidant enzymes, increased the uptake and translocation of mineral elements, and decreased H2O2 and MDA accumulation in both shoots and roots of Cd-stressed plants. Addition of 200 μM SA not only decreased the Cd uptake in ryegrass, but also decreased the root-to-shoot translocation of Cd and changed its subcellular distribution in plants. Addition of 200 μM SA increased Cd concentrations in soluble fraction and cell wall in both shoots and roots markedly, with the majority of Cd associated with the cell wall and the soluble fraction and a minor part of Cd present in the cell organelle. Based on these results, we conclude that the optimal concentrations of exogenous SA could alleviate Cd-induced stress and promote ryegrass plant growth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is a widespread pollutant with a long biological half-life; it enters into the food chain and constitutes a potential risk for both animals and humans (Wagner 1993). Cd is a potential environmental phytotoxicant, the main sources in agricultural soils are from the use of phosphate fertilizers, dispersal of sewage sludge, mining and atmospheric deposition of industrial emission (Pinto et al. 2004). This metal is easily taken up by plant roots and can be loaded into the xylem for its transport into leaves. Furthermore, Cd induces complex changes in plants at genetical, biochemical and physiological levels, leading to phytotoxicity, the most obvious symptoms of which are: reduction of tissue and organ growth, leaf chlorosis and leaf and root necrosis (Hernandez and Cooke 1997). At cellular level, Cd toxicity enhanced oxidative stress by increased levels of reactive oxygen species (ROS) such as the superoxide anion (O ·−2 ), hydroxyl (Sharma and Dietz 2009). Accumulation of ROS, including H2O2, causes oxidative damage in plants because they oxidize organic compounds and induce membrane lipid peroxidation in the cellular environment (Schützendübel et al. 2002). However, plants have evolved a complex antioxidant system for protecting potential cells against oxidative injury caused by ROS. Mainly, the ROS-scavenging mechanisms are enzymatic consisting of peroxidase (POD), superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX) and glutathione reductase (GR), and non-enzymatic system consisting of glutathione (GSH) and ascorbic acid (Xu et al. 2010). To avoid Cd toxicity, plants adopt various defence strategies including phytochelatin-based sequestration and compartmentalization processes, as well as additional defence mechanisms, based on cell wall immobilization, plasma membrane exclusion, induction of stress proteins, etc. Other detoxification mechanisms that plants have developed to cope with damages caused by Cd are related with some stress signalling molecules, such as salicylic acid and nitric oxide.
Salicylic acid (SA) is an endogenous growth regulator of phenolic nature, which participates in physiological processes in plants, such as growth, photosynthesis, nitrate metabolism, ethylene production and flowering (Hayat et al. 2007). The effects of SA on abiotic stress tolerance in plants were reported by many authors (Borsani et al. 2001; Kang and Saltviet 2002). In the recent decades, an increasing number of articles have reported the effects of exogenous SA on alleviating heavy-metal toxicity in plants, for example in rice (He et al. 2010), barley (Metwally et al. 2003), soybean (Drazic and Mihailovic 2005) and pea (Popova et al. 2009). Guo et al. (2007) reported that pretreatment with SA alleviated Cd-induced inhibition of root growth and enhanced the antioxidant defense activities in Cd-stressed rice, thus alleviating Cd-induced oxidative damage and enhancing Cd tolerance. A similar situation was described in Matricaria chamomilla plants under Cd stress (Kováčik et al. 2009). Cd-induced oxidative stress on rice and Arabidopsis thaliana was also alleviated by the addition of SA (Chao et al. 2010; Zawoznik et al. 2007). Similarly, Cd-induced oxidative stress on bean plants was alleviated by supplementation with SA (Saidi et al. 2013). All of these results indicated the importance of SA in protection against deleterious effects of heavy metals, and the mechanisms by which SA might help plants resist heavy-metal stress also were postulated. First of all, SA could be an effective preventive agent against certain compounds, which may induce oxidative stress directly (Ananieva et al. 2002). Secondly, low concentrations of SA might cause enhanced tolerance toward most kinds of abiotic stresses due primarily to enhanced antioxidative capacity (Horváth et al. 2007). Thirdly, SA could function as a signaling molecule in the induction genes responsible for protective mechanisms (Horváth et al. 2007).
Perennial ryegrass is an important and widespread perennial cool-season turf grass due to its massive root system, superior regeneration and tillering ability (Hannaway et al. 1999). As well, ryegrass was used since it represented a kind of considerable important forage crop. It can accumulate metals in its biomass, and commonly used as a suitable species for revegetation of metalliferous wastes (Arienzo et al. 2004). In addition, our previous studies have demonstrated that perennial ryegrass has the potential for rehabilitation of Cd stress (Wang et al. 2013). Based on the above studies, we hypothesized that SA may ameliorate Cd-induced toxic effects on perennial ryegrass. Therefore, the influence of different concentrations of SA on Cd-induced changes in growth and antioxidant system, as well as on Cd content, Cd subcellular distribution and micronutrient distribution in ryegrass seedlings has been investigated. Furthermore, screened out the optimal concentrations of SA in alleviating Cd stress and illuminated the physiological mechanism of SA on alleviating cadmium stress in perennial ryegrass.
