Abstract
Brassica species, capable of heavy metals (HMs) hyperaccumulation, differ in their ability to accumulate and tolerate metals present in their environment. In this comparative study, the accumulation, morphological, and physiological responses of three Brassica species i.e., Brassica juncea, B. napus, and B. campestris, against lead (Pb) were examined. Plants were grown in pots under greenhouse conditions and subjected to 0, 50, 100, 150 mM concentrations of Pb for 14 days. The study revealed that 150 mM Pb concentration reduced the plant length and biomass in all the species and this decline was more obvious in B. napus. At 100 mM Pb concentration, plant length increased 3.5% in B. juncea, while decreased by 8 and 36% in B. campestris and B. napus, respectively. B. campestris and B. napus suffered from more pronounced Pb-accumulation in the root followed by shoot as compared to B. juncea. Pb-accumulation in 100 mM treated root of B. campestris and B. napus increased 29 and 80%, respectively as compared to B. juncea Pb treated root. Antioxidant enzyme catalase (CAT) activity was increased in B. juncea and B. campestris up to 150 mM concentration, while in B. napus activity of enzyme decreased at 100 and 150 mM Pb concentration. Phenylalanine ammonia-lyase (PAL) and nitrate reductase activity increased at 50 mM, while the polyphenol oxidase (PPO) and nitrite reductase significantly increased at 150 mM. Brassica species also showed more significant accumulation of amino acid, inhibition of proteins and total sugar content at 100 and 150 mM concentrations. Although all species exhibited enhanced antioxidant activity, activation in B. juncea was relatively higher. These results suggest that B. juncea is relatively more tolerant towards Pb stress as compared to B. campestris and B. napus due to reduced metal uptake and enhanced antioxidant enzyme activities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Concentration of HMs in the soil has considerably increased after the industrial revolution (Zaidi et al. 2005). The parent material, from which the soil is formed, also contains HMs but its concentration is relatively low (Herawati et al. 2000). Soil and the atmosphere are the main sources of HMs through which they enter the plant system (Uzu et al. 2010; Arshad et al. 2008). HMs accumulate in crops grown in metal polluted soil and cause harmful effects on human health after being incorporated into the food chain (Fu et al. 2008). Among the HMs that are damaging to plants, Pb is the most toxic and frequently occurring metal (Shahid et al. 2011). Anthropogenic sources of Pb include vehicles, mining, industrial activities, and agricultural activities such as use of fertilizers and pesticides. Pb adversely affects seed germination, root elongation, cell division, transpiration, and chlorophyll development (Sharma and Dubey 2005; Krzeslowska et al. 2009; Gupta et al. 2009; Gupta et al. 2010; Maestri et al. 2010). Binding of HMs ions to the sulfhydryl groups of proteins and replacement of essential cations from specific binding sites, causes inactivation of enzymes and production of reactive oxygen species (ROS), which in turn cause oxidative damages to lipids, proteins, and nucleic acids (Sharma and Dietz 2009). Enzymatic defence mechanism contains several enzymes which work together and protect the plant from harmful effects of ROS. Superoxide dismutase (SOD), ascorbate peroxidase (APX), CAT, guaiacol peroxidase (GPOD), and glutathione activities generally increase under metal stress (Mittler 2002; Feigl et al. 2013). Overall Pb induces harmful effects on plants at higher concentrations and decreases the crop yield and productivity.
The genus Brassica contains over 150 species of annual, biennial, or rarely perennial herbs mostly in North temperate parts of the world. Several species are used for human consumption, animal fodder, condiments, biofuel, and for oil production (Bancroft 2011). B. juncea, which is also known as mustard greens, Indian mustard, Chinese mustard, or leaf mustard, is an oilseed crop. After soybean and oil palm, mustard oil is the third most important vegetable oil. It is also widely used as a vegetable (Anuradha et al. 2012). Biodiesel production potential of B. juncea has recently been explored by Jham et al. (2009). Mustard seeds are used for the treatment of abdominal pain, anorexia, tumours, and diabetes (Grover et al. 2002). Extract obtained from leaf has antioxidant potential and reduces lipid peroxidation under diabetic oxidative stress (Yokozawa et al. 2003). The plant also has phytoremediation ability and removes the HMs, such as Pb from contaminated sites (Naser et al. 2012). B. napus or rapeseed is mainly cultivated for its oil rich seeds, but nowadays it is also grown to produce animal feeds and edible vegetable oils. Its oil is used as an effective lubricating agent and to produce soaps and plastics (Johnson 1999). B. napus colonizes the disturbed areas (Warwick 2010) and may increase the density of plants in ruderal habitats but it reduces crop yields when growing as a weed in agricultural fields (Gulden and Warwick 2008). B. campestris, or field mustard is winter annual or rotational cover crop. B. campestris prevents soil erosion, decreases weeds growth and soil borne pests, increases soil compaction and scavenge nutrients. It can grow under drought conditions, moderate heat, and soil with low fertility (Clark 2007). Due to agricultural and medicinal importance Brassica is the most economically important genus of Brassicaceae family.
Brassica species have a key role in phytoremediation as they can accumulate relatively higher amounts of toxic matter without showing any observable symptoms. In recent times, extensive studies have been conducted on the effects of HMs stress on Brassica species. The first visible symptoms related to Pb toxicity include stunted growth, and changes in root growth and morphology (Feigl et al. 2013). Higher concentrations of Pb significantly decreased the plant length and biomass in B. juncea by affecting the metabolic processes (Cu 2015; Sheetal et al. 2016; Kaur 2018). Contrary to these findings, at 250 mg/kg Pb concentrations, B. juncea shoot and root length increased significantly (Naaz and Chauhan 2019). Pb toxicity drastically reduces the water content of B. juncea plant. HMs affect the uptake of other essential elements, but B. juncea is able to selectively absorb essential nutrients and maintain adequate nutrition of their organs (Zaier et al. 2010). Chlorophyll content and PSII activity were increased in B. juncea under Cr stress due to Cr-induced stabilization of the oxygen evolving complex. In contrast to B. juncea, Pb stress significantly reduced the plant growth and biomass in the B. napus and B. campestris plant (Anjum et al. 2008; Ali et al. 2014a). HMs (Cd, Cr, Cu, Ni, Pb and Zn) accumulation were more pronounced in B. napus and B. campestris shoot than root (Brunetti et al. 2011). The Pb application significantly increased the ROS as well as malondialdehyde (MDA) in the leaves and roots of B. napus plant (Ali et al. 2014b). The essential elements were also significantly reduced after HMs toxicity (Ebbs and Kochian 1997). Reduction in the supply of these important elements can also lead to the inhibition of important enzymes used in chlorophyll biosynthesis (Ali et al. 2014c; Ahmad et al. 2015). Like any other plant, Brassica species also have an array of different layers of defence mechanisms comprising both enzymatic and non-enzymatic substances that reduce the HMs availability and toxicity (Mourato et al. 2012). The enzymatic system consists of several enzymes that work together to avoid the deleterious effects of ROS and other toxic species. The chelating of HMs seems to be another most important mechanism for the tolerance of Brassica species (Mourato et al. 2015). HMs are damaging to plants at higher concentration, but Brassica species have ability to combat the metal-induced toxicity by induction of different detoxification systems.
