Abstract
Heavy metal contamination is a major trouble across the world. In India, there have been many reports of heavy metal pollution due to speedy industrialization and urbanization. The Indian brown mustard is an important oil yielding crop. However, the response of Indian mustard at germination and early seedlings stages to heavy metals like cadmium (Cd) stress is not clear. Current work renders a perceptivity into the part played by enzymatic and non-enzymatic antioxidants towards differential response of Cd (0, 0.5, and 1.0 mM doses) stress in mustard cultivars (Pusa bold, Pusa bahar, and Pusa agrani). The results show that irrespective of dose, Cd severely hamper germination and retard the early seedling growth in mustard cultivars. Pusa bold showed comparatively less reduction in seedling growth as compared to Pusa bahar and Pusa argani. Oxidative stress as measured by lipid peroxidation (MDA), hydrogen peroxide (H2O2), lipoxygenase (LOX), and cell death was significantly less in Pusa bold than Pusa agrani. Chlorophyll and carotenoids’ content was significantly reduced in Pusa agrani compared to Pusa bold. On the other hand, antioxidant metabolites (proline, ascorbate, and glutathione) showed increased accumulation under Cd stress in Indian mustard; also was the case with antioxidant enzymes (superoxide dismutase, catalase, glutathione-s-transferase, glutathione reductase, ascorbate peroxidase, and peroxidase), which significantly (p < 0.001) increased in Pusa bold when compared to other two. This work brings into limelight the significant role of enzymatic and non-enzymatic antioxidants in three varieties of Indian mustard under Cd stress during germination and early seedling growth. The three cultivars in order of decreasing sensitivity to Cd: Pusa agrani > Pusa bahar > Pusa bold
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal pollution in soil is a very serious concern for the living world. Speedy industrialization and urbanization have contributed to exponential increase of heavy metal concentrations in the soil which is ultimately affecting living organisms. It directly poses a negative impact on plant growth, mineral balance, metabolic processes, and yield. Cd is a non-essential heavy metal and is also considered as one of the most potent among top ten toxic heavy metals. Cd is normally noticed in the earth's crust along with zinc, lead, and copper ores. The chief source of Cd contamination in soil is excess use of fertilizer and pesticides (Cheng et al. 2014; Fagerberg et al. 2015), mining, metallurgy, electroplating, etc. When crops are cultivated in Cd contaminated soil, it easily gets absorbed due to its high mobility features and gets accumulated in different parts of plants (Aery and Rana 2003; Lux et al. 2010). Once Cd enter into plant, it reduces growth, and causes mineral nutrition imbalance and photosynthesis inhibition.
Seed germination is a crucial stage in the life cycle of plants. Cd surplus in the soil, which induces diminution in germination rate and seedling growth (Heidari and Sarani 2011; Shanmugaraj et al. 2013; Bohra and Sanadhya 2015; He et al. 2014; Chen et al. 2011). Cd toxicity stimulates different repercussions at physiological, biochemical, morphological, and molecular levels (Shanmugraj et al. 2013; Daud et al. 2013; Fojtová and Kovãrik 2000; Kapoor et al. 2014). Cd toxicity hastens ROS synthesis in plants. In response to this oxidative burst, many non-enzymatic (proline, ascorbate, and glutathione) and enzymatic (superoxidase dismutase, catalase, peroxidase, glutathione reductase, glutathione-s-transferase, and ascorbate peroxidase) systems are induced in plants, for scavenging of these ROS moieties (Mobin and Khan 2007; Li et al. 2013). Previous works have mentioned that the activities of antioxidant enzymes are directly involved with plant’s resistance against Cd stress (Ekmekci et al. 2008; Shah et al. 2001; Lannelli et al. 2002). Superoxide anion (O2−) is converted into hydrogen peroxide (H2O2) with the help of SOD, whereas POD acts to convert H2O2 into water (H2O), and CAT breaks H2O2 into oxygen (O2) and water (H2O) molecules. On the other hand, metabolites, viz., proline, ascorbate, and glutathione, accumulate in plants in response to stressors not only to regulate osmolarity, but also assist in ROS scavenging activities (Apel and Hirt 2004; Guo et al. 2019; Murtaza et al. 2019).
Most of the work, reaction of mustard to toxicity of heavy metal has been done on late seedling stages (Vatehova et al. 2012; Gill et al. 2011a, b; Nouairi et al. 2009). A few studies were done at seed germination and early seedling growth, but no detailed studies in Indian mustard are present (Bohra and Sanadhya 2015; Marchiol et al. 2006; Bauddh and Singh 2011). Our studies provide a detailed knowledge of physiological, morphological, and biochemical changes due to Cd toxicity in three cultivated genotypes during germination and early seedling stage.
Materials and methods
Three popular varieties of Indian brown mustard (Brassica juncea), viz., Pusa bold, Pusa bahar, and Pusa agrani, were acquired from IARI (Indian Agricultural Research Institute) Regional station, Karanal, India. Surface sterilized seeds (in 1% (w/v) NaOCl solution for 15 min followed by washing with distilled water) were put to germination in petri-plate (Borosil, 9.0 cm in diameter) with cotton embedding and treated with solution of Cd (CdCl2 salt, Sigma-Aldrich, molecular weight—183.32 and purity of 99.99%). In this experiment, four concentrations of Cd; 0.5 mM, 1.0 mM, 2.0 mM, and 4.0 mM excluding control and three varieties were selected. During the course of the experiment, 25 seeds were placed in each plate and 10.0 ml of solution was introduced into the petri-plates. Plants were grown in a growth chamber below white light with photon flux density of 52 µmol m−2 s−1 (PAR) along with a mean day and night temperature of 22/14 ± 3 °C and relative humidity of 62° ± 5%. In this experiment, total fifteen treatment combinations were repeated thrice in 45 petri-plates in a stochastic fashion.