Materials and methods
Plant material and culture conditions
Ryegrass seeds were first sterilized with 5 % sodium hypochlorite for 15 min and washed extensively with distilled water, then germinated on moist filter paper in the dark at 26° for 3 days. Initially, seedlings of uniform size were transferred to plastic pots (volume 500 mL) filled with perlite (50 plants per pot) and watered with half-strength Hoagland nutrition solution for 7 days. The seedlings were then watered with full-strength Hoagland solution. Three-week-old uniform seedlings were transferred into 1,000 mL black plastic containers with 50 seedlings per container. The nutrient solution was renewed every 2 days. These treatments contain: CK: Hoagland’s solution; Cd: 100 μM Cd-treated nutrient solutions; T1: 100 μM SA added into 100 μM Cd-treated nutrient solutions; T2: 200 μM SA added into 100 μM Cd-treated nutrient solutions; T3: 300 μM SA added into 100 μM Cd-treated nutrient solutions; T4: 400 μM SA added into 100 μM Cd-treated nutrient solutions.
The treatments were arranged in a randomized block design with three replicates. The experiment was carried out under a controlled-environment chamber at 14/10 light/dark photoperiod and photon flux density 150 µmol m−2 s−1 at the leaf level, day/night temperature of 25/18 °C and 65 ± 5 % relative humidity. After 2 weeks of growth with the above conditions, the plants were harvested and the roots and leaves were separated and washed with 5 mM CaCl2 first and then repeatedly washed with deionized distilled water. For the estimation of plant dry matter, Cd and mineral nutrients content, the plants were dried at 80 °C for 48 h. For the enzyme determination, fresh plant material was frozen in liquid nitrogen and stored at −70 °C until use.
Determination of plant growth and root activity
Seedlings heights were determined immediately after harvesting. At harvest, the roots and leaves were separated and oven-dried for 30 min at 105 °C, then at 70 °C till the materials reach their constant weights. Plant height, fresh weight and dry weight were measured. Root activity was determined by TTC method according to Zhang and Di (2003). It was expressed as absorbance per unit gram root fresh weight.
Determination of chlorophyll content
The chlorophyll content was determined according to the method of Knudson et al. (1977). Fresh ryegrass leaf (0.5 g) was extracted in 2 mL 95 % ethanol for 24 h in the dark, and the extracted solution was analyzed. The amounts of chlorophyll a, b and carotenoid were determined using a spectrophotometer (SHIMADZU UV-2450, Kyoto, Japan), by reading the absorbance at 665, 649 and 470 nm. The chlorophyll content results are expressed as unit’s mg per gram-fresh weight (mg g−1 FW).
Determination of antioxidant enzyme activities
For extraction of antioxidative enzymes, leaves and roots were homogenized with 50 mM Na2HPO4-NaH2PO4 buffer (pH 7.8) containing 0.2 mM EDTA and 2 % insoluble polyvinylpyrrolidone in a chilled pestle and mortar. The homogenate was centrifuged at 12,000×g for 20 min and the resulted supernatant was used for determination of enzyme activities. The whole extraction procedure was carried out at 4 °C. All spectrophotometric analysis was conducted on a SHIMADZU UV-2450 spectrophotometer (Kyoto, Japan). SOD activity was assayed by measuring its ability to inhibit the photochemical reduction of nitroblue tetrazolium following the method of Stewart and Bewley (1980). CAT activity was measured as the decline in absorbance at 240 nm due to the decrease of extinction of H2O2 according to the method of Patra et al. (1978). POD activity was measured by the increase in absorbance at 470 nm due to guaiacol oxidation (Nickel and Cunningham 1969).
Determination of O ·−2 generation rate
The production rate of O ·−2 was measured as described by Elstner and Heupel (1976). Fresh leaves (0.2 g) were homogenized in 1 mL of 50 mM phosphate buffer (pH 7.8), and the homogenate was centrifuged at 10,000×g for 10 min. Then 0.5 mL of the supernatant was added to 0.5 mL 50 mM phosphate buffer (pH 7.8) and 0.1 mL of 10 mM hydroxylamine hydrochloride. After 1 h reaction at 25°, the mixture was added to 1 mL 17 mM sulfanilamide and 1 mL 7 mM a-naphthylamine at 25° for 20 min. The specific absorbance at 530 nm was determined. Sodium nitrite was used as a standard solution to calculate the production rate of O ·−2 .
Determination of H2O2 concentration and lipid peroxidation
Fresh samples (1.0 g) were homogenized in 2 mL ice-cold acetone. Titanium reagent (2 % TiCl2 in conc. HCl) was added to a known volume of extract supernatant to give a Ti (IV) concentration of 2 %. The Ti–H2O2 complex, together with unreacted Ti, was then precipitated by adding 0.2 mL 17 M ammonia solution for each 1 mL of extract. The precipitate was washed five times with ice acetone by resuspension, drained, and dissolved in 1 M H2SO4 (3 mL). The absorbance of the solution was measured at 410 nm against blanks, which had been prepared similarly but without plant tissue (Patterson et al. 1984). The level of lipid peroxidation in fresh leaf was measured in terms of malondialdehyde (MDA) concentration by the thiobarbituric acid reaction method (Heath and Packer 1968). MDA concentration was expressed as nmol g−1 FW.
Determination of soluble protein and ascorbic acid (AsA) content
Protein was estimated by the method of Bradford (1976). Fresh leaves (0.5 g) were homogenized in 1 mL phosphate buffer (pH 7.0). The crude homogenate was centrifuged at 5,000×g for 10 min. Half milliliter of freshly prepared trichloroacetic acid (TCA) was added and centrifuged at 8,000×g for 15 min. The debris was dissolved in 1 mL of 0.1 N NaOH and 5 mL Bradford reagent was added. Absorbance was recorded at 595 nm using a spectrophotometer (SHIMADZU UV-2450, Japan).