Different species of Brassica exhibit varying levels of HMs tolerance. However, no single experiment has been reported till date to observe the comparative tolerance level of Brassica species under Pb stress. This study was designed to investigate the effects of Pb on the growth of three different Brassica species and to assess the potential of relative tolerance of these species. Biochemical analysis and the activity of antioxidant enzymes was also checked in control and treated plants.
Material and methods
Growth conditions
Seeds of three Brassica species (B. juncea, B. campestris and B. napus) were obtained from National Agricultural Research Centre (NARC), Islamabad. Morphologically healthy seeds of three different Brassica species were sterilized using 25% sodium hypochlorite for 2 min. Distilled water was used for washing the seeds 3 times after the sterilization process. Seeds were sown in earthen pots filled with soil and sand in a 3:1 ratio. The pots were placed in the greenhouse under relative humidity 55–60%, temperature 23 ± 3 °C and 16/8 day/night conditions. Three independent biological replicates were used for each experiment. For each replicate, five plants were selected for every experimental analysis.
B. juncea can tolerate as high as 250 mg/kg Pb therefore in this study sufficiently higher levels of Pb were selected to compare the extent of tolerance of these selected species. After 45 days seedlings were treated with Pb acetate Pb(CH3COO)2 as T0: Control, T1: Pb (50 mM), T2: Pb (100 mM) and T3: Pb (150 mM).
Morphological measurements
Morphological parameters including fresh and dry weights (g), shoot length, root length and number of leaves were measured after fourteen days of the treatment. Shoot lengths and root length (cm) were measured manually using a scale. For each treatment point five plants were measured.
Metal analysis
Metal uptake by plant was measured by wet acid digestion method (Wan et al. 2012). Three independent replicates were grown for metal analysis. Plant was harvested and root and shoot were separately oven dried at 60 °C for 72 h. 100 mg dried plant material was grinded into fine powder with the help of pestle and mortar and added in to 50 mL conical flask. 10 mL mixture of per chloric acid (HCLO4) and nitric acid (HNO3) was added into flask in 1:3 ratio and then left for overnight. Partially digested material was transferred to the fume hood and heated at 150 °C until brown fumes turned into white fumes. Distilled water was added to the mixture to cool and dilute it. Fully digested mixture was filtered with Whatman filter paper No. 42. Afterwards, the final volume was adjusted to 50 mL by distilled water, and the solution was used to determine the desired metal concentration.
Biochemical assay
Quantitative estimation of biochemical contents (Total soluble proteins, total free amino acids, total soluble sugar contents, activity of CAT, nitrate reductase, nitrite reductase, PAL and PPO) of Brassica species were determined by using UV-1100 absorption spectrophotometer. After 2 weeks of Pb application, leaves of Brassica species were collected. 1.0 gm leaves of each species were crushed in pestle and mortar using liquid nitrogen followed by the addition of 10 mL of 0.02 M phosphate buffer having 7 pH. Slurry was transferred into Eppendorf tubes and was centrifuged at 8000X rpm for 10 min in Hettich Zentrifugen to separate the supernatant. The supernatant was transferred to another Eppendorf tube and was used for further analysis.
Estimation of free amino acids
Total free amino acids contents were estimated by mixing 1 mL extract, 2% ninhydrin reagent (2% ninhydrin dissolved in 98 mL distilled water) and 10% pyridine (10 mL of pyridine mix in 90 mL of distilled water). The tubes were then heated in a water bath for 30 min. The optical densities were measured at 570 nm using UV-spectrophotometer (Roensen, and Johnson 1961).
Estimation of protein
Biuret method was used for the estimation of total soluble protein contents. 1 mL of enzyme extract was mixed with 1 mL of biuret reagent (mixture of reagent CuSO4, NA-EDTA and KI in 5 N NaOH). After adding the required chemical and shaking vigorously, the tubes were incubated at room temperature for 25 min. The absorbance was measured at 545 nm in a spectrophotometer against an appropriate blank (Hamilton and Slyke 1943).
Estimation of total sugar
Anthrone reagent was used for estimating sugar contents in Brassica species. 1 mL of plant extract was mixed with 3 mL of anthrone reagent (0.2% anthrone, 80 mL H2SO4 and 20 mL distilled water). Test tube was heated in a water bath for 10 min, and then cooled in ice water. The optical density was observed at 620 nm using a spectrophotometer (Yemm and Willis 1954).
Estimation of nitrate reductase activity
1 mL of plant extract was added in 5 mL of 0.2 M Phosphate buffer (pH 7.0) containing 0.02 M KNO3 and incubated at 30 °C for 30 min. Then 0.5 mL of 1% sulphonilamide and 0.5 mL of 0.02% N Ethylene diamine dihydrochloride was added and left for 20 min after which the colour was noted. Optical density was measured by spectrophotometer at 542 nm (Sym 1984).
Estimation of nitrite reductase activity
1 mL of plant extract was added in 5 mL of 0.2 M Phosphate buffer (pH 7.0) containing 0.02 M KNO2 and incubated at 30 °C for 30 min. Then 0.5 mL of 1% sulphonilamide and 0.5 mL of 0.02% N Ethylene diamine dihydrochloride was added and left for 20 min after which the colour was noted. Optical density was measured by spectrophotometer at 542 nm (Sym 1984).
Estimation of CAT activity
Plant extract 0.01–0.04 mL was mixed with 3 mL of H2O2-phosphate buffer (35% H2O2, 0.067 phosphate buffer pH 7). After mixing the reagent in a tube the optical density was measured at 240 nm. Time was also observed for a decrease in absorbance from 0.45 to 0.40. Blank tube contained 3 mL of phosphate buffer. More concentrated sample solution should be used if the time of decrease is greater than 60 s (Luck 1974).
Estimation of PAL activity
The method was described by Zucker and then modified by Pendharker and Nair. Plant extract (0.3 mL) was mixed with 1 mL of 0.5% 30 mM phenylalanine. After adding 1 mL of 0.07% 200 µM Borate buffer, tubes were incubated at 40 ℃ for 1 h, afterwards 0.2 mL of 5 N HCl was added for termination of the reaction. Optical density was observed at 290 nm (Zucker 1968; Pendharkar and Nair 1975).
Estimation of PPO oxidase activity
The Decker method was used for the analysis of PPO activity. 0.1 mL of enzyme extract was mixed with 1 mL of 0.5 M phosphate buffer (K2HPO4, KH2PO4 and H2O). Then 1 mL of 0.018% of 0.00 M tyrosine and 0.9 mL water was added. Optical density was measured at 280 nm with the help of a spectrophotometer (Decker 1977).
Statistical analysis
One-way ANOVA was used to check the statistical significance of comparisons between multiple groups. A p value of < 0.05 was considered as statistically significant. All statistical analyses were performed using SPSS (version 12.0 J; IBM Corp. Armonk, NY, USA).
