Abstract
To assess the role of salicylic acid (SA) in alleviating cadmium (Cd) toxicity in hemp (Cannabis sativa L.) plants, the growth parameters, Cd accumulation, photosynthetic performance and activities of major antioxidant enzymes were investigated in hemp seedlings treated with 500 μM SA, under 0, 25, 50, and 100 mg Cd kg−1 sands (DW) conditions, respectively. Cd exposure resulted in a small reduction in biomass (12.0–26.9% for root, and 8.7–29.4% for shoot, respectively), indicating hemp plants have innate capacity to tolerant Cd stress. This was illustrated by little inhibition in photosynthetic performance, unchanged malondialdehyde content, and enhancement of superoxide dismutase (SOD) and peroxidases (POD) activities in hemp plants. Cd content in root is 25.0–29.5 times’ higher than that in shoot, suggesting the plant can be classified as a Cd excluder. It is concluded that SA pretreatment counteracted the Cd-induced inhibition in plant growth. The beneficial effects of SA in alleviating Cd toxicity can be attributed to the SA-induced reduction of Cd uptake, improvement of photosynthetic capacity, and enhancement of SOD and POD activities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is a highly toxic trace element that enters the environment mainly from industrial processes and phosphate fertilizers. It can reach high levels in agricultural soils and is easily accumulated in plants. Excess Cd induces complex changes in plants at genetical, biochemical and physiological levels, leading to phytotoxicity. The most obvious symptoms are (1) depression of plant growth and even resulting in plant death by disturbing the uptake of nutrients (Sandalio et al. 2001; Drazkiewicz et al. 2003); (2) destruction of photosynthesis via degradation of chlorophyll (Somashekaraiah et al. 1992; Sandalio et al. 2001) and inactivation of enzymes involved in CO2 fixation (Greger and Ogren 1991; De Filippis and Ziegler 1993) or the aggregation of pigment protein complexes of the photosystems (Horvath et al. 1996); (3) interaction with the water balance of the plant, and inhibiting stomatal opening (Barcelo and Poschenrieder 1990), (4) alteration of proline and polyamine contents (Sharma and Dietz 2006), and (5) changes in the content of reactive oxygen species (ROS) and activity of antioxidant enzymes (Sandalio et al. 2001; Schützendübel and Polle 2002).
Salicylic acid (SA) is considered as a hormone-like substance, which plays a key role in plant disease resistance (Klessig and Malamy 1994; Durner et al. 1997; Alvarez 2000). SA has been reported to induce a number of defense responses to abiotic stress, such as low temperature (Janda et al. 1999; Kang and Saltveit 2002; Tasgin et al. 2003), thermo-stress (Chen et al. 1997; Dat et al. 1998a, b), salt and osmotic stresses (Borsani et al. 2001), ozone or UV light (Sharma et al. 1996), and drought stress (Senaratna et al. 2000). In addition, SA is also known to be involved in plant protection against heavy metals (Mishra and Choudhuri 1999).
Although several studies have showed that SA could alleviate Cd toxicity (Metwally et al. 2003; Drazic and Mihailovic 2005; Dražić et al. 2006; Zawoznik et al. 2007), the mechanism is varied according to different species. Metwally et al. (2003) concluded that SA alleviated Cd toxicity to barley roots not at the level of antioxidant defense, but by affecting other mechanisms of Cd detoxification. Drazic and Mihailovic (2005) and Dražić et al. (2006) reported that the influence of SA on the alleviation of toxic effects of Cd is probably indirect, through maintenance of ionic homeostasis. More recently, Zawoznik et al. (2007) and Guo et al. (2007, 2009) suggested that SA may function as a signaling molecule necessary to generate, to sustain or to amplify Cd-induced oxidative stress. Krantev et al. (2008) suggested that SA may protect cells against oxidative damage and photosynthesis against Cd toxicity. Thus, a deeper understanding of the roles of SA in alleviating Cd toxicity is necessary.
Hemp (Cannabis sativa L.), a fast growing and high-biomass producing plant, has been traditionally grown as fiber crop and recently considered as an energy crops (Venendaal et al. 1997). Previous studies demonstrated that hemp is a metal-tolerant organism, and the amount of Cd accumulated in its shoot was not negligible (Linger et al. 2002, 2005; Citterio et al. 2003). However, little is known about the mechanism of Cd tolerance.
This study was undertaken (1) to assess Cd tolerance capacity of hemp plants according to their growth and physiological changes; (2) determine the effects of SA pretreatment on growth, Cd uptake, and physiological changes in hemp plants during Cd-induced stress; and (3) investigate whether this regulator is involved in the induction of defense responses.
