Abstract
The reported study investigated the interaction between salicylic acid (SA) and citric acid (CA) in cadmium (Cd)-stressed Brassica juncea plants. Seedling received Cd (0.6 mM) stress through soil at 5-day stage of growth. SA (0.01 mM) and CA (0.6 mM) treatments were applied at 25 days after sowing. Growth, photosynthesis, oxidative burst, and antioxidant systems were examined at 30-day stage of growth. Growth and photosynthetic parameters reduced significantly in the presence of Cd, and elevated levels of H2O2 were indicative of oxidative burst which resulted in decline of cell viability. Foliar spray of SA and CA alone or in combination mitigated the toxic effects generated by Cd and enhanced plant growth parameters. The inhibitory effects of Cd toxicity on width of stomatal pore resulted in reduced internal CO2 concentration and carbonic anhydrase activity which consequently limited the photosynthetic rate. SA and CA alleviated the inhibitory effect of Cd on photosynthesis by stimulating the stomatal activity and pore size. The Cd-generated oxidative burst was reduced via enhanced antioxidant activity (catalase, peroxidase, and superoxide dismutase) upon follow-up treatment with SA and CA alone or in combination. A combined dose of SA and CA countered Cd-induced damage by reducing levels of reactive oxygen species and strengthening plant antioxidant defense systems, which resulted in membrane stabilization and recovery from stress. Combined dose of SA and CA proved more effective than their individual application towards Cd stress which suggests an effective synergism between the two acids.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rapidly increasing population, urbanization, industrial discharges, and use of chemical fertilizers in agriculture have contaminated the soil environment. Heavy metals are among the most significant threats to plant growth and development due to their non-biodegradability. It is, therefore, essential to maintain a healthy soil environment for plant growth. Cadmium (Cd), lead, chromium, and mercury are among the most notorious soil and water pollutants and their concentrations are increasing as a result of anthropogenic activities including mining and smelting operations, and use of metals and metal-containing compounds for domestic as well as agricultural purposes (Nagajyoti et al. 2010). Heavy metals occur in the natural environment, albeit in trace quantities. Increased concentrations above certain levels results in serious adverse effects on plant physiology and productivity (Mahmud et al. 2018). Cd is one of the most toxic heavy metals which enters agricultural fields via excessive use of phosphatic fertilizers, uncontrolled releases from industry, and by land application of Cd-enriched wastewater treatment solids (Murtaza et al. 2015). Cd is readily transferred through the food chain due to its high mobility (Gallego et al. 2012). Humans may ultimately become exposed to Cd as a result of uptake by crop plants (Clemens et al. 2013).
Specific effects of Cd to plants include reduced nutrient uptake, decreased photosynthetic activity, altered nitrogen and sulfur metabolism, and changes in levels of antioxidant enzymes (Benavides et al. 2005; Gill and Tuteja 2011; Zong et al. 2017; Pereira de Araújo et al. 2017). Cd induces an increase in levels of reactive oxygen species (ROS) by disrupting the electron transport chain (Wang et al. 2004). Chlorophyll is denatured, which disrupts enzymes of the Calvin cycle, ultimately decreasing the rates of both photosynthesis and gas exchange (Silveira et al. 2015).
Salicylic acid (SA) acts as a plant growth regulator and stress messenger (Ghazijahani et al. 2014). The role of SA in mitigating biotic stress is well established. SA induces a hypersensitive response within the plant upon encountering an invading entity. The response acts as a secondary messenger and increases the resistance in plants to external stimuli (Klessig and Malamy 1994). The protective role of SA against abiotic stress has been reported in various plants (Gondor et al. 2016; Kohli et al. 2018a, b, 2019). Metwally et al. (2003) reported SA-mediated alleviation of Cd toxicity in barley seedlings. SA provides stress tolerance in plants by inducing the expression of genes that encode defense-related compounds like jasmonic acid (JA) and proline (Wani et al. 2017).
Citric acid (CA) is an intermediate product formed during tricarboxylic acid cycle that supplies energy to cells during respiration and in many other biochemical pathways (Hu et al. 2016). Various reports suggest that CA plays a role during phytoremediation of several heavy metals—it detoxifies metal-induced oxidative stresses in the plant by improving antioxidant capabilities (Gao et al. 2010; Yeh et al. 2012; Vagner et al. 2013). Involvement of CA in aluminum detoxification, iron-generated stress, and tolerance towards heavy metal and salinity stress has been reported (Ma and Furukawa 2003; Shlizerman et al. 2007; Gao et al. 2010; Sun and Hong 2011).
Several studies have assessed the role of SA or CA in the presence of specific abiotic stresses, especially heavy metals; however, the combined effects of SA and CA under stressful conditions remain unexplored (Sun and Hong 2011; Ghazijahani et al. 2014; Gondor et al. 2016; Liu et al. 2016; Hassan et al. 2016).
Cd is among the most common toxic metals present in many soil environments and is not readily removed from soil; it is therefore necessary to reduce its phytotoxicity. By virtue of the protective role of SA and CA in response to heavy metal stress, the present experiment was designed with the objective of assessing the interactive role of SA and CA in enhancing plant growth, photosynthesis rate, and antioxidant defense systems. The study would establish the relation between the two acids, i.e., whether they act synergistically or antagonistically to each other.
Materials and Methods
Plants Used for Study
Seeds of Brassica juncea var. RGN-48 were obtained from the Indian Agricultural Research Institute (IARI), New Delhi, India. Healthy uniform-size seeds were tested for percent viability before the start of the experiment. Seeds were surface-sterilized with 5% sodium hypochlorite for 2 min followed by washing at least thrice with distilled water.
Treatment Pattern and Experimental Design
The experiment was conducted using a simple randomized complete block design. Seeds were sown in earthen pots filled with soil and farmyard manure in the ratio of 6:1 and subsequently placed under natural environmental conditions in an open area. Average temperature and humidity were 25 °C and 55%, respectively. Concentrations used in this experiment were selected according to the data provided in Supplementary Material Fig. 1.
Cd (0.6 mM) was applied to soil on the fifth day after sowing (DAS). Twenty-five day-old seedlings were sprayed with either double distilled water (DDW), designated as control; 0.01 mM salicylic acid (SA); or 0.6 mM citric acid (CA) on foliage in the evening. The sprayer nozzle was adjusted so that each plant received a total of 3 mL DDW, SA, or CA. Each treatment was replicated five times. Plants were sampled at 30 DAS in order to determine various growth and biochemical parameters.
Growth Biomarkers
Plants were gently removed from each pot along with soil, immersed in DDW and rinsed gently to remove adhering soil particles. Root and shoot lengths were measured using meter scale. Samples were weighed to obtain their fresh and dry mass. Plant tissue was oven-dried at 70 °C for 48 h to measure dry biomass.
Chlorophyll (SPAD Value) and Total Carotenoid Contents
Chlorophyll values were measured using an SPAD chlorophyll meter (SPAD-502; Konica, Minolta Sensing, Inc., Japan), under natural lighting “at mid-day.”