Morphometric attributes
7 DAS (days after sowing) petri-plates were evaluated to assess the final germination percentage (FGP), germination index (GI), seedling vigor index (SVI), and moisture content percentage (MCP) along with seedling length were also measured by adopting the protocols given by Li (2008), Baki and Anderson (1973), and Moulick et al. (2016) respectively, all in triplicates.
Biochemical attributes
During the course of biochemical and thereafter for metal content analysis, doses of 2.0 and 4.0 mM Cd were not considered, for being extremely lethal to all the chosen three varieties (Chowardhara et al. 2019). At 7DAS, intact seedlings from each treatment were evaluated for chlorophyll a, chlorophyll b, total chlorophyll, and carotenoids following Arnon (1949). Lipid peroxidation/malondialdehyde (MDA), lipoxygenase (LOX), and hydrogen peroxide content (H2O2) complying with Heath and Packer (1968), Williams et al. (2000), and Alexiva et al. (2001) respectively, whereas proline, glutathione, and ascorbate estimation were done according to Bates et al. (1973), Oser and Hawks (1985) and Anderson (1985), respectively.
Besides these, antioxidant enzyme profiles of seedlings germinated under Cd stress were also elucidated. To prepare enzyme extract for the same, tissue samples were grounded in 0.1 M phosphate buffer supplemented with 1 mM EDTA and 1% PVP. The grounded product was centrifuged at 13,000 rpm for 20 min at 4 °C. The supernatant was employed to determine enzyme activity. For ascorbate peroxidase (APX) extraction, buffer additionally contained 2 mM ascorbic acid. Enzyme activity was estimated for superoxide dismutase (SOD; EC.1.15.1.1), guaiacol peroxidase (POX; EC.1.11.1.7), catalase (CAT; EC.1.11.1.6), glutathione S transferase (GST; EC.2.5.1.18), glutathione reductase (GR; EC.1.8.1.7), polyphenol oxidase (PPO; EC.1.14.18.1), and ascorbate peroxidase (APX; EC.1.11.1.11) following the protocols of Gupta et al. 1993; Chance and Maehly 1955; Habig and Jakoby, 1981; Smith et al. 1988; Mayer et al. 1966; Nakano and Asada 1981, respectively.
Estimation of membrane injury index/cell death
Cell viability was estimated spectrophotometrically by measuring Evan’s blue uptake following Yamamoto et al. (2001) protocol. The intact seedling was infiltrated with 0.25% Evan’s blue solution for 30 min. After that, seedlings were rinsed with 100 µM CaCl2 three times to remove excess stain. The stained seedling was homogenized in 1% SDS solution and centrifuged at 12,000 rpm for 20 min. The supernatant was quantitated for OD at 600 nm.
Elemental profile of intact Brassica seedlings
By complying with the protocol depicted by Gill et al. (2011a, b), Cd contents of intact seedlings of three tested varieties were analyzed. At 7 DAS, intact seedlings were collected from each treatment and washed with tap water succeeded by distilled water to assure the absence of any kind of metal deposition. Intact seedlings were then oven-dried at 72 °C for 48 h and then grounded to fine powder. Before acid digestion, all apparatus (glass-wares and stainless spatulas) were dipped in freshly prepared chromic acid solution for 24 h and again oven-dried. Accurately 0.1 g of plant material from each treatment were acid digested by adding 5.0 ml of a di-acid mixture (containing perchloric acid and nitric acid) in 1:3 ratios along with reagent blank (5.0 ml of acid mixture only) replicated thrice by adopting block digestion method and then quantified the elements using atomic absorption spectrophotometer (AAS-ICE 3500).
Statistical analysis
All the obtained information was evinced as mean (n = 3) followed by standard error (mean ± SE) format using SPSS 21 (Windows version) software. Furthermore, difference among the various treatments was determined by employing two-way ANOVA (analysis of variance) and post hoc Tukey’s HSD (honest significant difference) test at 0.05 level of significance. Origin Pro 8.5 software was employed for plotting graphs.
Results
Consequence of cadmium on germination and seedling growth
Cd stress exposure led to reduction in the final germination % (significant at p < 0.001 and 0.01 levels respectively) was observed, in the examined varieties in accordance with the strength of Cd. Within the three tested cultivars, Pusa bold had the highest rate of germination percentage (76%) even in the highest dose (4.0 mM) of Cd stress, as compared to the Pusa agrani and Pusa bahar varieties. Similarly, the germination index was significantly reduced (at p < 0.001 level) in all varieties under Cd. The seedling vigor index also showed gradual reduction dose-dependently. Pusa bold showed the highest seedling vigor index. The seedling length undergoes prominent (at p < 0.001 level) diminution with increase in Cd dose. Among the varieties, the growth of Pusa bold was found to be least affected (Table 1).
Measurement of Cd-induced oxidative stress and its impact
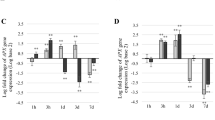
Among the varieties considered here, when analysed with respect to controls (grown in absence of Cd), Pusa bold showed least MDA accumulation (by 48.36%) than Pusa bahar (53.31%) and Pusa agrani (52.26%), respectively, grown at 1.0 mM of Cd stress (Fig. 1a). The lipoxygenase content increased profoundly with time and dose of heavy metals. The lowest activity was found in Pusa bold and highest in Pusa agrani. It showed 5.37-, 4.79-, and 3.13-fold increase in Pusa agrani, Pusa bahar, and Pusa bold at 1.0 mM of Cd, respectively (Fig. 1b). Findings from the current experiment suggest that besides MDA, LOX content, H2O2 was also found to increase in a linear fashion under Cd stress. At 7 DAS, the maximum level of H2O2 was observed in Pusa agrani (67.11%) over control under 1.0 mM Cd stress (Fig. 1c). The ion leakage phenomenon was used as cell death marker. Pusa agrani showed highest ion leakage (210%) after Pusa bahar and Pusa bold, respectively, at 1.0 mM Cd concentration (Fig. 1d).
