Abstract
The present study is the first report of isolation and characterization of endophytic actinobacteria from cactus (Opuntia ficus-indica). A total of 179 morphologically distinct endophytic actinobacterial isolates were purified from the roots of two different genetic accessions of cactus. All these isolates were screened for their plant growth promotion traits, namely, growth on N-free medium, P-solubilization, siderophore production, ACC deaminase activity and auxin production. A majority of the endophytic actinobacterial isolates (85%) exhibited their potential for plant growth promotion under in vitro conditions. Ten among the isolates were selected based on their multi-PGP traits and were identified as Streptomyces sp. following the 16S rRNA gene sequencing and phylogenetic analysis. Plant growth promotion potential of these selected endophytic Streptomyces was studied in wheat seedlings. All these selected isolates significantly enhanced the growth parameters such as seedling length and rootlets number compared to the uninoculated control. The wheat seeds inoculated with Streptomyces tuirus VL-70-IX exhibited maximum number of rootlets (6.33) compared to uninoculated control (3.67). The inoculation of endophytic actinobacteria Streptomyces levis VL-70-XII caused maximum seedling length (20.53 cm) and root length (8.26 cm), while the inoculation of S. radiopugnans HV-VIII resulted in highest shoot length (12.33 cm). These endophytic actinobacteria isolated from the roots of cactus accessions showed potential PGP traits. This work lays foundation for characterization and selection of endophytic actinobacteria from the under-exploited, drought tolerant species such as cactus with potential cross-compatibility for the improvement of plant growth of field crops especially under abiotic stress conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding and managing the plant–microbial interactions will accord considerable benefits especially in improving the crop production under stressed environments. Among these plant–microbe interactions, endophytes are the microbes inhabiting inner tissues of a plant and confer neutral, positive or negative effects to the hosts. Endophytic microbes that live within plant tissues without causing any visible damage to the host and promote plant growth directly or indirectly through a combination of mechanisms are considered as plant-beneficial endophytes (Rosenblueth and Martínez-Romero 2006; Compant et al. 2010). The ecological role of beneficial endophytes is more conspicuous due to the positive impacts such as enhanced nutrient use efficiency, biotic or abiotic stress tolerance of plants. All the three domains of life viz., Bacteria, Archea and Eukarya are reported to form endophytic association with various plant parts under different climatic conditions (Hirsch and Mauchline 2012; Govindasamy et al. 2018). The structural composition of endophytic bacterial communities depends on the host genotype, plant tissue and its vegetation stage. In addition, the microbial species composition is significantly influenced by the plant stress and soil types (Reinhold-Hurek and Hurek 2011; Govindasamy et al. 2017; Leite et al. 2017).
Members of one the dominant phyla, Actinobacteria, are found widely distributed in terrestrial (in soil) and aquatic ecosystems and play a significant role in decomposition process and humus formation. They are commonly referred as “actinomycetes” and are Gram-positive having high G + C content in their genome. This phylum comprises wide array of bacterial diversity such as those residing in the soil (Streptomyces), N-fixing symbiont of non-leguminous plants (Frankia), an important plant pathogen (Streptomyces scabies) among others (Stackebrandt 2000). Diverse group of endophytic actinobacterial species such as Streptomyces spp., Microbispora, Micromonospora, Nocardioides, Streptosporangium, Actinoplanes, Aeromicrobium, Arthrobacter, Brevibacterium, Corynebacterium, Microbacterium and new genera including Jatrophihabitans, Herbiconiux, Jishengella, Koreibacter, Phytohabitans, Phytomonospora, Flindersiella, Actinophytocola and Allonocardiopsis etc. were isolated and characterised from various plant species conferring a myriad of ecological advantages (Govindasamy et al. 2014, 2018; De Meyer et al. 2015; Zhao et al. 2016). Actinobacteria are known to exhibit diverse physiological and biochemical properties, such as production of extracellular enzymes and formation of a wide variety of secondary metabolites. In recent years, research pertaining to endophytic actinobacteria has gained immense attention attributed mainly to their plant growth promoting (PGP) properties. Endophytic actinobacteria were reported from various plants including wheat, lucerne, tomato, jatropha etc. across the world (Govindasamy et al. 2014; Qin et al. 2015; Franco et al. 2016; Le et al. 2016; Passari et al. 2016). Endophytic actinobacteria are known to confer ecological advantages to the crop plants grown under abiotic stresses, even though the plant–microbe interactions under these adverse environmental conditions are still inadequately understood (Govindasamy et al. 2014; De Meyer et al. 2015; Zhao et al. 2016).
Cactus (Opuntia ficus-indica), commonly known as prickly pear, belongs to the plant family Cactaceae. It uses the Crassulacean acid metabolism (CAM) for photosynthesis. It is highly water-use efficient and adapted to arid and semiarid environments (Singh 2003; Mangalassery et al. 2017). Cacti develop an association with niche soil microbes which could also potentially contribute to overcome these stress conditions (Fonseca-García et al. 2016). Endophytes of cacti are comparatively less explored and actinobacterial inhabitants of cactus endosphere in particular are yet to be characterized for their PGP activities. Hence, the present study was planned to isolate root-endophytic actinobacteria from the cactus plants using different enrichment media combinations and to characterize them for various PGP traits.
Materials and methods
Collection of Cactus-root samples and isolation of endophytic actinobacteria
Two genetic accessions (Acc. No. 1280 and 1287) of cactus (Opuntia ficus-indica) were explored for the isolation of root-endophytic actinobacteria. These accessions were obtained from the cactus germplasm collection of ICAR-Central Soil Salinity Research Institute, Karnal, India. Cactus Acc. No. 1280 is a thorn-less type bearing yellow fruits and Cactus Acc. No. 1287 is a thorny type with pink fruits. Root samples were collected from a depth of 15–30 cm of the cactus plants which were grown in murrum soil of the research farm at ICAR-National Institute of Abiotic Stress Management, Baramati, Maharashtra, India. Collected roots were washed in running tap water for 5 min to remove the soil debris. The air dried root samples were then surface sterilized following standard procedures as stated below: 1 min. initial wash in 90% ethanol; 4–5 min. in 4% (v/v) NaOCl; 30 s in 90% ethanol; Samples were washed twice in the sterile water followed by 5 min. wash in 5% Na2S2O3 and the final rinse in sterile water for 5 times. The surface sterilization procedure was further validated by examining the final washed solution for no bacterial growth on the tryptone soy agar (TSA) medium (Govindasamy et al. 2017). The surface sterilized roots were air dried and cut it in to small fragments (0.5–1 cm) under aseptic conditions.