Materials and methods
Plant growth and treatment with Cd
Seeds of hemp were sterilized and divided into two groups. One group of the seeds was soaked in 500 μM SA solution for 6 h, while the other group was soaked in distilled water (control). Subsequently, seeds were sown in pot (16 × 18 cm) filled with acid washed sands, additionally supplemented with Cd as CdCl2·2.5 H2O at levels of 0, 25, 50, and 100 mg Cd kg−1 sands (DW) (three replications). After germinating, five uniform plants were allowed to grow in each pot, at uniform spacing. Pot cultivation was carried out in greenhouse, the average temperature was between 25.4 ± 0.5°C (day) and 22.6 ± 0.4°C (night), and the relative humidity was 60.5 ± 1.2% (day) and 68.4 ± 1.7 % (night). The pots were watered daily to 60% water holding capacity of the sand, and fertilized twice a week with 100 mL of Hoagland’s nutrient solution. The 40-day seedlings were first used to measure gas exchange and chlorophyll fluorescence, and then harvested to determine the other morphological and physiological parameters.
Evaluation of plant growth and Cd accumulation in plants
At harvest, the plants were washed with running tap water to remove any sand particles attached to the plant surfaces. The roots were soaked in 20 mM Na2-EDTA for 15 min to remove the metal ions adhering to root surfaces (Yang et al. 2004). The roots and shoots were separated and oven-dried for 30 min at 105°C, then at 70°C till the materials reach their constant weights. The dried tissues were weighed and grinded into powder for the determination of Cd concentration, which was measured by flame-atomic absorbance spectrometry after digested with mixed acid [HNO3 + HClO4 (3:1, v/v)]. The translocation factors (TF) were calculated as: TF = [Cd]shoot/[Cd]root.
Chlorophyll concentration analysis
Mature leaves (0.2 g) were extracted in dark at 4°C with 5 ml mixture of acetone and ethanol (v/v = 1:1) until color disappeared completely. Light absorbance at 663, 645, and 470 nm was determined using spectrophotometry. The concentrations of chlorophyll (a, b) and carotenoids were calculated using adjusted extinction coefficients (Lichtenthaler 1987).
Measurement of chlorophyll fluorescence
Chlorophyll fluorescence parameters were performed using the Mini PAM (Walz, Effeltrich, Germany). After dark-adapted for 30 min, leaves were used to determine the F o (the minimal fluorescence after the dark adaptation), F m (the maximal fluorescence after the pulse of red light), and F v/F m (the ratio of variable to maximal fluorescence). Steady-state chlorophyll a fluorescence yield (F) was monitored under ambient irradiation (500 μmol quanta m−2 s−1). A saturating light pulse (5,000 μmol quanta m−2 s−1; 1 s) was applied to measure the maximal fluorescence of light-adapted leaf (F m′). The effective quantum yield of PS II (ΦPS II) was calculated as follows: ΦPS II = ΔF/F m = (F m′ − F)/F m′.
Gas-exchange measurements
The net photosynthetic rate (P n), transpiration rate (E), stomatal conductance (G s), intercellular CO2 concentration (C i) and dark respiration (R d) were determined with a portable photosynthesis system (Li-6400; LiCor Inc. Lincoln, Nebraska, USA) and an LED light source (6400–02). This was conducted at light intensity of 1,000 μmol m−2 s−1 (for dark respiration, the light intensity is 0 μmol m−2 s−1), leaf temperature of 25°C, and constant CO2 of 380 ± 5 μmol CO2 mol−1 in the sample chamber provided with buffer volume.
Malondialdehyde (MDA) determination
The MDA determination was followed the method described by Li (2000). Fresh leaf material (0.2 g) from different plants was homogenized in 5 ml of 10% trichloroacetic acid. The homogenate was centrifuged at 10,000×g for 20 min, and then 2 ml supernatant of sample was reacted with 2 ml of 0.6% 2-thiobarbituric acid. The absorbance was monitored at 600, 532, and 450 nm, respectively. Calculation of MDA was based on the following formula: C (μM) = 6.45(A 532 − A 600) − 0.56 A 450.
Enzyme extraction and enzyme assays
A 0.2 g of leaf tissue was used to extract soluble protein by homogenizing in 5 ml of 50 mM phosphate buffer (pH 7.0) containing 1 mM EDTA and 1% polyvinylpolypyrrolidone. The homogenate was centrifuged at 10,000×g at 4°C for 20 min, and the supernatant was used for the following enzyme assays. Protein content was determined according to the method of Bradford (1976) with bovine serum albumin as standard.
The superoxide dismutase (SOD, EC 1.15.1.1) activity was assayed according to Beauchamp and Fridovich (1971). One unit of SOD was defined as the amount of enzyme that caused a 50% decrease of the SOD-inhibited nitroblue tetrazolium chloride reduction. Peroxidases (POD, EC 1.11.1.7) activity was based on the determination of guaiacol oxidation (ε = 26.6 mM cm−1) at 470 nm by H2O2 (Putter 1974). Catalase (CAT, EC1.11.1.6) activity was determined by the consumption of H2O2 conversion at 240 nm (ε = 39.4 mM cm−1) (Aebi 1984).