Results
Plant growth characteristics
To investigate the effects of Pb on three different Brassica species, morphological changes (plant length and plant biomass) were analysed by treating the 45 days old plant with Pb (Figs. 1, 2). Exposure of Brassica species to 50, 100, and 150 mM concentrations of Pb showed significant and visible symptoms of toxicity at higher concentrations. Various growth parameters exhibit different behaviour under different Pb concentrations in all three species.
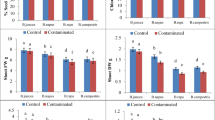
Effects of different concentrations of Pb Control (0 mM), T1: Pb (50 mM), T2: Pb (100 mM) and T3: Pb (150 mM) on growth parameters. A Shoot length B root length and C no of leaf/plant in B. juncea, B. campestris and B. napus. Values are shown as the mean of three replicates ± SE. Means followed by the same small letters are not significantly different at p ≤ 0.05
Effects of different concentrations of Pb Control (0 mM), T1: Pb (50 mM), T2: Pb (100 mM) and T3: Pb (150 mM) on growth parameters. A Shoot length B Root length C Leaf weight and D, E Fresh and Dry weight in B. juncea, B. campestris and B. napus. Values are shown as the mean of three replicates ± SE. Means followed by the same small letters are not significantly different at p ≤ 0.05
Plant morphology
Pb caused damaging effects on plants and decreased the shoot length (Fig. 1A). At 50 mM Pb concentration, shoot length exhibited non-significant changes in Brassica species compared to control plant. The maximum shoot length was 38.16 cm in B. juncea 50 mM treated plant exhibiting a 7% increase. As the Pb concentration increased, the shoot length significantly decreased in B. napus plants, while in B. juncea and B. campestris non-significant changes occur at 100 mM. A decrease of 31% in shoot length was recorded in B. juncea and 21% in B. campestris only at 150 mM Pb concentration. A similar pattern was recorded in the root length (Fig. 1B). Root length was increased non-significantly at 50 mM Pb concentration as compared to control plant. Maximum root length was 10.05 cm in B. napus. Root length was significantly decreased by 40 and 58% in B. napus and 28% and 37% in B. campestris at 100 and 150 mM Pb concentrations, respectively. While in B. juncea root length decreased only at 150 mM Pb concentration by 28%. Pb also affected the development of leaves in Brassica species (Fig. 1C). Leaf number significantly increased in B. juncea i.e., 20% at 50 mM Pb. As the concentration of Pb increased from 50 to 150 mM a significant decrease of 31% in leaf number was recorded. While, in B. campestris and B. napus leaf number was significantly decreased at 100 and 150 mM Pb treated plants i.e., 23% and 46% in B. campestris and 31% and 39% in B. napus, respectively.
Plant biomass
At higher concentration of Pb, plant biomass was significantly decreased (Fig. 2). The maximum shoot weight was 1.59 g in 50 mM Pb treated B. juncea plant (Fig. 2A). Shoot weight exhibited non-significant changes at 50 and 100 mM Pb concentration in B. juncea and B. campestris but it significantly decreased at higher concentration in both species. In B. napus shoot weight significantly reduced by 39% at 100 and by 55% at 150 mM Pb treated plant. Root weight exhibited tolerant behaviour in B. juncea plants (Fig. 2B). Therefore, non-significant changes were observed in root weight of B. juncea at three different stress levels. While, in B. napus and B. campestris root weight was significantly decreased as compared to the control. A similar behaviour was also exhibited by leaf weight (Fig. 2C). Maximum leaf weight was 1.5 g in 50 mM Pb treated B. juncea plant. Leaf weight was significantly affected by 51 and 52% in 150 mM Pb treated B. napus and B. campestris plants, respectively. Pb caused non-significant changes in B. juncea plant fresh and dry weight (Fig. 2D, E). Fresh and dry weight of 50 and 100 mM Pb treated B. juncea plants were greater than control plants. Fresh and dry weight was significantly affected at 100 and 150 mM Pb concentrations in B. campestris and B. napus.
Pb uptake
To investigate the causative agent for the different responses of Brassica species Pb uptake by plants were measured after fourteen days of Pb treatment (Fig. 3). Pb uptake by plant root and its transportation towards shoot significantly affected the plant growth and metabolism. Uptake of Pb was increased in plant roots as the concentration of Pb was increased from 50 to 150 mM (Fig. 3A). B. juncea exhibit a non-significant difference at 50 and 100 mM but at 150 mM, Pb concentration increased significantly in plant roots as compared to other two Pb treatments. While, in B. campestris and B. napus Pb uptake was increased significantly as the concentration of Pb increased. A Similar behaviour was also observed in the transportation of Pb from root to shoot (Fig. 3B). Maximum Pb contents were present in 150 mM Pb treated B. napus plant.
Biochemical traits
Biochemical traits were analysed to investigate the role of enzymatic and non-enzymatic antioxidant under the Pb stress (Figs. 4, 5) Total amino acid contents are one of the most important Physiological traits. Amino acid content significantly increased in B. campestris and B. napus at all three different treatments (Fig. 4A). While, in B. juncea treated plant non-significant changes occurred in the amino acid contents as compared to control plant. Maximum increase was at 150 mM i.e., 75.6% in B. juncea, 65% in B. campestris and 30% in B. napus. Pb significantly decreased the total soluble protein contents in the B. napus plant at all three different stress levels (Fig. 4B). While in B. juncea and B. campestris Pb induced non-significant changes at 50 and 100 mM Pb concentrations as compared to control. At 150 mM Pb concentration, protein contents decreased by 24% in B. juncea and 26% in B. campestris. Total sugar contents also exhibited similar behaviour (Fig. 4C). Maximum sugar content was recorded in the control plants of Brassica species. Sugar content was decreased under the stress conditions. In 50 and 100 mM B. juncea and B. campestris treated plants non-significant changes were observed in sugar content. While at 150 mM, sugar contents were significantly decreased. In B. napus, sugar content at 100 and 150 mM Pb concentrations was significantly lower than the control.
Effects of different concentrations of Pb Control (0 mM), T1: Pb (50 mM), T2: Pb (100 mM) and T3: Pb (150 mM) on A amino acid content B protein content and C sugar contents in B. juncea, B. campestris and B. napus. Values are shown as the mean of three replicates ± SE. Means followed by the same small letters are not significantly different at p ≤ 0.05
Effects of different concentrations of Pb Control (0 mM), T1: Pb (50 mM), T2: Pb (100 mM) and T3: Pb (150 mM) on enzyme A, B nitrate and nitrite reductase activity C catalase activity and D, E PAL and PPO activity in B. juncea, B. campestris and B. napus. Values are shown as the mean of three replicates ± SE. Means followed by the same small letters are not significantly different at p ≤ 0.05
Activity of enzymes
Pb induced significant changes in antioxidant enzyme activities under different concentrations in all three species. Pb significantly decreased the activity of nitrate and nitrite reductase in B. napus at 100 and 150 mM concentrations (Fig. 5A, B). While, in B juncea and B. campestris activity of nitrate and nitrite reductase decreased only at higher concentration. The nitrite activity reduced by 78% in B. juncea at 150 mM whereas it reduced up to 44 and 46% in B. campestris and B. napus, respectively. At lower concentration, the decrease in enzyme activity was non-significant. CAT is the major enzyme under stress conditions. Its concentration was significantly increased in B. juncea and B. campestris (Fig. 5C). While, in B. napus concentration of enzyme decreased non-significantly at higher Pb concentrations. At 150 mM the CAT activity increased by 81% in B. juncea, 68% in B. campestris, and 10% in B. napus. PAL activity also increased significantly in all three Brassica species whereas a non-significant increase in PPO activity was observed at 50 mM Pb concentration (Fig. 5d, e). As the concentration of Pb increased, a non-significant decrease in PPO activity was observed in B. juncea and B. campestris. While, in B. napus PPO activity significantly decreased at higher Pb concentration (Fig. 6).
