Abstract
Growth performance, chromium (Cr) accumulation potential and induction of antioxidative defence system and phytochelatins (PCs) were studied in hydroponically grown Brassica juncea (Indian mustard) and Vigna radiata (mungbean) at various levels of Cr treatments (0, 50, 100, 200 μM Cr). B. juncea accumulated twofolds and threefolds higher Cr in root and shoot, respectively than in V. radiata. Compared to B. juncea, V. radiata was found to be particularly sensitive to Cr as observed by the severity and development of Cr toxicity symptoms and decreased growth. Induction of PC and enzymes of antioxidant defence system were monitored as plant’s primary and secondary metal detoxifying responses, respectively. There was induction of PC and enzymes of antioxidant defence system in both the plants. PCs were induced significantly in roots and shoot of both the plants at all the levels of Cr treatments. Significantly higher activities of superoxide dismutase (SOD), ascorbate peroxidase (APX), catalase (CAT) and glutathione reductase (GR) were observed in shoot of B. juncea than V. radiata at all the levels of Cr treatments. Induction of PCs along with antioxidant defence system in response to Cr stress suggests the cumulative role of PCs and antioxidants in conferring tolerance against accumulated Cr in B. juncea, and thereby signifies the suitability of this plant as one of the potential remediators of Cr.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pollution of the biosphere with heavy metal has accelerated dramatically during the last century due to mining, smelting, amendment of agricultural soils with municipal sewage sludge, and waste disposal practices. Chromium (Cr) is one of several heavy metals (HM) that causes serious environmental contamination in soil, sediments, and groundwater (Bartlett 1991; Witmer et al. 1991; Diwan et al. 2010). Wastes coming from Cr-related industries such as electroplating, leather tanning, metal finishing, corrosion control and pigment manufacturing industries (Sklar 1980; Pawlisz et al. 1997; Shukla et al. 2007) have severely contaminated sites around the world. Among the various forms of chromium, Cr(VI) is considered as highly toxic and carcinogenic, producing cardiovascular shock affecting kidney, liver and blood-forming organs (Shanker et al. 2005, 2009). It is a very toxic, powerful epithelial irritant and an established human carcinogen by the Environmental Protection Agency (1984) and the World Health Organization (1988). One potential strategy to efficiently remove HMs from contaminated sites is the use of plants with potential of heavy metal accumulation and tolerance capacity (Salt et al. 1995). This strategy, called phytoremediation, has several advantages over physical remediation methods in costs, practice and the scale at which the processes operate (Salt et al. 1995; Khan et al. 2009). Suitable plants for phytoremediation purpose are identified on the basis of biochemical mechanisms related to heavy metal tolerance and accumulation. Important biological mechanisms of HM detoxification involves the induction of metal-binding ligand and antioxidative defence mechanisms.
Phytochelatins (PCs) are a family of peptides with the general structure (γ-Glu-Cys)n-Gly (n = 2–11). PCs are synthesized enzymatically from glutathione by the constitutive enzyme PC synthase, which requires posttranslational activation (Grill et al. 1989; Zenk 1996; Cobbett 2000; Schat et al. 2002). Heavy metals do not bind directly to the enzyme to activate PC biosynthesis, but instead act as substrate ligands for a bisubstrate-substituted enzyme transpeptidation reaction in which free glutathione and its corresponding heavy metal thiolate are cosubstrates (Vatamaniuk et al. 2001). PCs are rapidly induced in a wide range of plant species by heavy metal ions, such as As5+, Cd2+, Cu2+, Ag+, Hg2+, and Pb2+ (Zenk 1996; Rauser 1999; Cobbett 2000; Schmoger et al. 2000; Hall 2002; Raab et al. 2004). Induction of phytochelatin in response to Cr exposure has not been reported so far in any plant species.
Heavy metals induce oxidative stress by generating free radicals and toxic reactive oxygen species (ROS) (Sanita-di-toppi et al. 2002; Arvind and Prasad 2003). ROS are partially reduced forms of atmospheric oxygen and under normal conditions their production in cells is low and tightly controlled (Dat et al. 2000). Heavy metal toxicity enhances the production of ROS up to 30-fold (Mittler 2002). These species react with lipids, proteins, pigments and nucleic acids and cause lipid peroxidation, membrane damage and inactivation of enzymes, thus affecting the cell viability. The deleterious effects resulting from the cellular oxidative state may be alleviated by the enzymatic and non-enzymatic antioxidant machinery of the plant (Halliwell 1987). The antioxidant protection in plant cells is complex and highly compartmentalized. Superoxide dismutases (SODs) are a family of metalloenzymes catalyzing the dismutation of O2 − to H2O2 (Alscher et al. 2003). A fine regulation of the H2O2 level is achieved by the enzymes and metabolites of the ascorbate–glutathione cycle (Shigeoka et al. 2002). The balance between the activities of SOD and enzymes of ascorbate–glutathione cycle may be crucial for determining the steady-state level of O2 − and H2O2. This balance, together with the sequestration of metal ions, is considered to be important in preventing the formation of the ROS via the metal-dependent Haber–Weiss or Fenton reactions (Mittler 2002).