Statistical analysis
Data were subjected to a two-way ANOVA using the SPSS Version 11.5 software (SPSS Inc.). Multiple comparisons of means among different Cd treatments with or without SA pretreatment were performed using Duncan’s test at the 0.05 significance level.
Results
Growth parameters
Elevated Cd reduced root and shoot growth in hemp plants. Shoot biomass decreased proportionally with increasing Cd concentration, and the reduction in the values of this parameter under 25, 50, and 100 mg Cd kg−1 sand was 8.7, 18.7, and 29.4%, respectively, compared with the control. For root biomass, the reduction was 12.0, 26.3, and 26.9%, respectively (Table 1).
In the absence of Cd, SA pretreatment decreased the biomass of shoots and roots by 8.6 and 8.1%, respectively. However, when plants were exposed to Cd stress, SA pretreatment alleviated Cd toxicity; this was illustrated by the enhancement of biomass in SA pretreated plants, in comparison with the non-pretreated one at the same Cd level. The beneficial effect of SA was statistically significant in shoot biomass, but less pronounced on root biomass.
Cd accumulation in plants
Cd accumulation in shoots and roots was high, and it was found to be dose-dependent (Table 1). Cd content ranged from 44.6 to 79.7 mg kg−1 (DW) in shoot, and from 1,115 to 2,148 mg kg−1 (DW) in root, respectively. Cd content in roots exposed to 25, 50, and 100 mg Cd kg−1 sand, were about 25.0-, 29.5, and 27.0-fold higher than that in shoots, respectively. SA pretreatment decreased Cd accumulation by 9.0, 8.6, and 19.1% in shoots, and by 17.4, 17.4, and 39.0% in roots under 25, 50, and 100 mg Cd kg−1 sand conditions, respectively. TF was unaffected neither by Cd nor by SA treatments (Table 1).
Photosynthetic pigment contents
Cd exposure caused little inhibition in photosynthetic pigment in hemp plants. No pronounced changes were found in chlorophyll (a, b, a + b) and carotenoids (Car) content, as well as the Chl a/b and Car/Chl ratios (Table 2). SA pretreatment did not affect the Chl a, Chl b, Chl a + b, Chl a/b, and Car/Chl, but enhanced Car in the control and 25 mg kg−1 Cd treatment. There were Cd × SA interactions on Chl a, Chl a + b, and Car/Chl (Table 2).
Chlorophyll fluorescence
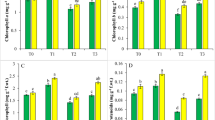
As shown in Fig. 1a, the F v/F m ratio, ranged from 0.80 to 0.81, did not differ in all Cd and SA treatments. The ΦPS II was the same in different Cd treatments, but pretreatment with SA resulted in an increase in ΦPS II under Cd stress. Two-way ANOVA of ΦPS II indicated a significant main effect of Cd (P < 0.05) and SA (P < 0.01), as well as Cd by SA interaction (P < 0.01) (Fig. 1b).
The effect of SA and Cd on chlorophyll fluorescence in hemp seedlings subjected to Cd stress. The different letters above the bars (open square SA − or filled square SA+) of the same SA treatment are significantly different according to the Duncan’s test (P < 0.05). n.s. not significant, *P < 0.05, **P < 0.01, ***P < 0.001
Gas exchange
No significant reductions were detected in photosynthetic parameters of plants treated with Cd (Table 3). Furthermore, low Cd treatment (25 mg kg−1) result in a significant enhancement in P n and G s. SA pretreatment caused a significant increase in P n, E, and G s under Cd stress. In comparison with the control, SA presoaking increased P n by 61.9, 24.3, and 46.0%, G s by 23.1, 2.4, and 62.0%, and E by 27.1, 20.2, and 78.8%, respectively, under 25, 50, and 100 mg Cd kg−1 sand conditions. SA pretreatment decreased C i was also observed in the 25 and 50 mg kg−1 Cd treatments. There were significant main effects of SA, as well as Cd × SA interactions for P n, E, G s, and C i, Cd had a significant main effect on P n and E, but not on G s and C i. The R d did not change in different Cd and SA treatments (Table 3).
MDA content and SOD, CAT, and POD activities
Figure 2 compares MDA contents and activity of antioxidant enzymes of leaves from hemp plants subjected to Cd with or without presoaking in SA. The presence of Cd in sands did not lead to disturbances in the MDA level, however, a significant reduction of MDA was observed in SA-treated plants (Fig. 2a). The POD activity was increased with Cd levels in both the control and SA-treated plants, and it was significantly high in plants subjected to Cd with SA presoaking, compared with the control (Fig. 2b). Similar pattern was also observed in the changes of SOD activity, and it was significantly affected by Cd and SA, as well as Cd by SA interaction (Fig. 2c). No major changes were found in CAT activity in both Cd and SA treatments (Fig. 2d).
