Abstract
The effect of cadmium (Cd) on growth, accumulation, and antioxidative response was studied in Sesbania drummondii callus, cultivated on different concentrations of Cd (0–250 μM) for four weeks. Callus growth was comparable to that of the control for concentrations up to 50 μM Cd; however, concentrations higher than 50 μM affected growth. A concentration of 100 μM Cd inhibited growth by 16%, with respect to control. Cd concentration in callus increased with increasing Cd concentrations in the growth medium. Callus accumulated 530 mg Cd kg−1 of their dry weight at 100 μM Cd concentration. Sesbania callus responded to Cd-induced oxidative stress by modulating antioxidants (glutathione and other non-protein thiols) level and antioxidative enzymes: superoxide dismutase (SOD), ascorbate peroxidase (APX), and glutathione reductase (GR). The content of the glutathione (GSH) and GSH/GSSG ratio first increased up to a concentration of 50 μM Cd and then decreased. The content of other non-protein thiols significantly increased with increasing Cd concentrations in the growth medium. The activities of antioxidative enzymes, SOD, APX, and GR, followed the same trends as antioxidants first increasing up to a concentration of 50 μM Cd and then decreasing. These results suggest that antioxidative defense mechanisms play a significant role in Cd detoxification and accumulation in Sesbania drummondii.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Cadmium (Cd) is one of the most toxic heavy metals due to its high toxicity and great solubility in water (Lockwood 1976). It is supplied to soil and water mainly by effluent from industries, mining, burning, and leakage of waste, fertilization with phosphate, and sewage sludge (Nriagu and Pacyna 1988).
One possible mechanism, in which elevated concentrations of heavy metals may damage plant tissues, is the stimulation of free radical production by imposing oxidative stress (Foyer et al. 1997). Heavy metals like copper (Cu) and iron (Fe) can be toxic because of their participation in redox cycles producing hydroxyl radicals (.OH) which are extremely toxic to living cells (Stohs and Bagchi 1995). By contrast with those metals, Cd is a non-redox metal that is strongly phytotoxic and causes growth inhibition and plant death. It produces alterations in the functionality of membranes by inducing changes in lipid composition (Quariti et al. 1997) and by affecting the enzymatic activities associated with membranes, such as the H+- ATPase (Fodor et al. 1995). Cd is also reported to damage the photosynthetic apparatus (Krupa 1988; Sidlecka and Baszynsky 1993), decrease chlorophyll content (Stobart et al. 1985; Larsson et al. 1998), and inhibit the stomatal opening (Barcelo and Poschenrieder 1990).
Plants possess several antioxidative defense systems to scavenge toxic free radicals in order to protect themselves from the oxidant stress including that caused by heavy metals. The antioxidative defense system falls into two general classes: (1) low molecular weight antioxidants, which consist of lipid-soluble membrane-associated antioxidants (e.g., α-tocopherol and β-carotene), and water-soluble reductants (e.g., glutathione and ascorbate); and (2) antioxidative enzymes: superoxide dismutase (SOD) ascorbate peroxidase (APX), catalase (CAT), and glutathione reductase (GR).
Glutathione (GSH), a sulfur containing tripeptide, is considered to be a very important antioxidant involved in cellular defense against toxicants (Scott et al. 1993). It can react chemically with singlet oxygen, superoxide and hydroxyl radicals, and, therefore, function directly as a free radical scavenger. It is also the precursor for the phytochelatins that act as heavy metal binding peptides in plants (Rosen 2002). GSH levels in plant tissues are known to change under metal stress (Koricheva et al. 1997). While the role of GSH as an important cellular antioxidant is generally known, several aspects of the functions of this compound remain debatable (Bartosz 1996). SOD, the first enzyme in the detoxifying process, converts O ·2 radicals to H2O2 (Polle and Rennenberg 1994). In the ascorbate-glutathione cycle, the enzymatic action of APX reduces H2O2 using ascorbate as an electron donor. Oxidized ascorbate is then reduced by GSH, which is generated from oxidized glutathione (GSSG) by GR at the expense of NADPH. GR also plays an important role in protecting against oxidative damage by maintaining a high GSH/GSSG ratio (Broadbent et al. 1995; Foyer et al. 1995, 1997). Increased activities of these enzymes have been reported in plants exposed to metals (Chaoui et al. 1997; Koricheva et al. 1997).