Over 200 terrestrial species are endemic to metalliferous soils and can tolerate and accumulate high amounts of heavy metals such as Cd, Zn, Cu, and Ni in their organs (Baker et al. 2000; Kumar et al. 1995; Hajiboland 2005). Species from the genus Brassica are good toxic metal accumulators, allocating large amounts of Cd, Pb, and Ni into the shoots (Salt et al. 1995, 1996; Jiang et al. 2000; Panwar et al. 2002; Ghosh and Singh 2005; Diwan et al. 2010). V. radiata (Mung bean) is one of the major grain legume crops in the poorer countries of Asia (Lawn and Ahn 1985). For their high nutritive value, they are important agricultural crops as a food source of proteins, calcium, phosphorus, and certain vitamins, and some cultivars possess excellent aroma (Gupta 1983; Simonová et al. 2007). On the other hand, the seedlings of V. radiata can possibly be used as accumulators of arsenic (Van den Broeck et al. 1998), lead (Rout et al. 2001; Singh et al. 2003), and chromium (Samantary 2002). Since, plants are known to respond differently in their heavy metal accumulation and tolerance levels, the present study was undertaken to assess the induction of phytochelatin and antioxidant defence system in response to Cr stress in B. juncea and V. radiata.
Materials and methods
Plant material
Seeds of Brassica juncea (L.) Czern. Coss. cv. Pusa Jai Kisan and Vigna radiata L. cv. Pusa Ratna were surface sterilized with 0.5% (w/v) mercuric chloride for 10 min. These seeds were sown in soilrite and kept for germination for 4 days. Seedlings were transferred to 2.5-l plastic pots with nutrient solution with the following composition: 2 mmol l−1 Ca(NO3)2·4H2O, 1 mmol l−1 MgSO4·7H2O, 0.9 mmol l−1 K2SO4, 0.2 mmol l−1 KH2PO4, 10−6 mol l−1 H3BO3, 2 × 10−7 mol l−1 MnSO4·H2O, 10−6 mol l−1 ZnSO4·7H2O, 2 × 10−7 mol l−1 CuSO4·5H2O, 2 × 10−8 mol l−1 (NH4)6Mo7O24·4H2O and 10−4 mol l−1 C10H12FeN2NaO8 and allowed to grow in growth chambers under controlled environmental conditions (20°C in the light and 18°C in the dark, 16 h light/8 h dark photoperiod with a photon flux density of 380 μmol m−2 s−1 and 60% humidity). After 7 days of growth, Cr in the form of K2Cr2O7 was supplied at concentrations of 50 (T1), 100 (T2) and 200 μM (T3). The concentration of these treatments was chosen because these are the environmentally relevant concentrations of Cr commonly found in polluted soils (Dheri et al. 2007). A control treatment contained only nutrient medium. The treated and control plants were analysed at 1 Day After Treatment (DAT), 3 DAT, 5 DAT and 7 DAT.
Measurement of growth and chromium accumulation
Fresh weight was recorded on an electronic top pan balance (Sartorius BL-210S, Germany) and expressed in g per plant. For dry weight determination, the plants were dried separately in a hot air oven at 65 ± 2°C for 72 h. Dried samples were weighed and expressed in g per plant. Plants were cut at the root-shoot junction and the root and shoot lengths were recorded. For Cr accumulation, chromium concentration of the dried root and the shoot samples was measured by atomic absorption spectrometer (Model ZEEnit 600/650, Analytik Jena, Germany) after wet digestion of samples with concentrated HNO3 at 300°C for 1 h.
Estimation of lipid peroxidation
The level of lipid peroxidation in the shoot was determined through estimation of malodealdehyde (MDA), a major 2-thiobarbaturic acid reactive substances (TBARS), by the method of Heath and Packer (1968). The MDA level was expressed in nmol g−1 fresh weight.
Assay of antioxidative enzymes
The shoot was ground with a mortar and pestle under chilled conditions in homogenization buffer (2 ml) containing phosphate buffer (0.1 M, pH 7.5), and ethylene diamine tetraacetic acid (EDTA, 0.5 mM). The homogenate, filtered through four layers of muslin cloth, was centrifuged at 12,000g for 10 min at 4°C. The resulting supernatant was used for the assay of different enzymes. Soluble protein estimation was carried out by the method of Bradford (1976) using bovine serum albumin as the standard.
For estimation of SOD (EC 1.15.1.1) activity, method of Beyer and Fridovich (1987) was followed. SOD activity was assayed by measuring the inhibition of photo-reduction of nitroblue tetrazolium (NBT) at 560 nm using UV–Vis spectrophotometer (Model λ-Bio-20, Perkin-Elmer, Germany). One unit of SOD is defined as that being present in the volume of extract that caused inhibition of the photo-reduction of NBT by 50%, and was expressed in enzyme units (mg−1 protein h−1).
Activity of APX (EC 1.11.1.11) was measured by following the rate of hydrogen peroxide-dependent oxidation of ascorbate in a reaction mixture that contained 0.5 M phosphate buffer (pH 7.0), 0.5 mM ascorbic acid and enzyme extract (Nakano and Asada 1981). The reaction was initiated by addition of 10 μl of 10% (v/v) H2O2 and the oxidation rate of ascorbic acid was estimated by following the decrease in absorbance at 290 nm for 3 min by using UV–vis spectrophotometer (Model λ-Bio-20, Perkin-Elmer, Germany). APX activity was calculated by using extinction coefficient 2.8 mM−1 cm−1 and expressed as enzyme units (mg protein)−1. One unit of enzyme (EU) is the amount necessary to decompose 1 μmol H2O2 per min at 25°C.
Catalase (CAT) activity was determined by monitoring the disappearance of H2O2, measuring a decrease in the absorbance at 240 nm (Aebi 1984). The reaction was carried in a reaction mixture containing 1.0 ml of the 0.5 M (pH 7.2) phosphate buffer, 3 mM EDTA, 0.1 ml of the enzyme extract and 0.3% H2O2, and allowed to run for 3 min. The enzyme activity was calculated using the extinction coefficient 0.036 mM−1 cm−1. One enzyme unit (EU) determines the amount of enzyme necessary to decompose 1 μmol of H2O2 per mg protein per min at 25°C and expressed as EU mg−1 protein.