Discussion
Heavy metals are continuously being incorporated into the environment due to rapid industrialization and urbanization. This raises serious concerns owing to their toxicity (Sethy and Shyamasree 2013). Pb is one of the most toxic HMs and its concentration in soil continues to increase as it is extensively use in various industries (Hamid et al. 2010). It is toxic even at low concentrations as its exposure causes serious physiological, biochemical, and morphological changes in plants (Ali et al. 2013, 2014b). The present study was aimed to compare the response of three species of Brassica i.e., Brassica juncea, Brassica napus, and Brassica campestris against various treatments of Pb using morphological and biochemical markers.
Morphology
Shoot length decreased at 150 mM in all three species i.e., 51% in B. napus, 30% in B. juncea, and 20% in B. campestris. In B. napus and B. campestris shoot length also decreased at 100 mM concentration. Pb-induced reduction in shoot length of Brassica species at higher concentration was also reported by Pratima and Mathad (2016), Kaur (2018), Sheetal et al. (2016) and Helal et al. (2016). Higher concentrations of Pb effect the mitotic process which may result in the decrease in shoot length (Srivastava et al., 2011). Root to shoot transportation of Pb was higher in B. napus and B. campestris as compared to B. juncea which resulted in the reduction in shoot length being more pronounced in the former two species. On the other hand, in B. juncea, shoot length increased at 50 and 100 mM Pb concentration because of low Pb concentration in shoot. Presence of relatively lower concentration of Pb in shoot also indicates towards a tolerant behaviour in B. juncea as Pb was not actively translocated from root to shoot. Cu (2015) also reported an increase in shoot length of B. juncea at lower Pb concentration.
Root is adversely affected under Pb stress as it is the first organ which comes in direct contact with the components present in the soil (Kumar et al. 1995). The root growth was reduced in B. napus and B. campestris at 100 and 150 mM Pb concentrations i.e., 58.5% in B. napus and 37% in B. campestris. Reduction in root length of Brassica species was also reported by Ali et al. (2015), Helal et al. (2016) and Li et al. (2018) under Pb, Cd and Cr stress. This decrease in root length was due to higher Pb uptake by plant roots, which disturbs the barrier function and selective permeability of plasmalemma, and tonoplast (Seregin et al. 2004). After entering the root Pb also affects the mitotic apparatus and decrease root length. On the contrary in B. juncea, lower uptake of Pb by plant roots caused the root length to increase at 50 and 100 mM Pb concentration. The root endodermis acts as a barrier in the transport of Pb from root to shoot (Seregin and Lvaniov 1997). The callose present between cell wall and plasma membrane act as an additional barrier against Pb uptake.
The decrease in the number of leaves was also more pronounced in B. napus and B. campestris as compared to B. juncea particularly at 100 mM Pb concentration. This decrease in leaf number can be attributed to the toxic effects of HMs on chlorophyll contents, gas exchange parameters, stomatal conductance, and photosynthetic rate (Balakhnina et al. 2005; Wahid et al. 2007; Ali et al 2014c). Boroumand et al. (2012) and Kanwal et al. (2014) also reported that B. napus shows visible symptoms of toxicity when exposed to Pb.
Plant biomass has been considered as a prerequisite measurement to assess the extent of abiotic stress. Present study depicted that plant biomass decreased in B. napus and B. campestris at 100 and 150 mM Pb concentration. These findings are supported by Kanwal et al. (2014) who also reported a decrease in B. napus biomass under higher Pb exposure. The decrease in plant biomass under higher Pb exposure may be due to the reason that Pb affects root and mineral uptake which in turn affects plant metabolism and ultimately decreases biomass (Breckle 1991; Islam et al. 2008; Gopal and Rizvi 2008; Singh et al. 2010; Sharma and Dubey 2005). HMs stress might also inactivate the photosystem II, enzymes of carbon reduction cycles and cause photosynthesis inhibition which ultimately results into biomass reduction (Gill et al. 2015). While in B. juncea plant biomass was increased at 50 and 100 mM Pb concentration which indicates that B. juncea has more tolerance towards increasing Pb concentrations than other two species. This might be due to more efficient uptake of other metals in B. juncea under Pb stress.
Biochemical traits
Measurement of amino acid content is a useful tool to trace the toxicity of HMs, as they tend to accumulate under stress conditions and help in osmotic adjustments and stabilize the structure of macromolecules and organelles (Kasai et al. 1998). In present investigation, amino acid contents were increased in three Brassica species in response to increasing Pb concentration. Maximum amino acid content was recorded in 150 mM Pb treated B. juncea plants. Amino acid level increases under Pb exposure in accordance with the stress level (Ahmad and Jhon 2005; Ahmad et al. 2006; 2008). The increase is due to metal chelation in the cytosol by high affinity ligands which is a metal detoxification and tolerance mechanism. These ligands may be amino acids and organic acids (Hall 2002). It has been also suggested that amino acids have a role in osmotic adjustment at the cellular level and enzyme protection by stabilizing the structure of macromolecules and organelles. Storage proteins also have an important role in growth and development of seedling. During the seed germination, variety of proteases degrade the storage protein and convert them into soluble peptides and free amino acids to provide the energy and support the growth (Schlereth et al., 2001). Many researchers have reported decrease in protein content of Brassica species after exposure to HMs stress (Singh and Sinha 2005; John et al. 2009). In the present study, the protein content decreased in all three species, however, it was more pronounced in B. napus at higher Pb concentration. Under Pb stress protein content decreased due to increased protease enzyme activity, which induced lipid peroxidation and fragmentation of protein under oxidative stress (Stiborova et al1987; Palma et al. 2002). Protein degradation also contributes to amino acid accumulation in metal stressed plant (Chen et al. 2003). Soluble sugar is the major constituent that helps in direct detoxification of ROS and maintaining the osmotic potential (Sharma and Dietz 2006; Kavi-Kishor and Sreenivasulu 2013; Keunen et al. 2013). Ali et al. (2015) also confirmed the protective role of total sugar content in B. napus under the Cd stress. HMs are known to affect plant sugar content through ROS induced oxidative stress. Monireh et al. (2011) found that increasing concentration of Pb significantly decreased the total sugar content in B. napus. In the current study, total sugar content also decreased in all Brassica species but it was more pronounced in B. napus due to stimulation of respiration rate and photosynthesis inhibition (Ouzounidou 1995).