Carotenoid contents were estimated by the method of Maclachlan and Zalik (1963). Fresh leaves (0.1 g) from the interveinal region were ground in 10 mL of 80% acetone using a mortar and pestle. The suspension was decanted and filtered through Whatman no. 1 filter paper into a Buchner funnel. The optical density (OD) of the solution was read at 480 and 510 nm for carotenoid estimation using a UV–Vis spectrophotometer. Total carotenoid contents were calculated using the formula:
where OD is the optical density of the extract at the given wavelength (480 or 510 nm).
Scanning Electron Microscopy
Stomatal apertures from leaf abaxial surfaces were observed under a scanning electron microscope (JEOL, JSM 6510). Fresh leaves were fixed with 2% paraformaldehyde, 2.5% glutaraldehyde, and 0.1 M sodium cacodylate buffer (pH 7.3) for 2 h. The leaves were transferred to petri plates to run an ethanol-graded series (50%, 70%, 80%, 90%, and 100%). After dehydrating, the samples were coated with gold–palladium in a sputter coater (JEOL JFC-1600).
Leaf Gas Exchange Traits
Stomatal conductance, intracellular CO2 concentration, transpiration rate, and net photosynthetic rate were analyzed using a portable infrared gas analyzer (IRGA) photosynthetic system (LI-COR 6400, LI-COR, Lincoln, NE, USA) between 11:00 and 12:00 h under clear skies. Relative humidity, CO2 concentration, air temperature, and photosynthetic photon flux density (PPFD) were maintained at 85%, 600 μmol mol−1, 25 °C, and 800 μmol mol−2 s−1, respectively.
Nitrate Reductase Activity
Nitrate reductase (NR) was measured (Jaworski 1971) in fresh leaf samples that were cut into small pieces and dipped into a solution containing phosphate buffer (pH 7.5), KNO3, and isopropanol followed by incubation at 30 °C for 2 h. Sulfanilamide and N-1-naphthylethylenediamine hydrochloride solutions were added afterwards. Absorbance was read at 540 nm on a UV–Vis spectrophotometer. NR activity was expressed on a fresh mass (FM) basis as n mol (NO2) g−1 (FM) s−1.
Carbonic Anhydrase Activity
Carbonic anhydrase (CAase) activity was determined by the method of Dwivedi and Randhawa (1974). Fresh leaf samples were cut into small pieces and suspended in a solution of cysteine hydrochloride. Samples were incubated at 4 °C for 20 min and then transferred to test tubes containing phosphate buffer (pH 6.8) followed by addition of alkaline bicarbonate solution and bromothymol blue indicator. The test tubes were incubated at 5 °C for 20 min and titrated against 0.05 N HCl after adding 0.2 ml of methyl red indicator. CAase activity was expressed on a FM basis as mol CO2 g−1 (FM) s−1.
Proline Content
The proline content of fresh leaves was determined by the method of Bates et al. (1973). Samples were placed in sulfosalicylic acid followed by addition of an equal volume of glacial acetic acid and ninhydrin. The samples were heated at 100 °C for 2 h and subsequently transferred to an ice bath. 5 ml of toluene was added to the reaction mixture. The absorbance of the aspirated toluene layer formed was read at 520 nm. Proline content was expressed as mol g−1 (FM).
H2O2 Content
The content of H2O2 in fresh leaves was determined according to the method of Mukherjee and Choudhuri (1983). Leaves of B. juncea (0.5 g) were homogenized using a cold mortar and pestle in pre-cooled acetone (5 mL) and the homogenate was centrifuged at 12,000×g for 5 min. 1 mL of supernatant was mixed with 0.1 mL of 5% Ti(SO4)2 and 0.2 mL of 19% ammonia after a precipitate was formed. The reaction mixture was centrifuged at 12,000×g for 5 min. The resulting pellet was dissolved in 3 mL of 2 M H2SO4 and absorbance was read at 415 nm. The H2O2 concentration was calculated according to a standard curve of H2O2 ranging from 0 to 10 μM. H2O2 content was expressed as n mol g−1 (FW).
Antioxidant Enzyme Activity
Antioxidant enzyme activities were determined by the method of Khan et al. (2015). Fresh leaves were homogenized in a pre-cooled mortar and pestle with phosphate buffer (pH 7), 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM ethylenediaminetetraacetic acid (EDTA), 0.5% Triton X-100, and 2% polyvinylpyrrolidone (PVP). This homogenate was subjected to centrifugation at 12,000×g at 4 °C for 20 min and the supernatant was held at − 20 °C for analysis of catalase (CAT 1.11.1.6), peroxidase (POX 1.11.1.7), and superoxide dismutase (SOD 1.15.1.1). Enzyme activities were expressed on a fresh mass basis as n mol (H2O2) decomposed g−1 (FM) for CAT, U g−1 (FM) for POX, and U g−1 (FM) for SOD.
Compound Microscopy
Compound microscopy (Nikon ECLIPSE Ci-E equipped with Nikon digital camera DS-Fi1c) was used for detection of stomatal activity (Siddiqui et al. 2018). At least three leaves were collected from each treatment and immersed in a solution containing NaOH to promote removal of the epidermis. The abaxial surface was exposed under a cover slip to view stomatal aperture under a compound microscope.
Confocal Laser Scanning Microscopy
Cell viability was determined by the method of Siddiqui et al. (2018). Fresh roots were immersed in a solution containing 25 µM propidium iodide (PI) for 10 min. They were subsequently washed twice with DDW and placed on glass slides for viewing under a confocal laser scanning microscope (Zeiss, LSM 780) at × 20 magnification with maximum excitation at 535–617 nm.
Statistical Analysis
Data were statistically analyzed using SPSS 17.0 for Windows (SPSS, Chicago, IL, USA). Standard error was calculated and analysis of variance (ANOVA) performed to determine the least significance difference (LSD) between treatment means with a level of significance at p ≤ 0.05.
Results
Growth Biomarkers
Values of all the growth biomarkers (i.e., root and shoot length; fresh and dry biomass) increased markedly after exogenous application of SA (0.01 mM) and CA (0.6 mM) to the Cd-stressed plants as compared to control plants (Fig. 1). Role of SA in promoting growth of B. juncea has been established in our previous studies (Hayat et al. 2009, 2012; Fariduddin et al. 2003; Yusuf et al. 2008); hence, the sole treatment of SA was omitted in the present study.