Activity of GR (EC 1.6.4.2) was determined by the method of Foyer and Halliwell (1976) and modified by Rao (1992). The supernatant was immediately used to assay GR activity through glutathione-dependent oxidation of NADPH at 340 nm. About 1 ml reaction mixture, containing 0.2 mM NADPH, 0.5 mM GSSG and 50 μl of enzyme extract, was run for 5 min at 25°C by using UV–vis spectrophotometer (Model λ-Bio-20, Perkin-Elmer, Germany). The activity was calculated by using extinction coefficient 6.2 mM−1 cm−1 and expressed in enzyme unit (mg protein)−1. One unit of enzyme is the amount necessary to decompose 1 μmol of NADPH per min at 25°C.
Estimation of GSH content
Glutathione (GSH) content was determined by the recycling method of Anderson (1985). Fresh plant material (0.5 g) was homogenized in 3.0 ml of 5% (w/v) sulfosalicylic acid under cold conditions and was centrifuged at 10,000 rpm for 10 min. Half ml aliquot was taken in a microfuge tube, to which 0.5 ml reaction buffer [0.1 M phosphate buffer (pH 7.0), 3 mM ethylenediaminetetraacetic acid (Na2EDTA)] and 50 μl of 5′ dithio-bis-(2-nitrobenzoic acid) (0.15% DTNB) were added. After 5 min, absorbance for determination of GSH was read at 412 nm using UV–Vis spectrophotometer (Model λ Bio-20, Perkin-Elmer, Germany). The level of GSH were expressed in nmol g−1 fresh weight.
Estimation of phytochelatins
Phytochelatins were analyzed by pre-column derivatization using monobromobimane (mBBr). About 500 mg of tissue (each of roots and shoot) was frozen in liquid nitrogen, pulverized and transferred to a microfuge tube. 1 N NaOH containing 1 mg of sodium borohydride (NaBH4) per ml was added. After thorough mixing the solution was centrifuged at 11,000g for 5 min at 4°C. Supernatant was collected and acidified with 3.6 N HCl (ratio 5:1). The tubes were incubated in ice bath for 15 min followed by centrifugation at 11,000g for 5 min at 4°C. The 200 μl of this supernatant was diluted with 400 μl of 200 mM HEPES buffer (pH 8.2), then derivatized by adding 10 μl of 25 mM mBBr and incubated for 30 min in dark. The reaction was stopped by adding 60 μl of 10 mM acetic acid. The final mixture was filtered through 0.45 μm filter and used for analysis of PCs. Separation and analysis of PCs was carried on reverse phase HPLC (Waters, model Water Corp, Milford, USA) with purospher RP-18e column (Merck) using a gradient of solution A and B (A containing 0.05% trifluoroacetic acid and B containing 26% acetonitrile in solution A) at a flow rate of 1.5 ml min−1. Fluorescence intensity with an excitation wavelength of 380 nm and an emission wavelength of 470 nm was recorded using a fluorescence detector. Concentration of PCs was expressed as nanomoles of GSH equivalent g−1 fw.
Statistical analysis
Each treatment was analyzed using three replicates of each treatment (n = 3). To confirm the variability of data and validity of results, analysis of variance (ANOVA) was conducted. To determine whether differences between treatments were significant as compared to control, least significant difference (LSD) was determined (Cochram and Cox 1957).
Results
Fresh weight (FW) and dry weight (DW) of the B. juncea was not significantly affected by any level and duration of the Cr treatments. There was significant reduction in the FW and DW of V. radiata at all levels of Cr with maximum decline with T3 (200 μM). There was 5–16% reduction in the root length of B. juncea by Cr treatments. However, the significant reduction was reported with T3 only. The per cent reduction in root length of V. radiata was 5–26% over various days of Cr treatments with significant difference observed with T2 (100 μM) and T3 (200 μM)Cr treatments on 5 and 7 DAT. Similarly, shoot length of B. juncea declined slightly (3–13%) but non-significantly at all levels and duration of Cr treatments. In case of V. radiata, all the Cr treatments significantly decreased the shoot length with increasing levels and durations of Cr. Maximum decline (12.5-25%) occurred with T3 treatment (Fig. 1).
Effect of chromium treatments on the fresh weight (g), dry weight (g), root length (cm) and shoot length (cm) of the Brassica juncea and Vigna radiata at various days after treatment (DAT). All the values are mean of triplicates. Different letters indicate significantly different values at a particular DAT (P ≤ 0.05)
Cr accumulation in the roots of B. juncea was in the range of 100–300, 224–450, 398–754 and 550–972 μg g−1 DW, respectively at 1, 3, 5 and 7 day of Cr treatments (T1-T3). The corresponding figures in shoot were 80–120, 152–289, 274–419 and 414–504 μg g−1 DW. In V. radiata, the roots accumulated Cr in the range of 23–296, 31–301, 51–189 and 144–224 μg g−1 DW at 1, 3, 5 and 7 days of Cr treatments (T1–T3). The corresponding figures in shoot were 113–241, 120–238, 106–241 and 141–238 μg g−1 DW (Fig. 2).
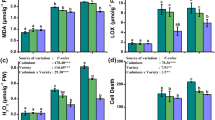
Malondialdehyde (MDA) level in the shoot of B. juncea was enhanced by 8–34, 9–85, 26–70 and 21–58% at 1, 3, 5, and 7 days of Cr treatments (T1–T3). However, the enhancement in MDA was non-significant with T1. In V. radiata, there was drastic increase in the level of MDA following exposure to Cr. The percent increase in MDA level was 50–80, 243–275, 125–149 and 116–164% respectively at 1, 3, 5 and 7 days of Cr treatments (T1–T3) (Table 1).