Ascorbic acid (AsA) concentration was measured according to Tonamura (1978). Fresh leaves (0.2 g) were homogenized in ice-cold 2 mL 10 % metaphosphoric acid. After centrifugation at 15,000×g for 10 min, to 0.5 mL of supernatant was added 1 mL citric acid-phosphoric acid buffer (pH 2.3) and 1 mL 2, 6-dichlorophenol indophenol (30 mg L−1). After 30 s, the absorbance was determined at 524 nm. AsA concentration was expressed as mg g−1 FW.
Determination cadmium and mineral element concentrations
The dried tissues were weighed and grinded into powder for the determination of cadmium and mineral element concentrations, which was measured by flame atomic absorbance spectrometry (SHIMADZU AA-6300, Kyoto, Japan) after digested with mixed acid [HNO3 + HClO4 (3:1, v/v)] (Ali et al. 2002).
Subcellular distribution study
Leaf and root samples (0.2 g) were crushed into powder by adding 10 ml of extraction solution containing 0.25 mM sucrose, 50 mM Tris–HC1 buffer solution (pH 7.5), and 1.0 mM DL-dithioerythritol. According to the method of Weigel and Jäger (1980) with some modification, cells were separated with different centrifugation into three fractions, i.e. the cell wall, the cell organelle, and the soluble fraction. All operations were undertaken at 4 °C. The cell wall and cell organelle fractions were transferred to 100 ml Erlenmeyer conical flasks with deionized water, evaporated to dryness, and digested with 5 ml of HNO3. Cd concentrations of the soluble fraction and digested samples were analyzed by AAS.
Statistical analysis
The experiment was a completely random design with three replications. Statistical analyses were carried out by analysis of variance (ANOVA) using SAS software (SAS Institute, Cary NC). Differences between treatments were separated by the least significant difference (LSD) test at a 0.05 probability level.
Results
Plant growth and root activity
Cd exposure significantly decreased shoot height, root length, fresh weight, dry weight, root/shoot ratio and root activity of ryegrass seedlings (Table 1). However, this inhibition was significantly alleviated by 100, 200 and 300 μM SA, especially 200 μM SA. Compared with Cd treatment, the shoot height, root length, fresh weight, dry weight and root/shoot ratio of T2 treatment were increased by 17.20, 31.06, 34.08, 33.34 and 34.28 %, but T4 treatment had no significant effect. Furthermore, the root activity of ryegrass seedlings under Cd stress also increased markedly with the addition of SA. Taken together, the alleviating effect of SA was found in a general trend of T2 > T3 > T1 > T4.
Chlorophyll content
Table 2 showed that total chlorophyll, chl a, chl b, and car contents were markedly decreased under Cd treatment (41.94, 38.27, 51.42 and 52.71 % respect to CK). With adding different concentrations of SA under Cd stress, the chlorophyll content has different changes. The T1, T2, T3 and T4 treatments increased total chlorophyll by 34.51, 85.74, 33.02 and 29.67 % than Cd treatments. And the best of alleviating effect on increasing total chlorophyll content was T2 treatment. Similar findings were found for the chl a, chl b and car contents.
Antioxidant enzymes
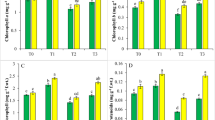
The effects of Cd and SA on activities of antioxidant enzymes were found in Fig. 1. Cd inhibited in SOD activity, POD activity and CAT activity in both shoots and roots compared with CK. SOD activity (Fig. 1a) of shoots and roots decreased by 61.36 % and by 47.74 % under Cd stress, whereas they were significantly increased in Cd + SA treatment, respectively. The T1, T2, T3 and T4 treatments increased SOD activity by 105.98, 135.17, 97.03, 81.73 % in shoots and by 86.18, 95.51, 71.73, 69.63 % in roots than Cd treatment. And the best of alleviating effect was T2 treatment. It was similar in activities of POD (Fig. 1b) and CAT (Fig. 1c). The alleviating effect of SA was found in a general trend of T2 > T3 > T1 > T4.
Effects of different concentrations of SA on SOD (a), CAT (b) and POD (c) content in leaves and roots of 14 days treatment ryegrass plants under Cd stress grown in nutrient solutions. Values are the mean of three replicates. Each replicate has 50 plants. Bars with different letters are significantly different at P < 0.05
O ·−2 generation rate, H2O2 and MDA content
Compared with CK, Cd treatment increased O ·−2 generation rate (Fig. 2a) by 325.33 % in shoots and by 266.24 % in roots significantly. However, addition of SA significantly decreased O ·−2 generation rate in both shoots and roots under Cd stress. T1, T2, T3 and T4 treatments decreased O ·−2 generation rate by 42.82, 65.17, 60.62, 42.11 % in shoots and by 25.14, 36.35, 19.44, 23.33 % in roots than Cd treatment. T2 treatment had the lowest O ·−2 generation rate in both shoots and roots. Similar findings were found for the H2O2 content (Fig. 2b).
Effects of different concentrations of SA on O ·−2 generation rate (a), H2O2 (b) and MDA (c) content in leaves and roots of 14 days treatment ryegrass plants under Cd stress grown in nutrient solutions. Values are the mean of three replicates. Each replicate has 50 plants. Bars with different letters are significantly different at P < 0.05
Figure 2c showed the effects of SA treatment and Cd exposure on the concentrations of MDA in ryegrass shoots and roots. MDA levels in Cd-stressed plants elevated significantly compared with CK. However, the addition of SA markedly reduced MDA content compared with Cd treatment alone. And the best of alleviating effect was T2 treatment.