The effect of SA pretreatment on MDA content, and POD, SOD, and CAT activity in hemp seedlings subjected to Cd stress. The different letters above the bars (open square SA − or filled square SA+) of the same SA treatment are significantly different according to the Duncan’s test (P < 0.05). n.s. not significant, *P < 0.05, **P < 0.01, ***P < 0.001
Discussion
Cd tolerance and accumulation in hemp plants
In this study, we demonstrated that hemp plants have innate resistance to Cd stress. This was in consistent to the results reported by Citterio et al. (2003) and Linger et al. (2005). The high tolerance of hemp plants to 25–100 mg kg−1 Cd stress was illustrated by the small reduction of roots and shoots biomass, little inhibition in photosynthesis, and less oxidative stress maintained by POD and SOD.
Generally, Cd caused the destruction of photosynthesis resulting from severe alterations in the chlorophyll level, chloroplast structure, photochemistry, and carboxylating enzyme activities, and the high extent of lipid peroxidation (Krantev et al. 2008). In the present study, excess Cd caused little inhibition in photosynthetic pigment contents (Chl a, Chl b, Chl, Chl a/b, Car, and Car/Chl), the chlorophyll fluorescence parameter (the F v/F m ratio and ΦPS II), and gas exchange parameters (P n, G s, E, and C i), these results suggest hemp plant had a stability in the PS II/PS I ratio (Varadi et al. 2003) and in the antennae composition (Durnford et al. 2003). Thus, the plants did not suffer Cd stress in their photosynthetic apparatus.
Although Cd does not generate ROS directly, it generates oxidative stress via interference with the antioxidant defense system (Foyer and Noctor 2005). MDA content is an index of the membrane integrity and lipid peroxidation. Cd-induced increase in MDA content were reported for many plant species, including bean (Chaoui et al. 1997), pea (Lozano-Rodriguez et al. 1997; Metwally et al. 2005), rice (Chien et al. 2001), sunflower (Gallego et al. 1996), mustard (Mobin and Khan 2007), and maize (Pál et al. 2007; Krantev et al. 2008). In the present study, however, the MDA was similar in different Cd treatments, indicating the plants not suffering a Cd-induced oxidative damage.
The obtained results showed that the ROS scavenging in hemp plants may be contributed by the dramatic enhancement of SOD and POD activity (Fig. 2b, c). SOD catalyzes the conversion of superoxide anion to O2 and H2O2 (Sudhakar et al. 2001), its activity reflects the O2 radical-eliminating ability of plants (Azevedo Neto et al. 2006; Ekmekçi et al. 2008). POD catalyzes H2O2-dependent oxidation of substrate, while CAT eliminates H2O2 by breaking it down directly to form water and oxygen. CAT is less efficient than POD in H2O2 scavenging because of its low-substrate affinity, so as long as the stress is not too strong for the plant defense capacity, the main response to heavy metal is an increase in SOD and POD activities along with a decrease in CAT (Siedlecka and Krupa 2002).
The mechanism of metal tolerance in plants includes metal exclusion and accumulation (Baker 1987). Metal exclusion is the avoidance of absorption and restriction of translocation to shoot. Metal accumulation is an extreme type of physiological response that plants absorb and accrete high concentration of metal (Dahmani-Muller et al. 2000). As showed in Table 1, most of Cd absorbed in hemp plant was retained in the root, reaching 25–29.5 times higher values than that in shoots. The results obtained in this study suggest that hemp plant strongly limited translocation of Cd to shoot. Such metal immobilisation in root cells is related to an exclusion strategy.
Effects of SA on Cd tolerance of hemp plant
Our results showed that, although treated with SA or Cd alone both caused an inhibition in plant growth, when applied simultaneously, they act antagonistically on shoot and root biomass. Such SA-mitigated Cd toxicity has been shown in other plant species, including barley (Hordeum vulgare) (Metwally et al. 2003), soybean (Glycine max) (Drazic and Mihailovic 2005), alfalfa (Dražić et al. 2006), maize (Krantev et al. 2008), and rice (Choudhury and Panda 2004; Guo et al. 2007, 2009).
A moderate resistance to heavy metals can be realized by selective Cd exclusion, lowered uptake, or active efflux from the roots, i.e. by mechanisms leading to lower cytoplasmic Cd contents (Hall 2002). Our results showed that presoaking hemp seeds for 6 h with SA before exposure to Cd diminishes the Cd accumulation, This may be one of the important causes for SA-induced Cd tolerance in hemp plants. Similar results was also found in rice (Choudhury and Panda 2004; Guo et al. 2009) and maize (Pál et al. 2002; Krantev et al. 2008). However, in barley seedlings, Cd contents in tissue were unaltered (Metwally et al. 2003). Furthermore, the cases of SA increased Cd accumulation were observed in soybean (Drazic and Mihailovic 2005) and alfalfa (Dražić et al. 2006). Hence, the effects of SA on Cd accumulation depend on its concentration, treatment duration, plant species, age at treatment, and plant organ examined.