Impact of Cd stress on a MDA, b LOX, c H2O2, and d cell death at 7 DAS. Each vertical column represents mean ± SE (n = 3) value. Column-bearing same letter cases are not significantly different at p˂ 0.05 level. *, **, and *** indicate that the F values are significant at 0.05, 0.01, and 0.001 levels, respectively
Effect of cadmium on photosynthetic pigments
Pusa agrani showed the highest reduction (57.15%) of chlorophyll a, and then Pusa bahar (42.97%) and Pusa bold (33.74%), respectively, to 1.0 mM Cd stress at 7DAS (Fig. 2a). Similar phenomenon was observed in case of chlorophyll b, total chlorophyll, and carotenoids on exposure to Cd stress (Fig. 2b–d).
Impact of Cd stress on a chlorophyll a, b chlorophyll b, c total chlorophyll, and d cartenoids at 7DAS. Each vertical column represents mean ± SE (n = 3) value. Column-bearing same letter cases are not significantly different at p˂ 0.05 level. *, **, and *** indicate that the F values are significant at 0.05, 0.01, and 0.001 levels, respectively
Effect of Cd on proline accumulation
The proline content significantly increased in all the three varieties of Indian mustard compared to respective controls on Cd treatment. Here, Pusa bold (4.62 fold) showed the highest accumulation, whereas Pusa agrani (2.54-fold) less amount of proline under 1.0 mM Cd stress at 7DAS (Fig. 3a).
Impact of Cd stress on a proline, b ascorbate, c reduced glutathione, d oxidized glutathione, e total glutathione, and f ration of GSH and GSSG at 7 DAS. Each vertical column represents mean ± SE (n = 3) value. Column-bearing same letter cases are not significantly different at p˂ 0.05 level. *, **, and *** indicate that the F values are significant at 0.05, 0.01, and 0.001 levels, respectively
Effects on ascorbate and glutathione accumulation
Ascorbate content heightened with the enhancement in Cd dose in all the studied varieties. Among the varieties considered here, Pusa bold experienced the highest increase in ascorbate content in a dose-dependent manner whereas, Pusa agrani showed the least ascorbate concentration (Fig. 3b). Reduced and oxidized glutathione showed significant increase with increase in concentration of Cd. Pusa agrani, Pusa bahar, and Pusa bold showed 4.68-, 7.36-, and 7.95-fold increase in reduced glutathione, respectively, under 1.0 mM of Cd treatment with respect to control (Fig. 3c, d). The results of oxidized glutathione and total glutathione showed similar trend as of reduced glutathione. GSH/GSSG ratio under 1.0 mM of cadmium stress did not show much deviation from what observed in control condition (Fig. 3f).
Effects of cadmium stress on antioxidant enzymes activities
The antioxidant enzymes have crucial roles to play in precluding oxidative stress by detoxification of free radicals. Higher activity denotes better ROS scavenging and, hence, better survival instincts. SOD activity increased by 1.59-, 2.02-, and 2.34-fold in Pusa agrani, Pusa bahar, and Pusa bold, respectively, at 1.0 mM Cd stress, with respect to controls (Fig. 4a). POX activity was also found to increase in a dose-dependent and variety irrespective manner in a highly significant way (at p < 0.001 level) for Pusa bold. Among the varieties, an enhancement by 78.34%, 69.49%, and 42.02% in Pusa bold, Pusa bahar, and Pusa agrani can be seen under 1.0 mM Cd stress, respectively, at 7 DAS (Fig. 4b). Similar to previous trend, a moderate-to-high significant (at p < 0.01–0.001 level) enhancement in CAT activity can be seen in all the studied varieties in a concentration-dependent mode under Cd stress. At 7 DAS, highest activity of CAT was recorded in Pusa bold (2.04 fold) and least in Pusa agrani (1.38 fold) at 1.0 mM Cd stress (Fig. 4c). The Cd stress on mustard seedlings increased GST activities at early seedling stage. The highest activities were recorded in Pusa bold and Pusa bahar varieties. At 7 DAS, the relative activity of GST was recorded as 69.91%, 74.83%, and 75.13% at 1.0 mM of Cd for Pusa agrani, Pusa bahar, and Pusa bold, respectively (Fig. 4d). GR activities in Pusa agrani, Pusa bahar, and Pusa bold increased by 1.6-, 1.69-, and 2.14-fold, respectively, at 1.0 mM dose of Cd. Pusa agrani relatively showed lesser increase in GR activity (Fig. 5a). Exposure to heavy metals significantly enhanced the functioning of PPO in all tested cultivars. Among the three varieties, though Pusa bold experienced highest PPO activity in a dose-dependent manner, but it was not significant compared to other two. The APX activity significantly heightened with enhancement in the concentrations of Cd. Among the three varieties, Pusa bold showed the highest activity of APX on Cd treatment at 7 DAS (Fig. 5c).
Impact of Cd stress on a SOD, b POX, c CAT, and d GST at 7 DAS. Each vertical column represents mean ± SE (n = 3) value. Column-bearing same letter cases are not significantly different at p˂ 0.05 level. *, **, and *** indicate that the F values are significant at 0.05, 0.01, and 0.001 levels, respectively
Determination of cadmium content in plant tissue
The atomic absorption spectrometry data for all the varieties showed an enhancement in Cd content when exposed to stress (Table 1). Accumulation of Cd was observed to be more in 1.0 mM Cd treatments. Whereas, among the varieties considered here, the order of Cd content lies in the order Pusa bold < Pusa bahar < Pusa agrani.