Effect of 0.01 mM salicylic acid (SA), 0.6 mM citric acid (CA), SA (0.01 mM) + CA (0.6 mM) in the presence of 0.6 mM cadmium (Cd) stress on the length of shoot (a) and root (b) fresh mass of shoot (c) and root (d), dry mass of shoot (e) and root (f) of mustard at 30 days after sowing (DAS). All the data are the mean of five replicates (n = 5) and vertical bars show standard error (± SE). Values of bars with the same letter are not significantly different, P < 0.05 Duncan’s multiple range test
Shoot length declined by 20% upon exposure to Cd as compared to control plants. However, treatment with SA or CA alleviated Cd-generated toxicity; growth of plants treated with SA and CA improved by 14.9% and 11.9%, respectively, over the control. The combined dose of SA and CA proved the most beneficial in mitigating toxicity symptoms by enhancing shoot length by 30.9% as compared to the control plants and 63.8% when compared to the sole Cd-treated plants (Fig. 1A). A 20% decline in root length occurred in Cd-treated plants. CA enhanced root length by 20.9% in non-stressed plants and improved growth by 38.8% in Cd-stressed plants as compared to sole Cd-treated ones. Similarly, SA improved root length by 43.8% in Cd-stressed plants as compared to the control. The combined SA and CA treatment protected plants from Cd stress and improved root length by 61.2% when compared to those treated with Cd (Fig. 1B). Shoot fresh mass (SFM) declined by 22.2% in Cd-treated plants and an increase of 23.7% was observed in CA-treated plants. SA and CA improved growth in Cd-stressed plants by 15.9% and 9%, respectively, over the control. The combined SA and CA treatment proved beneficial and completely neutralized toxicity effects (Fig. 1C). Cd reduced root fresh mass (RFM) by 30.6%; however, follow-up treatment with SA or CA improved RFM by 3.4% and 1.1%, respectively, over the control. The combined SA and CA treatment completely neutralized Cd-generated toxic effects by increasing RFM by 23.8% as compared to the control (Fig. 1D). A decrease of 25.3% in shoot dry mass (SDM) occurred in Cd-treated plants, whereas a 24% increase was observed in CA-treated plants compared to the control. When SA or CA were applied as a follow-up treatment to Cd-stressed plants, a recovery of 8.3% and 1.9%, respectively, over the control, was observed. The combined SA and CA treatment yielded the best response and increased SDM by 40.9% compared to the control (Fig. 1E). A decrease of 27.7% of root dry mass was observed in Cd-treated plants. The SA and CA treatments completely neutralized the toxic effects of Cd and increased root dry mass (RDM) of Cd-treated plants by 13.8% and 3.9% compared to the control (Fig. 1F). Decrease in leaf area was significant in the presence of Cd as compared to control plants (Fig. 2A). Foliar application of SA, CA, and SA + CA to Cd-stressed plants resulted in increased leaf area by 36.4, 34 and 49.4%, respectively, over Cd-stressed plants. Highest leaf area values were noted in the plants treated with combined SA and CA.
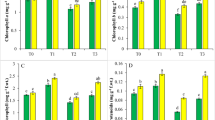
Effect of 0.01 mM salicylic acid (SA), 0.6 mM citric acid (CA), SA (0.01 mM) + CA (0.6 mM) in the presence of 0.6 mM cadmium (Cd) stress on the a leaf area and b chlorophyll content (SPAD value), c total carotenoid content at 30 days after sowing (DAS). All the data are the mean of five replicates (n = 5) and vertical bars shows standard error (± SE). Values of bars with the same letter are not significantly different, P < 0.05 Duncan’s multiple range test
Chlorophyll and Carotenoid Contents
Cd-treated plants experienced a marked reduction in chlorophyll content; however, foliar application of SA and/or CA to the plants significantly increased chlorophyll content (SPAD value) by 41.7% and 35.2%, respectively (Fig. 2B). Therefore, the Cd-induced reduction was completely overcome by the follow-up treatment with SA and/or CA alone and in combination.
Cd-generated stress decreased total carotenoid contents by 20% (Fig. 2C). The follow-up treatment of SA and/or CA mitigated the toxic effects of Cd. Treatment with the combined SA and CA resulted in highest chlorophyll values in plants treated with Cd only and also in water-treated plants.
Leaf Gaseous Exchange, CAase, and NR Activity
Application of CA enhanced the photosynthetic rate (PN) as compared to the control. The presence of Cd in the soil reduced PN and stomatal conductance (Gs) in the plants as compared to control (Fig. 3A, B), with losses of 18 and 19.5%, respectively. Treatment of Cd-stressed plants with SA and CA significantly increased values of PN by 52.1% and 42.44%, respectively, over sole Cd-treated plants. The values for Gs increased by 42.42% and 36.36% upon SA and CA treatment, respectively, with reference to the plants that received only Cd treatment. Combined dose of SA and CA enhanced PN (37.9%) and Gs (57.5%) in Cd-stressed plants as compared to plant treated with only Cd. The presence of Cd in the soil resulted in decreases of both internal CO2 concentration (Ci) and transpiration rate (E); however, subsequent application of SA, CA, and SA + CA improved Ci by 17, 14, and 30%, and E by 16, 12, and 31% over the control, respectively (Fig. 3C, D). The treatments, therefore, resulted in complete recovery of the plants from Cd stress.
Effect of 0.01 mM salicylic acid (SA), 0.6 mM citric acid (CA), SA (0.01 mM) + CA (0.6 mM) in the presence of 0.6 mM cadmium (Cd) stress on the a net photosynthetic rate (PN), b stomatal conductance (Gs), c internal CO2 concentration (Ci), d transpiration rate (E), e carbonic anhydrase (CAase) activity, and f nitrate reductase (NR) activity of mustard at 30 days after sowing (DAS). All the data are the mean of five replicates (n = 5) and vertical bars shows standard error (± SE). Values of bars with the same letter are not significantly different, P < 0.05 Duncan’s multiple range test
CAase and NR activity declined in plants that received Cd only as compared to the control (Fig. 3E, F) but improved by subsequent application of SA and CA. Highest values for CAase and NR activities were recorded in plants supplemented with SA and CA (27 and 31%, respectively, greater than the control).
Antioxidants and H2O2 Content
Activities of CAT, POX, and SOD increased under Cd stress and increased further upon exposing plants to SA and/or CA (Fig. 4A–C). Values for CAT, POX, and SOD in plants treated with SA and CA were 51, 63, and 50% greater than those of the control plants. Proline content increased in leaves of plants that received Cd, SA, and CA (Fig. 4D). Values were 42.4, 37.5, and 63% greater than the control by application of SA, CA, and SA + CA, respectively. The SA + CA treatment resulted in highest values for proline content. Increased H2O2 content was determined in Cd-treated plants (Fig. 4E). However, treatment of plants with CA and/or SA reduced H2O2 generated by Cd. The maximum decrease in H2O2 content (19%) was recorded in plants in the combined SA and CA treatment, compared with stressed plants treated with Cd only.