Activity of SOD in both the plants increased by Cr treatments, when compared with control. There were fourfolds to sevenfolds increase in SOD activity by Cr treatments, when compared with the control. In V. radiata, the range of significant enhancement in SOD activity was 57–127, 47–120 and 42–101% at 1, 3 and 5 days of Cr treatments, respectively. At 7 DAT, no significant increase in SOD activity was observed in V. radiata (Fig. 3).
Effect of chromium treatments on the activities SOD, CAT, APX and GR (EU mg protein−1) in the shoot of the Brassica juncea and Vigna radiata at various days after treatment (DAT). All the values are mean of triplicates. Different letters indicate significantly different values at a particular DAT (P ≤ 0.05)
Catalase activity was significantly enhanced (up to sixfold over the control) in B. juncea by Cr treatments. In V. radiata, the CAT activity increased in the range of 13–80% in a dose dependent manner. At 7 DAT, however, increase in CAT was non-significant (Fig. 3).
There was a induction of APX activity (up to eightfold) in the B. juncea by Cr treatments. Maximum induction, however, was reported at T2. In V. radiata, significant increase (8–30%) in the APX activity was observed at 1 DAT only (Fig. 3).
Glutathione reductase activity in the B. juncea was increased in the range of 111–178% with all the Cr treatments, when compared with control. Maximum increase, however, was observed with T2. In V. radiata, the GR activity was increased significantly at 1 DAT only, which was 56–77% over control (Fig. 3).
The content of GSH in V. radiata was higher than B. juncea. Chromium treatments increased the GSH content in the B. juncea, compared to control. In V. radiata, on the contrary, GSH content decreased with increase in the Cr treatments (Fig. 4).
Analysis of PCs was performed after 7 days of Cr treatments. PC2 and PC3 in shoot and roots of treated as well as control plants were identified and there were also some unidentified thiol peaks. Induction of PCs was higher in roots than shoot in both the plants at all the Cr treatments, and in all observations PC2 was more dominant Cr-binding ligand. With the increase in the level of Cr treatments, there was increase in the induction of PCs in B. juncea. Contrary to this, the induction of PCs in V. radiata increased with 50 and 100 μM Cr treatments. However, the induction of PCs was lesser with 200 μM Cr treatment than that observed with 50 and 100 μM Cr treatments in both shoot and root (Tables 2, 3).
Discussion
Plant biomass, root length and shoot length are used as indices of growth performance. In our study B. juncea and V. radiata highlighted significant differences in their responses to the exposure of various levels and durations of Cr treatments. In V. radiata, there was a significant reduction in the fresh weight (FW) and dry weight (DW) in response to levels and durations of Cr treatments. On the other hand, B. juncea highlighted complete tolerance to Cr regimes. Decrease in the FW may be the outcome of a decreased water uptake or enhanced water loss, both of which may occur following membrane damage since plant cell membranes are generally considered as the primary sites of metal injury (Barcelo and Poschenrieder 1990). The decline in the biomass production in V. radiata might be due to reasons like increased tissue permeability, inhibition of cell division resulting in the decline in growth or accumulation of Cr in different plant parts leading to retarded growth (Dube et al. 2003). Root growth has been traditionally considered as a powerful trait for scoring HM tolerance in plants. Results of our study indicated higher degree of Cr tolerance in B. juncea, compared to V. radiata. A non-significant effect was observed on the root length of B. juncea at lower (T1–T2) Cr doses, showing the ability of B. juncea to resist any damage by Cr accumulation. However, the root development was affected at the highest level of Cr treatment (200 μM). Root growth arrest caused by Cr at this treatment can be considered as a toxicity symptom. Significant decline in the root length in V. radiata was observed at 100 and 200 μM of Cr as the exposure time was increased. This reduction could be due to the accumulation of high HM concentrations in roots, and/or a non-existence of any defined HM-translocation mechanism, thereby enhancing the HM sequestration in the tissue and, thus, inhibiting the root development (Lu et al. 2004). The direct contact of roots with metals also results in a collapse of roots and their inability to absorb water from the media (Barcelo et al. 1986). Shoot development of B. juncea and V. radiata was affected differentially by Cr treatments. B. juncea was able to tolerate the effect of 50 and 100 μM of Cr. In V. radiata, a more pronounced effect was observed, probably reflecting its higher degree of sensitivity towards Cr. A reduced shoot length has also been reported in pea, tomato, cauliflower, maize and green gram under Cr stress (Sanita-di-Toppi et al. 2002; Shanker et al. 2004a).
The removal of HMs by plants is based on their ability to take up these potentially harmful heavy metals into their tissues (roots/aerial parts) and modulate their defence system so as to develop optimum tolerance for the accumulated heavy metal as well. In our studies, B. juncea accumulated 972 μg Cr g−1 DW in roots and 504 μg Cr g−1 DW in shoot, whereas V. radiata accumulated in the range of 301 and 259 μg Cr g−1 DW in the root and the shoot, respectively. Roots accumulated higher amount of Cr in both the plants studied. It could be because the roots serve as an interface between the soil, rhizosphere and the plant and being in direct contact with the metal they quench most of it, thus restricting its mobility to higher plant parts (Barcelo et al. 1986). Poor translocation to the shoot could also be due to the vacuolar sequestration of Cr in the root cells in order to render it non-toxic, which may be a natural anti-toxicity response of this plant against Cr stress.