Discussion
Germination of seed is a crucial phase in the life cycle of any plant which is highly dependent on variety of environmental factors (Seneviratne et al. 2017; Anjum et al. 2015). Suppression of seed germination under Cd stress may be considered as the absence of necessary/sufficient protective arrangement during this particular stage (germination) of plants’ life cycle. At this junction (during germination), for the first time, plants come into contact with external environment. If any kind of stressor exists (biotic/abiotic such as Cd here), it makes germination and seedling growth (early developmental stage) more prone to inhibition (Liu et al. 2012a, b). Generally, heavy metals bear toxic effects on ecosystem, especially in agro-ecosystem. Cd had direct impression on germination, growth, and development of mustard plants. The consequences of Cd on germination were scored as FGP, GI, and SVI which had been earlier recorded in different plants, e.g., mulberry (Chen et al.2019), bread wheat (Bouziani et al. 2019), Ocimum basilicum (Singh and Lal, 2018), Oryza sativa (He et al. 2014), Picea omorika (Prodanovic et al. 2016), and Suaeda salsa (Liu et al. 2012a, b) with similar responses. Current findings regarding a decline in FGP, GI, and SVI in all the tested varieties in accordance with stress indicate significant phytotoxicity due to Cd.
If ROS persists for longer duration within the plant cell, it results in undesired consequences like intensification in MDA content (lipid peroxidation), and subsequent loss of ions from the cell which ultimately results in cell death. MDA, H2O2, and LOX (associated with lipid peroxidation) content/activity has been employed as a reliable indicant of stress (Aravind et al. 2003; Zhou et al. 2008; Zhang et al. 2016; Samma et al. 2017; Borgohain et al. 2019). The results show marked enhancement in MDA, H2O2, and LOX content/activity irrespective of varietal and stressor (Cd) differences in a linear fashion, indicating that Cd have the potential to significantly disrupt the ROS homeostasis in all the tested varieties. Results also show that, from varietal prospect, MDA, H2O2, and LOX content/activity follows the order Pusa bold < Pusa bahar < Pusa agrani. Cell death due to loss of membrane integrity has been depicted through enhancement in uptake of EB staining here. EB staining is a commonly employed tool to measured cell death for membrane degradation in numerous crops like Oryza sativa (Choudhury and Panda 2004), Pisum sativum (Yamamoto et al., 2001), and Nicotiana tabacum when exposed to aluminum (Zhang et al. 2016). With respect to control, cell death was more prominent when exposed to Cd in all the cultivars. Among the varieties considered here, the effect was more striking in Pusa agrani whereas least in Pusa bold.
Plant pigments (Chl a, b and carotenoids) are stress sensitive. Various reports have mentioned that pigments are highly sensitive to heavy metals (Lu and Zhang 2000; Ekmekci et al. 2008). Chlorophyll content has been considered as important stress stimulated biomarker to measure heavy metal phytotoxicity in various crops (Moulick et al. 2017, 2018). Cd stress can lead to a decrease in chlorophyll content in a linear fashion in all the tested varieties, as earlier observed by Shi and Cai (2008).
The prominent diminution in chlorophyll capacity might be the consequence of aggregation of Cd in seedling leaves, which later inhibit the chlorophyll biosynthesis process, stimulate chlorophyll reduction, and cause alternation of magnesium bi-valent ion from chlorophyll with Cd, as it bears alike oxidation state or even by facilitating membrane (thylakoid) damage (Parmar et al. 2013; Kupper and Andresen 2016). The reduction of photochemical function ultimately leads to diminution in seedling growth in all cultivars due to the degradation of chlorophyll content. Besides these, a significant increment in the functioning of non-enzymatic antioxidants and carotenoids was found. Previous statement was supported by Dias et al. 2013; Jali et al. 2016; Nath et al. 2017 detecting that greater efforts of plants towards ROS quenching activity to withstand excess Cd-induced imbalance of cellular machinery in a significant manner, applicable to all the tested varieties.
Metabolites plays a crucial part in plant abiotic stress responses. Proline, ascorbate, and glutathione are three main metabolites which plays a crucial role during heavy metals stress in plants. Sun et al. (2007) mentioned that free proline combines with Cd to form a non-toxic Cd proline complex. Our results showed that enhancement in Cd dose causes a significant increase in proline content under Cd stress. Similar result was found in different plants under Cd stress, i.e., Solanum melongena (Sun et al. 2007), Malva parviflora (Zoufan et al. 2018), Arachis hypogsea (Dinakar et al. 2009), and Groenlandia densa (Yilmaz and Parlak 2011).
GSH-AsA cycle is a major antioxidant system in plants which is responsible for neutralization of ROS moieties (Khan et al. 2019). Our finding shows a significant enhancement in AsA-GSH under Cd stress especially in Pusa bold, as compared to other two cultivated Indian mustard varieties.