One of the important results in this study is that SA pretreatment significantly enhanced P n in hemp plants subjected to Cd stress. This was accompanied with an increase in G s and E. The parallel changes of P n and G s suggested a SA-induced stomatal effect existed in SA-treated plants (Chartzoulakisa et al. 2002). Previous studies suggested that SA induce a reversal of inhibited transpiration rate in zucchini yellow mosaic virus-infected leaves (Radwan et al. 2006) and a reversal of ABA-induced stomatal closure (Rai et al. 1986), hence, the positive effect of SA pretreatment on G s and E in hemp plant might be accounted for by the SA-reversed stomatal closure. In addition, SA pretreatment caused a decrease of C i at low Cd concentrations (25 and 50 mg kg−1), indicating that enzymatic dark reaction of photosynthesis may be affected (Vaillant et al. 2005; Hura et al. 2007). This was strengthened by Krantev et al. (2008), who found that SA pretreatment alleviate the inhibitory effect of Cd on the activity of RuBPC and PEPC in maize seedlings.
Generally, transpiration is the most important mechanism for the uptake of nutrient elements by its gradient of water potential, which can drive them to move into roots and upward to shoots (Novák and Vidovič 2003; Tani and Barrington 2005; Liao et al. 2006; Guo et al. 2009). A previous study with Indian mustard showed that Cd accumulation in shoots was strongly influenced by transpiration (Salt et al. 1995). However, in the present study, a high transpiration along with lower Cd accumulation in shoot and root was observed in SA-treated plants. Moreover, it was also observed that the TF was similar in control and SA-treated plants. Therefore, low Cd uptake in SA-treated plants may be result from the decreasing of uptake area or/and inhibition of metal transporters.
Chlorophyll content and chlorophyll fluorescence are considered as indicators of damages to photosynthetic system induced by environmental stressors (Lichtenthaler and Miehe 1997; Maxwell and Johnson 2000). The present study showed that SA pretreatment increased Car and ΦPS II in absent of Cd or present of low Cd level (25 mg kg−1), indicating SA takes a positive role in protecting photosynthetic pigment and apparatus.
It has been recognized that SA acts as a signaling molecule necessary to generate, to sustain or to amplify Cd-induced oxidative stress (Zawoznik et al. 2007). In the present study, SA pretreatment alleviated Cd-induced oxidative stress as evidenced by a decrease in MDA content, and a sustain increase in SOD and POD activity. Our result was in consistent with those reported by Guo et al. (2007, 2009) and Krantev et al. (2008), but disagreed with Metwally et al. (2003).
In conclusion, hemp has innate capacity of Cd tolerance. Cd exposure caused little inhibition in photosynthesis, but result in an increase in activities of SOD and POD. Most of the Cd uptake by plants was located in roots, which indicated that the plant as a Cd excluder. SA pretreatment alleviate Cd toxicity in hemp seedlings, which result from the reduction of Cd uptake, the improvement of photosynthesis, and the enhancement of SOD and POD activities.
Abbreviations
- Car:
-
Carotenoids
- CAT:
-
Catalase
- Chl:
-
Chlorophyll
- C i :
-
Intercellular CO2 concentration
- E :
-
Transpiration rate
- F :
-
Steady-state chlorophyll fluorescence yield
- F o :
-
The minimal fluorescence
- F m :
-
The maximal fluorescence
- F m′:
-
The maximal fluorescence of light-adapted leaf
- F v :
-
The variable fluorescence
- F v/F m :
-
Maximal quantum yield of PS II
- G s :
-
Stomatal conductance
- MDA:
-
Malondialdehyde
- PS II:
-
Photosystem II
- PS I:
-
Photosystem I
- P n :
-
Net-photosynthetic rate
- POD:
-
Peroxidases
- ΦPS II:
-
The effective quantum yield of PS II
- R d :
-
Dark respiration
- ROS:
-
Reactive-oxygen species
- SOD:
-
Superoxide dismutase
- TF:
-
Translocation factor
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. doi:10.1016/S0076-6879(84)05016-3
Alvarez ME (2000) Salicylic acid in the machinery of hypersensitive cell death and disease resistance. Plant Mol Biol 44:429–442. doi:10.1023/A:1026561029533
Azevedo Neto AD, Prisco JT, Eneas-Filho J, Abreu CEB, Gomes-Filho E (2006) Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ Exp Bot 56:87–94. doi:10.1016/j.envexpbot.2005.01.008