Oxidative burst
Plants have developed antioxidant defence mechanisms to decrease the oxidative damage caused by HMs including Pb (Ruciska-Sobkowiak and Pukacki 2006). Pb toxicity can either induce their synthesis or may decrease the activity of these enzymes. This Pb induced inhibition of enzyme activity depends upon plant species, duration of treatment, and the Pb concentration (Islam et al. 2008; Gopal and Rizvi 2008). Decrease in the enzymatic activity is due to affinity of -SH group for Pb (Gupta et al. 2009; Sharma and Dubey 2005). Similarly in the present study nitrate activity was significantly decreased in B. napus at higher concentration due to disorganization of chloroplast structure. Metal stress at enzyme production sites causes water stress, which in turn, either reduces NADH supply or causes reduction in NO (Kumar et al. 2008). The nitrite reductase was significantly decreased in Brassica species under Pb toxicity due to reduced carbon fixation, low uptake of NO3− by roots and translocation in the xylem (Rai et al. 2004). CAT is the major enzyme which reduce the oxidative stress by converting the H2O2 into water and oxygen (Miller et al. 2008). A significant correlation was found between increase in the CAT activity and metal stress. In our findings, the CAT activity was significantly increased in B. juncea and B. campestris under Pb stress. Szollosi et al. (2009), Nouairi et al. (2006) and Goncalves et al. (2013) also reported increase in CAT activity in B. juncea at higher metal exposure. HMs induce increased transcription of CAT gene which results in increased synthesis of CAT enzyme. Contrarily, CAT activity was decreased in B. napus which might be responsible for its susceptible behaviour against Pb stress. PAL is one of the branch point enzyme and functions in the plant phenyl propanoid biosynthetic pathway to deaminate the amino acid L-phenylalanine forming trans-cinnamic acid and ammonia (McInnis et al. 2009). PAL activity was significantly increased in three Brassica species with the increasing Pb concentration. The increased PAL activity was due to enhanced phenolic metabolism, which produces precursors for antioxidant phenolics and lignin, to reduce the oxidative stress caused by Pb (Dai et al. 2006; Kovacik et al. 2007). PPO is another enzyme associated with defence mechanism and catalyses the oxidation of phenols to quinones (Martins and Mourato 2006). Activity of PPO non-significantly changed in B. juncea and B. campestris at all Pb concentrations which indicate a tolerant mechanism in these species. While in B. napus, which exhibited a susceptible behaviour, the PPO activity was significantly changed suggesting its role in the synthesis of phenolic compounds. These compounds play an important role in metal detoxification.
Conclusions
The current study indicate that prominent differences were observed in three Brassica species in response to Pb stress. Higher Pb concentrations negatively affected the different morphological characteristics in all three Brassica species. At low concentration B. juncea showed the highest tolerance level as compared to the other two Brassica species. The major tolerance strategy of B. juncea relies on low uptake of Pb by root and its translocation towards the shoot. While the other two species are less tolerant to Pb due to higher accumulation of Pb in the root and its translocation toward the shoot. Different biochemical parameters also varied in Brassica species under Pb stress. Higher Pb concentration caused protein degradation which resulted in an increase in free amino acid level. Pb also affected photosynthetic processes, ultimately reducing sugar contents. To cope with this stressful condition, all species exhibited higher antioxidant enzyme activity. Our study highlights the significance of enzymatic and non-enzymatic antioxidant activities in Brassica species under Pb stress. Nevertheless, for a better understanding of this tolerance mechanism, further investigations on genomic and proteomic level will be deeply insightful.
References
Ahmad P, Jhon R (2005) Effect of salt stress on growth and biochemical parameters of Pisum sativum L .(Einfluss von Salzstress auf Wachstum und biochemische Parameter von Pisum sativum L.). Arch Agron Soil Sci 51(6):665–672. https://doi.org/10.1080/03650340500274151
Ahmad P, Sharma S, Srivastava PS (2006) Differential physio-biochemical responses of high yielding varieties of mulberry (Morus alba) under alkalinity (Na~ 2CO~ 3) Stress in vitro. Physiol Mol Biol Plants 12(1):59
Ahmad P, Jhon R, Sarwat M, Umar S (2008) Responses of proline, lipid peroxidation and antioxidative enzymes in two varieties of Pisum sativum L. under salt stress. Int J Plant Prod 2:353–366. https://doi.org/10.22069/ijpp.2012.626
Ahmad P, Sarwat M, Bhat NA, Wani MR, Kazi AG, Tran LSP (2015) Alleviation of cadmium toxicity in Brassica juncea L. (czern. & coss.) calcium application involves various physiological and biochemical strategies. PLoS ONE 10:e0114571. https://doi.org/10.1371/journal.pone.0114571
Ali B, Jin PQR, Ali S, Khan M, Aziz R, Tian T, Zhou W (2013) Morphophysiological and ultra-structural changes induced by cadmium stress in seedlings of two cultivars of Brassica napus. L Biol Plant 58(1):131–138
Ali B, Mwamba TM, Gill RA, Yang C, Ali S, Daud MK, Wu Y, Zhou W (2014a) Improvement of element uptake and antioxidative defense in Brassica napus under lead stress by application of hydrogen sulfide. Plant Growth Regul 74(3):261–273. https://doi.org/10.1007/s10725-014-9917-9
Ali B, Song WJ, Hu WZ, Luo XN, Gill RA, Wang J, Zhou WJ (2014b) Hydrogen sulfide alleviates lead-induced photosynthetic and ultrastructural changes in oilseed rape. Ecotoxicol Environ Saf 102:25–33. https://doi.org/10.1016/j.ecoenv.2014.01.013
Ali B, Xu X, Gill RA, Yang S, Ali S, Tahir M, Zhou W (2014c) Promotive role of 5-aminolevulinic acid on mineral nutrients and antioxidative defense system under lead toxicity in Brassica napus. Ind Crops Prod 52:617–626. https://doi.org/10.1016/j.indcrop.2013.11.033
Ali B, Gill RA, Yang S, Gill MB, Farooq MA, Liu D, Daud MK, Ali S, Zhou W (2015) Regulation of cadmium-induced proteomic and metabolic changes by 5-aminolevulinic acid in leaves of Brassica napus L. PLoS ONE 10:e0123328. https://doi.org/10.1371/journal.pone.0123328
Anjum NA, Umar S, Ahmad A, Iqbal M, Khan NA (2008) Ontogenic variation in response of Brassica campestris L. to cadmium toxicity. J Plant Interact 3(3):189–198. https://doi.org/10.1080/17429140701823164
Anuradha M, Pragyandip D, Murthy PN, Siddique HH, Poonam K (2012) A classical review on Rajika (Brassica juncea). Res Rev J Biol Sci 1(1):18–23
Arshad M, Silvestre J, Pinelli E (2008) A field study of lead phytoextraction by various scented Pelargonium cultivars. Chemosphere 71:2187–2192. https://doi.org/10.1016/j.chemosphere.2008.02.013
Balakhnina TI, Kosobryukhov AA, Ivanov AA, Kreslavskii VD (2005) The effect of cadmium on CO 2 exchange, variable fluorescence of chlorophyll, and the level of antioxidant enzymes in pea leaves. Russ J Plant Physiol 52(1):15–20. https://doi.org/10.1007/s11183-005-0003-z
Bancroft I (2011) Genetics and Genomics of the Brassicaceae. Springer, Germany
Boroumand JS, Ranjbar M, Lari YH (2012) Damaging effects of lead on plant growth parameters of brassica napus, and the effect of salicylic acid on reducing the harmful effects of lead. Findings Science 8(1):19–27
Breckle SW, Kahle H (1991) Ecological geobotany/autecology and ecotoxicology. Progress in botany. Springer, Berlin, Heidelberg, pp 391–406. https://doi.org/10.1007/978-3-642-76293-2
Brunetti G, Farrag K, Soler-Rovira P, Nigro F, Senesi N (2011) Greenhouse and field studies on Cr, Cu, Pb and Zn phytoextraction by Brassica napus from contaminated soils in the Apulia region, southern Italy. Geoderma 160:517–523. https://doi.org/10.1016/j.geoderma.2010.10.023
Chen F, Wu FB, Dong J, Vincze E, Zhang GP, Wang F, Huang YZ, Wei K (2003) Cadmium translocation and accumulation in developing barley grains. Planta 227:223–232. https://doi.org/10.1007/s00425-007-0610-3
Clark A (2007) Managing Cover Crop Profitability, National SARE Outreach Handbook Series Book 9. National Agric. Laboratory, Beltsville, MD Beltsville, MD.