That chromium produces oxidative stress is evident from enhancement in lipid peroxidation. Heavy metals may cause molecular damage to plant cells either directly or indirectly through the formation reactive oxygen species (Qureshi et al. 2005; Diwan et al. 2008), which include free radicals as well as non-radical molecules of high reactivity such as H2O2, singlet oxygen (1O2). Membrane lipids are especially prone to attack by free radicals. Protonation of superoxide radical can produce hydroperoxyl radical (∙OH, H2O2), which can convert fatty acids to toxic lipid peroxides, destroying the biological membranes (Foyer et al. 1994a). Since lipid peroxidation is ascribed to oxidative damage (Zenk 1996), measurement of MDA levels, a common product of lipid peroxidation, is routinely used as sensitive index of oxidative stress (Smirnoff 1993; Metwally et al. 2005; Choudhary et al. 2007). In this study, MDA level rose significantly high when plants were subjected to high level of Cr. This suggests that high level of endogenous Cr induced production of superoxide radicals, leading to increased lipid peroxidation. Increased MDA levels in shoot of V. radiata as compared to B. juncea indicated an increased lipid peroxidation of cell membrane. The relatively low MDA content in B. juncea in contrast to V. radiata suggested low oxidative injury and better stress abating tendency of B. juncea, which in turn accounted for the limited inhibition of growth. The possible tolerance mechanism operative in both the plants is described below.
ROS production in plants is regulated by the activities of a particular group of enzymes (Van Assche and Clijsters 1990; Gratao et al. 2005). Inadequate regulation of ROS generation potentially leads to oxidative damage. The first line of defence against ROS-mediated toxicity is achieved by SOD that catalyzes the dismutation of superoxide radicals to H2O2 and O2. Enhanced SOD activity in both the plants suggested that Cr caused oxidative stress. The data, however, also revealed that SOD activity was higher in B. juncea, indicating that this plant can efficiently detoxify the toxic superoxide radicals produced by the accumulated Cr when compared to V. radiata. High SOD activity has been associated with stress tolerance in plants because it neutralizes the reactivity of O2 −, which is overproduced under oxidative stress. It has been well documented that SOD activity has a protective role in heavy metal plants. In response to higher levels of Cr, however, the increase in SOD activity was not comparable to that evident at lower Cr levels as there might be inactivation of the enzyme by H2O2 (Yamaguchi et al. 1995). On the other hand, the up-regulation in the SOD activity in V. radiata in response to Cr stress was not strong enough to detoxify the superoxide radicals completely, thus reflecting lesser tolerance towards Cr stress, which also indicated that O2 − scavenging function of SOD was impaired with duration and levels of Cr treatments. Similar results have emerged from several other studies on plant responses to environmental stress (Labra et al. 2006; Zhang et al. 2005, 2007). H2O2, a product of SOD activity, is also toxic to cells and has to be further detoxified by CAT and the ascorbate–glutathione cycle. In the present study, CAT activity was increased in both the plants in response to Cr treatments. However, compared to B. juncea, low activity of CAT was observed in V. radiata. This may be due to inhibition of enzyme synthesis or change in assembly of enzyme subunits in the later (Ogawa et al. 1997).
Ascorbate–glutathione cycle in chloroplasts is the major defence system for scavenging H2O2, which finally converts H2O2 to H2O and O2. The cycle involves mainly ascorbate peroxidase and glutathione reductase enzymes, ascorbate and glutathione as oxireductants, H2O2 as an electron acceptor, and NADPH as an H+ donor, which are strictly compartmentalized and act in a highly coordinated manner (Asada 1992; Foyer et al. 1994b). In our study, APX activity significantly increased with Cr treatments in both the plants. However, this induction of APX activity was much higher in B. juncea (up to eightfolds) than V. radiata (8–30%). Increase in APX activity suggested a role of APX in the detoxification of H2O2 and its up-regulation under Cr-induced oxidative stress as established earlier with reference to many other heavy metals (Qureshi et al. 2005; Israr et al. 2006; Diwan et al. 2008; Khan et al. 2009). Importance of APX as a limiting factor of defence against photo-oxidative stress has been confirmed in transgenic tobacco plants (Rizhsky et al. 2002; Yabuta et al. 2002). Both the plants showed an initial increase in GR activity. However, the decrease in GR activity with duration of Cr exposure in V. radiata may be the result of a direct reaction of metal with sulfhydryl groups interfering with glutathione cycle since Cr is known to bind to the thiol group and thereby inactivate the thiol-containing enzymes such as GR. Sustained activity in B. juncea with duration and levels of Cr treatments proved the ability of hypertolerance in B. juncea toward oxidative stress induced by chromium. On exposure to HMs, increased GR activity has been reported in Phaseolus vulgaris and Alyssum (Srivastava et al. 2004). It was also observed that GR activity in B. juncea did not allow any fall in the GSH content as evident from the enhancement of GR enzyme upon exposure to Cr stress. Elevated GSH concentration is correlated with the ability of plants to withstand a metal-induced oxidative stress (Freeman et al. 2004). In case of V. radiata, decline in the GSH content was observed which could possibly point towards the formation of PC since GSH serves as the precursor for PC synthesis. We reported the induction of PCs in B. juncea and V. radiata in response to Cr. Increase in GSH content with a concomitant increase in phytochelatins in B.juncea signify that B. juncea besides showing an induction in PC generation, simultaneously managed to restore the GSH levels also. This could be explained by an augmented sulphur uptake, which eventually led to GSH biosynhesis, thus maintaining both GSH and PC concentration in this plant (Sharma et al. 2004). Induction of PCs in response to Cr stress has not been reported earlier. However, expression of metallothioneins (MTs)-like proteins has been reported under Cr stress in sorghum (Shanker et al. 2004b). The role of PCs in regulating metal toxicity has been reported earlier for As, Cd, Cu, Ag, Hg and Pb in a wide range of plant species (Cobbett 2000; Cobbett and Goldsbrough 2002; Schmoger et al. 2000; Hall 2002; Raab et al. 2004), and are known to play a major role in the detoxification of metals in plant cells (Shanker et al. 2004b; Srivastava et al. 2007). A sustained increase in PCs level in B. juncea with Cr treatments compared to V. radiata showed stronger tolerance capacity of the former in detoxification of accumulated Cr than the latter which showed a comparatively higher but inconsistent increase in PCs induction. In both the plants, the induction of PCs in shoot was lesser than those observed in roots. This may be due to binding of metal to other ligands (Salt et al. 1995) or to cell wall (Vecchia et al. 2005) or due to binding with GSH. In V. radiata, there was lesser induction of PCs at 200 μM Cr than at 50 and 100 μM Cr treatments. The decrease in the PCs at higher concentration in V. radiata may be due to its transport to shoot or due to the fact that PCs might have got degraded due to excessive Cr accumulation (Harmens et al. 1993). The results point towards the putative role of PCs along with antioxidant enzymes in conferring tolerance in plant cells subjected to Cr stress.