Remediation of metal-contaminated soils became a goal for many research laboratories in the world. Hyperaccumulator plants represent a resource for phytoremediation of metal-polluted soils, as they are able to extract metals from the soils and to concentrate them in their upper parts (Brooks 1998). Most of the hyperaccumulators are restricted to metal-enriched soils and also have the character of metal tolerance (Brooks 1998). Sesbania drummondii is a perennial shrub with high biomass productivity and distributed in southern coastal areas of the United States and known to hyperaccumulate Pb (Sahi et al. 2002; Ruley 2004).
Tissue cultures of plant species such as tobacco, sunflower, soybean, and Cuscuta reflexa have been used to understand the mechanism of metal resistance (Bueno and Piqueras 2002; Gallego et al. 2002; Sobkowiak et al. 2004; Srivastava et al. 2004). In vitro culture of plant cells in the presence of high concentrations of metals provides a useful tool to study the adaptive mechanism of plants living in adverse environments. The objectives of the present investigation were to study (1) the effects of Cd on the growth of Sesbania callus, (2) the uptake of Cd by Sesbania callus, and (3) the effects of Cd on the level of antioxidants (GSH and other non-protein thiols) and activities of antioxidative enzymes (SOD, APX, and GR).
Materials and Methods
Establishment of Sesbania Suspension Culture
Callus was induced from hypocotyls or cotyledonary leaf segments of Sesbania seedlings (Cheepala et al. 2004). After 4–6 weeks of callus proliferation, 10 g of callus mass was homogenized aseptically in 50 ml of the Murashige-Skoog (MS) medium (Murashige and Skoog 1962) and used as a stock culture to initiate suspension cultures in 250-ml flasks. Equal volumes (5 ml) of the stock cultures were transferred to 100 ml growth medium [MS + 2 mg L−1 Benzyladenine (BA) + 1.5 mg L−1 naphthalene acetic acid (NAA)]. Flasks containing cultures were placed on a shaker (125 rpm) and incubated for 4–5 weeks at 25 ± 2°C in 16-h photoperiod of 50 μmol m−2 s−2 irradiance.
Cadmium Treatment and Growth Assay
Exponentially growing cells were filtered through three layers of sterile cheese cloth and resuspended in fresh growth medium at a concentration of 200 mg cells ml−1. The cell suspension was continuously stirred while pipetting 0.5ml aliquots onto the surface of 8cm discs of Whatman No. 2 qualitative filter paper that had been placed on 25 ml of agar (0.8%) medium in 100 mm × 25 mm plastic petriplates. The agar-growth medium contained MS + 2 mg L−1 BA + 1.5 mg L−1 NAA and different concentrations of CdCl2 (0– 250 μM). Fresh weight of culture was measured using a filter paper growth assay (Horsch et al. 1980). Culture growth was recorded at every week for four weeks and expressed in % increase in fresh weight.
Estimation of Cd
Callus was harvested after four weeks of growth under control and Cd media, thoroughly washed with deionized water, and oven-dried at 70°C for two days. Sample was weighed and transferred to a pre-cleaned screw-capped Teflon beaker. Two ml of concentrated HNO3 (16N) was added to the sample and the beaker was placed on a hot plate at a temperature of 100°C overnight, then evaporated to dryness. The residue was dissolved in 1 ml of 8N HNO3 and then transferred to a Nalgene LDPE bottle and diluted to 20 ml using deionized water. The analysis for Cd was carried out using the Element 2 (Finnegan MAT, Bremen, Germany) sector field high resolution Inductively Coupled Plasma Mass Spectrometry (ICP-MS). Analysis was performed using an external calibration procedure and indium was used as an internal standard to correct for instrumental drift and matrix effects (Schneegurt et al. 2001). Procedural blanks were analyzed to check for any contribution from the reagents.