Antioxidant enzymes also have a crucial part in ROS scavenging and quenching activities to mitigate oxidative damage caused by too heavy metals. A marked increase in antioxidant enzymes under Cd toxicity irrespective of varietal differences suggests that the studied varieties employ a considerable effort to detoxify ROS induced upon exposure to Cd stress. The present study detected enhancement in functioning of SOD under Cd stress, matches with the findings of Srivastava et al. (2014); Zayneb et al. (2015) who reported about similar observations in Oryza sativa (L.) and Trigonella foenumgraecum respectively. Irfan et al. (2014) also reported identical enhancement in SOD activity in Brassica juncea, but the stress was important at late seedling stage. CAT is generally situated in peroxisomes and mitochondria, while POX is located cytoplasm, membrane, vacuole, apoplast, extracellular space, and cell wall. Wang et al. (2008) depicted that POX is activated by heavy metal hastened oxidative stress and is more efficient then CAT which was also observed in this study. Prodanovic et al. (2016) and Mohamed et al. (2012) also detected enhancement in CAT activity when exposed to Cd stress in Picea omorika and Brassica juncea spp., respectively. Cd stress led to enhancement in APX functioning in the three cultivated varieties. Along with the above-mentioned antioxidant enzymes involved directly to combat ROS induced fluctuation in various cellular domains, a considerable increase (compare to the control) in the activity of PPO was also observed. The enhancement in the functioning of PPO in all the studied cultivars exposed to Cd suggests that these varieties employ phenolic compound and metal chelators to withstand heavy metal-induced toxicity. PPO activity in all three varieties increased dose-dependently. Some plant species showed that the activity of PPO under heavy metals stress significantly increased when compared to control (Wang et al. 2008; D’souza and Devaraj 2012). PPO is not directly involved in stress response, but helps in the synthesis of key phenolic compounds, which acts as ROS removers and metal chelators. GST catalyzes GSH binding to xenobiotic and thus plays a vital role in detoxification processes (Davis and Swanson 2001). Several endogenously produced reactive metabolites react with GSH in the presence of GST to produce a conjugate (Nagalakshmi et al. 2001). These conjugates are transported into vacuoles for further degradation and thus protect the plants from oxidative injury (Mohanpuria et al. 2007). GST activity increased with increment in dose of Cd in a dose-dependent and variety independent fashion. GST activity in Cd-induced stress has also been found to increase in Eichhornia crassipes and Salvinia auriculata (Vestena et al. 2011). Similar to GST, GR activity was also found to increase under Cd stressed condition in all the three varieties during germination and early stage seedlings as compared to control. Similar result was found by Panda et al. (2011) in Oryza sativa and Mishra et al. (2008) in Ceratophyllum demersum L., but oppose the results of Mobin et al. (2007) who detected a decrease in the activity of GR in Brassica juncea (L.).
Conclusion
Our experiment demonstrated differential stress response in the three genotypes of Indian mustard under Cd exposure on the basis of morphological, physiological, and biochemical mechanisms at germination stage. The findings depicted that Cd toxicity led to heavy injury in Indian mustard seedlings and also that some defense mechanisms were activated to protect from damages. Pusa bold exhibited more tolerance among the three cultivated varieties. The main reason behind the Cd stress tolerance for Pusa bold was less oxidative stress due to increased enzymatic and non-enzymatic antioxidants. Our results finding give a broad range of implications of Cd stress on Indian mustard at germination as well as early seedling growth stage.
References
Abdul-Baki AA, Anderson JD (1973) Vigor determination in soybean seed by multiple criteria 1. Crop Sci 13:630–633. https://doi.org/10.2135/cropsci1973.0011183X001300060013x
Aery NC, Rana DK (2003) Growth and cadmium uptake in barley under cadmium stress. J Environ Biol 24:117–123
Alexieva V, Sergiev I, Mapelli S, Karanov E (2001) The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ 24:1337–1344. https://doi.org/10.1046/j.1365-3040.2001.00778.x
Anderson ME (1985) Determination of glutathione and glutathione disulfide in biological samples. In: Meister A (ed) Methods in enzymology. Academic Press, Cambridge, pp 548–555
Anjum SA, Tanveer M, Hussain S, Bao M, Wang L, Khan I, Ullah E, Tung SA, Samad RA, Shahzad B (2015) Cadmium toxicity in Maize (Zea mays L.): consequences on antioxidative systems, reactive oxygen species and cadmium accumulation. Environ Sci Pollut Res 22:17022–17030. https://doi.org/10.1007/s11356-015-4882-z
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399. https://doi.org/10.1146/annurev.arplant.55.031903.141701
Aravind P, Prasad MN (2003) Zinc alleviates cadmium-induced oxidative stress in Ceratophyllum demersum L.: a free floating freshwater macrophyte. Plant Physiol Biochem 41(4):391–397. https://doi.org/10.1016/S0981-9428(03)00035-4
Arnon DI (1949) Copper enzymes in isolated chloroplast of polyphenoloxidase in Beta Vulgaris. Plant Physiol 24:1–1