The dry root bits from the cactus plants were separately placed on Petri plates containing 5 different actinobacterial-specific isolation media, namely, Humic acid vitamin-B (HV) agar (Hayakawa and Nonomura 1987), Tap water yeast extract (TWYE) agar (Crawford et al. 1993), Mannitol soya (MS) agar, VL-70agar (Joseph et al. 2003) and VL-70 + Cactus extract (VLCE) agar (reported first in this study). Briefly, cactus extract was prepared by grinding the fresh cactus roots followed by filtration of the extract through a muslin cloth. Ten mL of the filter sterilized cactus extract was added in to 1L of sterile VL-70 agar and used as VL-70 + Cactus extract (VLCE) agar medium. The chemical composition of VLCE agar medium developed in this study is provided in Supplementary Table 1. Each sterile medium was supplemented with benomyl (50 mg L−1) to inhibit the fungal growth. The wax/parafilm-sealed plates were incubated for 3 months at 28 °C and 37 °C in closed plastic boxes. Plates were observed regularly for actinobacterial colonies and the emerging colonies were regularly picked and purified on the half strength potato dextrose agar (HPDA) plates (Franco et al. 2016). Morphological features/cultural characteristics of these isolates were documented.
Screening of endophytic actinobacterial isolates for plant growth promotion traits
Qualitative determination of PGP traits
All the isolates were screened for PGP traits, such as N-fixation, phosphate solubilization (Pikovskaya 1948) and siderophore production (Schwyn and Neilands 1987). N-fixation was determined by streak inoculation of individual cultures on N-free medium (Jensen’s N-free medium, HiMedia, India) and incubation at 28 °C for 5–6 days (Qin et al. 2015). The presence of mucoid and slimy growth of actinobacterial isolates on N-free culture plates was considered as putative N-fixers and the cultures were further subjected to confirmatory analysis through acetylene reduction activity. Solubilization of phosphate was determined by spot inoculation of the actinobacterial isolates on Pikovskaya’s agar (HiMedia) followed by incubation at 28 °C for 6 days. Actinobacterial isolates exhibiting clear zones were considered to possess P-solubilization trait (Pikovskaya 1948). Bacterial isolates were assayed for their ability to produce siderophores on Chrome Azurol S (CAS) agar medium (Schwyn and Neilands 1987) following spot inoculation of individual actinobacterial isolates and incubation at 28 °C for 6 days. Development of a yellow–orange halo zone around the bacterial growth was construed as a potential for siderophore production.
Quantitative estimation of nitrogenase activity by acetylene reduction assay
All the endophytic actinobacterial isolates showing growth on the N-free Jensen medium were streaked onto N-free Jensen medium slants in glass tubes and were incubated at 28 °C for 7 days. In the total headspace, 10 percent volume was exchanged with an equal amount of acetylene and were sealed with stoppers, the culture tubes were further incubated for 24 h. Reduction of acetylene to ethylene by the nitrogenase enzyme was measured with a gas chromatograph (Agilent Technologies 7890A) using a flame ionization detector. Non-streaked slants injected with acetylene served as a negative control, and Azotobacter chroococcum isolate (Ac-EPS-1) was used as positive control. The experiment was conducted twice and each time in triplicates (Hardy et al 1968; Dahal et al. 2017).
Quantitative estimation of auxin production
Auxin production was quantitatively determined following the method suggested by Gordon and Weber (1951) and Bric et al. (1991). Actinobacterial isolates were grown in International Streptomyces Project-2 (ISP-2) medium (Le et al. 2016) supplemented with L-tryptophan (100 mg mL−1)-a precursor/inducer of IAA (auxin) synthesis. Cultures grown for 5 days were centrifuged at 8000 rpm at room temperature (25 °C) for 10 min and the supernatant obtained was mixed with Salkowski’s reagent (50 mL 35% of perchloric acid, 1 mL 0.5 M FeCl3 solution) in the ratio of 2:1 and kept in dark for 30 min. The pink colour developed was measured at 530 nm using spectrophotometer (Shimadzu, Japan). The concentration of auxins produced by the individual bacterial isolates was determined from a standard curve prepared using known concentrations of IAA (Hi-media, India).
Quantitative estimation of 1-aminocyclopropane-1-carboxylic acid (ACC) consumption
ACC deaminase activity of actinobacteria was indirectly estimated by measuring the consumption of ACC-provided as a sole N-source in the medium (Li et al. 2011). Briefly, actinobacteria were inoculated in ISP-2 broth and incubated in a refrigerated incubator shaker (180 rpm) at 28 °C for 5 days. The fully grown cultures were centrifuged at 8000 rpm at room temperature for 10 min and actinobacterial cell pellets were washed thrice with sterile DF medium. Cell pellets were re-suspended in DF medium supplemented with ACC (3 mmol L−1) and incubated at 30 °C in incubator shaker at 200 rpm for 48 h. From each of these cultures, 1 mL of culture fluid was centrifuged at 8000 rpm at room temperature (25 °C) for 10 min and 100 µL of supernatant was diluted to 1 mL with DF medium. To this, 2 mL of ninhydrin reagent was mixed in the test tubes and kept in boiling water bath for 15 min. The tubes were cooled to room temperature for 10 min, and absorbance was measured spectrophotometrically at 570 nm. Leftover ACC in the bacterial grown DF liquid medium was quantitatively estimated by developing a standard curve for ACC (Sigma-Aldrich, USA). The amount of ACC consumption (mmol L−1) by the individual actinobacterial isolates was calculated from the initial ACC concentration (3.0 mmol L−1) of DF medium.