Estimation of Glutathione and Other Non-Protein Thiols Contents
Reduced (GSH), oxidized (GSSG), and total glutathione contents were determined by the recycling method according to Anderson (1985). Fresh callus (0.5 g) was harvested after four weeks of growth under control and Cd media and homogenized in 3.0 ml of 5% sulfosalicylic acid under cold conditions. The homogenate was centrifuged at 10,000 rpm for 10 min. A 0.5ml aliquot was taken in a microfuge tube, to which 0.5 ml reaction buffer [0.1 M phosphate buffer (pH 7.0), 0.5 mM ethylenediaminetetraacetic acid (EDTA)] and 50 μl of 3 mM 5′ dithio-bis-(2-nitrobenzoic acid) (DTNB) were added. After 5 min, absorbance for determination of GSH was read at 412 nm using UV-vis spectrophotometer (Model Ultrospec 3000, Pharmacia Biotech, USA). To the same tube, 100 μl of NADPH (0.4 mM) and 2 μl GR was added for the determination of total glutathione; the reaction was allowed to run for 20 min. The amount of GSSG was calculated by subtracting GSH from total glutathione concentrations. A standard curve was prepared from varying concentrations of reduced glutathione.
Other non-protein thiols were determined as described by Del Longo et al. (1993). One hundred μl of the aliquot (described above) was taken in the microfuge tube, to which 0.5 ml reaction buffer [0.1 M phosphate buffer (pH 7.0), 0.5 mM EDTA] and 0.5 ml of DTNB (1 mM) were added. The reaction mixture was incubated for 10 min and absorbance was read at 412 nm using UV-vis spectrophotometer (Model Ultrospec 3000, Pharmacia Biotech). Values were corrected for the absorbance by preparing a blank without extract. A standard curve was prepared from varying concentrations of cysteine to calculate the other non-protein thiols content in samples.
Antioxidative Enzymes
Extraction of Enzymes
Fresh callus (1.0 g) was harvested after four weeks of growth in different Cd concentrations, and homogenized under ice-cold conditions in 5.0 ml of extraction buffer, containing 50 mM phosphate buffer (pH 7.5), 1% polyvinylpyrrolidone (PVP), 0.5% Triton X−100, and 1 mM EDTA. The homogenate was centrifuged at 10,000 rpm for 20 min at 4°C. The supernatant was used to measure the activities of SOD, APX, and GR. Protein content in the supernatant was estimated by the method of Bradford (1976) using bovine albumin as a standard.
Assay of Superoxide Dismutase
SOD activity was measured according to the method of Beauchamp and Fridovich (1971). The reaction mixture (1.5 ml) contained 100 mM potassium phosphate buffer (pH 7.8), 0.1 mM EDTA, 13 mM methionine, 2.25 mM nitroblue tetrazolium (NBT), 60 μM riboflavin, and enzyme extract. After mixing, the contents in the cuvet were illuminated for 10 min. A tube with enzyme extract kept in the dark served as a blank, while the control tube contained no enzyme extract and was kept in light. The absorbance was measured at 560 nm against blank using UV-vis spectrophotometer (Model Ultrospec 3000, Pharmacia Biotech). NBT reduction in the light was measured in the presence and absence of enzyme extract. SOD activity is presented as absorbance of control minus absorbance of sample, giving the total inhibition. One unit of activity is the amount of enzyme required for 50% reduction in color and was expressed in units of the enzyme (mg−1 protein h−1).
Assay of Ascorbate Peroxidase
Ascorbate peroxidase activity was measured by the method of Nakano and Asada (1987). The reaction mixture (1 ml) contained 100 mM potassium phosphate buffer (pH 7.0), 0.5 mM ascorbate, 0.3 mM H2O2 and enzyme extract. The oxidation of ascorbic acid was measured by the decrease in absorbance at 290 nm for 3 min using UV-vis spectrophotometer (Model Ultrospec 3000, Pharmacia Biotech). The enzyme activity was calculated using the extinction coefficient 2.8 mM−1 cm−1 and expressed in enzyme units (mg protein)−1. One unit of enzyme is the amount necessary to decompose 1 μmol of substrate per min at 25°C.
Assay of Glutathione Reductase
GR activity was determined as described by Rao et al. (1996). GR activity was measured by monitoring the glutathione-dependant oxidation of NADPH at 340 nm. The reaction mixture (1 ml) contained 100 mM potassium phosphate buffer (pH 7.5), 1 mM EDTA, 0.2 mM NADPH, 0.5 mM oxidized glutathione, and enzyme extract. The reaction was allowed to run for 3 min using UV-vis spectrophotometer (Model Ultrospec 3000, Pharmacia Biotech). The enzyme activity was calculated using extinction coefficient 6.2 mM−1 cm−1 and expressed in enzyme units (mg protein)−1. One unit of enzyme is the amount necessary to decompose 1 μmol of NADPH per min at 25°C.