Cu NX (2015) Effect of Heavy Metals on plant growth and ability to use fertilizing substances to reduce heavy metal accumulation by Brassica juncea L. Czern. Global J Sc Front Res 15(3):35–41
Dai LP, Xiong ZT, Li HY, MJ (2006) Cadmium-induced changes in pigments, total phenolics, and phenylalanine ammonia-lyase activity in fronds of Azolla imbricata. Environ Toxicol 21(5):505–512. https://doi.org/10.1002/tox.20212
Decker LA (1977) Worthington enzyme manual. Freehold, NJ. https://doi.org/10.1002/tox.20212
Ebbs SD, Kochian LV (1997) Toxicity of zinc and copper to brassica species: Implications for phytoremediation. J Environ Qual 26:776–781. https://doi.org/10.2134/jeq1997.00472425002600030026x
Feigl G, Kumar D, Lehotai N, Tugyi N, Molnár Á, Ördög A, Kolbert Z (2013) Physiological and morphological responses of the root system of Indian mustard (Brassica juncea L. Czern.) and rapeseed (Brassica napus L.) to copper stress. Ecotoxicol Environ Saf 94:179–189. https://doi.org/10.1016/j.ecoenv.2013.04.029
Fu J, Zhou Q, Liu J, Liu W, Wang T, Zhang Q, Jiang G (2008) High levels of heavy metals in rice (Oryza sativa L.) from a typical E-waste recycling area in southeast China and its potential risk to human health. Chemosphere 71(7):1269–1275. https://doi.org/10.1016/j.chemosphere.2007.11.065
Gill RA, Zang L, Ali B, Farooq MA, Cui P, Yang S, Ali S, Zhou W (2015) Chromium-induced physio-chemical and ultrastructural changes in four cultivars of Brassica napus L. Chemosphere 120:154–164. https://doi.org/10.1016/j.chemosphere.2014.06.0290
Gonçalves EM, Alegria C, Abreu M (2013) Benefits of Brassica nutraceutical compounds on human health. In: Lang M (ed) Brassicaceae, Nova Science Publishers, Inc, p 19
Gopal R, Rizvi AH (2008) Excess lead alters growth, metabolism and translocation of certain nutrients in radish. Chemosphere 70(9):1539–1544. https://doi.org/10.1016/j.chemosphere.2007.08.043
Grover JK, Yadav S, Vats V (2002) Hypoglycemic and antihyperglycemic effect of Brassica juncea diet and their effect on hepatic glycogen content and the key enzymes of carbohydrate metabolism. Mol Cell Biochem 241(1–2):95–101. https://doi.org/10.1023/a:1020814709118
Gulden RH, Warwick SI, Thomas AG (2008) The biology of Canadian weeds Brassica napus L. and B. rapa L. Can J Plant Sci 88(5):951–996. https://doi.org/10.4141/CJPS07203
Gupta DK, Nicoloso FT, Schetinger MRC, Rossato LV, Pereira LB, Castro GY, Tripathi RD (2009) Antioxidant defense mechanism in hydroponically grown Zea mays seedlings under moderate lead stress. J Hazard Mater 172(1):479–484. https://doi.org/10.1016/j.jhazmat.2009.06.141
Gupta DK, Huang HG, Yang XE, Razafindrabe BHN, Inouhe M (2010) The detoxification of lead in Sedum alfredii H. is not related to phytochelatins but the glutathione. J Hazard Mater 177(1–3):437–444. https://doi.org/10.1016/j.jhazmat.2009.12.052
Hall JÁ (2002) Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot 53(366):1–11. https://doi.org/10.1093/jexbot/53.366.1
Hamid N, Bukhari N, Jawaid F (2010) Physiological responses of Phaseolus vulgaris to different lead concentrations. Pak J Bot 42(1):239–246
Hamilton PB, Van Slyke DD (1943) Amino acid determination with ninhydrin. J Biol Chem 150(1):231–250
Helal NM, Shaaban H, Dessoky EDS (2016) Effect of some heavy metals stress on micropropagated plantlets of canola plant (brassica napus l.). Egypt J Exp Biol (bot) 12(1):67–77
Herawati N, Suzuki S, Hayashi K, Rivai IF, Koyama H (2000) Cadmium, copper, and zinc levels in rice and soil of Japan, Indonesia, and China by soil type. B Environ Contam Toxicol 64(1):33–39. https://doi.org/10.1007/s001289910006
Islam E, Liu D, Li T, Yang X, Jin X, Mahmood Q, Li J (2008) Effect of Pb toxicity on leaf growth, physiology and ultrastructure in the two ecotypes of Elsholtzia argyi. J Hazard Mater 154(1–3):914–926. https://doi.org/10.1016/j.jhazmat.2007.10.121
Jham GN, Moser BR, Shah SN, Holser RA, Dhingra OD, Vaughn SF, Walter EL (2009) Wild Brazilian mustard (Brassica juncea L.) seed oil methyl esters as biodiesel fuel. J Am Oil Chem Soc 86(9):917–926. https://doi.org/10.1007/s11746-009-1431-2
John R, Ahmad P, Gadgil K, Sharma S (2009) Cadmium and lead-induced changes in lipid peroxidation, antioxidative enzymes and metal accumulation in Brassica juncea L. at three different growth stages. Arch Agric Soil Sci 55:395–405. https://doi.org/10.1080/03650340802552395
Johnson DL (1999) High performance 4-cycle lubricants from canola. In: Janick J (ed) Perspectives on New Crops and New Uses. ASHS Press, Alexandria, pp 247–250
Kanwal U, Ali S, Shakoor MB, Farid M, Hussain S, Yasmeen T, Abbas F (2014) EDTA ameliorates phytoextraction of lead and plant growth by reducing morphological and biochemical injuries in Brassica napus L. under lead stress. Environ Sci Pollut Res 21(16):9899–9910. https://doi.org/10.1007/s11356-014-3001-x
Kasai Y, Kato M, Aoyama J, Hyodo H (1998) Ethylene production and increase in 1-amino-cyclopropane-1-carboxylate oxidase activity during senescence of broccoli florets. In: International Postharvest Science Conference Postharvest 96(464):153–158. https://doi.org/10.17660/ActaHortic.1998.464.20
Kaur L (2018) Accumulation potential of Indian mustard (Brassica juncea var. arawali) and fenugreek (Trigonella foenum-graecum L.) planted on Lead and Nickel contaminated soil. Trop Plant Res 5(2):217–223. https://doi.org/10.22271/tpr.2018.v5.i2.027
Kavi-Kishor PB, Sreenivasulu N (2013) Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ 43:300–311. https://doi.org/10.1111/pce.12157