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Bauddh K, Singh RP (2011) Differential toxicity of cadmium to mustard (Brassica juncia L.) genotypes under higher metal levels. J Environ Biol 32:355
Bohra A, Sanadhya D (2015) Phytotoxic effects of cadmium on seed germination and seedling growth of Brassica juncea L. Czern Coss cv. Int Res J Biol Sci 4:80–86
Borgohain P, Saha B, Agrahari R, Chowardhara B, Sahoo S, van der Vyver C, Panda SK (2019) SlNAC2 overexpression in Arabidopsis results in enhanced abiotic stress tolerance with alteration in glutathione metabolism. Protoplasma 256(4):1065–1077
Bouziani Y, Degaichia H, Benmoussa M (2019) Effect of cadmium on the germinative parameters of bread wheat. Rev Mex Cienc Agríc 10:301–309. https://doi.org/10.29312/remexca.v10i2.1476
Chance B, Maehly AC (1955) Assay of catalases and peroxidases. Methods Enzymol. https://doi.org/10.1016/S0076-6879(55)02300-8
Chen L, Wang X, Zhang F, Xing D, Zhang M (2019) Effects of cadmium stress on seed germination of ten mulberry varieties. J South Agric 50:257–263
Chen X, Wang J, Shi Y, Zhao MQ, Chi GY (2011) Effects of cadmium on growth and photosynthetic activities in pakchoi and mustard. Bot Stud 52(1):41–46
Cheng K, Tian HZ, Zhao D, Lu L, Wang Y, Chen J, Liu XG, Jia WX, Huang Z (2014) Atmospheric emission inventory of cadmium from anthropogenic sources. Int J Environ Sci Technol 11:605–616. https://doi.org/10.1007/s13762-013-0206-3
Choudhury S, Panda SK (2004) Role of salicylic acid in regulating cadmium induced oxidative stress in Oryza sativa L. roots. Bulg J Plant Physiol 30:95–110
Chowardhara B, Borgohain P, Saha B, Awasthi JP, Moulick D, Panda SK (2019) Phytotoxicity of Cd and Zn on three popular Indian mustard varieties during germination and early seedling growth. Biocatal Agric Biotechnol 21:101349
D’souza RM, Devaraj VR (2012) Induction of oxidative stress and antioxidative mechanisms in hyacinth bean under zinc stress. Afr Crop Sci J 20:17–19
Daud MK, Ali S, Variath MT, Zhu SJ (2013) Differential physiological, ultramorphological and metabolic responses of cotton cultivars under cadmium stress. Chemosphere 93(10):2593–2602
Davis DG, Swanson HR (2001) Activity of stress-related enzymes in the perennial weed leafy spurge (Euphorbia esula L.). Environ Exp Bot 46(2):95–108. https://doi.org/10.1016/S0098-8472(01)00081-8
Dias MC, Monteiro C, Moutinho-Pereira J, Correia C, Gonçalves B, Santos C (2013) Cadmium toxicity affects photosynthesis and plant growth at different levels. Acta Physiol Plant 35:1281–1289. https://doi.org/10.1007/s11738-012-1167-8
Dinakar N, Nagajyothi PC, Suresh S, Damodharam T, Suresh C (2009) Cadmium induced changes on proline, antioxidant enzymes, nitrate and nitrite reductases in Arachis hypogaea L. J Environ Biol 30:289–294
Ekmekçi Y, Tanyolac D, Ayhan B (2008) Effects of cadmium on antioxidant enzyme and photosynthetic activities in leaves of two maize cultivars. J Plant Physiol 165:600–611. https://doi.org/10.1016/j.jplph.2007.01.017
Fagerberg B, Barregard L, Sallsten G, Forsgard N, Östling G, Persson M, Borné Y, Engström G, Hedblad B (2015) Cadmium exposure and atherosclerotic carotid plaques—results from the Malmö diet and Cancer study. Environ Res 136:67–74. https://doi.org/10.1016/j.envres.2014.11.004
Fojtová M, Kovařík A (2000) Genotoxic effect of cadmium is associated with apoptotic changes in tobacco cells. Plant Cell Environ 23:531–537
Gill SS, Khan NA, Tuteja N (2011a) Differential cadmium stress tolerance in five Indian mustard (Brassica juncea L.) cultivars: an evaluation of the role of antioxidant machinery. Plant Signal Behav 6:293–300. https://doi.org/10.4161/psb.6.2.15049
Gill SS, Khan NA, Tuteja N (2011b) Differential cadmium stress tolerance in five Indian mustard (Brassica juncea L.) cultivars: an evaluation of the role of antioxidant machinery. Plant Signal Behav 6:293–300. https://doi.org/10.4161/psb.6.2.15049
Guo J, Qin S, Rengel Z, Gao W, Nie Z, Liu H, Li C, Zhao P (2019) Cadmium stress increases antioxidant enzyme activities and decreases endogenous hormone concentrations more in Cd-tolerant than Cd-sensitive wheat varieties. Ecotoxicol Environ Saf 172:380–387
Gupta AS, Webb RP, Holaday AS, Allen RD (1993) Overexpression of superoxide dismutase protects plants from oxidative stress (induction of ascorbate peroxidase in superoxide dismutase-overexpressing plants). Plant Physiol 103:1067–1073. https://doi.org/10.1104/pp.103.4.1067
Habig WH, Jakoby WB (1981) Assays for differentiation of glutathione S-transferases. Methods in enzymology. Academic Press, Cambridge, pp 398–405. https://doi.org/10.1016/S0076-6879(81)77053-8
He J, Ren Y, Chen X, Chen H (2014) Protective roles of nitric oxide on seed germination and seedling growth of rice (Oryza sativa L.) under cadmium stress. Ecotoxicol Environ Saf 108:114–119. https://doi.org/10.1016/j.ecoenv.2014.05.021
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198. https://doi.org/10.1590/S1677-04202011000200005
Heidari M, Sarani S (2011) Effects of lead and cadmium on seed germination, seedling growth and antioxidant enzymes activities of mustard (Sinapis arvensis L.). ARPN J Agric Biol Sci 6:44–47
Iannelli MA, Pietrini F, Fiore L, Petrilli L, Massacci A (2002) Antioxidant response to cadmium in Phragmites australis plants. Plant Physiol Biochem 40:977–982