Soluble protein and AsA content
Cd treatment decreased soluble protein content (Fig. 3a) and AsA content (Fig. 3b) in shoots and roots dramatically. However, these inhibitions were significantly alleviated by 100, 200 and 300 μM SA, especially 200 μM SA. Compared with Cd treatment, the soluble protein and AsA content of T2 treatment were increased by 49.58, 68.44 % in shoots and by 124.09, 32.80 % in roots.
Effects of different concentrations of SA on soluble protein (a) and AsA (b) content in leaves and roots of 14 days treatment ryegrass plants under Cd stress grown in nutrient solutions. Values are the mean of three replicates. Each replicate has 50 plants. Bars with different letters are significantly different at P < 0.05
Cd concentrations
Figure 4a showed Cd accumulation was significantly higher in roots than in shoots. However, Cd accumulation was decreased not only in shoots but also in roots with addition of SA, and T2 treatment had the lowest Cd content both in shoots and roots. Moreover, T1, T2, T3 and T4 treatments decreased Cd content by 58.39, 67.42, 51.92, 21.46 % in shoots and by 4.25, 19.46, 7.95, 3.28 % in roots than treatment with Cd. And the best of alleviating effect was T2 treatment.
Effects of different concentrations of SA on Cd content (a) in leaves and roots and Cd subcellular distribution in leaves (b) and roots (c) of 14 days treatment ryegrass plants under Cd stress grown in nutrient solutions. Values are the mean of three replicates. Each replicate has 50 plants. Bars with different letters are significantly different at P < 0.05
Cd subcellular distribution
In order to investigate the mechanisms of SA in alleviating Cd toxicity, the subcellular distribution of Cd was examined carefully. In the shoots and roots of Cd-treated plants, the majority of Cd accumulated in the cell wall (Fig. 4b, c). Cd accumulation in the soluble fraction was less than in the cell wall, and only a minority of Cd accumulation was in cell organelles. The results indicated that the subcellular distribution of Cd was quite different in lower concentrations (100, 200 and 300 μM) of SA with Cd treatments. Treatment with 200 μM SA increased Cd accumulation in the cell walls of the ryegrass plant shoots and roots almost by 36.27 and 30.80 %, and Cd accumulation in the organelles of the shoots and root cells decreased significantly, although Cd accumulation in the soluble fraction of the shoots and roots increased almost by 47.27 and 50.22 %.
Ca, Fe, Mg, Cu, Zn and Mn contents
As shown in Table 3, Cd treatment significantly decreased Ca, Fe, Mg, Cu, Zn and Mn contents in shoots and Ca, Fe, Zn and Mn contents in roots. In contrast, Mg and Cu contents were significantly increased under Cd stress in roots. The application of SA significantly increased Ca, Fe, Mg, Cu, Zn and Mn contents in shoots, and 200 μM SA had the most obvious promotion on mineral concentrations. In addition, compared with Cd treatment, SA supplementation also increased mineral concentrations in root, but with the increased concentration of SA, the absorption of mineral contents also increased. So, it needed proper concentration.
Discussion
Salicylic acid, a naturally occurring plant hormone, influences various physiological and biochemical functions in plants, acts as an important signaling molecule and has diverse effects on tolerance to biotic stress (Horváth et al. 2007). However, studies have shown that different concentrations of SA have both promotive and inhibitory effects on germination, depending on the concentration applied and the assay conditions (Guo et al. 2013). In the present experiment, compared with CK, the growth of ryegrass plants in the presence of Cd was delayed significantly. However, the inhibitory effects were significantly alleviated by low concentrations of SA (100, 200 and 300 μM). The mitigation effect of higher concentrations of SA (400 μM) on ryegrass reduced significantly (Table 1). These results demonstrated the dual nature of SA on plants that were consistent with the results of Maslenkova and Toncheva (1998). The optimum concentration of SA can increase root activity (Table 1) which can reflect the situation of root growth and the level of metabolite in plant, and directly affect the shoot growth, nutrition status and production of plant. Therefore, the alleviation of inhibitory growth by SA may depend on increasing chlorophyll content, changing in mineral nutrition, induction of antioxidant response and protective role of membranes that increase the tolerance of plant to damage.
Symptoms of Cd toxicity such as chlorosis and necrotic spots were considerably visible in ryegrass plants treated with Cd alone (Table 2). Several authors reported decreased chlorophyll content in the leaves of Cd-treated plants (Drazic and Mihailovic 2005; Djebali et al. 2005), such chlorosis could result from Fe deficiency and the inhibition of chlorophyll synthesis (Prasad et al. 1999). And this is primarily because Cd destroyed the structure of chloroplast, inhibited synthesis of chlorophyll and increased the rate of chlorophyll degradation (Djebali et al. 2005). The decrease in chlorophyll content may lead to the reduced in dry biomass of shoot and root tissues. However, the addition of SA increased chlorophyll content dramatically, and the best of alleviating effect was T2 treatment. Similar results were reported in Kentucky bluegrass leaves under Cd stress (Guo et al. 2013) and hemp leaves under Cd stress (Shi et al. 2009). In this experiment, 100 μM SA, 300 μM SA, and especially 200 μM SA significantly increased Fe concentration in both the shoots and roots of ryegrass and then increased chlorophyll content. In addition, SA may effectively reduce the level of ROS generated during stress resulting in alleviating the oxidative negative effects of ROS on growth and chlorophyll content.