Statistical Analysis
Values in figures and tables are mean values of four independent replicates. Significant differences in each treatment relative to control were analyzed by Student’s t-test using SYSTAT (version 9 for Windows, 1999, Systat software Inc., Richmond, CA).
Results
Effect of Cd on Growth of Sesbania Callus
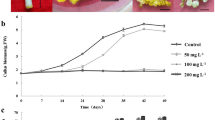
As shown in Figure 1, Sesbania callus grew and proliferated with time on increasing Cd concentrations up to 100 μM. The growth of callus was comparable to that of the control, up to 50 μM Cd concentration. A 16% reduction in callus growth was observed at a concentration of 100 μM Cd, with respect to the control. However, callus growth was negligible at 250 μM Cd concentration.
Accumulation of Cd in Sesbania Callus
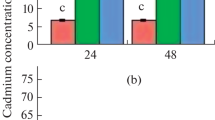
Cadmium concentration in Sesbania callus increased with increasing concentrations of Cd in the growth medium (Fig. 2). The callus accumulated 260 and 530 mg Cd kg−1 (tissue dry weight) at the concentration of 50 and 100 μM Cd, respectively. At a concentration of 250 μM of Cd, Sesbania callus accumulated 1678 mg Cd kg−1 dry weight (DW) (data not presented) but growth at this concentration was negligible.
Effect of Cd on Glutathione and Non-Protein Thiols Levels in Sesbania Callus
Cd treatments altered the levels of GSH and GSSG level in Sesbania callus (Table 1). Cd at a concentration of 50 μM significantly increased GSH level (94.7%) and GSH/GSSG ratio (36.7%), with respect to the control. A slight reduction in GSH as well as in GSH/GSSG ratio was observed at a concentration of 100 μM Cd.
Levels of other non-protein thiols also showed a significant increase with increase in concentrations of Cd in media (Fig. 3). Cd produced an increase in non-protein thiols level by 122.2 and 144.4% at 50 and 100 μM concentrations, respectively, when compared with the control. At 250 μM, callus growth was negligible, thus no antioxidative data was collected at this concentration.
Effect of Cd on Superoxide Dismutase Activity of Sesbania Callus
The effect of varying concentrations of Cd on SOD activity of callus of Sesbania showed an increase in activity in a concentration-dependent manner up to 50 μM Cd (Fig. 4). The activity slightly decreased at the concentration of 100 μM Cd; however, the activity was appreciably higher with respect to the control. The SOD activity in the presence of 50 and 100 μM Cd was 2.83- and 2.70-fold higher, respectively, with respect to the control.
Effect of Cd on Ascorbate Peroxidase Activity of Sesbania Callus
The effect of Cd on APX activity of callus of Sesbania exhibited a pattern similar to that of SOD (Fig. 5). The APX activity in the presence of 50 and 100 μM Cd was 3.28- and 3.21-fold higher, respectively, with respect to the control.
Effect of Cd on Glutathione Reductase Activity of Sesbania Callus
GR activity also increased significantly up to 50 μM Cd and then decreased (Fig. 6). The activity in the presence of 50 and 100 μM Cd increased by 62.5 and 56.2%, respectively, with respect to the control. However, no significant change in GR activity was observed among the different treatments of Cd. The enzyme activity was significantly higher at each treatment when compared to control.
Discussion
The present study shows that the antioxidative defense system plays an important role in Cd tolerance and accumulation in Sesbania drummondii callus. Sesbania drummondii has been shown as an accumulator of Pb (Sahi et al. 2002; Ruley 2004) but its response to Cd was not known. Therefore, Sesbania cell cultures were established in the presence of increasing concentrations of Cd to evaluate its defense mechanism at a cellular level. Sesbania callus grew well up to 50 μM Cd while the growth was significantly inhibited at a concentration of 100 μM Cd, with respect to the control. The growth was negligible at a concentration of 250 μM Cd (Fig. 1). Therefore, the antioxidative study was focused at or below the concentration of 100 μM Cd. The reduction of callus growth at high levels of Cd may be correlated to high Cd accumulations by callus (Fig. 2). In that case, cells might have to spend extra energy to cope with the high Cd concentration in the tissues (Gregger 1999). The growth pattern of Sesbania callus in the presence of Cd was similar to Helianthus annuus L. (Gallego et al. 2002) and Cuscuta reflexa callus (Srivastava et al. 2004), in which callus growth was significantly inhibited at higher concentration of Cd (> 50 μM). However, growth response in Sesbania is different from soybean cell cultures, where lower concentrations (≥ 6 μM) of Cd significantly inhibit the soybean cells growth (Sobkowiak et al. 2004).
