Abstract
The impact of different levels of dietary β-carotene to alleviate the effect of mercuric chloride toxicity in Nile tilapia was assessed. Semi-purified diets containing 0, 40, and 100 mg β-carotene kg−1 dry diet were fed for 21 days, which were subjected to sublethal concentration of mercuric chloride (0.05 ppm). Hematological and biochemical parameters, lipid profile, and antioxidant response were examined. All hematological parameters of tilapia fish starting from second week of toxicity were significantly decreased. A significant increasing trend in liver enzymes (ALT and AST) were observed parallel to the time of toxicity and peroxide radicals (MDA) appearing significantly increased in toxicated group without carotene supplement, although carotene supplementation return all parameters within the control levels. Mercury accumulated significantly in fish liver and white muscles in toxicated group while it showed a significant reduction in dietary β-carotene-treated group. Overall, it can be used as immunostimulant and alleviate the suppression effect resulted from immune depressive stressful condition in farmed Nile tilapia.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With increasing intense activity of the industrial and agriculture sectors, water pollution has become of great concern to aquatic organisms like fish. Heavy metal pollution is one of the most important water pollutants (Singer et al. 2005) that are persistent in the environment and have the ability to accumulate by aquatic organisms (Veena et al. 1997). Cultured fish live in intimate contact with their environment with constant exposure to stressors, like handling, crowding, and pathogens beside the pollution; notably, heavy metals that lead to several physiological changes that eventually lead to immune depression and increase the infections outbreaks (Low and Sin 1998).
Mercury (Hg), one of the most toxic heavy metals, it is considered as blacklisted pollutants due to their persistence and accumulation in the ecosystems (Moore and Ramamoorthy 1984). It takes many form in the environment, the most common ones are elemental mercury, inorganic mercury, and organic compounds as methyl mercury (MeHg). Organic mercury is the most toxic form; however, the inorganic mercury is the most common form that present in the aquatic environment with subsequent effect on fish (de Oliveira Ribeiro et al. 2002). Toxicity of fish by mercury has been studied in several Egyptian lakes (Adel 2003; Ahmed and Hussein 2004). Recently, mercury toxicity have been linked to generation of reactive oxygen species (ROS) resulted in changes in the antioxidant defense systems and oxidative damage induction such as lipid peroxidation leading to cell death (Berntssen et al. 2003; Elia et al. 2003; Milaeva 2006; Larose et al. 2008; Verlecar et al. 2008, 2007) and different pathologic processes involved as a causative agents of many fish diseases (Kehrer 1993; Banerjee et al. 1999).
Defenses against ROS include scavenger compounds, enzymatic, and non-enzymatic antioxidants are required to maintain the redox status of fish cells and act as an important biological defense against oxidative stress (Monteiro et al. 2010).
Carotenoids constitute a widespread group of plant-synthesized polyene pigments, which vary in color from yellow and orange to red (Tacon 1981). Carotenoids have several beneficial effects on aquatic animals; they enhance larval growth and survival (Torrissen 1984) and improve the performance of brood stock (Watanabe et al. 1991; Verakunpiriya et al. 1997), as well as increase resistance to diseases (Tachibana et al. 1997).
β-Carotene is a non-toxic carotenoid with immune modulatory effect on animals and humans (Garewal et al. 1992). Moreover, carotene has been revealed to be an efficient scavenger of free radicals, notably under low partial pressure of oxygen (Burton and Ingold 1984).
Carotenoids are one of the non-enzymatic groups of the antioxidant system protecting against oxidative damage through two mechanisms: (1) quenching of singlet oxygen, and (2) scavenging of radicals. Therefore, carotenoids have multi-functions as antioxidants (Wang et al. 2006).
This study was, therefore, undertaken to investigate if dietary supplementation of β-Carotene at various concentrations would affect the mercury bioaccumulation in tissue, the oxidative stress response and oxidative damage induced through mercuric chloride toxicity in Nile tilapia.
Materials and methods
Experimental fish
One hundred and twenty-eight Nile tilapia (Oreochromis niloticus) weighing 80–100 g were obtained from a private fish farm in Gamasa, Dakhlyia, Egypt. Fish were acclimated in indoor fiberglass tanks at 25–27 °C for 2 weeks prior to beginning the experiment. During that time, fish were fed ad lib twice daily with standard commercially prepared pellets at 3 % of their body weight. Fecal matters were siphoned out once daily. Ammonia, nitrite, and pH were monitored weekly. Fish were maintained on a photoperiod of 12-h light/12-h dark.