SOD, POD and CAT are all important enzymes involved in antioxidation processes and are present in different organelles in plants, protecting plants from oxidative stress. In the present study, the presence of Cd reduced the activities of these antioxidant enzymes (Fig. 1). This result was in agreement with Guo et al. (2007) who reported that exposure to 50 μΜ Cd significantly decreased activities of SOD, CAT and POD. These enzymes contain Fe in their structure. Since high concentrations of Cd may decrease Fe content in plant tissues, it can be supposed that the reduction in their activities in shoots and roots of ryegrass plants subjected to excess Cd may result from deficiency of Fe for the biosynthesis of these enzyme molecules. However, when SA was applied to the nutrient solution, SOD, POD and CAT activities (Fig. 1) increased, whereas H2O2 content (Fig. 2b) and MDA content (Fig. 2c) decreased. Similar results of increasing SOD, POD and CAT activities by pretreatment with SA have been demonstrated in Cd-stressed rice plants (Guo et al. 2007). In addition, SA increased Fe uptake also can increase SOD, POD and CAT activities. This suggested that stimulation of antioxidant production might be achieved not only by SA-induced protein synthesis, but also by SA had an ameliorating effect on heavy-metal toxicity and thus protected against oxidative damage, then improved Cd tolerance.
Cd stress activates a common mechanism involving the production of ROS like O ·−2 and H2O2 in plant cells (Hsu and Kao 2004; Laspina et al. 2005). MDA content was measured as an index of lipid peroxidation. In this experiment, the markedly increase in contents of O ·−2 , H2O2 and MDA under Cd stress were observed (Fig. 2). Similar results have been observed in ryegrass and lettuce (Wang et al. 2013; Xu et al. 2013). The growth inhibition might be partly due to enhanced production and accumulation of ROS. Furthermore, leaf MDA accumulation appeared to be a suitable indicator of heavy-metal stress experienced by plants and indicates oxidative damage to membranes (Guo et al. 2007). However, SA-treated plants counteracted oxidative damages and had protective effect against Cd stress, which was concomitant with the decreased production rate of O ·−2 (Fig. 2a) and the concentration of H2O2 (Fig. 2b). This influence can protect cell membrane from oxidative injury and alteration in cell structure, and then decreased the accumulation of MDA (Fig. 2c). In particular, the best of alleviating effect was T2 treatment. This could be SA inhibited the plants from oxidation damage by the regulating general mechanisms for cellular redox homeostasis and promoting the transformation of O ·−2 to H2O2 and O2 and also by enhancing the H2O2-scavenging enzymes activities. Moreover, the chelating action of SA on metals may be responsible for decreasing MDA content in plants under heavy-metal stress.
It is well known that abiotic stress may inhibit a synthesis of some proteins and promote others (Ericson and Alfinito 1984) with a general trend of decline in the overall content (Fig. 3a). This study coincided with Reinheckel et al. (1998) who also reported that Cd stress caused a decrease in soluble protein content in isolated mitochondria. It is likely that heavy metals have induced lipid peroxidation and fragmentation of proteins due to the toxic effects of ROS, which led to the reduction of protein content (Davies et al. 1987). However, SA supplementation improved Cd-decreased soluble protein content. The probable reason was that the SA-induced protein kinases may also function as central convergence points in stress signaling in abiotic stresses (Jonak et al. 2002), accompanied by the improvement of protein content. And T2 treatment had the highest soluble protein content. In addition, ascorbate acid is an important antioxidant in plants. In the present study, ryegrass plants responded to Cd stress with decrease AsA content in both shoots and roots (Fig. 3b). Mostofa and Fujita (2013) also demonstrated Cu decreased AsA in rice leaves and roots. However, Cd-caused decrease of AsA content was reversed by the addition of SA. This suggested SA mediated the enhancement of AsA levels in ryegrass plants. Moreover, AsA also might help the plant to cope with the Cd-induced oxidative damage, and AsA can directly detoxify oxygen free radicals, and then improved the tolerance of Cd toxicity.
Plant root is the main part direct contact with Cd in soil and the cell walls in roots plays a significant role in heavy metal tolerance in plants (Xiong et al. 2009). As shown in Fig. 4a, Cd concentrations in different ryegrass tissues decreased following the order of roots > shoots, which implies that the translocation of Cd to the shoots part was restricted by internal barriers to defend the above-ground part. Shi et al. (2009) had also reported that most Cd was accumulated in roots of hemp plants. Therefore, higher Cd concentration in root than in other tissues could be considered as an important tolerance mechanism of ryegrass. As a non-essential element, Cd inside root cells by loading from the symplasm into the xylem (Clemens 2006), but the progress that Cd ions trapped inside cells by the formation of metal-chelating molecules and transporter-mediated vacuolar sequestration may restrict Cd delivery to the xylem from the symplast, therefore contribute to the change of Cd distribution between roots and shoots observed in ryegrass. However, SA decreased the uptake of Cd and the root-to-shoot translocation of Cd, thus resulting in low Cd accumulation in shoots. Concurred to our finding, exogenous SA decreased the root-to-shoot translocation of Cd of ryegrass plants under Cd stress (Wang et al. 2013). Raza and Shafiq (2013 ) also indicated that foliar application of SA substantially retarded Cd uptake in radish plants thereby improved root vegetative characters resulting in amelioration of Cd-toxicity. The reasons for this result may be the high proportion of Cd in the soluble fraction limited root-to-shoot translocation of Cd. Integrated the findings, we conclude that, SA might play an important role in reducing Cd uptake and root-to-shoot translocation of Cd in ryegrass plants subjected to Cd stress, thereby contributing to improve the resistance to Cd stress and explaining the ameliorating effect of SA on the growth.