16S rRNA gene sequencing and phylogenetic analysis
Genomic DNA was extracted from the selected isolates following the standard methods (Charles and Nester 1993; Sambrook and Russell 2001) with slight modifications (Coombs and Franco 2003). Quantity and purity of isolated genomic DNA was ascertained by gel electrophoresis. The 16S rRNA genes from the genomic DNA of the actinobacterial isolates were PCR amplified. The universal bacterial primers 8F (5ʹ-AGAGTTTGATCCTTGGCTCAG-3ʹ) and 1492R (5ʹ-GGTTACCTTGTTACGACTT-3ʹ) were used for the amplification of 16S rRNA genes (Lane 1991). The resulting PCR products were analyzed by performing electrophoresis in 1.2% agarose gel followed by observation in a UV trans-illuminator. The PCR products were sequenced at Sci-Genome Pvt. Ltd. Kochin, India. The Seq-Man software version 4.1 (DNASTAR.) was used to compile the 16S rRNA gene sequences and individual isolates were identified based on a BLAST search. The 16S rRNA gene sequences were submitted to NCBI GenBank repository and the accession numbers were assigned. For the phylogenetic analysis, 16S rRNA gene sequences derived from the type strains of Streptomyces spp were obtained from the List of Prokaryotic names with Standing in Nomenclature (LPSN) database (https://lpsn.dsmz.de/genus/streptomyces) at DSMZ (Parte et al., 2020). The phylogenetic tree was generated using Nocardia casuarinae BMG51109a (KF924767)-an endophytic, actinobacteria characterized from root nodules of Casuarina as an out group. In addition, the type species of Streptomyces genera S. albus sub sp. albus strain NBRC 13014 was included in the molecular evolutionary tree analysis. The sequences were aligned in ClustalW using CLC Genomics Workbench 20.0 software (https://digitalinsights.qiagen.com) and a Maximum-Likelihood (ML) phylogenetic tree with a bootstrap value of 1000 replicates was generated.
PCR-based detection of nifH gene
The genomic DNA isolated from the actinobacterial isolates was used as a template to ascertain the amplification of nifH gene using the primers IGK3/DVV and PCR conditions as enumerated in Ando et al. (2005) as well as by Gaby and Buckley (2012).
Wheat seedling growth assay
Wheat seeds (cultivar—Nethravati) obtained from Wheat Crop Improvement Project, Mahatma Pule Krishi Vidyapeeth, Rahuri, Maharashtra, India were used for the seedling vigour assays. Wheat seeds were surface sterilized by soaking in ethanol (70%) for 30 s followed by 2–3 min in 4% (v/v) NaOCl and eventually performing multiple washes of seeds in the sterile water. Selected endophytic actinobacterial spores were collected by growing respective cultures on mannitol soy agar (MS agar) for 5–7 days following the method of spore preparation suggested by Conn and Franco (2004). Briefly, when the sporulation was adequate, the spores of respective culture were harvested from MS agar medium and suspended in 0.3% sterilized Xanthan gum which was used for inoculating plants or treating of wheat seeds. Surface sterilized seeds were immersed in respective actinobacterial spore suspension (~ 108 cells mL−1) for 4 h. The spore coated seeds were air dried and transferred in to Petri plates containing two sheets of sterile filer papers moistened with 10 mL of sterile distilled water. Seeds added with sterile water served as the control and all the Petri plates were incubated in a plant growth chamber (25 °C, 60% RH). After 10 days of incubation without external supply of water, number of rootlets, root length, shoot length and total seedling length were measured.
Statistical analysis
Statistical analysis was carried out using the SPSS statistical software package version 16.0 (IBM SPSS, USA). Data regarding plant growth measurements on wheat seedlings were analyzed by performing analysis of variance (ANOVA) and the treatment means were subjected to the least significant difference (LSD) followed by Duncan’s Multiple-Range Test (DMRT) post-hoc analysis. All the hypotheses were tested at the 95% confidence interval (α = 0.05).
Results
Isolation of endophytic actinobacteria
Endophytic actinobacterial colonies growing around the root bits placed in actinobacterial isolation media were carefully transferred to purification medium (Supplementary Fig. 1). A total of 179 phenotypically distinct endophytic actinobacterial isolates were purified on HPDA medium. The number of isolates obtained from each of the actinobacterial isolation medium (incubated at two different temperatures) is given in Table 1. HV agar medium was found to be more effective in yielding morphologically diverse endophytic actinobacteria (62 nos.) from both the cactus accessions. Incubation temperature also affected the number of actinobacterial isolates obtained from the specific isolation media as well from the two different accessions of cactus. Results showed that 122 isolates (68%) (71 and 51 isolates from the cactus accessions 1280 and 1287, respectively) were purified from different isolation media while incubating at 37 °C. The selected endophytic actinobacterial isolates purified from the surface sterilized roots of cactus accessions exhibiting different colony morphology on HPDA medium are presented in Supplementary Fig. 1. The endophytic actinobacterial isolates were named according to the media and cactus accessions that yielded them. Isolates named after isolation medium followed by Roman numerals indicate their origin from the cactus accession 1280, while isolates appended with Arabic numerals indicate their origin from the cactus accession 1287.
Screening of endophytic actinobacteria for PGP traits
Most of the isolates of endophytic actinobacteria showed at least one of the PGP characters studied; however, 26 isolates did not show any of the PGP traits tested. Of the endophytic actinobacterial isolates from the cactus accessions 1280 and 1287, 73% and 92% of them exhibited the putative N-fixing ability based on their growth in the N-free Jensen medium, respectively (Supplementary Fig. 3). Whereas, 68% and 65% of the endophytic actinobacterial isolates from the cactus accession 1287 showed siderophore (Supplementary Fig. 4) and auxin production ability, respectively. All the PGP traits, except ACC deaminases activity, was found high in the endophytic actinobacterial isolates purified from the Cactus accession 1287 compared to the isolates derived from the accession 1280 (Fig. 1).