Similar to the earlier report of Cd accumulation by whole plants (Jiang et al. 2001; Schickler and Caspi 1999), the Cd concentration in callus increased with increasing Cd concentrations in the growth media (Fig. 2). Enormous Cd accumulation (1678 mg kg−1) in cells was noticed at 250 μM Cd in growth medium; this explains why callus registers a negligible growth at this concentration. At a concentration of 100 μM Cd, Sesbania callus accumulated 530 mg Cd kg−1 dry weight, which is a level well above the threshold for Cd hyperaccumulation (100 mg Cd kg−1) (Brooks 1998).
Accumulations of Cd in Sesbania callus were accompanied by concomitant induction in the levels of antioxidants (Glutathione and other non-protein thiols) and activities of antioxidative enzymes such as SOD, APX, and GR. In this study a significant increase in GSH level was observed with Cd treatments (Table 1). This suggests active participation of GSH in detoxification of oxygen species and free radicals (Asada and Takahashi 1987). This result was in agreement with those observed in Brassica (Zhu et al. 1999), Chinese brake fern (Pteris vittata L.) (Cao et al. 2004), and Thlaspi caerulescens (Freeman et al. 2004), in which enhanced levels of GSH improved Cd, As, and Ni tolerance and accumulation, respectively. An increase in the GSH pool was correlated with increasing concentrations of Zn in Brassica juncea (Prasad et al. 1999). However, responses in Sesbania differs from those observed in Helianthus annuus L. (Gallego et al. 1996) and Scenedesmus bijugatus (Nagalakshmi and Prasad 2001), in which decreased levels of GSH were recorded in response to Cd and Cu, respectively.
Intracellular concentration of GSSG increases at the expense of GSH under severe stress conditions (Strid 1993). A high GSH/GSSG ratio is necessary to sustain the role of glutathione as an antioxidant and a reductant (Foyer et al. 1997). It is also essential to keep glutathione in its reduced form in order for its incorporation into phytochelatins (Cobbett 2000). Cd treatments in this study significantly enhanced the GSH/GSSG ratio up to a concentration of 50 μM. This indicates the potential of Sesbania callus to tolerate Cd stress. GR catalyzes the reduction of GSSG to GSH at the expense of NADPH; this is an important determinant in the maintaining of the glutathione pool. In this study, a considerable change in GR activity proves the above hypothesis and is consistent with findings on Cuscuta reflexa callus (Srivastava et al. 2004). An increase in GR activity was also reported in seedlings of bean (Chaoui et al. 1997) and Brassica juncea (Prasad et al. 1999) exposed to Cd and Zn. Conversely, a decrease in GR activity has also been reported in response to Cu and Cd stress (Mazhoudi et al. 1997; Patra and Panda 1998).
Cd treatments also induced the contents of other non-protein thiols in Sesbania callus. The increase in other non-protein thiols was greater than that in GSH as a result of Cd treatments. At a concentration of 50 μM Cd, the contents of other non-protein thiols increased by 122.2%, while GSH increased by 94.7%, compared to the control (Fig. 3). Based on the above trends, this study suggests that other non-protein thiols also play an important role in Cd detoxification.
Similar to antioxidants, a significant change in the activity of antioxidative enzymes was recorded in Sesbania exposed to Cd. SOD activity in the present study was elevated in most of the treatments. An increase of 283% with respect to the control was observed at a 50μM Cd concentration (Fig. 4), though a slight decrease was recorded at 100 μM Cd. This trend was compatible to SOD activity reported in the adapted calli of sunflower, cultivated under Cd, Al, and Cr treatments (Gallego et al. 2002). Other studies also indicated Cd induced SOD activity in a number of plant systems (Okamoto et al. 1996; Chaoui et al. 1997; Schickler and Caspi 1999). Though some reports also refer to a decrease or no change in the activity of SOD with Cd (Kato and Shimizu 1987).