Preparation of diet and feeding
Existing information on the nutritional requirement of O. niloticus was used for formulation of the diets (National Research Council 1993). Casein-based semi-purified diets were prepared contain 0 mg (control) and M groups (HgCl2 0.05 ppm), 40 mg (M + C40 group) and 100 (M + C100 group) of β-carotene kg−1 dry diet (Table 1). β-carotene (Carotene®, kahira pharmaceuticals and chemical industrial Co., Cairo, Egypt) were incorporated to provide the respective carotenoids at levels of 40–100 mg kg−1 diet according to Amar et al. (2004, 2000b), β-carotene-supplemented diets did not contain vitamins A or any other sources of carotenoids included in the mix. The ingredients were mixed thoroughly, pelletized, vacuum freeze-dried, and the prepared diets were stored at −20 °C or kept at 4 °C in between feedings. The diets were offered at 0900–1700 hours to apparent satiation.
Experimental design
Sixteen fish were placed in each of eight, 60-L freshwater aquaria (total N = 128). All fish began feeding normally. At day 1, all fish were switched to semi-purified diets (Table 1) prepared to be control (1st group), and 2nd group (M group) was exposed throughout the experiment to sublethal concentration of mercuric chloride (HgCl2) (0.05 ppm) according to previous toxicological assay for 96 h LC50 of (Monteiro et al. 2010), and the 3rd (M + C40 group) and 4th (M + C100 group) were simultaneously fed with 40–100 mg β-carotene kg−1 dry diet and exposed to HgCl2 for 21 days, respectively. Duplicate aquaria were assigned to each one of four treatments. During the experiment, 75 % water changes were done twice weekly and replaced with fresh dechlorinated water with same toxicant concentration; water quality was measured (ammonia, nitrite, nitrate, pH, and dissolved oxygen) twice weekly via water quality test kits (Aquarium Pharmaceuticals, Inc.). The daily water mercury concentration was monitored at the testing level.
Preparation of mercuric chloride stock solution
Stock solution of mercuric chloride (HgCl2 from Adwek company, Egypt) was prepared by dissolving the analytical grade mercuric chloride in double-distilled water, then desired HgCl2 concentration in part per million (ppm) was prepared from the stock solution.
Sample collection
Four fish from each aquarium (8 fish/group) were sampled at week 1–3. Each aquarium was sampled one at a time; the fish were sedated with a low dose of buffered tricane (30 mg L−1 tricane + 60 mg L−1 sodium bicarbonate), and each fish was then euthanized one at a time in a separate container having a high dose of buffered tricane (200 mg L−1 tricane + 400 mg L−1 sodium bicarbonate). Heparinized blood samples were collected from the caudal vein to determine the hematological data. Moreover, plasma was collected by centrifugation for 5 min at 3,000 rpm and store frozen until biochemical assay.
Tissue samples
At the end of the trial period, fish of all experimental groups were taken, liver and white muscle were carefully excised and washed with physiological saline (0.9 % NaCl) and immediately kept frozen at −80 °C until the mercury analysis assay.
Biochemical and hematological assay
Test kits of StanBio (USA) were used for determination of plasma lipid profile including total lipid TL, total cholesterol, Triglycerides TG, high-density lipoprotein HDL, and low-density lipoprotein LDL according to Fossati and Principe (1982).
Colorimetric determination of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) was done according to the method of Burtis and Ashwood (1999), using commercial kits supplied by Bio-diagnostic Egypt.
The plasma levels of Malondialdehyde (MDA) as a lipid peroxidation marker were measured by colorimetric method of (Ohkawa et al. 1979) using kits supplied by Bio-diagnostic (Egypt).
Blood hemoglobin (Hb) was assessed by Drabkin (l964). Red blood cell count (RBCs), hematocrit value (PCV), blood indices {mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), and total white blood cell count (WBC) were determined according to (Jain 1986).
Mercury analysis in the water and tissue
The analysis of water samples was carried out according to APHA (1985) using cold vapor atomic absorption spectrophotometer (Model 3100, Perkin-Elmer, Norwalk, Conn, USA). The quantification limit for the total mercury was 0.1 μg L−1.
The total mercury in the tissue was analyzed according to APHA (1985) using a PSA atomic fluorescence spectrophotometer (Model, Merlin, P.S. Analytical LTC, and Princeton, NJ, USA) equipped with a PSA random access auto sampler. The quantification limit for the total mercury was 0.05 mg kg.
Statistical analysis
All data were analyzed using SPSS version 17.0 (SPSS Inc, Chicago, IL). Data were analyzed using one-way ANOVA with Duncan’s post hoc test LSD multiple comparison test, to determine the significant differences between data in this study. The differences between means were analyzed at the 5 % probability level (P value <0.05 was considered statistically significant). All parameters were expressed as mean ± standard error.