Cd sequestration is one of the principle mechanisms employed to avoid free Cd in plasmatic compartments. In the present study, the distributions of Cd were different in cells; the majority of Cd accumulated in the cell wall; Cd accumulated in the soluble fraction was less than in the cell wall, and a minority of Cd accumulated in cell organelle (Fig. 4b, c). The distribution of Cd in these subcellular fractions was considered a crucial mechanism for Cd tolerance (Gallego et al. 2012). Cd retention in cell wall might be due to cross-linking of Cd to carboxyl groups of the cell wall (Barceló and Poschenrieder 1990) and/or to an interaction with thiol residues of soluble proteins (Leita et al. 1993). This was in agreement with Ma et al. (2005) who indicated Cd was mostly found in cell wall and in soluble fractions. However, SA supply restricted Cd in cell organelle, but increased Cd in soluble fraction and cell wall (Fig. 4b, c). This difference may be due to coupling to components of cell wall and vacuole compartmentalization. Plant cell walls are mainly composed of polyose (including cellulose, hemicellulose and pectin) and protein (Hayens 1980) which can bind Cd ions and restrict their transportation across cytomembrane. Xiong et al. (2009) also indicated that treatment with SNP increased Cd accumulation in the cell walls of rice roots due to SNP increased pectin and hemicellulose content and decreased cellulose content significantly in the cell walls. In addition, the cell wall is the main Ca2+ store in plant cells and addition of SA increased the uptake of Ca2+, so this can protect the integrity of the cell function and the cell walls may accumulate more Cd. Moreover, earlier studies showed that the vacuole is the site for the accumulation of a number of heavy metals, including Cd (De 2000; Ernst et al. 1992), which acts as the subdominant site of preferential Cd binding in all test tissues. This result may be that the prevailing part of cell Cd is bound to phytochelatins (PCs) and transported into vacuole as PC-Cd-S complex (Hall 2002). PCs may increase Cd content in soluble fraction under Cd stress and increased plants resistance under Cd stress. This strategy could further decrease the amount of Cd interfering with the organelles. Moreover, the reduction of Cd content in cell organelle fractions may be an important mechanism of SA-induced Cd tolerance. Based on these findings, we conclude that the inhibition of Cd in the cell wall and compartmentalization of Cd in the vacuole of both shoots and roots was responsible for SA-increased Cd tolerance in ryegrass plants.
The beneficial effect of SA on plant growth could also be attributed to the maintenance of optimal mineral nutrition. In ryegrass plants, Cd toxicity inhibited the uptake of Ca, Zn, Mn and Fe by plants, disturbing intracellular ion homeostasis and exerting a toxic effect on plants, and SA treatment was found to induce its transport from nutrient solution to plants (Table 3). Changes of Ca, Fe, Mg and Mn concentrations indicated that Cd significantly disturbed ionic homeostasis, and that SA stimulated its maintenance, especially in leaves. Positive effect of SA on the ion uptake and inhibitory effect of SA on Cd uptake should also be reliable for managing Cd of ryegrass plants and contributing to ionic homeostasis maintenance. In addition, it is well known that H+-ATPase in plasma membrane plays an important role in the transport of multiple ions (Palmgren and Harper 1999), and there are investigations indicate that SA-mediated could induce H+-ATPase activity (Gordon et al. 2004), which might be responsible for SA increasing absorption of Ca, Fe, Mg and Zn under Cd toxicity (Table 3). These results indicated that SA could ameliorate ion equilibrium in ryegrass cells under Cd stress.
Conclusion
In conclusions, Cd exposure alone depressed plant growth, reflected by the inhibition of chlorophyll synthesis, increase of oxidative stress and the inhibitory effect on uptake of some mineral nutrient elements. 100, 200, 300 μM SA could attenuate Cd toxicity in ryegrass plants exposed to Cd stress, which probably includes not only the regulation of the chloroplast and antioxidant system, but also the reduction of Cd uptake and translocation of Cd from roots to shoots and the improvement of mineral nutrient absorption. And 200 μM SA had the best of alleviating effect against Cd toxicity. Furthermore, lower Cd-induced stress in SA-treated plants may be related to lower Cd levels in cell organelle due to the inhibition of the cell wall, as well as the compartmentalization of the vacuole that reduce Cd toxicity. This suggests that an appropriate concentration of SA could be used as a potential growth regulator to improve plant growth under Cd stress.