Effect of 0.01 mM salicylic acid (SA), 0.6 mM citric acid (CA), SA (0.01 mM) + CA (0.6 mM) in the presence of 0.6 mM cadmium (Cd) stress on a catalase activity, b peroxidase activity, c superoxide dismutase, d proline, and e hydrogen peroxide content of mustard at 30 days after sowing (DAS). All the data are the mean of five replicates (n = 5) and vertical bars shows standard error (± SE). Values of bars with the same letter are not significantly different, P < 0.05 Duncan’s multiple range test
Microscopic Studies
Compound and scanning electron microscopic studies showed clear evidence of Cd toxicity to stomatal openings by affecting stomatal pore size (Figs. 5 and 6). However, recovery was observed in the SA + CA treatment as evidenced from increases in stomatal pore size. Confocal microscopic study of roots revealed that Cd stress resulted in membrane damage as expressed by increase numbers of stained nuclei (DNA). However, plants treated with SA and CA exhibited recovery from Cd-induced toxicity as revealed by the reduction in number of stained nuclei as compared to sole Cd-treated plants (Fig. 7a–c).
Discussion
Cd is recognized as one of the most toxic heavy metals in soil that destabilizes membrane integrity and nutrient status; moreover, it inhibits chlorophyll biosynthesis (Benavides et al. 2005) which ultimately results in reduced plant growth (Wahid et al. 2007). Cd toxicity has been reported among various plant groups (Gallego et al. 2012; Ahmad et al. 2015; Al Mahmud et al. 2017; Kaur et al. 2017; Lu et al. 2018; Kaya et al. 2019).
Growth parameters in B. juncea such as root and shoot length, plant fresh and dry weights, and leaf area decreased upon exposure to Cd (Figs. 1A–F and 2A), which is in agreement with the findings of Irfan et al. (2014). Ahmad et al. (2015) reported that Cd adversely affects plant growth by interfering with mineral uptake, plant water relations, and photosynthesis. However, SA and/or CA application promoted the recovery of the Cd-stressed plants and the SA + CA spray proved to be most effective in mitigating Cd-related toxicity symptoms. Lu et al. (2013) reported higher biomass production by CA treatment in Sedum alfredii plants growing under Cd stress. Positive results of SA and CA under salinity, heat, and alkalinity stress have also been reported (Ahmad et al. 2018; Sun and Hong 2011; Hu et al. 2016). Alleviation of Cd toxicity by application of SA in rice, mustard, and flax has also been documented (Guo et al. 2007; Belkhadi et al. 2010; Ahmad et al. 2011).
The photosynthetic apparatus is a primary target of Cd toxicity to plants (Balakhnina et al. 2005). Chlorophylls and carotenoids are important photosynthetic pigments involved in the light-harvesting process, and carotenoids also protect chlorophyll from photo oxidative damage by reducing levels of ROS (Behera et al. 2002). It is essential to maintain the proper balance of these pigments for optimal light energy capture required to initiate photosynthesis (Wahid and Ghazanfar 2006). Estimating leaf chlorophyll content is useful in predicting rate of photosynthesis (Dalio et al. 2011). Cd application (0.6 mM) decreased SPAD chlorophyll and carotenoid levels in B. juncea; however, foliage-applied CA and/or SA not only neutralized the toxic effects of Cd but also increased chlorophyll and carotenoid contents even beyond those of the control (Fig. 2B, C) which contributed in improving photosynthesis rate and related parameters. Similar trends of recovery upon exposure to heavy metal stress were reported in B. juncea (Kaur et al. 2018; Parashar et al. 2014; Yusuf et al. 2012), where SA and CA alleviated the deleterious effects of heavy metals on photosynthetic pigments. The loss of chlorophyll content may be due to Cd-mediated inhibition of amino levulinate synthesis that acts as one of the precursors of chlorophyll biosynthesis and is responsible for photoreduction of protochlorophyllide to chlorophyllide (Parmar et al. 2013).
Cd-induced decrease in chlorophyll and carotenoid contents (Fig. 2B, C) might have resulted in decreased PN; moreover, the decrease in Gs and pore size further slowed PN (Fig. 3A–C). However, application of CA and/or SA mitigated the harmful effects of Cd stress on mustard plants. The combined dose of CA and SA proved optimal in mitigating the toxic effects of Cd as compared to plants treated with either SA or CA. Similar effects of SA and CA towards heavy metal stress have been reported in B. juncea (Kaur et al. 2018; Parashar et al. 2014; Yusuf et al. 2012).
Metals impart toxic effects to photosynthetic rate as they inhibit chlorophyll synthesis and induces its degradation (Zengin and Munzuroglu 2005; Parmar et al. 2013; Liu et al. 2014). Figure 3A–D reveals lower values for gas exchange parameters PN, Gs, E, and Ci due to Cd; however, this damage was mitigated by follow-up treatment with CA and/or SA, which is supported by the results of Ghazijahani et al. (2014). The decline of photosynthesis upon Cd exposure may be the expression of (i) closing of stomata; (ii) increase in stomatal resistance; and/or (iii) decrease in stomatal density which lowers the rate of gas exchange (Ying et al. 2010). A similar decrease in the size of stomatal pores in the presence of Cd was observed in the present study. The follow-up treatment with CA + SA, however, mitigated the effects of Cd by increasing pore size (Fig. 5a–f), which is in agreement with the findings of Kaur et al. (2018) and Sandalio et al. (2001). SA increases leaf potassium ion concentration in the presence of abiotic stress (Delavari et al. 2010). Changes in stomatal aperture upon CA application may be a result of changes in K+ levels in and around the guard cells as K+ is known to play a major role in the opening and closing of stomata (Smith and Stewart 1990).
Plants exhibit tolerance to heavy metals via activation of compounds involved in metal chelation such as phytochelatins (PCs), metallothioneins (MT), organic acids (citrate and malate), and amino acids (Anjum et al. 2015; Manara 2012; Rauser and Curvetto 1980). Salicylic acid enhances tolerance towards oxidative stress by increasing the phytochelatin content in Zea mays via regulation of glutathione reductase and phytochelatin synthase activity (Szalai et al. 2013). Citrate is itself a ligand and exhibits strong affinity for Cd as compared to other organic acids (Anjum et al. 2015). CA suppresses Cd transport to above-ground plant parts by forming complexes and facilitating Cd uptake from solution to root, followed by storage of Cd–citric complexes in vacuoles. PC synthesis terminates as soon as those metal ions activating a PC synthase are bound to a chelator. Citric acid reduces PC content by forming Cd–citrate complexes, leading to decline in Cd ion concentrations (Loeffler et al. 1989).