Thus, it can be inferred that cumulative effect of both antioxidant enzymes and PCs probably accounted for enhanced tolerance in B. juncea over V. radiata in combating Cr toxicity. It can be concluded that B. juncea, as a hyperaccumulator of Cr, could be potentially used for remediation of Cr contaminated soils and V. radiata as a fodder and food under our environmental and climatic conditions.
Abbreviations
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- Cr:
-
Chromium
- DTNB:
-
5′ Dithio-bis-(2-nitrobenzoic acid)
- Fw:
-
Fresh weight
- GR:
-
Glutathione reductase
- GSH:
-
Reduced glutathione
- LSD:
-
Least significant difference
- MT:
-
Metallothioneins
- MDA:
-
Malondialdehyde
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- TBA:
-
Thiobarbituric acid reactive substances
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Alscher RG, Erturk N, Heath LS (2003) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53:1131–1341
Anderson ME (1985) Determination of glutathione and glutathione disulfides in biological samples. Methods Enzymol 113:548–554
Arvind P, Prasad MNV (2003) Zinc alleviates cadmium-induced oxidative stress in Ceratophyllum demersum L., a free floating freshwater macrophyte. Plant Physiol Biochem 41:391–397
Asada K (1992) Ascorbate peroxidase: a hydrogen peroxide-scavenging enzyme in plants. Physiol Plant 85:235–241
Baker AJM, Mc Grath SP, Reeves RD, Smith JAC (2000) Metal hyperaccumulator plants: a review of the ecology and physiology of a biological resource for phytoremediation of metal-polluted soils. In: Terry N, Banuelos G (eds) Phytoremediation of contaminated soil and water. Lewis Publishers, Boca Raton, pp 85–107
Barcelo J, Poschenrieder C (1990) Plant-water relations as affected by heavy metal stress: a review. J Plant Nutr 13:1–37
Barcelo J, Poschenrieder C, Gunse B (1986) Water relations of chromium (VI) treated Bush bean plants (Phaseolus vulgaris L. cv Contender) under both normal and water stress conditions. J Exp Bot 37:178–187
Bartlett RJ (1991) Chromium cycling in soils and water: links, gaps, and methods. Environ Health Perspect 92:17–24
Beyer WF, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161:559–566
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 2:248–254
Choudhary M, Jetley UK, Khan MA, Zutshi S, Fatma T (2007) Effect of heavy metal stress on proline, malondialdehyde, and superoxide dismutase activity in the cyanobacterium Spirulina platensis-S5. Ecotoxicol Environ Saf 66:204–209
Cobbett CS (2000) Phytochelatin biosynthesis and function in heavy-metal detoxification. Curr Opin Plant Biol 3:211–216
Cobbett CS, Goldsbrough P (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol 53:159–182
Cochram WG, Cox GM (1957) Experimental designs. Wiley, New York
Dat J, Vandanabeele S, Vranova E, Van Montagu M, Inze D, Van Breusegem F (2000) Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci 57:779–795
Dheri GS, Brar MS, Malhi SS (2007) Comparative phytoremediation of chromium contaminated soil by fenugreek, spinach and raya. Commun Soil Sci Pl Anal 38:1655–1672
Diwan H, Ahmad A, Iqbal M (2008) Genotypic variation in the phytoremediation potential of Indian mustard for chromium. Environ Manag 41:734–741
Diwan H, Ahmad A, Iqbal M (2010) Chromium-induced modulation in the antioxidant defense system during phenological growth stages of Indian mustard. Int J Phytol 12:142–158
Dube BK, Tewari K, Chatterjee J, Chatterjee C (2003) Excess chromium alters uptake and translocation of certain nutrients in citrullus. Chemosphere 53:1147–1153
EPA US (1984) Health assessment document for chromium. Environmental Criteria and Assessment Office, EPA 600/8-83-014F. NTIS PB 85-115905. US Environmental Protection Agency, Research Triangle Park
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Foyer CH, Deascouveries P, Kunert KJ (1994a) Protection against oxygen radicals: important defense mechanisms studied in transgenic plants. Plant Cell Environ 17:507–523
Foyer CH, Lelandais M, Kunert KJ (1994b) Photooxidative stress in plants. Physiol Plant 92:696–717
Freeman JI, Persans MW, Nieman K, Albrecht C, Peer W, Pickering IJ, Salt DE (2004) Increased glutathione biosynthesis plays a role in nickel tolerance in Thalpsi nickel hyperaccumulator. Plant Cell 16:2176–2191
Ghosh M, Singh SP (2005) A comparative study of cadmium phytoextraction by accumulator and weed species. Environ Pollut 133:365–371
Gratao PL, Polle A, Lea PJ, Azevedo RA (2005) Making the life of heavy metal-stressed plants a little easier. Funct Plant Biol 32:481–494
Grill E, Thumann J, Winnacker EL, Zenk MH (1989) Phytochelatins, the heavy metal binding peptides of plants are synthesised from glutathione by a specific gamma glutamylcysteine dipeptide transpeptidase (phytochelatin synthase). Proc Natl Acad Sci USA 86:6838–6842