Experiment to quantify putative nitrogenase activity of the selected root-endophytic actinobacterial isolates by performing the acetylene reduction assay did not confirm nitrogen fixation trait, since no detectable nitrogenase activity was observed except A. chroococcum isolate (Ac-EPS-1), which served as a positive control (1032.28 nmol C2H5. h−1 mg protein−1) (Supplementary Fig. 5). In addition, none of selected actinobacterial DNA samples yielded a PCR amplification specific for nifH gene with the IGK3/DVV primers (Supplementary Fig. 6). The non-specific PCR bands amplified in some of the endophytic actinobacterial isolates were gel eluted and sequenced, but does not show any sequence similarity with nifH gene (data not shown). The auxin production capability of the isolates in the presence of precursor L-tryptophan varied from 10 to 200 µg/mL of the ISP-2 broth. The maximum auxin production was exhibited by the isolate VL-70-PIII (200.82 µg/mL) followed by the isolate HV-18 (170.80 µg/mL) (Fig. 2a). These two isolates were obtained from the roots of cactus accessions 1280 and 1287 using VL-70 + Cactus extract agar (VLCEA) and Humic acid Vitamin-B agar (HVA) medium, respectively. Quantitative measurement of auxin production by the selected root-endophytic actinobacterial isolates having multi-PGP traits is shown in Fig. 3a. Endophytic actinobacterial isolates from the cactus accession 1280 showed a relatively high ACC consumption, than the isolates obtained from the accession 1287. ACC consumption ranged from 0.018 to 2.3 mmol L−1. The endophytic actinobacterial isolate HV-VIII exhibited a maximum consumption of 2.3 m mol L−1 of ACC after 48 h of incubation (Fig. 2b) which was followed by the isolate VL-70-PIII (1.754 mmol L−1). These two isolates originated from the roots of cactus accession 1280 in HVA and VLCEA medium, respectively. Quantitative measurement of ACC consumption by the selected root-endophytic actinobacterial isolates having multi-PGP traits is shown in Fig. 2b.
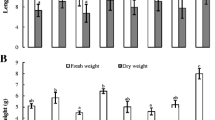
Quantitative estimation of auxin production (a) and ACC consumption (b) traits of the selected root-endophytic actinobacteria of Cactus. Isolates named with Roman and Arabic numerals are obtained from the cactus accessions 1280 and 1287, respectively. Values are the mean of three replications ± standard error
Maximum-likelihood (ML) phylogenetic tree based on the 16S rRNA gene sequences of selected Cactus-root endophytic actinobacteria possessing multiple PGP traits and 16S rRNA gene sequences of type strains of Streptomyces spp.. Nocardia casuarinae BMG51109 (KF924767) was used as an outgroup taxon. Cactus-root endophytic actinobacteria reported in this work are highlighted with arrows. The numbers at the nodes indicate the percentage of bootstrap support, based on the analysis of 1000 replicated data sets. Bootstrap values indicated next to the branches. The actinobacterial identity was performed based on BLAST analysis of 16S rRNA gene sequences of the isolates reported herein against the sequences derived from the type strains and hence the use of species name is tentative
Identification of endophytic actinobacteria and their phylogenetic analysis
Based on the above PGPR properties, ten superior endophytic actinobacterial isolates, having multiple PGP traits among others, were selected for further studies (Table 2). Morphological features of the selected root-endophytic actinobacteria in the purification medium are given in Table 2. These selected endophytic actinobacterial isolates were identified using 16S rRNA gene sequence and sequence-based phylogenetic analysis. BLAST analysis of sequences revealed that all the ten isolates belong to different species of the genus Streptomyces. The closest molecular identity and 16S rRNA gene sequence features of each endophytic actinobacteria are given in Table 3. The phylogenetic tree of the endophytic actinobacterial isolates was rooted to Nocardia sp. strain K78 (MT422810) as an out-group taxon and evolutionary history was inferred based on the Maximum-Likelihood (ML) method (Fig. 3). The molecular phylogeny revealed that all the ten isolates reported in this study formed constituents of distinct clades of Streptomyces spp. quite away from the actinobacterial genera Nocardia casuarinae (Fig. 3). Molecular evolutionary lineage analysis discloses that the isolate Streptomyces mutabilis isolate HV-I reported in this study exhibited closest phylogenetic relationship with other two isolates of Streptomyces mutabilis (HV-VIII and HVA-18) forming a distinct sub-clade I (of Clade I) suggesting their monophyletic origin. A distinct sub-clade II (Clade I) comprised Streptomyces tuirus VL-70-IX showing closest phylogenetic relationship with type strain Streptomyces tuirus NBRC 15617. The basal sub-clade III within the main clade I comprise Streptomyces levis VL-70-XII (KU885914.2) showing close genetic relationship with type strain Streptomyces levis NBRC 15423. Quite interestingly, the isolate S. rameus VL-70-PIII (KU885916.2) formed a distinct clade (Clade II) along with a type strain S. coelicolour NBRC 12854. However, the type strain S. rameus NBRC 3782 formed a component of Clade IV along with isolates of Streptomyces deccanensis HV-19 (KU885911.2) and the latter’s type strain DAS-139. The distinct clades viz., III and IV of the phylogenetic tree were formed of isolates Streptomyces canarius MS-10 (KU885912.2) and Streptomyces flavovariabilis VL-70-XIII (KU550047.2) along with their corresponding type strains, respectively. Sequence-based identity revealed that the isolate VL-70-XIII (KU550047.2) could be Streptomyces flavovariabilis (> 99.14%) which was further corroborated in the phylogeny as it clustered with type strain Streptomyces flavovariabilis NBRC 100764 with an appreciable boot strap value of 80% (Clade V). Similarly, the isolate HV-19 (KU885911.2) showed its genetic affinity with type strain Streptomyces deccanensis DAS-139 (EF219459) in both the BLAST and phylogenetic analysis (Clade IV). Analyzing the phylogenetic relationship among the endophytic actinobacterial isolates in the context of PGP traits disclose that non-P-solubilizers, namely, HV-19, VL-70-XIII, are genetically distinct (clades IV and V) from the poor P-solubilizer VL-70-IX forming a component of Clade I (Fig. 3).