Keunen E, Peshev D, Vangronsveld J, van den Wim E, Cuypers A (2013) Plant sugars are crucial players in the oxidative challenge during abiotic stress: extending the traditional concept. Plant Cell Environ 36:1242–1255. https://doi.org/10.1111/pce.12061
Kováčik J, Bačkor M (2007) Phenylalanine ammonia-lyase and phenolic compounds in chamomile tolerance to cadmium and copper excess. Water Air Soil Pollut 185(1–4):185–193. https://doi.org/10.1007/s11270-007-9441-x
Krzesłowska M, Lenartowska M, Mellerowicz EJ, Samardakiewicz S, Woźny A (2009) Pectineus cell wall thickenings formation—a response of moss protonemata cells to lead. Environ Exp Bot 65(1):119–131. https://doi.org/10.1016/j.envexpbot.2008.05.006
Kumar S, Joshi UN (2008) Nitrogen metabolism as affected by hexavalent chromium in sorghum (Sorghum bicolor L.). Environ Exp Bot 64(2):135–144. https://doi.org/10.1016/j.envexpbot.2008.02.005
Kumar PN, Dushenkov V, Motto H, Raskin I (1995) Phytoextraction: the use of plants to remove heavy metals from soils. Environm Sci Technol 29(5):1232–1238. https://doi.org/10.1016/B978-0-12-803158-2.00015-1
Li L, Zhang K, Gill RA, Islam F, Farooq MA, Wang J, Zhou W (2018) Ecotoxicological and interactive effects of copper and chromium on physiochemical, ultrastructural, and molecular profiling in Brassica napus L. Biomed Res Int 16:87–98. https://doi.org/10.1155/2018/9248123
Luck H (1974) Catalase in methods of enzymatic analysis, Bergmeyer. Academic press, New York, pp 885–890
Maestri E, Marmiroli M, Visioli G, Marmiroli N (2010) Metal tolerance and hyperaccumulation: costs and trade-offs between traits and environment. Environ Exp Bot 68(1):1–13. https://doi.org/10.1016/j.envexpbot.2009.10.011
Martins LL, Mourato MP (2006) Effect of excess copper on tomato plants: growth parameters, enzyme activities, chlorophyll, and mineral content. J Plant Nutr 29(12):2179–2198. https://doi.org/10.1080/01904160600972845
McInnis S, Clemens S, Kermode AR (2009) The ornamental variety, Japanese striped corn, contains high anthocyanin levels and PAL specific activity: establishing the potential for development of an oral therapeutic. Plant Cell Rep 28(3):503–515. https://doi.org/10.1007/s00299-008-0650-6
Miller G, Shulaev V, Mittler R (2008) Reactive oxygen signaling and abiotic stress. Physiol Plantarum 133(3):481–489. https://doi.org/10.1111/j.1399-3054.2008.01090.x
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7(9):405–410. https://doi.org/10.1016/S1360-1385(02)02312-9
Monireh R, Hossien LY, Sheida BJ (2011) The effect of salicylic acid on photosynthetic pigments, contents of sugar and antioxidant enzyme under lead stress in Brassica. Iran J Plant Biol 9:39–52
Mourato M, Reis R, Martins L (2012) Characterization of plant antioxidative system in response to abiotic stresses: A focus on heavy metal toxicity. In: Montanaro G, Dichio B (eds) Advances in Selected Plant Physiology Aspects. Intech, Rijeka, Croatia, pp 23–44
Mourato MP, Moreira IN, Leitão I, Pinto FR, Sales JR, Martins LL (2015) Effect of heavy metals in plants of the genus Brassica. Int j Mole Sci 16(8):17975–17998. https://doi.org/10.3390/ijms160817975
Naaz G, Chauhan KL (2019) Lead tolerance and accumulation potential of B. juncea L. in imitatively contaminated soil. Res J Life Sci Bioinform Pharm Chem Sci 5(2):436–447. https://doi.org/10.26479/2019.0502.31
Naser HM, Sultana S, Gomes R, Noor S (2012) Heavy metal pollution of soil and vegetables grown near roadside at Gazipur. Bangladesh J Agr Res 37(1):9–17. https://doi.org/10.3329/bjar.v37i1.11170
Nouairi I, Ammar WB, Youssef NB, Daoud DBM, Ghorbal MH, Zarrouk M (2006) Antioxidant defense system in leaves of Indian mustard (Brassica juncea) and rape (Brassica napus) under cadmium stress. Plant Sci 170(3):511–519. https://doi.org/10.1007/s11738-008-0224-9
Ouzounidou G (1995) Responses of maize (Zea mays) plant to copper stress, growth mineral content and ultrastructure of roots. Environ Exp Botany 35:167–176. https://doi.org/10.1016/0098-8472(94)00049-B
Palma JM, Sandalio LM, Corpas FJ, Romero-Puertas MC, McCarthy I, Luis A (2002) Plant proteases, protein degradation, and oxidative stress: role of peroxisomes. Plant Physiol Biochem 40(6–8):521–530. https://doi.org/10.1016/S0981-9428(02)01404-3
Pendharkar MB, Nair PM (1975) Induction of phenylalanine ammonia lyase (PAL) in gamma irradiated potatoes. Radiat Bot 15(2):191–197. https://doi.org/10.1016/S0033-7560(75)80007-X
Pratima H, Pratima M (2016) Lead-induced oxidative stress and metabolic alteration in seedlings of Brassica Juncea L. Res J Environ Sci 5(3):37–41
Rai V, Vajpayee P, Singh SN, Mehrotra S (2004) Effect of chromium accumulation on photosynthetic pigments, oxidative stress defense system, nitrate reduction, proline level and eugenol content of Ocimum tenuiflorum L. Plant Sci 167(5):1159–1169. https://doi.org/10.1016/j.plantsci.2004.06.016
RoensenJohnson DB (1961) Estimation of protein in cellular material. Nature 91:492–493
Ruciska-Sobkowiak R, Pukacki PM (2006) Antioxidative defense system in lupin roots exposed to increasing concentrations of lead. Acta Physiol Plant 28(4):357–364. https://doi.org/10.1007/s11738-006-0032-z
Schlereth A, Standhardt D, Mock HP, Muntz K (2001) Stored cysteine proteinases start globulin breakdown in protein bodies of embryonic axis and cotyledons of germinating vetch (Vicia sativaL.) seeds. Planta 212:718–727. https://doi.org/10.1007/s004250000436
Seregin IV, Ivanov VB (1997) Histochemical investigation of cadmium and lead distribution in plants. Russ J Plant Physiol 44(6):791–796. https://doi.org/10.1134/S1021443711040133