Baker AJM (1987) Metal tolerance. New Phytol 106:93–111
Barcelo J, Poschenrieder C (1990) Plant water relations as affected by heavy metal Stress: A review. J Plant Nutr 13:1–37. doi:10.1080/01904169009364057
Beauchamp C, Fridovich I (1971) Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287. doi:10.1016/0003-2697(71)90370-8
Borsani O, Valpuesta V, Botella MA (2001) Evidence for a role of salicylic acid in the oxidative damage generated by NaCl and osmotic stress in Arabidopsis seedlings. Plant Physiol 126:1024–1030. doi:10.1104/pp.126.3.1024
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Chaoui A, Mazhoudi S, Ghorbal M, El Ferjani E (1997) Cadmium and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in bean (Phaseolus vulgaris L.). Plant Sci 127:139–147. doi:10.1016/S0168-9452(97)00115-5
Chartzoulakisa K, Patakasb A, Kofidisc G, Bosabalidisc A, Nastoub A (2002) Water stress affects leaf anatomy, gas exchange, water relations and growth of two avocado cultivars. Sci Hortic (Amsterdam) 95:39–50. doi:10.1016/S0304-4238(02)00016-X
Chen Z, Iyer S, Caplan A, Klessig DF, Fan B (1997) Differential accumulation of salicylic acid and salicylic acid-sensitive catalase in different rice tissues. Plant Physiol 114:193–201. doi:10.1104/pp.114.1.265
Chien H, Wang J, Lin C, Kao C (2001) Cadmium toxicity of rice leaves is mediated through lipid peroxidation. Plant Growth Regul 33:205–213. doi:10.1023/A:1017539616793
Choudhury S, Panda SK (2004) Role of salicylic acid in regulating cadmium induced oxidative stress in Oryza sativa. Bulg J Plant Physiol 30:95–110
Citterio S, Santagostino A, Fumagalli P, Prato N, Ranalli P, Sgorbati S (2003) Heavy metal tolerance and accumulation of Cd, Cr and Ni by Cannabis sativa L. Plant Soil 256:243–252. doi:10.1023/A:1026113905129
Dahmani-Muller H, van Oort F, Gélie B, Balabane M (2000) Strategies of heavy metal uptake by three plant species growing near a metal smelter. New Phytol 109:231–238
Dat JF, Foyer CH, Scott IM (1998a) Changes in salicylic acid and antioxidants during induced thermo tolerance in mustard seedlings. Plant Physiol 118:1455–1461. doi:10.1104/pp.118.4.1455
Dat JF, Lopez-Delgado H, Foyer CH, Scott IM (1998b) Parallel changes in H2O2 and catalase during thermo tolerance induced by salicylic acid or heat acclimation in mustard seedlings. Plant Physiol 116:1351–1357. doi:10.1104/pp.116.4.1351
De Filippis LF, Ziegler H (1993) Effect of sublethal concentrations of zinc, cadmium and mercury on the photosynthetic carbon reduction cycle of Euglena. J Plant Physiol 142:167–172
Drazic G, Mihailovic N (2005) Modification of cadmium toxicity in soybean seedlings by salicylic acid. Plant Sci 168:511–517. doi:10.1016/j.plantsci.2004.09.019
Dražić G, Mihailović N, Lojić M (2006) Cadmium accumulation in Medicago sativa seedlings treated with salicylic acid. Biol Plant 50:239–244. doi:10.1007/s10535-006-0013-5
Drazkiewicz M, Tukendorf A, Baszynski T (2003) Age-dependent response of maize leaf segments to cadmium treatment: effect on chlorophyll fluorescence and phytochelatin accumulation. J Plant Physiol 160:247–254. doi:10.1078/0176-1617-00558
Durner J, Shah J, Klessig DF (1997) Salicylic acid and disease resistance in plants. Trends Plant Sci 2:266–274. doi:10.1016/S1360-1385(97)86349-2
Durnford DG, Price JA, McKim SM, Sarchfield ML (2003) Light harvesting complex gene expression is controlled by both transcriptional and post-transcriptional mechanisms during photoacclimation in Chlamydomonas reinhardtii. Physiol Plant 118:193–205. doi:10.1034/j.1399-3054.2003.00078.x
Ekmekçi Y, Tanyolaç D, Ayhan B (2008) Effects of cadmium on antioxidant enzyme and photosynthetic activities in leaves of two maize cultivars. J Plant Physiol 165:600–611. doi:10.1016/j.jplph.2007.01.017
Foyer C, Noctor G (2005) Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ 28:1056–1071. doi:10.1111/j.1365-3040.2005.01327.x
Gallego S, Benavides M, Tomaro M (1996) Effect of heavy metal ion excess on sunflower leaves: evidence for involvement of oxidative stress. Plant Sci 121:151–159. doi:10.1016/S0168-9452(96)04528-1
Greger M, Ogren E (1991) Direct and indirect effects of Cd2+ on photosynthesis in sugar beet (Beta vulgaris). Physiol Plant 83:129–135. doi:10.1111/j.1399-3054.1991.tb01291.x