Effect of endophytic actinobacteria on the growth of wheat seedlings
Significant differences were observed in the growth parameters such as root length and rootlet numbers of wheat seedlings following root inoculation of these endophytic actinobacteria compared to the un-inoculated control (Fig. 4a). The highest number of rootlets (6.33) was recorded in the seedlings coated with Streptomyces tuirus VL-70-IX. Root length was maximum (8.26 cm) in wheat seedlings inoculated with S. levis VL-70-XII followed by the inoculation with S. tuirus VL-70-IX (8.14 cm). The inoculation of wheat seeds with S. mutabilis HV-VIII resulted in maximum shoot length of 12.33 cm after 10 days of incubation (Fig. 4b) followed by the inoculation with S. tuirus VL-70-IX that increased the shoot length to 12.667 cm. The total seedling length was increased by 67% (20.53 cm) with the inoculation of S. tuirus VL-70-IX over the uninoculated control (12.27 cm). All the wheat seedlings treated with endophytic actinobacteria maintained green and healthy growth even after 10 days of incubation without the supply of external moisture compared to the control seedlings (Fig. 5).
Growth promoting effects of selected root-endophytic actinobacteria on wheat seedlings: a root and b length parameters. Isolates named with Roman and Arabic numerals originate from the cactus accessions 1280 and 1287, respectively. Values are the mean of three replications ± standard error. The bars in graph denoted by the same alphabet indicate non-significance at P ≥ 0.05 based on Duncan’s Multiple-Range Test (DMRT)
Discussion
Cactus (Opuntia ficus-indica) is one of the most drought tolerant plants growing in arid environments (Singh 2003; Mangalassery et al. 2017). Although cactus species are adapted to desert conditions, diverse endophytic bacterial groups are found to inhabit their roots (Fonseca-García et al. 2016; deCarvalho Costa and de Melo 2012). Prominent among them are actinobacteria belonging to Gram positive bacteria with high DNA G + C content exhibiting filamentous growth and formation of spores. Furthermore, members of the phylum Actinobacteria are the largest ecological resource for secondary metabolites (plant hormones, antibiotics and other bioactive compounds), with potential biotechnological applications in agriculture, industry and medicine (Govindasamy et al. 2014, 2018). Actinobacteria could withstand extreme desiccation conditions and hence they are ecologically significant in imparting abiotic stress tolerance among the crop plants (You et al. 2007; Vílchez et al. 2016. In this context, this is the first report on the isolation and characterization of endophytic actinobacteria from the roots of cactus (Opuntia ficus-indica) plants. Herein, 179 actinobacteria species were isolated from the surface sterilized roots of two cactus accessions using various growth media and incubation temperature combinations. Congruent with the findings of Zhao et al. (2009), humic acid–vitamin agar (HV agar) medium, having soil humic acid as a sole carbon and nitrogen source, supported the maximum number of endophytes (35% of the total isolates). This media was developed for the selective isolation of soil actinomycetes supporting the growth of largest number of actinobacteria such as Streptomyces, Micromonospora, Microbispora, Nocardia, etc. (Hayakawa and Nonomura 1987).
Actinobacteria have been reported to possess PGP traits in addition to their ability to produce other secondary metabolites. Further, their endophytic nature confers them with relative efficiency in promoting the plant growth and crop yield (Franco et al. 2007). Endophytic actinobacteria adapt a wide range of mechanisms including nutrient acquisition, phytohormone production, removal of contaminants, and direct suppression of pathogens via antibiosis or competition, and induction of plant defence responses to promote the plant growth. Biological N-fixation (BNF) is one of the most common plant beneficial mechanisms shown by endophytic PGPRs (Govindasamy et al. 2017; George et al. 2018) which ensure the supply of considerable quantum of N for the diverse agronomically important crops (Puri et al. 2018). A majority of the endophytic actinobacteria of cactus root origin (85% of the total) exhibited growth on an N-free Jenson medium. Similarly, N-fixing ability of culturable endophytic actinobacteria associated with Jatropha curcas L. grown in Panxi dry-hot valley soil based on its growth in N-free medium was reported by Qin et al. (2015). However, the results of acetylene reduction assay and non-amplification of nifH gene product confirmed their inability to fix atmospheric nitrogen (with no detectable nitrogenase activity) (Supplementary Figs. 5 and 6). N-fixing ability based on growth in N-free medium and nitrogenase activity based acetylene reduction assay of an endophytic Streptomyces chartreusis strain WZS021 isolated from the sugarcane shown enhanced the crop biomass (Wang et al. 2017). Nonetheless, N-fixation ability of various other endophytic actinobacteria such as Arthrobacter, Mycobacterium, Propionibacteria and many other genera isolated from the root nodules of leguminous and actinorhizal plants are reported (Gtari et al. 2012; Sellstedt and Richau 2013). Streptomyces improve the plant growth promotion either by improving the nutrition acquisition or production of phytohormones or through the suppression of plant diseases (Amaresan et al. 2018). Here, the cactus root-derived endophytic actinobacteria exhibited other PGP traits, such as siderophore production (59% of the total), phytohormone (auxin) production (54% of the total), P-solubilization (53% of the total) and ACC deaminase activity (28% of the total). The ability to produce siderophores, the second most predominant plant growth promotional trait, by the endophytic actinobacteria could have helped the cactus plants to extract various micronutrients such as Fe, Zn, and Cu (Dimkpa et al. 2008). It appears that the rhizospheric relationship with these siderophore producing endophytes bestows the cactus with the ability to grow on any micro-nutrient deficient environments (Dimkpa et al. 2008; Qin et al. 2015).