Seregin IV, Shpigun LK, Ivanov VB (2004) Distribution and toxic effects of cadmium and lead on maize roots. Russ J Plant Physiol 51(4):525–533. https://doi.org/10.1023/B:RUPP.0000035747.42399.84
Sethy SK, Ghosh S (2013) Effect of heavy metals on germination of seeds. J Nat Sci Biol Med 4(2):272. https://doi.org/10.4103/0976-9668.116964
Shahid M, Pinelli E, Pourrut B, Silvestre J, Dumat C (2011) Lead-induced genotoxicity to Vicia faba L. roots in relation with metal cell uptake and initial speciation. Ecotox Environ Safe 74(1):78–84. https://doi.org/10.1016/j.ecoenv.2010.08.037
Sharma SS, Dietz KJ (2006) The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J Exp Bot 57:711–726. https://doi.org/10.1093/jxb/erj073
Sharma SS, Dietz KJ (2009) The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci 14(1):43–50. https://doi.org/10.1016/j.tplants.2008.10.007
Sharma P, Dubey RS (2005) Lead toxicity in plants. Braz j Plant Physiol 17(1):35–52. https://doi.org/10.1590/S1677-04202005000100004
Sheetal KR, Singh SD, Anand A, Prasad S (2016) Heavy metal accumulation and effects on growth, biomass and physiological processes in mustard. Indian J Plant Physiol 21(2):219–223. https://doi.org/10.1007/s40502-016-0221-8
Singh S, Sinha S (2005) Accumulation of metals and its effects in Brassica juncea (L.) Czern. (cv. Rohini) grown on various amendments of tannery waste. Ecotox Environ Safe 62(1):118–127. https://doi.org/10.1016/j.ecoenv.2004.12.026
Singh R, Tripathi RD, Dwivedi S, Kumar A, Trivedi PK, Chakrabarty D (2010) Lead bioaccumulation potential of an aquatic macrophyte Najas indica are related to antioxidant system. Bioresour Technol 101(9):3025–3032. https://doi.org/10.1016/j.biortech.2009.12.031
Srivastava RR, Khan SA, Nasim N, Manzoor M (2011) Cadmium treatment alters phytochemical and biochemical activity in glycine max L. Int J Bot 7(4):305–309
Stiborová M, Ditrichová M, BŘEzinová A (1987) Effect of heavy metal ions on growth and biochemical characteristics of photosynthesis of barley and maize seedlings. Biol Plant 29(6):453
Sym GJ (1984) Optimisation of the in-vivo assay conditions for nitrate reductase in barley (Hordeum vulgare L. cv. Igri). J Sci Food Agr 35(7):725–730. https://doi.org/10.1002/jsfa.2740350703
Szőllősi R, Varga IS, Erdei L, Mihalik E (2009) Cadmium-induced oxidative stress and antioxidative mechanisms in germinating Indian mustard (Brassica juncea L.) seeds. Ecotoxicol Environ Saf 72(5):1337–1342. https://doi.org/10.1016/j.ecoenv.2009.04.005
Uzu G, Sobanska S, Sarret G, Munoz M, Dumat C (2010) Foliar lead uptake by lettuce exposed to atmospheric fallouts. Environ Sci Technol 44(3):1036–1042. https://doi.org/10.1021/es902190u
Wahid A, Perveen M, Gelani S, Basra SM (2007) Pretreatment of seed with H2O2 improves salt tolerance of wheat seedlings by alleviation of oxidative damage and expression of stress proteins. J Plant Physiol 164(3):283–294. https://doi.org/10.1016/j.jplph.2006.01.005
Wan Y, Luo S, Chen J, Xiao X, Chen L, Zeng G, Liu C, He Y (2012) Effect of endophyte-infection on growth parameters and Cd-induced phytotoxicity of Cd-hyperaccumulator Solanum nigrum L. Chemosphere 89(6):743–750. https://doi.org/10.1016/j.chemosphere.2012.07.005
Warwick S (2010) Flora of North America Editorial Committee. In: Napus BL (ed) Flora of North America North of Mexico, vol 422. Oxford, New York, p 7
Yemm EW, Willis A (1954) The estimation of carbohydrates in plant extracts by anthrone. Biochem j 57(3):508–514. https://doi.org/10.1042/bj0570508
Yokozawa T, Kim HY, Cho EJ, Yamabe N, Choi JS (2003) Protective effects of mustard leaf (Brassica juncea) against diabetic oxidative stress. J Nutr Sci Vitaminol 49(2):87–93. https://doi.org/10.3177/jnsv.49.87
Zaidi MI, Asrar A, Mansoor A, Farooqui MA (2005) The heavy metal concentration along roadside trees of Quetta and its effects on public health. JApSc 5(4):708–711. https://doi.org/10.3923/jas.2005.708.711
Zaier H, Mudarra A, Kutscher D, de la Campa MRF, Abdelly C, Sanz-Medel A (2010) Induced lead binding phytochelatins in Brassica juncea and sesuvium portulacastrum investigated by orthogonal chromatography inductively coupled plasma-mass spectrometry and matrix assisted laser desorption ionization-time of flight-mass spectrometry. Anal Chim Acta 671:48–54. https://doi.org/10.1016/j.aca.2010.04.054
Zucker M (1968) Sequential induction of phenylalanine ammonia-lyase and a lyase-inactivating system in potato tuber disks. Plant Physiol 43(3):365–374. https://doi.org/10.1104/pp.43.3.365
Acknowledgements
The authors thanks to Saba Manzoor and Aneela Nijabat Awan for their valuable suggestions regarding research work.
Author information
Authors and Affiliations
Contributions
Junaid Shehzad: Methodology, Formal analysis and investigation, Writing - Original Draft Ghazala Mustafa *: Conceived and designed the research, Supervision, Conceptualization, Resources Huma Arshad: Involved in final manuscript draft preparation and revisions. Aamir Ali: Critical review Naima Huma Naveed: Data interpretation Zarqa Riaz: Data curation and Visualization Ilham Khan: Critical revisions.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by W. Zhou.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shehzad, J., Mustafa, G., Arshad, H. et al. Morpho-physiological and biochemical responses of Brassica species toward lead (Pb) stress. Acta Physiol Plant 45, 8 (2023). https://doi.org/10.1007/s11738-022-03493-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-022-03493-5