Guo B, Liang YC, Zhu YG, Zhao FJ (2007) Role of salicylic acid in alleviating oxidative damage in rice roots (Oryza sativa) subjected to cadmium stress. Environ Pollut 147:743–749. doi:10.1016/j.envpol.2006.09.007
Guo B, Liang Y, Zhu Y (2009) Does salicylic acid regulate antioxidant defense system, cell death, cadmium uptake and partitioning to acquire cadmium tolerance in rice? J Plant Physiol 166:20–31. doi:10.1016/j.jplph.2008.01.002
Hall JL (2002) Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot 53:1–11. doi:10.1093/jexbot/53.366.1
Horvath G, Droppa M, Oraveez A, Raskin V, Marder JB (1996) Formation of the photosynthetic apparatus during greening of cadmium poisoned barley leaves. Planta 199:238–243. doi:10.1007/BF00196564
Hura T, Hura K, Grzesiak M, Rzepka A (2007) Effect of long-term drought stress on leaf gas exchange and fluorescence parameters in C3 and C4 plants. Acta Physiol Plant 29:103–113. doi:10.1007/s11738-006-0013-2
Janda T, Szalai G, Tari I, Paldi E (1999) Hydroponic treatment with salicylic acid decreases the effects of chilling in maize (Zea mays L.) plants. Planta 208:175–180. doi:10.1007/s004250050547
Kang HM, Saltveit M (2002) Chilling tolerance of maize, cucumber and rice seedling leaves and roots are differentially affected by salicylic acid. Physiol Plant 115:571–576. doi:10.1034/j.1399-3054.2002.1150411.x
Klessig DF, Malamy J (1994) The salicylic acid signal in plants. Plant Mol Biol 26:1439–1458. doi:10.1007/BF00016484
Krantev A, Yordanova R, Janda T, Szalai G, Popova L (2008) Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J Plant Physiol 165:920–931. doi:10.1016/j.jplph.2006.11.014
Li HS (2000) Principles and techniques of plant physiological biochemical experiment. Higher Education Press, Beijing, pp 260–263
Liao YC, Chang Chien SW, Wang MC, Shen Y, Hung PL, Das B (2006) Effect of transpiration on Pb uptake by lettuce and on water soluble low molecular weight organic acids in rhizosphere. Chemosphere 65:343–351. doi:10.1016/j.chemosphere.2006.02.010
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic membranes. Methods Enzymol 148:350–382. doi:10.1016/0076-6879(87)48036-1
Lichtenthaler HK, Miehe JA (1997) Fluorescence imaging as a diagnostic tool for plant stress. Trends Plant Sci 2:316–320. doi:10.1016/S1360-1385(97)89954-2
Linger P, Müssig J, Fischer H, Kobert J (2002) Industrial hemp (Cannabis sativa L.) growing on heavy metal contaminated soil: fibre quality and phytoremediation potential. Ind Crops Prod 16:33–42. doi:10.1016/S0926-6690(02)00005-5
Linger P, Ostwald A, Haensler J (2005) Cannabis sativa L growing on heavy metal contaminated soil: growth, cadmium uptake and photosynthesis. Biol Plant 49:567–576. doi:10.1007/s10535-005-0051-4
Lozano-Rodriguez E, Hernàndez L, Bonay P, Carpena-Ruiz R (1997) Distribution of cadmium in shoot and root tissues of maize and pea plants: physiological disturbances. J Exp Bot 48:123–128. doi:10.1093/jxb/48.1.123
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51:659–668. doi:10.1093/jexbot/51.345.659
Metwally A, Finkemeier I, Georgi M, Dietz KJ (2003) Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol 132:272–281. doi:10.1104/pp.102.018457
Metwally A, Safronova VI, Belimov AA, Dietz KJ (2005) Genotypic variation of the response to cadmium toxicity in Pisum sativum L. J Exp Bot 56:167–178
Mishra A, Choudhuri MA (1999) Effects of salicylic acid on heavy metal-induced membrane deterioration mediated lipoxygenase in rice. Biol Plant 42:409–415. doi:10.1023/A:1002469303670
Mobin M, Khan NA (2007) Photosynthetic activity, pigment composition and antioxidative response of two mustard (Brassica juncea) cultivars differing in photosynthetic capacity subjected to cadmium stress. J Plant Physiol 164:601–610. doi:10.1016/j.jplph.2006.03.003
Novák V, Vidovič J (2003) Transpiration and nutrient uptake dynamics in maize (Zea mays L.). Ecol Modell 166:99–107. doi:10.1016/S0304-3800(03)00102-9
Pál M, Szalai G, Horváth E, Janda T, Páldi E (2002) Effect of salicylic acid during heavy metal stress. Acta Biol Szeged 46:119–120
Pál M, Leskó K, Janda T, Páldi E, Szalai G (2007) Cadmium-induced changes in the membrane lipid composition of maize plants. Cereal Res Commun 35:1631–1642. doi:10.1556/CRC.35.2007.4.10