Another important PGP trait of the cactus-origin endophytic actinobacteria is the production of phytohormone, auxins. Indole 3-acetic acid (IAA) is one of the most physiologically important auxins, having pivotal functions in the lateral, adventitious root formation and in root elongation. Though this investigation detected only total auxin production capability of Cactus-derived endophytes, HPLC-based identification and quantification of IAA production ability of these endophytes is worth exploring in future studies. Rhizo-microbial auxin synthesis contributes to the enhanced total plant auxin pool thereby influencing the overall root growth and plant development (Idris et al. 2007). Similarly, root endophytes assist the plants in the uptake of soil mineral nutrients. In this study, almost half of the cactus root-actinobacterial endophytes exhibited in vitro P-solubilization activity. It suggests that the cactus accessions depend on these endophytes for their phosphorus requirement as the available P is very low in nutrient poor soils, such as murrum (Govindasamy et al. 2017). Exploration of ACC deaminase activity of the actinobacterial endophytes (Penrose and Glick 2003; Li et al. 2011) revealed that all the isolates exhibited ACC deaminase activity, which is considered a very potent PGP trait as it enhances the plant growth by overcoming the deleterious effects of ethylene-induced abiotic stress responses. ACC deaminase producing PGP rhizobacteria have been shown to mitigate the adverse effects of drought in plants suggesting the possibility of isolates reported herein to confer abiotic stress tolerance (Danish et al. 2020). In this study, the cactus accessions were grown in native murrum soil characterized with relatively low nutrient content (Govindasamy et al. 2017) and devoid of external supply of nutrients in the form of fertilizers. Consequently, it is rational to assume that these adverse plant growth conditions caused the cactus plants to accommodate/recruit as many PGP endophytic microbes as possible in its exo- and endo-rhizosphere which helps to promote its growth in this nutrient deficient soil.
The 16S rRNA gene sequence-based identification of endophytic actinobacterial isolates revealed the predominance of the genus Streptomyces. Similar preponderance of Streptomyces spp. among the actinobacterial endophytes in many other crop plants ecosystem was also reported (Franco et al. 2007; Qin et al. 2015; Zhao et al. 2016; Le et al. 2016). Phylogenetic studies of the selected isolates based on 16S rRNA gene sequences also reiterated that they belonged to the genus Streptomyces. In the phylogenetic tree, the endophytic actinobacterial isolates formed distinct clades. In addition, the correlation of phylogenetic relationship among the isolates with their multi-PGP traits divulged similar characters among the isolates of monophyletic origin with a notable exception of S. tuirus strain VL-70-IX. However, given the little analysis of 16S rRNA gene sequences employed in this study, the evolutionary or phylogenetic lineage of endophytic actinobacterial isolates requires further corroboration by performing multiple locus sequences analysis (MLSA) with many other conserved marker genes among the groups of actinobacteria (Govindasamy et al. 2014; Qin et al. 2015). Furthermore, the drawback of 16S rRNA gene sequences in separating the prokaryotes at finer taxonomic levels suggests the utilization of additional nearly universal marker genes in resolving the phylogeny of closely related species or strains of same species (Kitahara and Miyazaki 2013; Lan et al. 2016). Quite interestingly, all of these selected endophytic actinobacterial isolates reported herein were identified as Streptomyces sp. devoid of actual N-fixation ability. Nevertheless, the observed growth of some of these isolates on N-free agar medium could be attributed to the ability of these to utilize the traces of combined nitrogen from agar medium and also scavenge residual ammonia from the atmosphere (Yoshida et al. 2014). In this context, BNF capability of free-living Streptomyces was supported by nifH gene product amplification and through radio-isotope studies (Dahal et al. 2017). Nevertheless, there are no recent reports of Streptomyces sp. exhibiting nitrogen fixing ability including S. thermoautotrophicus (MacKellar et al. 2016).
Although endophytic actinobacteria are ubiquitous, their utilization as biofertilizer or PGPR is rather restricted. Hence, PGP traits and their effect/potential on the promotion of wheat seedling growth were evaluated. All the ten selected endophytic actinobacterial isolates significantly improved the seedling growth parameters over the uninoculated control. The isolates Streptomyces tuirus VL-70-IX and S. levis VL-70-XII significantly improved the root number (73% over control) and length (77% over control) of wheat seedlings upon 10 days of incubation without the supply of external moisture/water. The inoculation of wheat seeds with S. mutabilis HV-VIII resulted in 61% increase in shoot length over the uninoculated control. These isolates also possess multiple PGP traits such as siderophore production, N-fixation, auxin production and ACC deaminase activity significantly contributing to the improved growth of wheat seedlings. Similarly, harnessing of PGP effects of Streptomyces spp. isolated from the different plants species were reported (Gopalakrishnan et al. 2015; Toumatia et al. 2016; Qin et al. 2015, 2017). These endophytic actinobacteria reported herein were found to be promising and further investigations are required to explore their secondary metabolites production potential which influences the biotic and abiotic stress tolerance in the crop plants. However, this work provides the basis for characterization and selection of potential endophytic actinobacteria from the under-exploited, drought tolerant species such as cactus. Furthermore, it would add to the current state of knowledge regarding the development of an endophytic actinobacterial consortium from cactus plants with potential cross-compatibility for the improvement of plant growth of field crops especially under abiotic stress conditions.