Results
Hematological analysis
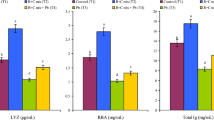
Regarding RBCs, Hb, PCV, MCHC, and WBCs values, there were significant decreases in the M group compared to the control and M + C100 group, while there were no significant differences with the M + C40 group at week1. However, by week 2, the M group was significantly decreased from all other groups. While, M + C40 returned to the value of the control one, and M + C100 exhibited higher value than control as well. The M group had a significant lower value than all other group. While, M + C100 restored the level to the control one by week 3, the M + C40 had a significant increase than both the control and M + C100 group by week 3 (Fig. 1a–c, e, f).
a–f Changes in different hematological parameters of Nile tilapia (O. niloticus) fed with 40–100 mg ß-carotene kg−1 dry diet (Mean ± SE) under HgCl2 exposure by a rate (0.05 ppm) for 21 days. N = 8 fish per sampling time. Values with different letters are significantly different between and within groups, **significant at (P < 0.001). M = HgCl2 only, M + C40 = HgCl2 with carotene 40 mg kg−1 diet, and M + C100 = HgCl2 with carotene 100 mg kg−1 diet
Generally, the HgCl2 level decreased these hematological parameters in fish while supplying carotene at dose 40 mg kg−1 could elevate the value again but not to the level of the control group, but on the contrary, supplying carotene at dose 100 mg kg−1 restores the level to the control one (Fig. 1a–c, e, f).
MCV encountered a significant increase in the M group compared to other groups. The M + C40 group getting significant lower level but still not at the level of the control group; while the M + C100 group had a significant decrease than M group which at or within the same level of the control one (Fig. 1d).
Biochemical analysis
Amino transferase levels exhibited different patterns between groups. A significant decrease in the M group compared to other groups at week 1. However, there were a significant increase compared to all other groups at week 2 and 3. The M + C40 and M + C100 group showed a significant increase compared to the M group and control group as well at week 1. While at week 2 and 3, they were significantly decreased from the M group and increased in comparison with the control group (Fig. 2a).
a–c Changes in ALT, AST, and MDA concentrations of Nile tilapia (O. niloticus) fed with 40–100 mg ß-carotene kg−1 dry diet (Mean ± SE) under HgCl2 exposure by a rate (0.05 ppm) for 21 days. N = 8 fish per sampling time. Values with different letters are significantly different between and within groups, **significant at (P < 0.001). M = HgCl2 only, M + C40 = HgCl2 with carotene 40 mg kg−1 diet, and M + C100 = HgCl2 with carotene 100 mg kg−1 diet
Aminotransferase levels showed a significant upregulation in the M group compared to all other groups at all time points. The M + C40 and M + C100 group showed a significant decrease compared to M group which exhibited at same time a significant increase from the control one too; but by week 3, both groups restore the AST level to the control one (Fig. 2b).
Malondialdehyde was significantly increased in the M group compared to the control one while it showed at same time a significant decrease from both M + C40 group and M + C100 group as well at week 1. Also, the M + C40 and M + C100 showed a significant rise from the control group at week 1. However, by week 2 and 3, MDA was significantly increased in the M group compared to all other groups, and both M + C40 and M + C100 restored significantly the higher MDA level of the M group to a little bit more than the control one (Fig. 2c).
In the present study, there were no significant differences in the plasma lipid parameters (TL, TG, Cholesterol, HDL, and LDL); however, levels were nominally significant and in the same trends like other Prameters tested; showing a decreased level in the M group, a little bit increase in the M + C40, but only in the M + C100 group; the levels were increased to be within the range of the control one (Table 2).
Mercury analysis in tissues
The total Hg concentrations in the liver and white muscle of Nile tilapia exposed to 0.05 ppm for three weeks were significantly increased in the M group in comparison with other group. Meanwhile both M + 40 and M + 100 groups encountered a significant decrease in Hg level. In the control group, no mercury was detected in any tissue (Fig. 3). Hg levels showed higher affinities to accumulate in liver than in white muscle.