Gupta YP (1983) Nutritive value of food legumes. In: Arora SK (ed) Chemistry and biochemistry of legumes. Edward Arnold, London, pp 287–328
Hajiboland R (2005) An evaluation of the efficiency of cultural plants to remove heavy metals from growing medium. Plant Soil Environ 51:156–164
Hall JL (2002) Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot 53:1–11
Halliwell B (1987) Oxidative damage, lipid peroxidation and antioxidant protection in chloroplasts. Chem Phys Lipids 44:327–340
Harmens H, DenHartog PR, ten Bookum WM, Verkleij JAC (1993) Increased zinc tolerance in Silene vulgaris (Moench) Garcke is not due to increased production of phytochelatins. Plant Physiol 103:1305–1309
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts In: Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Israr M, Sahi SV, Jain J (2006) Cadmium accumulation and antioxidative responses in the Sesbania drumondii callus. Arch Environ Contam Toxicol 50:121–127
Jiang W, Liu D, Hou W (2000) Hyperaccumulation of lead by roots, hypocotyls and shoots of Brassica juncea. Biol Plant 43:603–606
Khan I, Ahmad A, Iqbal M (2009) Modulation of antioxidant defense system for arsenic detoxification in Indian mustard. Ecotoxicol Environ Saf 72:626–634
Kumar PBAN, Dushenkov V, Motto H, Raskin I (1995) Phytoextraction—the use of plants to remove heavy metals from soils. Environ Sci Technol 29:1232–1238
Labra M, Gianazza E, Waitt R, Eberini I, Sozzi A, Regondi S, Grassi F, Agradi E (2006) Zea mays L. protein changes in response to potassium dichromate treatments. Chemosphere 62:1234–1244
Lawn RJ, Ahn CS (1985) Mung bean (Vigna radiata (L.) Wilczek/Vigna mungo (L.) Hepper. In: Summerfield RJ, Roberts EH (eds) Grain legume crops. Collins, London, pp 584–623
Lu X, Kruatrachue M, Pokethitiyook P, Homyok K (2004) Removal of cadmium and zinc by water hyacinth, Eichhornia crassipes. Sci Asia 30:93–103
Metwally A, Safronova VI, Belimov AA, Dietz KJ (2005) Genotypic variation of the response to cadmium toxicity in Pisum sativum L. J Exp Bot 56:167–178
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Ogawa K, Kanematsu S, Asada K (1997) Generation of superoxide anion and localization of Cu-Zn superoxide dismutase in the vascular tissue of spinach hypocotyls and their association with lignification. Plant Cell Physiol 38:1118–1126
Panwar BS, Ahmed KS, Mittal SB (2002) Phytoremediation of nickel contaminated soils by Brassica species. Environ Dev Sustainability 4:1–6
Pawlisz AV, Kent RA, Schneider UA, Jefferson C (1997) Canadian water quality guidelines for chromium. Environ Toxicol Water Qual 12:123–183
Qureshi MI, Israr M, Abdin MZ, Iqbal M (2005) Responses of Artemisia annua L. to lead and salt induced oxidative stress. Environ Exp Bot 53:185–193
Raab A, Feldmann J, Meharg AA (2004) The nature of arsenic-phytochelatin complexes in Holcus lanatus and Pteris cretica. Plant Physiol 134:1113–1122
Rao MV (1992) Cellular detoxification mechanisms to determine age dependent injury in tropical plant exposed to SO2. J Plant Physiol 140:733–740
Rauser WE (1999) Structure and function of metal chelators produced by plants. Cell Biochem Biophys 31:19–48
Rizhsky L, Hallak-Herr E, Van Breusegem F, Rachmilevitch S, Barr JE, Rodermel S, Inze D, Mittler R (2002) Double antisense plants lacking ascorbate peroxidase and catalase are less sensitive to oxidative stress than single antisense plants lacking ascorbate peroxidase or catalase. Plant J 32:329–342
Rout GR, Samantaray S, Das P (2001) Differential lead tolerance of rice and black gram genotypes in hydroponic culture. Rost Výroba (Praha) 47:541–548
Salt DE, Prince RC, Pickering IJ, Raskin I (1995) Mechanisms of cadmium mobility and accumulation in Indian mustard. Plant Physiol 109:1427–1433
Salt DE, Blaylock M, Kumar PBAN, Dushenkov V, Ensley BD, Chet I, Raskin I (1996) Phytoremediation: a novel strategy for the removal of toxic metals from the environment using plants. Biotechnology 13:468–474
Samantary S (2002) Biochemical responses of Cr-tolerant and Cr-sensitive mung bean cultivars grown on varying levels of chromium. Chemosphere 47:1065–1072
Sanita-di-toppi L, Fossati F, Musetti R, Mikerezi I, Favali MA (2002) Effect of hexavalent chromium on maize, tomato and cauliflower plants. J Plant Nutr 25:701–717
Schat H, Llugany M, Vooijs R, Hartley-Whitaker J, Bleeker PM (2002) The role of phytochelatins in constitutive and adaptive heavy metal tolerances in hyperaccumulator and non-hyperaccumulator metallophytes. J Exp Bot 53:2381–2392