References
Amaresan N, Kumar K, Naik JH, Bapatla KG, Mishra RK (2018) Streptomyces in plant growth promotion: mechanisms and role. New and future developments in microbial biotechnology and bioengineering. Elsevier publications, pp 125–135
Ando S, Goto M, Meunchang S, Thongra-ar P, Fujiwara T, Hayashi H, Yoneyama T (2005) Detection of nifH sequences in sugarcane (Saccharum officinarum L.) and pineapple (Ananas comosus [L.] Merr.) Soil Sci. Plant Nutr 51:303–308
Bric JM, Bostock RM, Silverstone SE (1991) Rapid in situ assay for indole acetic acid production by bacteria immobilized on a nitrocellulose membrane. Appl Environ Microbiol 57(2):535–538
Charles TC, Nester EW (1993) A chromosomally encoded two-component sensory transduction system is required for virulence of Agrobacterium tumefaciens. J Bacteriol 175(20):6614–6625
Compant S, Clément C, Sessitsch A (2010) Plant growth-promoting bacteria in the rhizo-and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol Biochem 42:669–678
Conn VM, Franco CM (2004) Effect of microbial inoculants on the indigenous actinobacterial endophyte population in the roots of wheat as determined by terminal restriction fragment length polymorphism. Appl Environ Microbiol 70(11):6407–6413
Coombs JT, Franco CM (2003) Isolation and identification of actinobacteria from surface-sterilized wheat roots. Appl Environ Microbiol 69(9):5603–5608
Crawford DL, Lynch JM, Whipps JM, Ousley MA (1993) Isolation and characterization of actinomycete antagonists of a fungal root pathogen. Appl Environ Microbiol 59:3899–3905
Dahal B, NandaKafle G, Perkins L, Brözel VS (2017) Diversity of free-Living nitrogen fixing Streptomyces in soils of the badlands of South Dakota. Microbiol Rep 195:31–39
Danish S, Zafar-ul-Hye M, Mohsin F, Hussain M (2020) ACC-deaminase producing plant growth promoting rhizobacteria and biochar mitigate adverse effects of drought stress on maize growth. PLoS One 15(4):e0230615
De Meyer SE, De Beuf K, Vekeman B, Willems A (2015) A large diversity of non-rhizobial endophytes found in legume root nodules in Flanders (Belgium). Soil Biol Biochem 83:1–11
deCarvalho Costa FE, de Melo IS (2012) Endophytic and rhizospheric bacteria from Opuntia ficus-indica mill and their ability to promote plant growth in cowpea, Vigna unguiculata (L.) Walp. Afr J Microbiol Res 6(6):1345–1353
Dimkpa CO, Svatos A, Dabrowska P, Schmidt A, Boland W, Kothe E (2008) Involvement of siderophores in the reduction of metal-induced inhibition of auxin synthesis in Streptomyces spp. Chemosphere 74(1):19–25
Fonseca-García C, Coleman-Derr D, Garrido E, Visel A, Tringe SG, Partida-Martínez LP (2016) The cacti microbiome: interplay between habitat-filtering and host-specificity. Front Microbiol 7:150
Franco C, Michelsen P, Percy N, Conn V, Listiana E, Moll S, Loria R, Coombs J (2007) Actinobacterial endophytes for improved crop performance. Austral Plant Pathol 36(6):524–531
Franco CM, Araujo R, Adetutu E, Tobe SS, Mallya S, Paul B, Satyamoorthy K (2016) Complete genome sequences of the endophytic Streptomyces strains EN16, EN23, and EN27, isolated from wheat plants. Genome Announce 4(6):e01342-e1416
Gaby JC, Buckley DH (2012) A comprehensive evaluation of pcr primers to amplify the nifH gene of nitrogenase. PLoS One 7(7):e42149
George P, Gupta A, Gopal M, Thomas L, Thomas GV (2018) Systematic screening strategies for identifying elite plant growth promoting rhizobacteria for coconut (Cocos nucifera L.). Int J Curr Microbiol App Sci 7(5):1051–1074
Gopalakrishnan S, Srinivas V, Alekhya G, Prakash B, Kudapa H, Rathore A, Varshney RK (2015) The extent of grain yield and plant growth enhancement by plant growth-promoting broad-spectrum Streptomyces sp. in chickpea. Springerplus 4(1):31
Gordon SA, Weber RP (1951) Colorimetric estimation of indole acetic acid. Plant Physiol 26(1):192
Govindasamy V, Franco CM, Gupta VV (2014) Endophytic actinobacteria: diversity and ecology. In: Verma VC, Gange AC (eds) Advances in endophytic research. Springer, New Delhi, pp 27–59
Govindasamy V, Raina SK, George P, Kumar M, Rane J, Minhas PS, Vittal KPR (2017) Functional and phylogenetic diversity of cultivable rhizobacterial endophytes of sorghum [Sorghum bicolor (L.) Moench]. Antonie Van Leeuwenhoek 110:925–943
Govindasamy V, George P, Raina SK, Kumar M, Rane J, Annapurna K (2018) Plant-associated microbial interactions in the soil environment: role of endophytes in imparting abiotic stress tolerance to crops. In: Bal SK, Mukherjee J, Choudhury BU, Dhawan AK (eds) Advances in crop environment interaction. Springer, Singapore, pp 245–284
Gtari M, Ghodhbane-Gtari F, Nouioui I, Beauchemin N, Tisa LS (2012) Phylogenetic perspectives of nitrogen-fixing actinobacteria. Arch Microbiol 194:3–11
Hardy RW, Holsten RD, Jackson EK, Burns RC (1968) The acetylene-ethylene assay for N2 fixation: laboratory and field evaluation. Plant Physiol 43:1185–1207
Hayakawa MT, Nonomura H (1987) Humic acid-vitamin agar, a new method for the selective isolation of soil actinomycetes. J Ferment Bioeng 65:501–509
Hirsch PR, Mauchline TH (2012) Who’s who in the plant root microbiome? Nat Biotechnol 30:961–962
Idris EE, Iglesias DJ, Talon M, Borriss R (2007) Tryptophan dependent production of indole-3-acetic acid (IAA) affects level of plant growth promotion by Bacillus amyloliquefaciens FZB42. Mol Plant Microbe Inter 20(6):619–626
Joseph SJ, Hugenholtz P, Sangwan P, Osborne CA, Janssen PH (2003) Laboratory cultivation of widespread and previously uncultured soil bacteria. Appl Environ Microbiol 69:7210–7215
Kitahara K, Miyazaki K (2013) Revisiting bacterial phylogeny: natural and experimental evidence for horizontal gene transfer of 16S rRNA. Mob Genet Elem 3(1):e24210
Lan Y, Rosen G, Hershberg R (2016) Marker genes that are less conserved in their sequences are useful for predicting genome-wide similarity levels between closely related prokaryotic strains. Microbiome 4:18
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, Chichester, pp 115–176