Activity of APX, another important enzyme of the antioxidative defense system, was also induced in response to Cd treatments in the Sesbania callus. APX protects the cell against oxidative damage by detoxifying toxic H2O2. An increase in APX activity under Cd stress suggests its role in the detoxification of H2O2 in Sesbania callus. As discussed earlier, APX reduces H2O2 into water using ascorbate as the electron donor, resulting in the formation of dehydroascorbate. It is recycled back to ascorbate using reduced GSH as an electron donor, and the oxidized glutathione (GSSG) is converted back to GSH by NADPH-dependent enzyme glutathione reductase (Asada and Takahashi 1987). The participation of GR in this pathway, which is activated upon Cd stress as observed in our study, is the best documented role for this enzyme (Chaoui et al. 1997; Stroinski et al. 1999). The increase in APX activity in this study is comparable to the activity in the adapted callus of sunflower (Gallego et al. 2002). The stimulation of APX activity has also been reported in several plants subjected to Cd, Zn, Cu, Pb, and Fe treatment (Karataglis et al. 1991, Kampfenkel et al. 1995, Weckx and Clijsters 1996, Patra and Panda 1998, Prasad et al. 1999, Rucinska et al. 1999). The increased activities of SOD and APX in Sesbania callus under Cd stress are circumstantial evidence for tolerance mechanisms developed by this plant.
In conclusion, the results clearly indicate that the upper limit of Cd tolerance in Sesbania drummondii callus is 100 μM and higher concentrations cause toxicity to the cells. Cd accumulation was significantly enhanced with increasing concentrations of Cd in the medium, suggesting its usefulness in phytoremediation research. A coordinated increase in non-enzymatic antioxidants (GSH) and enzymatic antioxidants (SOD, APX, and GR) was noted under Cd stress, consistent with callus Cd concentrations. This indicates the role of these enzymes and GSH in Cd tolerance and accumulation in Sesbania callus.
References
Anderson ME (1985) Determination of glutathione and glutathione disulfide in biological samples. Method Enzymol 113:548–554
Asada K, Takahashi M (1987) Production and scavenging of active oxygen in photosynthesis. In: Kyle DJ, Osmond CJ, Arntzen CJ (ed) Photo inhibition: Topics in Photosynthesis, Elsevier, Amsterdam, p 227
Barcelo J, Poschenrieder C (1990) Plant water relation as affected by heavy metal stress: review. J Plant Nutr 13:1–37
Bartosz G (1996) Glutathione as antioxidant and electrophile scavenger. Pol J Environ Stud 5:87–88
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Broadbent P, Creissen GP, Kular B, Wellburn AR, Mullineaux PM (1995) Oxidative stress responses in transgenic tobacco containing altered levels of glutathione reductase activity. Plant J 8:247–255
Brooks RR (1998) Plants that hyperaccumulate heavy metals. CAB International, Wallingford, UK
Bueno P, Piqueras A (2002) Effect of transition metals on stress, lipid peroxidation and antioxidant enzyme activities in tobacco cell cultures. Plant Growth Regul 36:161–167
Cao X, Ma LQ, Tu C (2004) Antioxidative responses to arsenic in the arsenic hyperaccumulator Chinese brake fern (Pteris vittata L.). Environ Pollut 128:317–325
Chaoui A, Mazhouri S, Ghorbal MH, Ferjani EE (1997) Cadmium and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in bean (Phaseolus vulgaris L.). Plant Sci 127:139–147
Cheepala SB, Sharma NC, Sahi SV (2004) Rapid in vitro regeneration of Sesbania drummondii. Biol Plant 48:13–18
Cobbett CS (2000) Phytochelatins and their roles in heavy metal detoxification. Plant Physiol 123:825–832
Del longo OT, Gonzalez CA, Pastori GM, Trippi VS (1993) Antioxidant defenses under hyperoxygenic and hyperosmotic conditions in leaves of two lines of maize with differential sensitivity to drought. Plant Cell Physiol 34:1023–1028
Fodor A, Szabo-Nagy A, Erdei L (1995) The effect of cadmium on the fluidity and H+- ATPase activity of plasma membrane from sunflower and wheat roots. J Plant Physiol 14:787–792
Foyer CH, Lopez-Delgado H, Dat JF, Scott IM (1997) Hydrogen peroxide and glutathione associated mechanisms of acclamatory stress tolerance and signaling. Physiol Plant 100:241–254