Metal-sensitive groups (SH– or histidyl groups) interact readily with heavy metals ultimately resulting in inactivation of enzyme catalytic activity (Kneer and Zenk 1992; De Miranda et al. 1990; Vallee and Ulmer 1972). Cd restricts the activity of carbonic anhydrase which is involved in the conversion of atmospheric CO2 to HCO3− during photosynthesis; concurrently, the value for NR (i.e., the enzyme responsible for assimilation of exogenous nitrate) activity declines (Fig. 3E, F). The presence of Cd in soil with consequent loss of CAase and NR activity among different plants has been reported numerous times (Hayat et al. 2007; Khan et al. 2008; Anuradha and Rao 2009; Erdal and Turk 2016). The reduced activity of CAase might be an outcome of stomatal closure and reduced Ci (Figs. 3C and 6). The decline in CAase activity along with lower Gs, E, and Ci values due to Cd stress reduces the availability of CO2 for rubisco (a major enzyme of the Calvin cycle) and marks the restriction of PN (Fig. 3A–E). Leaf NR activity is considered a useful indicator of nitrogen status of the plant (Srivastava 1980). As nitrogen is a critical component of phytochelatin structure (Zenk 1996), the enhanced activity of NR in the presence of the combined dose of SA and CA could eventually strengthen the heavy metal detoxification system and resultant growth of the plant.
Biotic and abiotic stress results in overproduction of ROS. The species OH·, O ·−2 , and H2O2 cause oxidative stress when present in excess quantities (Gossett et al. 1994; Meneguzzo et al. 1999). Hence, in order to promote normal cell function the balance between ROS generation and degradation must be maintained. Such balance is achieved through the actions of antioxidant machinery including enzymatic (superoxide dismutase, catalase, and peroxidase) and non-enzymatic (proline and carotenoids) antioxidants (Schutzendubel and Polle 2002; Ahmad et al. 2010). Cd induces the generation of free radicals and oxidative stress in plants (Khanna et al. 2019; Kaur et al. 2019; Alyemeni et al. 2018; Ahmad et al. 2016). It possesses the ability to boost H2O2 generation in plants (Fig. 4E) by suppressing or disrupting the antioxidant defense system (Benavides et al. 2005; Srivastava et al. 2004). SA and CA reduced H2O2 overproduction in Cd-stressed plants (Fig. 4E) by virtue of the enhanced activities of CAT and POX which convert toxic H2O2 to water (Fig. 4A–C). Our finding is supported by Zhang et al. (2011) who reported that exogenous application of SA reduces H2O2 accumulation in Phaseolus and Vicia sp. Reduced H2O2 accumulation in Cd-stressed plants by SA or CA application has also been reported by Belkadhi et al. (2014), Ehsan et al. (2014) and Mahmud et al. (2018).
Plants have evolved several defense systems to withstand stressful conditions, where antioxidant enzymes (e.g., SOD, POX, and CAT) act as a first line of defense. These enzymes catalyze the detoxification of ROS and minimize the adverse effects caused by abiotic stress (Hasanuzzaman et al. 2012). Therefore, in the present study (Fig. 4A–C) and in others (Meng et al. 2009; Ehsan et al. 2014; Irfan et al. 2014) the presence of Cd stimulated an increase in the activities of antioxidant enzymes. Moreover, the levels of these antioxidant enzymes increased further in plants supplied with CA and/or SA (Fig. 4E). Similarly, the use of CA/SA to minimize the toxic effects of Cd is reported in different plant species (Zhang et al. 2011; Afshan et al. 2015; Semida et al. 2015; Hassan et al. 2016; Lu et al. 2018).
Proline, a non-enzymatic antioxidant, supports organisms under adverse conditions in mitigating the harmful effects of ROS (Chen and Dickman 2005). Proline accumulation increases in the presence of stress (Hayat et al. 2007) which further increases upon SA and CA application, corroborating the findings of Kaur et al. (2017), Mahmud et al. (2018), and Parashar et al. (2014). Higher proline levels maintain the water balance in plants to withstand stress conditions (Zengin and Munzuroglu 2005). Increased SOD, CAT, and POX activities along with elevated proline content accelerate the detoxification of harmful H2O2 to non-harmful H2O thus reducing oxidative stress (Najeeb et al. 2011; Kang et al. 2013). SA also decreases the impact of oxidative stress caused by Cd by inhibiting accumulation of Cd in plant cells (Noriega et al. 2012).
Confocal microscopic study further confirmed that enhanced ROS levels caused membrane damage in Cd-stressed plants (Fig. 7a–c). However, SA and/or CA proved beneficial in mitigating Cd-induced damage by accelerating the antioxidant machinery and reducing the ROS levels.
Conclusion
Cd stress in B. juncea resulted in growth restriction via reduced photosynthesis and increased oxidative damage. However, the exogenous application of SA and/or CA to foliage of stressed plants minimized the harmful effects of Cd by enhancing the antioxidant machinery which attenuates the Cd-induced oxidative burst. Increased stomatal conductance and aperture improved the CO2 availability inside the cells and enhanced its capacity to bind with rubisco by elevating the carbonic anhydrase activity which ultimately resulted in enhanced photosynthetic rate. Moreover, the combined dose of SA and CA to standing mustard plants proved optimal in protecting plants from Cd stress.
References
Afshan S, Ali S, Bharwana SA et al (2015) Citric acid enhances the phytoextraction of chromium, plant growth, and photosynthesis by alleviating the oxidative damages in Brassica napus L. Environ Sci Pollut Res 22:11679–11689. https://doi.org/10.1007/s11356-015-4396-8
Ahmad P, Jaleel CA, Salem MA (2010) Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit Rev Biotechnol 30(3):161–175
Ahmad P, Nabi G, Ashraf M (2011) Cadmium-induced oxidative damage in mustard [Brassica juncea (L.) Czern. & Coss.] plants can be alleviated by salicylic acid. S Afr J Bot 77(1):36–44
Ahmad P, Sarwat M, Bhat NA et al (2015) Alleviation of cadmium toxicity in Brassica juncea L. (Czern. & Coss.) by calcium application involves various physiological and biochemical strategies. PLoS ONE 10:1–17. https://doi.org/10.1371/journal.pone.0114571
Ahmad P, Abdel Latef AA, Abd_Allah EF et al (2016) Calcium and potassium supplementation enhanced growth, osmolyte secondary metabolite production, and enzymatic antioxidant machinery in cadmium-exposed chickpea (Cicer arietinum L.). Front Plant Sci 7:513