Le XH, Franco CM, Ballard RA, Drew EA (2016) Isolation and characterization of endophytic actinobacteria and their effect on the early growth and nodulation of lucerne (Medicago sativa L.). Plant Soil 405(1–2):13–24
Leite J, Fischer D, Rouws LF, Fernandes-Júnior PI, Hofmann A, Kublik S, Schloter M, Xavier GR, Radl V (2017) Cowpea nodules harbor non-rhizobial bacterial communities that are shaped by soil type rather than plant genotype. Front Plant Sci 7:2064
Li Z, Chang S, Lin L, Li Y, An Q (2011) A colorimetric assay of 1-aminocyclopropane-1-carboxylate (ACC) based on ninhydrin reaction for rapid screening of bacteria containing ACC deaminase. Lett Appl Microbiol 53(2):178–185
MacKellar D, Lieber L, Norman JS, Bolger A, Tobin C, Murray JW, Oksaksin M, Chang RL, Ford TJ, Nguyen PQ, Woodward J, Permingeat HR, Joshi NS, Silver PA, Usadel B, Rutherford AW, Friesen ML, Prell J (2016) Streptomyces thermoautotrophicus does not fix nitrogen. Sci Rep 6:20086
Mangalassery S, Dayal D, Kumar A, Dev R (2017) Evaluation of cactus pear (Opuntia ficus-indica) accessions for various growth characteristics under arid region of north western India. Range Manag Agrofor 38(2):280–284
Parte AC, Sardà Carbasse J, Meier-Kolthoff JP, Reimer LC, Göker M (2020) List of Prokaryotic names with Standing in Nomenclature (LPSN) moves to the DSMZ. Int J Syst Evol Microbiol 70:5607–5612
Passari AK, Chandra P, Mishra VK, Leo VV, Gupta VK, Kumar B, Singh BP (2016) Detection of biosynthetic gene and phytohormone production by endophytic actinobacteria associated with Solanum lycopersicum and their plant-growth-promoting effect. Res Microbiol 167(8):692–705
Penrose DM, Glick BR (2003) Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol Plant 118(1):10–15
Pikovskaya RI (1948) Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya 17:362–370
Puri A, Padda KP, Chanway CP (2018) Nitrogen-Fixation by endophytic bacteria in agricultural crops: recent advances. In: Khan A, Fahad S (eds) Nitrogen in agriculture-updates. InTech, Rijeka, Croatia, pp 73–94
Qin S, Miao Q, Feng WW, Wang Y, Zhu X, Xing K, Jiang JH (2015) Biodiversity and plant growth promoting traits of culturable endophytic actinobacteria associated with Jatropha curcas L. growing in Panxi dry-hot valley soil. Appl Soil Ecol 93:47–55
Qin S, Feng WW, Wang TT, Ding P, Xing K, Jiang JH (2017) Plant growth-promoting effect and genomic analysis of the beneficial endophyte Streptomyces sp KLBMP 5084 isolated from halophyte Limonium sinense. Plant Soil 416(1–2):117–132
Reinhold-Hurek B, Hurek T (2011) Living inside plants: bacterial endophytes. Curr Opin Plant Biol 14:435–443
Rosenblueth M, Martínez-Romero E (2006) Bacterial endophytes and their interactions with hosts. Mol Plant Microbe Interact 19:827–837
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160(1):47–56
Sellstedt A, Richau KH (2013) Aspects of nitrogen-fixing Actinobacteria, in particular free-living and symbiotic Frankia. FEMS Microbiol Lett 342:179–186
Singh G (2003) General review of Opuntias in India. J Prof Assoc Cactus 1:30–46
Stackebrandt SP (2000) The prokaryotes: an evolving electronic resource for the microbiological community. Springer-Verlag, New York, NY
Toumatia O, Compant S, Yekkour A, Goudjal Y, Sabaou N, Mathieu F, Sessitsch A, Zitouni A (2016) Biocontrol and plant growth promoting properties of Streptomyces mutabilis strain IA1 isolated from a Saharan soil on wheat seedlings and visualization of its niches of colonization. S Afr J Bot 105:234–239
Vílchez JI, García-Fontana C, Román-Naranjo D, González-López J, Manzanera M (2016) Plant drought tolerance enhancement by trehalose production of desiccation-tolerant microorganisms. Front Microbiol 7:1577
Wang Z, Solanki MK, Pang F, Singh RK, Yang LT, Li YR, Li HB, Zhu K, Xing YX (2017) Identification and efficiency of a nitrogen-fixing endophytic actinobacterial strain from sugarcane. Sugar Tech 19(5):492–500
Yoshida N, Inaba S, Takagi H (2014) Utilization of atmospheric ammonia by an extremely oligotrophic bacterium, Rhodococcus erythropolis N9T–4. J Biosci Bioeng 117:28–32
You J, Xue X, Cao L, Lu X, Wang J, Zhang L, Zhou S (2007) Inhibition of Vibrio biofilm formation by a marine actinomycete strain A66. Appl Microbiol Biotechnol 76(5):1137–1144
Zhao XQ, Jiao WC, Jiang B, Yuan WJ, Yang TH, Hao S (2009) Screening and identification of actinobacteria from marine sediments: investigation of potential producers for antimicrobial agents and type I polyketides. World J Microbiol Biotechnol 25(5):859–866
Zhao K, Zhao C, Liao P, Zhang Q, Li Y, Liu M, Ao X, Gu Y, Liao D, Xu K, Yu X (2016) Isolation and antimicrobial activities of actinobacteria closely associated with liquorice plants Glycyrrhiza glabra L. and Glycyrrhiza inflate BAT. in Xinjiang, China. Microbiology 162(7):1135–1146
Acknowledgements
The authors express gratitude to ICAR-Central Soil Salinity Research Institute, Karnal, India for providing Cactus accessions. The authors are grateful to the Director, ICAR-National Institute of Abiotic Stress Management, Baramati, Maharashtra, India for the laboratory facilities and the Head, Division of Microbiology, ICAR-Indian Agricultural Research Institute, New Delhi, India for CLC Genomics analysis facility. The authors are also thankful to the Indian Council of Agricultural Research, New Delhi.
Funding
Indian Council of Agricultural Research (ICAR-NIASM Project Ref. No.: IXX08578).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have any conflict of interest.
Consent for publication
All authors have read and agreed on publication of this manuscript.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Govindasamy, V., George, P., Ramesh, S.V. et al. Characterization of root-endophytic actinobacteria from cactus (Opuntia ficus-indica) for plant growth promoting traits. Arch Microbiol 204, 150 (2022). https://doi.org/10.1007/s00203-021-02671-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-021-02671-2