Foyer CH, Souriau N, Perret S, Lelandais M, Kunert KJ, Pruvost C, Jouanin L (1995) Over-expression of glutathione reductase but not glutathione synthetase leads to increase in antioxidant capacity and improved photosynthesis in poplar (Populus tremula x P. alba ) tress. Plant Physiol 109:1047–1057
Freeman JL, Persans MW, Nieman K, Albrecht C, Peer W, Pickering I, Salt DE (2004) Increased glutathione biosynthesis plays a role in nickel tolerance in Thlaspi nickel hyperaccumulators. Plant Cell 16:2176–2191
Gallego SM, Benavides MP, Tomaro ML (1996) Effect of heavy metal ion excess on sunflower leaves: evidence for involvement of oxidative stress. Plant Sci 121:151–159
Gallego S, Benavides M, Tomaro M (2002) Involvement of an antioxidant defense system in the adaptive response to heavy metal ions in Helianthus annuus L. cells. Plant Growth Regul 36:267–273
Gregger M (1999) Metal availability and bioconcentration in plants. In: Hagemeyer J (ed) Heavy metals stress in plants: from molecules to ecosystems. Springer, Berlin, p 1
Horsch RB, King J, Jones GE (1980) Measurement of cultured plant cell growth on filter paper discs. Can J Bot 58:2402–2406
Jiang W, Liu D, Hou W (2001) Hyperaccumulation of cadmium by roots, bulbs and shoots of garlic (Allium sativum L.). Bioresource Technol 76:9–13
Kampfenkel K, Van Montagu M, Inze D (1995) Effects on iron excess on Nicotiana plumbaginifolia plants. Plant Physiol 107:725–735
Karataglis S, Moustakas M, Symeonidis L (1991) Effect of heavy metals on isoperoxidases of wheat. Biol Plant 33:3–9
Kato M, Shimizu S (1987) Chlorophyll metabolism in higher plants VII. Chlorophyll degradation in senscing tobacco leaves: phenolic dependant peroxidative degradation. Can J Bot 65:729–735
Koricheva J, Roy S, Vranjic JA, Haukioja E, Hughes PR, Hanninen O (1997) Antioxidants responses to stimulated acid rain and heavy metal deposition in birch seedlings. Environ Pollut 95:249–258
Krupa Z (1988) Cadmium induced changes in the composition and structure of the light harvesting complex II in radish cotyledons. Physiol Plant 73:518–524
Larsson EH, Bordman JF, Asp H (1998) Influence of UV-B radiation and Cd2+ on chlorophyll fluorescence, growth and nutrient content in Brassica napus. J Exp Bot 49:1031–1039
Lockwood MP (1976) Effects of pollutants on aquatic organisms. Cambridge University Press, New York
Mazhoudi S, Chaoui A, Ghorbal MH, Ferjani EE (1997) Response of antioxidant enzymes to excess copper in tomato (Lycopersicon esculentum, Mill.). Plant Sci 127:129–137
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15:473–497
Nagalakshmi N, Prasad MNV (2001) Responses of glutathione cycle enzymes and glutathione metabolism to copper stress in Scenedesmus bijugatus. Plant Sci 160:291–299
Nakano Y, Asada K (1987) Purification of ascorbate peroxidase in spinach chloroplasts: its inactivation in ascorbate depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol 28:131–140
Nriagu JO, Pacyna JM (1988) Quantitative assessment of world wide contamination of air, water and soils with trace metals. Nature 333:134–139
Okamoto OK, Asano CS, Aidar E, Colepicolo P (1996) Effects of cadmium on growth and superoxide dismutase activity of the marine microalga Tetraselmis gracilis (Prasinophaceae). J Phycol 32:74–79
Patra J, Panda BB (1998) A comparison of biochemical responses to oxidative and metal stress in seedlings of barley, Hordeum vulgare L. Environ Pollut 101:99–105
Polle A, Rennenberg H (1994) Photooxidative stress in trees. In: Foyer CH, Mullineaux PM (ed) Causes of photooxidative stress and amelioration of defense system in plants. CRC Press, Boca Raton FL, p 199
Prasad KVSK, Saradhi PP, Sharmila P (1999) Concerted action of antioxidant enzymes and curtailed growth under zinc toxicity in Brassica juncea. Environ Exp Bot 42:1–10
Quariti O, Boussama N, Zarrouk M, Cherif A, Ghorbal MH (1997) Cadmium and copper induced changes in tomato membrane lipids. Phytochemistry 45:1343–1350
Rao MV, Paliyath G, Ormrod DP (1996) Ultraviolet- B and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol 110:125–136
Rosen BP (2002) Biochemistry of arsenic detoxification. FEBS Lett 529:86–92
Rucinska R, Waplak S, Gwozoz EA (1999) Free radical formation and activity of antioxidant enzymes in lupin roots exposed to lead. Plant Physiol Biochem 37:187–194
Ruley AT (2004) Effects of accumulation of lead and synthetic chelators on the physiology and biochemistry of Sesbania drummondii. M.S. Thesis, Western Kentucky University, Bowling Green, KY.