Abbreviations
- SA:
-
Salicylic acid
- Cd:
-
Cadmium
- SOD:
-
Superoxide dismutase
- POD:
-
Peroxidase
- CAT:
-
Catalase
- O ·−2 :
-
Superoxide anion radical
- ROS:
-
Reactive oxygen species
- MDA:
-
Malondialdehyde
- AsA:
-
Ascorbic acid
- PCs:
-
Phytochelatins
References
Ali NA, Bernal MP, Ater M (2002) Tolerance and bioaccumulation of copper in Phragmites australis and Zea mays. Plant Soil 239:103–111
Ananieva EA, Alexieva VS, Popova LP (2002) Treatment with salicylic acid decreases the effects of paraquat on photosynthesis. J Plant Physiol 159:685–693
Arienzo M, Adamo P, Cozzolino V (2004) The potential of Lolium perenne for revegetation of contaminated soil from a metallur-gical site. Sci Total Environ 319:13–25
Barceló J, Poschenrieder CH (1990) Plant water relations as affected by heavy metal stress: a review. J Plant Nutr 13:1–37
Borsani O, Valpuesta V, Botella MA (2001) Evidence for a role of salicylic acid in the oxidative damage generated by NaCl and osmotic stress in Arabidopsis seedlings. Plant Physiol 126:1024–1030
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizating the principle of protein dyes binding. Anal Biochem 72:248–254
Chao YY, Chen CY, Huang WD, Kao CH (2010) Salicylic acid-mediated hydrogen peroxide accumulation and protection against Cd toxicity in rice leaves. Plant Soil 329:327–337
Clemens S (2006) Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 88:1707–1719
Davies CS, Nielsen SS, Nielsen NC (1987) Flavor improvement of soybean preparations by genetic removal of lipoxygenase-2. J Am Oil Chem Soc 64:1428–1433
De DN (2000) Plant Cell Vacuoles. CSIRO Publishing, Collingwood
Djebali W, Zarrouk M, Brouquisse R, El Kahoui S, Limam F, Ghorbel MH, Chaïbi W (2005) Ultrastructure and lipid alterations induced by cadmium in tomato (Lycopersicon esculentum) chloroplast membranes. Plant Biol 7:258–268
Drazic G, Mihailovic N (2005) Modification of cadmium toxicity in soybean seedlings by salicylic acid. Plant Sci 168:511–517
Elstner EF, Heupel A (1976) Inhibition of nitrite formation from hydroxyl ammonium-chloride: a simple assay for superoxide dismutase. Anal Biochem 70:616–620
Ericson MC, Alfinito AE (1984) Proteins produced during salt stress in tobacco cell cultures. Plant Physiol 74:506–509
Ernst WHO, Verkleij JAC, Schat H (1992) Metal tolerance in plants. Acta Bot Neerl 41:229–248
Gallego SM, Pena LB, Barcia RA, Azpilicueta CE, Iannone MF, Rosales EP, Zawoznik MS, Groppa MD, Benavides MP (2012) Unravelling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms. Environ Exp Bot 83:33–46
Gordon LK, Minibayeva FV, Rakhmatullina DF, Alyabyev AJ, Ogorodnikova TI, Loseva NL, Valitova YN (2004) Heat production of wheat roots induced by the disruption of proton gradient by salicylic acid. Thermoch Acta 422:101–104
Guo B, Liang YC, Zhu YG, Zhao FJ (2007) Role of salicylic acid in alleviating oxidative damage in rice roots (Oryza sativa) subjected to cadmium stress. Environ Pollut 147:743–749
Guo Q, Meng L, Mao PC, Jia YQ, Shi YJ (2013) Role of exogenous salicylic acid in alleviating cadmium-induced toxicity in Kentucky bluegrass. Biochem Syst Ecol 50:269–276
Hall J (2002) Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot 53:1–11
Hannaway D, Fransen S, Cropper J, Teel M, Chaney M, Griggs T, Halse R, Hart J, Cheeke P, Hansen D, Klinger R, Lane W (1999) Perennial ryegrass (Lolium perenne L.). In: A pacific northwest extension publication, vol PNW 502. Oregon State University, Washington State University, University of Idaho
Hayat S, Ali B, Ahmad A (2007) Salicylic acid: biosynthesis, metabolism and physiological role in plants. Salicylic acid: a plant hormone, pp 1–14
Hayens RJ (1980) Ion exchange properties of roots and ionic interactions within the root POPLsn: their role in ion accumulation by plants. Bot Rev 46:75–99
He JY, Ren YF, Pan XB, Yan YP, Zhu C, Jiang D (2010) Salicylic acid alleviates the toxicity effect of cadmium on germination, seedling growth, and amylase activity of rice. J Nutr Soil Sci 173(2):300–305
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I: kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hernandez LE, Cooke DT (1997) Modifications of the root plasma membrane lipid composition of cadmium-treated Pisum sativum. J Exp Bot 48:1375–1381
Horváth E, Szalai G, Janda T (2007) Induction of Abiotic Stress Tolerance by Salicylic Acid Signaling. J Plant Growth Regul 26:290–300
Hsu Y, Kao C (2004) Cadmium toxicity is reduced by nitric oxide in rice leaves. Plant Growth Regul 42:227–238
Jonak C, Őkrész L, Bögre L, Hirt H (2002) Complexity, cross talk and integration of plant MAP kinase signalling. Curr Opin Plant Biol 5:415–424
Kang HM, Saltviet M (2002) Chilling tolerance of maize, cucumber and rice seedling leaves and roots are differentially affected by salicylic acid. Physiol Plant 115:571–576
Knudson LL, Tibbitts TW, Edwards GE (1977) Measurement of ozone injury by determination of leaf chlorophyll concentration. Plant Physiol 60:606–608
Kováčik J, Grúz J, Bačkor M, Strnad M, Repčák M (2009) Salicylic acid-induced changes to growth and phenolic metabolism in Matricaria chamomilla plants. Plant Cell Rep 28:135–143
Laspina NV, Groppa MD, Tomaro ML, Benavides MP (2005) Nitric oxide protects sunflower leaves against Cd-induced oxidative stress. Plant Sci 169:323–330
Leita L, De Nobili M, Mondini C, Baca-García MT (1993) Response of leguminosae to cadmium exposure. J Plant Nutr 16:2001–2012