Irfan M, Ahmad A, Hayat S (2014) Effect of cadmium on the growth and antioxidant enzymes in two varieties of Brassica juncea. Saudi J Biol Sci 21:125–131. https://doi.org/10.1016/j.sjbs.2013.08.001
Jali P, Pradhan C, Das AB (2016) Effects of cadmium toxicity in plants: a review article. Sch Acad J Biosci 4:1074–1081. https://doi.org/10.21276/sajb.2016.4.12.3
Kapoor D, Kaur S, Bhardwaj R (2014) Physiological and biochemical changes in Brassica juncea plants under Cd-induced stress. BioMed Res Int 214:13
Khan MY, Prakash V, Yadav V, Chauhan DK, Prasad SM, Ramawat N, Singh VP, Tripathi DK, Sharma S (2019) Regulation of cadmium toxicity in roots of tomato by indole acetic acid with special emphasis on reactive oxygen species production and their scavenging. Plant Physiol Biochem. https://doi.org/10.1016/j.plaphy.2019.05.006
Küpper H, Andresen E (2016) Mechanisms of metal toxicity in plants. Metallomics 8:269–285. https://doi.org/10.1039/c5mt00244c
Li Y (2008) Effect of salt stress on seed germination and seedling growth of three salinity plants. Pak J Biol Sci 11:1268–1272. https://doi.org/10.3923/pjbs.2008.1268.1272
Li Y, Zhang S, Jiang W, Liu D (2013) Cadmium accumulation, activities of antioxidant enzymes, and malondialdehyde (MDA) content in Pistia stratiotes L. Environ Sci Pollut Res 20:1117–1123
Liu JG, Zhang YX, Shi PL, Chai TY (2012a) Effect of cadmium on seed germination and antioxidative enzymes activities in cotyledon of Solanum nigrum L. J Agro Environ Sci 31:880–884
Liu S, Yang C, Xie W, Xia C, Fan P (2012b) The effects of cadmium on germination and seedling growth of Suaeda salsa. Proc Environ Sci 16:293–298. https://doi.org/10.1016/j.proenv.2012.10.041
Lu C, Zhang J (2000) Photosynthetic CO2 assimilation, chlorophyll fluorescence and photoinhibition as affected by nitrogen deficiency in maize plants. Plant Sci 151:135–143. https://doi.org/10.1016/S0168-9452(99)00207-1
Lux A, Martinka M, Vaculík M, White PJ (2010) Root responses to cadmium in the rhizosphere: a review. J Exp Bot 62:21–37. https://doi.org/10.1093/jxb/erq281
Marchiol L, Assolari S, Fellet G, Zerbi G (2006) Germination and seedling growth of Indian mustard exposed to cadmium and chromium. Ital J Agron 31:45–50
Mayer AM, Harel E, Ben-Shaul R (1966) Assay of catechol oxidase—a critical comparison of methods. Phytochemistry 5:783–789. https://doi.org/10.1016/S0031-9422(00)83660-2
Mishra S, Srivastava S, Tripathi RD, Dwivedi S, Shukla MK (2008) Response of antioxidant enzymes in coontail (Ceratophyllum demersum L.) plants under cadmium stress. Environ Toxicol 23:294–301. https://doi.org/10.1002/tox.20340
Mobin M, Khan NA (2007) Photosynthetic activity, pigment composition and antioxidative response of two mustard (Brassica juncea) cultivars differing in photosynthetic capacity subjected to cadmium stress. J Plant Physiol 164:601–610. https://doi.org/10.1016/j.jplph.2006.03.003
Mohamed AA, Castagna A, Ranieri A, di Toppi LS (2012) Cadmium tolerance in Brassica juncea roots and shoots is affected by antioxidant status and phytochelatin biosynthesis. Plant Physiol Biochem 57:15–22. https://doi.org/10.1016/j.plaphy.2012.05.002
Mohanpuria P, Rana NK, Yadav SK (2007) Cadmium induced oxidative stress influence on glutathione metabolic genes of Camellia sinensis (L.) O. Kuntze. Environ Toxicol Int J 22(4):368–374. https://doi.org/10.1002/tox.20273
Moulick D, Ghosh D, Santra SC (2016) Evaluation of effectiveness of seed priming with selenium in rice during germination under arsenic stress. Plant Physiol Biochem 109:571–578. https://doi.org/10.1016/j.plaphy.2016.11.004
Moulick D, Santra SC, Ghosh D (2017) Seed priming with Se alleviate As induced phytotoxicity during germination and seedling growth by restricting As translocation in rice (Oryza sativa L cv IET-4094). Ecotoxicol Environ Saf 145:449–456. https://doi.org/10.1016/j.ecoenv.2017.07.060
Moulick D, Santra SC, Ghosh D (2018) Effect of selenium induced seed priming on arsenic accumulation in rice plant and subsequent transmission in human food chain. Ecotoxicol Environ Saf 152:67–77. https://doi.org/10.1016/j.ecoenv.2018.01.037
Murtaza B, Naeem F, Shahid M, Abbas G, Shah NS, Amjad M, Bakhat HF, Imran M, Niazi NK, Murtaza G (2019) A multivariate analysis of physiological and antioxidant responses and health hazards of wheat under cadmium and lead stress. Environ Sci Pollut Res 26:362–370
Nagalakshmi N, Prasad MN (2001) Responses of glutathione cycle enzymes and glutathione metabolism to copper stress in Scenedesmus bijugatus. Plant Sci 160:291–299. https://doi.org/10.1016/S0168-9452(00)00392-7
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Nath M, Bhatt D, Prasad R, Tuteja N (2017) Reactive oxygen species (ROS) metabolism and signaling in plant-mycorrhizal association under biotic and abiotic stress conditions. In: Varma A, Prasad R, Tuteja N (eds) Mycorrhiza—eco-physiology, secondary metabolites, nanomaterials. Springer, Cham, pp 223–232. https://doi.org/10.1007/978-3-319-57849-1-12
Nouairi I, Ammar WB, Youssef NB, Miled DD, Ghorbal MH, Zarrouk M (2009) Antioxidant defense system in leaves of Indian mustard (Brassica juncea) and rape (Brassica napus) under cadmium stress. Acta Physiol Plant 31:237–247. https://doi.org/10.1007/s11738-008-0224-9
Oser B, Hawks L (1985) Physiological chemistry. McGraw-Hill, New York
Panda P, Nath S, Chanu TT, Sharma GD, Panda SK (2011) Cadmium stress-induced oxidative stress and role of nitric oxide in rice (Oryza sativa L.). Acta Physiol Plant 33:1737–1747. https://doi.org/10.1007/s11738-011-0710-3