Sahi SV, Bryant NL, Sharma NC, Singh SR (2002) Characterization of a lead hyperaccumulator shrub, Sesbania drummondii. Environ Sci Technol 36:4676–4680
Schickler H, Caspi H (1999) Response of antioxidative enzymes to nickel and cadmium stress in hyperaccumulator plants of the genus Alyssum. Physiol Plant 105:39–44
Schneegurt MA, Jain JC, Menicuccu JA, Jr Brown S, Garafalo DF, Quallick M, Neal CR, Kulpa CF (2001) Biomass products for the remediation of wastewaters contaminated with toxic metals. Environ Sci Technol 35:3786–3791
Scott N, Hatlelid KM, MacKenzie NE, Carter DE (1993) Reactions of arsenic (III) and arsenic (V) species with glutathione. Chem Res Toxicol 6:102–106
Sidlecka A, Baszynsky T (1993) Inhibition of electron flow around photosystem I in chloroplast of cadmium treated maize plants is due to cadmium induced iron deficiency. Physiol Plant 87:199–202
Sobkowiak R, Rymer K, Rucinska R, Deckert J (2004) Cadmium-induced changes in antioxidant enzymes in suspension culture of soybean cells. Acta Biochim Polini 51:219–222
Srivastava S, Tripathi RD, Dwivedi UN (2004) Synthesis of phytochelatins and modulation of antioxidants in response to cadmium stress in Cuscuta reflexa-an angiospermic parasite. J Plant Physiol 161:665–674
Stobart AK, Griffiths WT, Ameen-Bukhari I, Sherwood RP (1985) The effect of Cd2+ on the biosynthesis of chlorophyll in leaves of barley. Physiol Plant 63:293–298
Stohs SJ, Bagchi D (1995) Oxidative mechanism in the toxicity of metal ions. Free Radical Biology and Medicine 18:321–336
Strid A (1993) Increased expression of defense genes in Pisum sativum after exposure to supplementary ultraviolet –B radiation. Plant Cell Physiol 34:949–953
Stroinski A, Kubis J, Zielezinska M (1999) Effect of cadmium on glutathione reductase in potato tubers. Acta Physiol Plant 21:201–207
Weckx J, Clijsters H (1996) Oxidative damage and defense mechanism in primary leaves of Phaseolus vulgaris as a result of root assimilation of toxic amounts of copper. Physiol Plant 96:506–512
Zhu YL, Pilon- Smits EAH, Jouanin L, Terry N (1999) Overexpression of glutathione synthetase in Indian mustard enhances cadmium accumulation and tolerance. Plant Physiol 119:73–80
Acknowledgments
The authors are thankful to Dr. Nilesh Sharma for technical help and critical review of this manuscript. Financial support from the Applied Research and Technology Program of Ogden College of Science and Engineering is greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Israr, M., Sahi, S.V. & Jain, J. Cadmium Accumulation and Antioxidative Responses in the Sesbania drummondii Callus. Arch Environ Contam Toxicol 50, 121–127 (2006). https://doi.org/10.1007/s00244-005-5029-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-005-5029-x