Ahmad P, Alyemeni MN, Ahanger MA et al (2018) Salicylic acid (SA) induced alterations in growth, biochemical attributes and antioxidant enzyme activity in faba bean (Vicia faba L.) seedlings under NaCl toxicity. Russ J Plant Physiol 65(1):104–114
Al Mahmud J, Hasanuzzaman M et al (2017) Relative tolerance of different species of Brassica to cadmium toxicity: Coordinated role of antioxidant defense and glyoxalase systems. Plant Omics 10:107–117. https://doi.org/10.21475/poj.10.02.17.pne409
Alyemeni MN, Ahanger MA, Wijaya L (2018) Selenium mitigates cadmium-induced oxidative stress in tomato (Solanum lycopersicum L.) plants by modulating chlorophyll fluorescence, osmolyte accumulation, and antioxidant system. Protoplasma 255(2):459–469
Anjum NA, Hasanuzzaman M, Hossain MA, Thangavel P, Roychoudhury A, Gill SS, Rodrigo MA, Adam V, Fujita M, Kizek R, Duarte AC (2015) Jacks of metal/metalloid chelation trade in plants—an overview. Front Plant Sci 6:192
Anuradha S, Rao SSR (2009) Effect of 24-epibrassinolide on the photosynthetic activity of radish plants under cadmium stress. Photosynthetica 47:317–320
Balakhnina TI, Kosobryukhov AA, Ivanov AA, Kreslavskii VD (2005) The effect of cadmium on CO2 exchange, variable fluorescence of chlorophyll, and the level of antioxidant enzymes in pea leaves. Russ J Plant Physiol 52(1):15–20
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Behera RK, Mishra PC, Choudhury NK (2002) High irradiance and water stress induce alterations in pigment composition and chloroplast activities of primary wheat leaves. J Plant Physiol 159:967–973. https://doi.org/10.1078/0176-1617-00823
Belkadhi A, De Haro A, Soengas P et al (2014) Salicylic acid increases tolerance to oxidative stress induced by hydrogen peroxide accumulation in leaves of cadmium-exposed flax (Linum usitatissimum L.). J Plant Interact 9:647–654. https://doi.org/10.1080/17429145.2014.890751
Belkhadi A, Hediji H, Abbes Z et al (2010) Effects of exogenous salicylic acid pre-treatment on cadmium toxicity and leaf lipid content in Linum usitatissimum L. Ecotoxicol Environ Saf 73:1004–1011
Benavides MP, Gallego SM, Tomaro ML (2005) Cadmium toxicity in plants. Braz J Plant Physiol 17(1):21–34
Chen C, Dickman MB (2005) Proline suppresses apoptosis in the fungal pathogen Colletotrichum trifolii. Proc Natl Acad Sci USA 102:3459–3464. https://doi.org/10.1073/pnas.0407960102
Clemens S, Aarts MG, Thomine S, Verbruggen N (2013) Plant science: the key to preventing slow cadmium poisoning. Trends Plant Sci 18(2):92–99
Dalio RJD, Pinheiro HP, Sodek L, Haddad CRB (2011) The effect of 24-epibrassinolide and clotrimazole on the adaptation of Cajanus cajan (L.) Millsp. to salinity. Acta Physiol Plant 33(5):1887–1896
De Miranda JR, Thomas MA, Thurman DA, Tomsett AB (1990) Metallothionein genes from the flowering plant Mimulus guttatus. FEBS Lett 260(2):277–280
Delavari PM, Baghizadeh A, Enteshari SH, Kalantari KM, Yazdanpanah A, Mousavi EA (2010) The effects of salicylic acid on some of biochemical and morphological characteristic of Ocimum basilicucm under salinity stress. Aust J Basic Appl Sci 4(10):4832–4845
Dwivedi RS, Randhawa NS (1974) Evaluation of a rapid test for the hidden hunger of zinc in plants. Plant Soil 40:445–451
Ehsan S, Ali S, Noureen S et al (2014) Citric acid assisted phytoremediation of cadmium by Brassica napus L. Ecotoxicol Environ Saf 106:164–172. https://doi.org/10.1016/j.ecoenv.2014.03.007
Erdal S, Turk H (2016) Cysteine-induced upregulation of nitrogen metabolism-related genes and enzyme activities enhance tolerance of maize seedlings to cadmium stress. Environ Exp Bot 132:92–99. https://doi.org/10.1016/j.envexpbot.2016.08.014
Fariduddin Q, Hayat S, Ahmad A (2003) Salicylic acid influences net photosynthetic rate, carboxylation efficiency, nitrate reductase activity, and seed yield in Brassica juncea. Photosynthetica 41(2):281–284
Gallego SM, Pena LB, Barcia RA et al (2012) Unravelling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms. Environ Exp Bot 83:33–46. https://doi.org/10.1016/j.envexpbot.2012.04.006
Gao Y, Miao C, Mao L et al (2010) Improvement of phytoextraction and antioxidative defense in Solanum nigrum L. under cadmium stress by application of cadmium-resistant strain and citric acid. J Hazard Mater 181:771–777. https://doi.org/10.1016/j.jhazmat.2010.05.080
Ghazijahani N, Hadavi E, Jeong BR (2014) Foliar sprays of citric acid and salicylic acid alter the pattern of root acquisition of some minerals in sweet basil (Ocimum basilicum L.). Front Plant Sci 5:1–7. https://doi.org/10.3389/fpls.2014.00573
Gill SS, Tuteja N (2011) Cadmium stress tolerance in crop plants. Plant Signal Behav 6:215–222. https://doi.org/10.4161/psb.6.2.14880
Gondor OK, Pál M, Darkó É et al (2016) Salicylic acid and sodium salicylate alleviate cadmium toxicity to different extents in maize (Zea mays L.). PLoS ONE 11:e0160157. https://doi.org/10.1371/journal.pone.0160157
Gossett DR, Millhollon EP, Lucas M (1994) Antioxidant response to NaCl stress in salt-tolerant and salt-sensitive cultivars of cotton. Crop Sci 34(3):706–714
Guo B, Liang Y, Li Z, Guo W (2007) Role of salicylic acid in alleviating cadmium toxicity in rice roots. J Plant Nutr 30:427–439. https://doi.org/10.1080/01904160601171835
Hasanuzzaman M, Hossain MA, da Silva JAT, Fujita M (2012) Plant response and tolerance to abiotic oxidative stress: antioxidant defense is a key factor. In: Venkateswarlu B, Shanker A, Shanker C, Maheswari M (eds) Crop stress and its management: perspectives and strategies. Springer, Dordrecht, pp 261–315
Hassan MS, Dagari MS, Babayo AU (2016) Effect of citric acid on cadmium ion uptake and stress response of hydroponically grown jute mallow (Corchorus olitorius). J Environ Anal Toxicol 6:375
Hayat S, Ali B, Hasan SA, Ahmad A (2007) Brassinosteroid enhanced the level of antioxidants under cadmium stress in Brassica juncea. Environ Exp Bot 60:33–41. https://doi.org/10.1016/j.envexpbot.2006.06.002
Hayat S, Masood A, Yusuf M, Fariduddin Q, Ahmad A (2009) Growth of Indian mustard (Brassica juncea L.) in response to salicylic acid under high-temperature stress. Braz J Plant Physiol 21(3):187–195
Hayat S, Maheshwari P, Wani AS, Irfan M, Alyemeni MN, Ahmad A (2012) Comparative effect of 28 homobrassinolide and salicylic acid in the amelioration of NaCl stress in Brassica juncea L. Plant Physiol Biochem 53:61–68