Putter J (1974) Peroxidases. In: Bergmeyer HU (ed) Methods of enzymatic analysis: II. Academic Press, New York, pp 685–690
Radwan DEM, Fayez AK, Mahmoud SY, Hamad A, Lu GQ (2006) Salicylic acid alleviates growth inhibition and oxidative stress caused by zucchini yellow mosaic virus infection in Cucurbita pepo leaves. Physiol Mol Plant Pathol 69:172–181. doi:10.1016/j.pmpp.2007.04.004
Rai VK, Sharma SS, Sharma S (1986) Reversal of ABA-induced stomatal closure by phenolic compounds. J Exp Bot 37:129–134. doi:10.1093/jxb/37.1.129
Salt DE, Prince RC, Pickering IJ, Raskin I (1995) Mechanisms of cadmium mobility and accumulation in Indian mustard. Plant Physiol 109:1427–1433
Sandalio LM, Dalurzo HC, Gómez M, Romero-Puertas MC, del Rio LA (2001) Cadmium-induced changes in the growth and oxidative metabolism of pea plant. J Exp Bot 52:2115–2126
Schützendübel A, Polle A (2002) Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot 53:1351–1365. doi:10.1093/jexbot/53.372.1351
Senaratna T, Touchell D, Bunns E, Dixon K (2000) Acetyl salicylic acid (aspirin) and salicylic acid induce multiple stress tolerance in bean and tomato plants. Plant Growth Regul 30:157–161. doi:10.1023/A:1006386800974
Sharma SS, Dietz KJ (2006) The significance of amino acids and amino acid-derived molecules in plant response and adaptation to heavy metal stress. J Exp Bot 57:711–726. doi:10.1093/jxb/erj073
Sharma YK, Leon J, Raskin I, Davis KR (1996) Ozone-induced responses in Arabidopsis thaliana—the role of salicylic acid in the accumulation of defence-related transcripts and induced resistance. Proc Natl Acad Sci USA 93:5099–5104. doi:10.1073/pnas.93.10.5099
Siedlecka A, Krupa Z (2002) Functions of enzymes in heavy metal treated plants. In: Prasad MNV, Kazimierz S (eds) Physiology and biochemistry of metal toxicity and tolerance in plants. Kluwer, The Netherlands, pp 314–317
Somashekaraiah BV, Padmaja K, Prasad ARK (1992) Phytotoxicity of cadmium ions on germinating seedlings of mung bean (Phaseolus vulgaris): involvement of lipid peroxides in chlorophyll degradation. Physiol Plant 85:85–89. doi:10.1111/j.1399-3054.1992.tb05267.x
Sudhakar C, Lakshmi A, Giridarakumar S (2001) Changes in the antioxidant enzymes efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Sci 161:613–619. doi:10.1016/S0168-9452(01)00450-2
Tani FH, Barrington S (2005) Zinc and copper uptake by plants under two transpiration rates Part I. Wheat (Triticum aestivum L.). Environ Pollut 138:538–547
Tasgin E, Attici O, Nalbantogly B (2003) Effect of salicylic acid and cold on freezing tolerance in winter wheat leaves. Plant Growth Regul 41:231–236. doi:10.1023/B:GROW.0000007504.41476.c2
Vaillant N, Monnet F, Hitmi A, Sallanon H, Coudret A (2005) Comparative study of responses in four Datura species to a zinc stress. Chemosphere 59:1005–1013. doi:10.1016/j.chemosphere.2004.11.030
Vaŕadi G, Polyańka H, Darkó È, Lehoczki E (2003) Atrazine resistance entails a limited xanthophylls cycle activity, a lower PS II efficiency and altered pattern of excess excitation dissipation. Physiol Plant 118:47–56. doi:10.1034/j.1399-3054.2003.00089.x
Venendaal R, Jorgensen U, Foster CA (1997) European energy crops: a synthesis. Biomass Bioenergy 13:147–185. doi:10.1016/S0961-9534(97)00029-9
Yang XE, Long XX, Ye HB, He ZL, Calvert DV, Stoffella PJ (2004) Cadmium tolerance and hyperaccumulation in a new Zn-hyperaccumulating plant species (Sedum alfredii Hance). Plant Soil 259:181–189. doi:10.1023/B:PLSO.0000020956.24027.f2
Zawoznik MS, Groppa MD, Tomaro ML, Benavides MP (2007) Endogenous salicylic acid potentiates cadmium-induced oxidative stress in Arabidopsis thaliana. Plant Sci 173:190–197. doi:10.1016/j.plantsci.2007.05.004
Acknowledgments
Financial support from the natural science foundation of Jiangsu province (BK2006148) and the natural science foundation for college of Anhui province (KJ2008B66ZC, KJ2009B073) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Klobus.
Rights and permissions
About this article
Cite this article
Shi, G.R., Cai, Q.S., Liu, Q.Q. et al. Salicylic acid-mediated alleviation of cadmium toxicity in hemp plants in relation to cadmium uptake, photosynthesis, and antioxidant enzymes. Acta Physiol Plant 31, 969–977 (2009). https://doi.org/10.1007/s11738-009-0312-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-009-0312-5