Ma JF, Ueno D, Zhao FJ, McGrath SP (2005) Subcellular localisation of Cd and Zn in the leaves of a Cd-hyperaccumulating ecotype of Thlaspi caerulescens. Planta 220:731–736
Maslenkova L, Toncheva S (1998) Salicylic acid induced changes in photosystem II reactions in barley plants. Compt Rend Acad Bulg Sci 51:101–104
Metwally A, Finkemeier I, Georgi M, Dietz KJ (2003) Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol 132:272–281
Mostofa MG, Fujita M (2013) Salicylic acid alleviates copper toxicity in rice (Oryza sativa L.) seedlings by up-regulating antioxidative and glyoxalase systems. Ecotoxicology 22:959–973
Nickel RS, Cunningham BA (1969) Improved peroxidase assay method using Ieuco 2, 3, 6-trichlcroindophenol and application to comparative measurements of peroxidase catalysis. Anal Biochem 27:292–299
Palmgren MG, Harper JF (1999) Pumping with plant P-type ATPases. J Exp Bot 50:883–893
Patra HL, Kar M, Mishre D (1978) Catalase activity in leaves and cotyledons during plant development and senescence. Biochem Pharmacol 172:385–390
Patterson BD, MacRae EA, Ferguson IB (1984) Estimation of hydrogen peroxide in plant extracts using titanium (IV). Anal Biochem 139:487–492
Pinto AP, Mota AM, de Varennes A, Pinto FC (2004) Influence of organic matter on the uptake of cadmium, zinc, copper and iron by sorghum plants. Sci Total Environ 326:239–247
Popova LP, Maslenkova LT, Yordanova RY, Ivanova AP, Krantev AP, Szalai G, Janda T (2009) Exogenous treatment with salicylic acid attenuates cadmium toxicity in pea seedlings. Plant Physiol Biochem 47:224–231
Prasad KVSK, Saradhi PP, Sharmila P (1999) Concerted action of antioxidant enzymes and curtailed growth under zinc toxicity in Brassica juncea. Environ Exp Bot 42:1–10
Raza SH, Shafiq F (2013) Exploring the role of salicylic acid to attenuate cadmium accumulation in radish (Raphanus sativus). Int J Agric Biol 15(3):547–552
Reinheckel T, Noack H, Lorenz S, Wiswedel I, Augustin W (1998) Comparison of protein oxidation and aldehyde formation during oxidative stress in isolated mitochondria. Free Radic Res 29:297–305
Saidi I, Ayouni M, Dhieb A, Chtourou Y, Chaïbi W, Djebali W (2013) Oxidative damages induced by short-term exposure to cadmium in bean plants:protective role of salicylic acid. S Afr J Bot 85:32–38
Schützendübel A, Nikolova P, Rudolf C, Polle A (2002) Cadmium and H2O2-induced oxidative stress in populus canescens roots. Plant Physiol Biochem 40:577–584
Sharma SS, Dietz KJ (2009) The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci 14:43–50
Shi GR, Cai QS, Liu QQ, Wu L (2009) Salicylic acid-mediated alleviation of cadmium toxicity in hemp plants in relation to cadmium uptake, photosynthesis, and antioxidant enzymes. Acta Physiol Plant 31:969–977
Stewart RC, Bewley JD (1980) Lipid peroxidation associated with accelerated aging of soybean axes. Plant Physiol 65:245–248
Tonamura B (1978) Test reactions for a stopped flow apparatus regulation of 2, 6-D and potassium ferricyanide by L-ascorbic acid. Anal Biochem 84:370–383
Wagner GJ (1993) Accumulation of cadmium in crop plants and its consequences to human health. Adv Agron 51:173–212
Wang QH, Liang X, Dong YJ, Xu LL, Zhang XW, Hou J, Fan ZY (2013) Effects of exogenous nitric oxide on cadmium toxicity, element contents and antioxidative system in perennial ryegrass. Plant Growth Regul 69:11–20
Weigel HJ, Jäger HJ (1980) Subcellular distribution and chemical form of cadmium in bean plants. Plant Physiol 65:480–482
Xiong J, An L, Lu H, Yhu C (2009) Exogenous nitric oxide enhances cadmium tolerance of rice by increasing pectin and hemicellulose contents in root cell wall. Planta 230:755–765
Xu J, Wang WY, Yin HX, Liu XJ, Sun H, Mi Q (2010) Exogenous nitric oxide improves antioxidative capacity and reduces auxindegradation in roots of Medicago truncatula seedlings under cadmium stress. Plant Soil 326:321–330
Xu LL, Dong YJ, Kong J, Liu S (2013) Effects of root and foliar applications of exogenous NO on alleviating cadmium toxicity in lettuce seedlings. Plant Growth Regul. doi:10.1007/s10725-013-9834-3
Zawoznik MS, Groppa MD, Tomaro ML, Benavides MP (2007) Endogenous salicylic acid potentiates cadmium-induced oxidative stress in Arabidopsis thaliana. Plant Sci 173:190–197
Zhang ZL, Di WJ (2003) Laboratory guide for plant physiology [M]. Higher Education Press, Beijing
Acknowledgment
The authors thank Pingping Yang (College of Animal Science and Technology, Shandong Agricultural University, China) for her supplying instruments and patient guidance. The authors also thank English Lecturer Mr Stuart Craig MA (England, Taishan University of china) and Dr. G. Jones (University of Florida, USA), for their critical reading and revision of the manuscript. Special acknowledgements are given to the editors and reviewers. This research work was financially supported by the Shandong Provincial Natural Science Foundation of China (ZR2013CM003) and a Project of Shandong Province Higher Educational Science and Technology Program (J14LF08).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bai, X., Dong, Y., Kong, J. et al. Effects of application of salicylic acid alleviates cadmium toxicity in perennial ryegrass. Plant Growth Regul 75, 695–706 (2015). https://doi.org/10.1007/s10725-014-9971-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-014-9971-3