Hu L, Zhang Z, Xiang Z, Yang Z (2016) Exogenous application of citric acid ameliorates the adverse effect of heat stress in tall fescue (Lolium arundinaceum). Front Plant Sci 7:1–11. https://doi.org/10.3389/fpls.2016.00179
Irfan M, Ahmad A, Hayat S (2014) Effect of cadmium on growth and antioxidant enzymes in two varieties of Brassica juncea. Saudi J Biol Sci 21:125–131
Jaworski EG (1971) Nitrate reductase assay in intact plant tissues. Biochem Biophys Res Commun 43:1274–1279
Kang GZ, Li GZ et al (2013) Exogenous salicylic acid enhances wheat drought tolerance by influence on the expression of genes related to ascorbate-glutathione cycle. Biol Plant 57:718–724. https://doi.org/10.1007/s10535-013-0335-z
Kaur R, Yadav P, Sharma A et al (2017) Castasterone and citric acid treatment restores photosynthetic attributes in Brassica juncea L. under Cd (II) toxicity. Ecotoxicol Environ Saf 145:466–475
Kaur R, Yadav P, Sharma A et al (2018) Castasterone and citric acid supplementation alleviates cadmium toxicity by modifying antioxidants and organic acids in Brassica juncea. J Plant Growth Regul 37(1):286–299
Kaur P, Bali S, Sharma A et al (2019) Cd induced generation of free radical species in Brassica juncea is regulated by supplementation of earthworms in the drilosphere. Sci Total Environ 655:663–675
Kaya C, Okant M, Ugurlar F (2019) Melatonin-mediated nitric oxide improves tolerance to cadmium toxicity by reducing oxidative stress in wheat plants. Chemosphere 225:627–638
Khan NA, Singh S et al (2008) Cadmium effects on carbonic anhydrase, photosynthesis, dry mass and antioxidative enzymes in wheat (Triticum aestivum) under low and sufficient zinc. J Plant Interact 3:31–37. https://doi.org/10.1080/17429140701724958
Khan TA, Fariduddin Q, Yusuf M (2015) Lycopersicon esculentum under low temperature stress: an approach toward enhanced antioxidants and yield. Environ Sci Pollut Res 22:14178–14188
Khanna K, Jamwal VL, Kohli SK (2019) Plant growth promoting rhizobacteria induced Cd tolerance in Lycopersicon esculentum through altered antioxidative defense expression. Chemosphere 217:463–474
Klessig DF, Malamy J (1994) The salicylic acid signal in plants. Plant Mol Biol 26:1439–1458. https://doi.org/10.1007/BF00016484
Kneer R, Zenk MH (1992) Phytochelatins protect plant enzymes from heavy metal poisoning. Phytochemistry 31(8):2663–2667
Kohli SK, Handa N, Sharma A et al (2018a) Combined effect of 24-epibrassinolide and salicylic acid mitigates lead (Pb) toxicity by modulating various metabolites in Brassica juncea L. seedlings. Protoplasma 255(1):11–24
Kohli SK, Handa N, Sharma A et al (2018b) Interaction of 24-epibrassinolide and salicylic acid regulates pigment contents, antioxidative defense responses, and gene expression in Brassica juncea L. seedlings under Pb stress. Environ Sci Pollut Res 25(15):15159–15173
Kohli SK, Bali S, Tejpal R et al (2019) In-situ localization and biochemical analysis of bio-molecules reveals Pb-stress amelioration in Brassica juncea L. by co-application of 24-epibrassinolide and salicylic acid. Sci Rep 9(1):3524
Liu L, Sun H, Chen J et al (2014) Effects of cadmium (Cd) on seedling growth traits and photosynthesis parameters in cotton. Plant Omics 7:284–290
Liu Z, Ding Y, Wang F et al (2016) Role of salicylic acid in resistance to cadmium stress in plants. Plant Cell Rep 35:719–731. https://doi.org/10.1007/s00299-015-1925-3
Loeffler S, Hochberger A, Grill E, Winnacker EL, Zenk MH (1989) Termination of the phytochelatin synthase reaction through sequestration of heavy metals by the reaction product. FEBS Lett 258(1):42–46
Lu L, Tian S, Yang X et al (2013) Improved cadmium uptake and accumulation in the hyperaccumulator Sedum alfredii: the impact of citric acid and tartaric acid. J Zhejiang Univ Sci B 14:106–114. https://doi.org/10.1631/jzus.B1200211
Lu Q, Zhang T, Zhang W et al (2018) Alleviation of cadmium toxicity in Lemna minor by exogenous salicylic acid. Ecotoxicol Environ Saf 147:500–508. https://doi.org/10.1016/j.ecoenv.2017.09.015
Ma JF, Furukawa J (2003) Recent progress in the research of external Al detoxification in higher plants: a minireview. J Inorg Biochem 97:46–51. https://doi.org/10.1016/S0162-0134(03)00245-9
Maclachlan S, Zalik S (1963) Plastid structure, chlorophyll concentration, and free amino acid composition of a chlorophyll mutant of barley. Can J Bot 41:1053–1062
Mahmud JA, Hasanuzzaman M, Nahar K et al (2018) Insights into citric acid-induced cadmium tolerance and phytoremediation in Brassica juncea L.: coordinated functions of metal chelation, antioxidant defense and glyoxalase systems. Ecotoxicol Environ Saf 147:990–1001. https://doi.org/10.1016/j.ecoenv.2017.09.045
Manara A (2012) Plant responses to heavy metal toxicity. In: Furini A (ed) Plants and heavy metals. Springer, Dordrecht, pp 27–53
Meneguzzo S, Navam-Izzo F, Izzo R (1999) Antioxidative responses of shoots and roots of wheat to increasing NaCl concentrations. J Plant Physiol 155(2):274–280
Meng H, Hua S, Shamsi IH et al (2009) Cadmium-induced stress on the seed germination and seedling growth of Brassica napus L., and its alleviation through exogenous plant growth regulators. Plant Growth Regul 58:47–59. https://doi.org/10.1007/s10725-008-9351-y
Metwally A, Finkemeier I, Georgi M et al (2003) Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol 132(1):272–281
Mukherjee SP, Choudhuri MA (1983) Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol Plant 58:166–170
Murtaza G, Javed W, Hussain A et al (2015) Metal uptake via phosphate fertilizer and city sewage in cereal and legume crops in Pakistan. Environ Sci Pollut Res 22:9136–9147. https://doi.org/10.1007/s11356-015-4073-y
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8:199–216. https://doi.org/10.1007/s10311-010-0297-8
Najeeb U, Jilani G, Ali S et al (2011) Insights into cadmium induced physiological and ultra-structural disorders in Juncus effusus L. and its remediation through exogenous citric acid. J Hazard Mater 186:565–574. https://doi.org/10.1016/j.jhazmat.2010.11.037
Noriega G, Caggiano E, Lecube ML et al (2012) The role of salicylic acid in the prevention of oxidative stress elicited by cadmium in soybean plants. Biometals 25:1155–1165. https://doi.org/10.1007/s10534-012-9577-z