Parmar P, Kumari N, Sharma V (2013) Structural and functional alterations in photosynthetic apparatus of plants under cadmium stress. Bot Stud 54:45. https://doi.org/10.1186/1999-3110-54-45
Prodanovic O, Prodanovic R, Pristov JB, Mitrovic A, Radotic K (2016) Effect of cadmium stress on antioxidative enzymes during the germination of Serbian spruce [Picea omorika (Pan.) Purkynĕ]. Afr J Biotechnol 11:11377–11385. https://doi.org/10.5897/AJB11.4114
Samma MK, Zhou H, Cui W, Zhu K, Zhang J, Shen W (2017) Methane alleviates copper-induced seed germination inhibition and oxidative stress in Medicago sativa. Biometals 30:97–111. https://doi.org/10.1007/s10534-017-9989-x
Seneviratne M, Rajakaruna N, Rizwan M, Madawala HM, Ok YS, Vithanage M (2017) Heavy metal-induced oxidative stress on seed germination and seedling development: a critical review. Environ Geochem Health 12:1–9. https://doi.org/10.1007/s106653-017-0005-8
Shah K, Kumar RG, Verma S, Dubey RS (2001) Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Sci 161:1135–1144
Shanmugaraj BM, Chandra HM, Srinivasan B, Ramalingam S (2013) Cadmium induced physio-biochemical and molecular response in Brassica juncea. Int J Phytoremediat 15:206–218. https://doi.org/10.1080/15226514.2012.687020
Shi GR, Cai QS (2008) Photosynthetic and anatomic responses of peanut leaves to cadmium stress. Photosynthetica 46:627–630
Singh AS, Lal EP (2018) Effect of Different Cadmium Concentrations on Seed Germination of Ocimum basilicum L. (Sweet Basil). Int J Sci Res Sci Technol 5:51–54
Smith IK, Vierheller TL, Thorne CA (1988) Assay of glutathione reductase in crude tissue homogenates using 5,5′-dithiobis (2-nitrobenzoic acid). Anal Biochem 175:408–413. https://doi.org/10.1016/0003-2697(88)90564-7
Srivastava RK, Pandey P, Rajpoot R, Rani A, Dubey RS (2014) Cadmium and lead interactive effects on oxidative stress and antioxidative responses in rice seedlings. Protoplasma 251:1047–1065. https://doi.org/10.1007/s00709-014-0614-3
Sun RL, Zhou QX, Sun FH, Jin CX (2007) Antioxidative defense and proline/phytochelatin accumulation in a newly discovered Cd-hyperaccumulator, Solanum nigrum L. Environ Exp Bot 60:468–476. https://doi.org/10.1016/j.envexpbot.2007.01.004
Vestena S, Cambraia J, Ribeiro C, Oliveira JA, Oliva MA (2011) Cadmium-induced oxidative stress and antioxidative enzyme response in water hyacinth and salvinia. Braz J Plant Physiol 23(2):131–139. https://doi.org/10.1590/S1677-04202011000200005
Wang Z, Zhang Y, Huang Z, Huang L (2008) Antioxidative response of metal-accumulator and non-accumulator plants under cadmium stress. Plant Soil 310:137. https://doi.org/10.1007/s11104-008-9641-1
Williams M, Sanchez JJ, Harwood JL (2000) Lipoxygenase pathway in olive callus cultures (Olea europaea). Phytochem 53:13–19. https://doi.org/10.1016/S0031-9422(99)00468-9
Yamamoto Y, Kobayashi Y, Matsumoto H (2001) Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiol 125:199–208. https://doi.org/10.1104/pp.125.1.199
Yılmaz DD, Parlak KU (2011) Changes in proline accumulation and antioxidative enzyme activities in Groenlandia densa under cadmium stress. Ecol Indic 11:417–423. https://doi.org/10.1016/j.ecolind.2010.06.012
Zayneb C, Bassem K, Zeineb K, Grubb CD, Noureddine D, Hafedh M, Amine E (2015) Physiological responses of fenugreek seedlings and plants treated with cadmium. Environ Sci Pollut Res 22:10679–10689. https://doi.org/10.1007/s113564270-8
Zhang M, Deng X, Yin L, Qi L, Wang X, Wang S, Li H (2016) Regulation of galactolipid biosynthesis by overexpression of the rice MGD gene contributes to enhanced aluminum tolerance in tobacco. Front in Plant Sci 30(7):337. https://doi.org/10.3389/fpls.2016.00337
Zhou ZS, Wang SJ, Yang ZM (2008) Biological detection and analysis of mercury toxicity to alfalfa (Medicago sativa) plants. Chemosphere 70:1500–1509. https://doi.org/10.1016/j.chemosphere.2007.08.028
Zoufan P, Jalali R, Hassibi P, Neisi E, Rastegarzadeh S (2018) Evaluation of antioxidant bioindicators and growth responses in Malva parviflora L. exposed to cadmium. Physiol Mol Biol Plants 24:1005–1016. https://doi.org/10.1007/s12298-018-0596-2
Acknowledgements
BC is grateful to University Grant Commission (UGC) for providing UGC non-NET fellowship (Award no: Ph.D./2126/2012). The help of Dr. Raj Kumar Chauhan, Indian Agricultural Research Institute (IARI) Regional station, Karnal, India in providing us with Indian brown mustard seeds is highly acknowledged. The authors are also grateful to SAIC, Tezpur University, India for providing us with Atomic Absorption spectrophotometer (AAS) facility.
Author information
Authors and Affiliations
Contributions
BC, BS, and SKP designed experiment. BC and PB performed the experiments. BC wrote manuscript. PB, BS, and JPA analyzed the data and edited manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No conflict among the authors.
Additional information
Communicated by P. Wojtaszek.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chowardhara, B., Borgohain, P., Saha, B. et al. Differential oxidative stress responses in Brassica juncea (L.) Czern and Coss cultivars induced by cadmium at germination and early seedling stage. Acta Physiol Plant 42, 105 (2020). https://doi.org/10.1007/s11738-020-03094-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-020-03094-0