Schmoger ME, Oven M, Grill E (2000) Detoxification of arsenic by phytochelatins in plants. Plant Physiol 122:793–801
Shanker AK, Djanaguiraman M, Sudhagar R, Chandreshekhar CN, Pathmanabhan G (2004a) Differential antioxidative response of ascorbate glutathione pathway enzymes and metabolites to chromium speciation stress in green gram [Vigna radiata (L.). Wilczek, cv CO4] roots. Plant Sci 166:1035–1043
Shanker AK, Djanaguiraman M, Sudhagar R, Jayaram R, Pathmanabhan G (2004b) Expression of metallothionein 3 (MT3) like protein mRNA in Sorghum cultivars under chromium (VI) stress. Curr Sci 86:901–902
Shanker AK, Cervantes C, Loza-Tavera H, Avudainayagam S (2005) Chromium toxicity in plants. Environ Int 31:739–753
Shanker AK, Djanaguiraman M, Venkateswarlu B (2009) Chromium interactions in plants: current status and future strategies. Metallomics 1:375–383
Sharma SS, Kaul S, Metwally A, Goyal KC, Finkemeier I, Dietz KJ (2004) Cadmium toxicity to barley (Hordeum vulgare) as affected by varying Fe nutritional status. Plant Sci 166:1287–1295
Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, Yabuta Y, Yoshimura K (2002) Regulation and function of ascorbate peroxidase isoenzymes. J Exp Bot 53:1305–1319
Shukla OP, Rai UN, Singh NK, Dubey S, Baghel VS (2007) Isolation and characterization of chromate resistant bacteria from tannery effluent. J Environ Biol 28:399–403
Simonová E, Henselová M, Masarovičová E, Kohanová J (2007) Comparison of tolerance of Brassica juncea and Vigna radiata to cadmium. Biol Plant 51:488–492
Singh RP, Tripathi RD, Dabas S, Rizvi SMH, Ali MB, Sinha SK, Gupta DK, Mishra S, Rai UN (2003) Effect of lead on growth and nitrate assimilation of Vigna radiate (L.) Wilczek seedlings in a salt affected environment. Chemosphere 52:1245–1250
Sklar FH (1980) A preliminary comparison of the uptake of chromium-51 and zinc-65 by three species of aquatic plants from Louisiana: Spirodela punctata, Eacopa caroliniana, Elodea Canadensis. Proc La Acad Sci 43:46–51
Smirnoff N (1993) The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol 125:27–58
Srivastava S, Tripathi RD, Dwivedi UN (2004) Synthesis of phytochelatins and modulation of antioxidants in response to cadmium stress in Cuscuta reflexa-an angiospermic parasite. J Plant Physiol 161:665–674
Srivastava S, Mishra S, Tripathi RD, Dwivedi S, Trivedi PK, Tandon PK (2007) Phytochelatins and antioxidant systems respond differentially during arsenite and arsenate stress in Hydrilla verticillata (L.f.) Royle. Environ Sci Technol 41:2930–2936
Van Assche F, Clijsters H (1990) Effects of metals on enzyme activity in plants. Plant Cell Environ 13:195–206
Van den Broeck K, Vandecasteele C, Geuns JMC (1998) Speciation by liquid chromatography-inductively coupled plasma-mass spectrometry of arsenic in mung bean seedlings used as a bio-indicator for the arsenic contamination. Anal Chim Acta 361:101–111
Vatamaniuk OK, Bucher EA, Ward JT, Rea PA (2001) A new pathway for heavy metal detoxification in animals—phytochelatin synthase is required for cadmium tolerance in Caenorhabditis elegans. J Biol Chem 276:20817–20820
Vecchia FD, Rocca NL, Moro I, De Faveri S, Andreoli C, Rascio N (2005) Morphogenetic, ultrastructural and physiological damages suffered by submerged leaves of Elodea canadensis exposed to cadmium. Plant Sci 168:329–338
WHO (1988) Chromium. Environmental health criteria. World Health Organization, Geneva
Witmer CM, Harris R, Shupack SI (1991) Oral bioavailability of chromium from a specific site. Environ Health Perspect 92:105–110
Yabuta Y, Motoki T, Yoshimura K, Takeda T, Ishikawa T, Shigeoka S (2002) Thylakoid membrane-bound ascorbate peroxidise is a limiting factor of antioxidative systems under photo-oxidative stress. Plant J 32:915–925
Yamaguchi K, Mori H, Nishimura M (1995) A novel isoenzyme of ascorbate peroxidase localized on glyoxysomal and leaf peroxisomal membranes in pumpkin. Plant Cell Physiol 36:1157–1162
Zenk MH (1996) Heavy metal detoxification in higher plants—a review. Gene 179:21–30
Zhang H, Jiang Y, He Z, Ma M (2005) Cadmium accumulation and oxidative burst in garlic (Allium sativum). J Plant Physiol 162:977–984
Zhang FQ, Wang YS, Lou ZP, Dong JD (2007) Effect of heavy metal stress on antioxidative enzymes and lipid peroxidation in leaves and roots of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza). Chemosphere 67:44–50
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Diwan, H., Khan, I., Ahmad, A. et al. Induction of phytochelatins and antioxidant defence system in Brassica juncea and Vigna radiata in response to chromium treatments. Plant Growth Regul 61, 97–107 (2010). https://doi.org/10.1007/s10725-010-9454-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-010-9454-0