Parashar A, Yusuf M, Fariduddin Q, Ahmad A (2014) Salicylic acid enhances antioxidant system in Brassica juncea grown under different levels of manganese. Int J Biol Macromol 70:551–558
Parmar P, Kumari N, Sharma V (2013) Structural and functional alterations in photosynthetic apparatus of plants under cadmium stress. Bot Stud 54(1):45. https://doi.org/10.1186/1999-3110-54-45
Pereira de Araújo R, Furtado de Almeida AA, Silva Pereira L et al (2017) Photosynthetic, antioxidative, molecular and ultrastructural responses of young cacao plants to Cd toxicity in the soil. Ecotoxicol Environ Saf 144:148–157. https://doi.org/10.1016/j.ecoenv.2017.06.006
Rauser WE, Curvetto NR (1980) Metallothionein occurs in roots of Agrostis tolerant to excess copper. Nature 287(5782):563
Sandalio LM, Dalurzo HC, Gomez M, Romero-Puertas MC, Del Rio LA (2001) Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J Exp Bot 52(364):2115–2126
Schutzendubel A, Polle A (2002) Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot 53(372):1351–1365
Semida WM, Rady MM, Abd El-Mageed TA et al (2015) Alleviation of cadmium toxicity in common bean (Phaseolus vulgaris L.) plants by the exogenous application of salicylic acid. J Hortic Sci Biotechnol 90:83–91. https://doi.org/10.1080/14620316.2015.11513157
Shlizerman L, Marsh K, Blumwald E, Sadka A (2007) Iron-shortage-induced increase in citric acid content and reduction of cytosolic aconitase activity in citrus fruit vesicles and calli. Physiol Plant 131:72–79. https://doi.org/10.1111/j.1399-3054.2007.00935.x
Siddiqui H, Ahmed KB, Hayat S (2018) Comparative effect of 28-homobrassinolide and 24-epibrassinolide on the performance of different components influencing the photosynthetic machinery in Brassica juncea L. Plant Physiol Biochem 129:198–212
Silveira FS, Azzolini M, Divan AM (2015) Scanning cadmium photosynthetic responses of Elephantopus mollis for potential phytoremediation practices. Water Air Soil Pollut 226(11):359
Smith S, Stewart GR (1990) Effect of potassium levels on the stomatal behavior of the hemi-parasite Striga hermonthica. Plant Physiol 94(3):1472–1476
Srivastava HS (1980) Regulation of nitrate reductase activity in higher plants. Phytochemistry 19(5):725–733
Srivastava S, Tripathi RD, Dwivedi UN (2004) Synthesis of phytochelatins and modulation of antioxidants in response to cadmium stress in Cuscuta reflexa—an angiospermic parasite. J Plant Physiol 161:665–674. https://doi.org/10.1078/0176-1617-01274
Sun YL, Hong SK (2011) Effects of citric acid as an important component of the responses to saline and alkaline stress in the halophyte Leymus chinensis (Trin.). Plant Growth Regul 64:129–139. https://doi.org/10.1007/s10725-010-9547-9
Szalai G, Krantev A, Yordanova R, Popova LP, Janda T (2013) Influence of salicylic acid on phytochelatin synthesis in Zea mays during Cd stress. Turk J Bot 37(4):708–714
Vagner E, Williams C, Souza A, Bruno F (2013) Citric acid-assisted phytoextraction of lead: a field experiment. Chemosphere 92:213–217. https://doi.org/10.1016/j.chemosphere.2013.01.103
Vallee BL, Ulmer DD (1972) Biochemical effects of mercury, cadmium, and lead. Annu Rev Biochem 41(1):91–128
Wahid A, Ghazanfar A (2006) Possible involvement of some secondary metabolites in salt tolerance of sugarcane. J Plant Physiol 163(7):723–730
Wahid A, Ghani A, Ali I, Ashraf MY (2007) Effects of cadmium on carbon and nitrogen assimilation in shoots of mungbean [Vigna radiata (L.) Wilczek] seedlings. J Agron Crop Sci 193(5):357–365
Wang Y, Fang J, Leonard SS, Rao KMK (2004) Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Free Radic Biol Med 36:1434–1443
Wani AB, Chadar H, Wani AH et al (2017) Salicylic acid to decrease plant stress. Environ Chem Lett 15:101–123. https://doi.org/10.1007/s10311-016-0584-0
Yeh TY, Lin CF, Chuang CC, Pan CT (2012) The effect of varying soil organic levels on phytoextraction of Cu and Zn uptake, enhanced by chelator EDTA, DTPA, EDDS and Citric Acid, in Sunflower (Helianthus annuus), Chinese Cabbage (Brassica campestris), Cattail (Typha latifolia), and Reed (Phragmites communis). Environ Anal Toxicol 2(5):2. https://doi.org/10.4172/2161-0525.1000142
Ying RR, Qiu RL, Tang YT et al (2010) Cadmium tolerance of carbon assimilation enzymes and chloroplast in Zn/Cd hyperaccumulator Picris divaricata. J Plant Physiol 167:81–87. https://doi.org/10.1016/j.jplph.2009.07.005
Yusuf M, Hasan SA, Ali B, Hayat S, Fariduddin Q, Ahmad A (2008) Effect of salicylic acid on salinity-induced changes in Brassica juncea. J Integr Plant Biol 50(9):1096–1102
Yusuf M, Fariduddin Q, Varshney P, Ahmad A (2012) Salicylic acid minimizes nickel and/or salinity-induced toxicity in Indian mustard (Brassica juncea) through an improved antioxidant system. Environ Sci Pollut Res 19(1):8–18
Zengin FK, Munzuroglu O (2005) Effects of some heavy metals on content of chlorophyll, proline and some antioxidant chemicals in bean (Phaseolus vulgaris L.) seedlings. Acta Biol Cracoviensia Ser Bot 47:157–164
Zenk MH (1996) Heavy metal detoxification in higher plants—a review. Gene 179(1):21–30
Zhang F, Zhang H, Xia Y et al (2011) Exogenous application of salicylic acid alleviates cadmium toxicity and reduces hydrogen peroxide accumulation in root apoplasts of Phaseolus aureus and Vicia sativa. Plant Cell Rep 30:1475–1483. https://doi.org/10.1007/s00299-011-1056-4
Zong H, Liu S, Xing R et al (2017) Protective effect of chitosan on photosynthesis and antioxidative defense system in edible rape (Brassica rapa L.) in the presence of cadmium. Ecotoxicol Environ Saf 138:271–278. https://doi.org/10.1016/j.ecoenv.2017.01.009
Acknowledgements
We thank the Department of Botany and Aligarh Muslim University for providing the platform to perform this experiment. We thank the authors for their contribution to this experiment. Thanks to Dr. John Pichtel, Ball State University, USA, for assistance with editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that this research work conducted without any financial relationship and authors have no conflict of interest among them.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Faraz, A., Faizan, M., Sami, F. et al. Supplementation of Salicylic Acid and Citric Acid for Alleviation of Cadmium Toxicity to Brassica juncea. J Plant Growth Regul 39, 641–655 (2020). https://doi.org/10.1007/s00344-019-10007-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-019-10007-0