Changes in hepatic and muscular mercury bioaccumulation of Nile tilapia (O. niloticus) fed with 40–100 mg ß-carotene kg−1 dry diet (Mean ± SE) under HgCl2 exposure by a rate (0.05 ppm) for 3 weeks. N = 8 fish per sampling time. Values with different letters are significantly different between and within groups, **significant at (P < 0.001). M = HgCl2 only, M + C40 = HgCl2 with carotene 40 mg kg−1 diet, and M + C100 = HgCl2 with carotene 100 mg kg−1 diet
Discussion
The discharge of effluents containing mercury (Hg) into the environment has caused grave contamination problems in communities of various ecosystems and in human populations. In the present study, the fish were adapted to commercial diet at 3 % bodyweight for 2 weeks before start of experiment. The data of hematological parameters of tilapia fish in this study showed significant decreases in all parameters in positive control group than other groups (Fig. 1a–e). These data were coincided with the previous results of Shakoori et al. (1994) in their experiment in grass carp, Ctenopharyngodon idella exposed to HgCl2 (0.005 mg L−1). Similar results were described for carp, Cyprinus carpio exposed to HgCl2 (0.30 mg L−1) for 90 h (Beena and Viswaranjan 1987). However, Ishikawa et al. (2007) reported no significant changes in Nile tilapia exposed to long-term mercuric chloride. Different and divergent results could occur due to various factors, such as differences in body size, species, concentration of the tested substance, and the time of exposure (Fletcher and White 1986). In Channa punctatus (Juneja and Mahajan 1983) and O. aureus (Allen 1994) exposed to sublethal concentration (0.034–0.136 and 0.5 mg L−1, respectively) of HgCl2 in water and reported increase of RBCs count and hematocrit value (Ht). O’Connor and Fromm (1975) observed at the beginning of their experiments a fall in Ht, and at the end of 12 weeks, the opposite effects occurred. This is may be attributed to the possibility that erythropoietic tissues could have been stimulated in response to the decline in RBCs caused by hemolysis as a consequence of exposure to HgCl2. In addition, Oliveira Ribeiro et al. (2000) described that fish exposed to inorganic Hg dissolved in the water causes hypoxia, as a result of cellular hyperplasia in the secondary lamellae of the gills, diminishing the surface area for gas exchange. In response to respiratory difficulty, the organism stimulates an increase in RBCs, Hb, and MCHC as mechanism to enhance oxygen transfer (Affonso et al. 2002).
In the present study, the WBCs count showed significant decreases in the positive control group as the result of 0.05 ppm mercuric chloride exposure. Similar finding was reported for C. punctatus exposed to 0.25 mg L−1 of HgCl2 (Misra and Behera 1992); Gill and Pant (1985) attributed the leucopenia to the stress caused by the bioassay type, which had also been demonstrated in several other vertebrates. Oncorhynchus mykis and Salvelinus fontinalis submitted to the stress of confinement and manipulation showed a reduction in number of WBCs (Benfey and Biron 2000). Then, the stress leads to a redistribution of lymphocytes, mainly to lymphoid organs (thymus and anterior kidney), diminishing in the blood circulation, or to the destruction of lymphocytes in response to high levels of cortisol.
Mercury is known to induce lipid peroxidation in fish. For instance, higher content of MDA was detected in snakehead tissue following 5 μg L−1 of HgCl2 exposure for 30 days (Rana et al. 1995). We found that peroxide radicals (MDA) appearing significantly increased in polluted group. Studies by others are supportive to our results regarding levels of MDA. Winston and Di Giulio (1991) reported increased selenium-dependent glutathione peroxidase (Se-GPX) activity and higher MDA levels in most tissues of carp exposed to Cu2+ while superoxide dismutase and catalase activities were decreased. Sánchez-Chardi et al. (2007) found that fish collected from a polluted stream had significant higher levels of thiobarbituric acid-reactive substances compared to those from an unpolluted site. On contrary, other studies revealed that mollusks (Cossu et al. 2000) and fish (Rodríguez-Ariza et al. 1993) from polluted areas exhibiting low MDA that may be attributed to animal adaptation to live in polluted areas.
In the present study, mercury exposure showed significant increases in MDA levels particularly in positive control group all over the period of experiment. MDA is frequently used methods to monitor lipid peroxidation and oxidative stress in fish (Lushchak 2011). Hepatotoxic effect of mercury exposure may be related to lipid peroxidation and significant increases of MDA (Slaninova et al. 2009). On the same respect, significant increases in the liver enzyme as appeared in the present study could approve the liver damage in positive control group. This damage could be prevented by β-carotene supplementation.
Our results showed a significant increase in ALT and AST enzyme activity in the toxicated group. In the same context, Torres et al. (2005) found elevated levels of both ALT and AST activities in carp (cyprinus carpio) exposed to waterborne cadmium. Also, Kumar et al. (2005) and Ghorpade et al. (2002) showed similar results with exposure to mercury. However, other studies were in contrast to ours. Gill et al. (1991) reported marked reduction in hepatic, branchial, and renal AST and ALT in rosy barb (Puntius conchonius) after toxication with mercuric chloride. In addition, Abu El-Ella (1996) and Shalaby (2000) found similar results with grass carp, Ctenopharyngodon idella, and common carp, Cyprinus carpio when exposed to cadmium (Cd), respectively. Since mercury can interact with biosynthesis of pyridoxal phosphate, an essential requirement for normal function of aminotransferases. At higher levels of mercury concentrations, the activities of ALT and AST may initially increase to counter the energy crises during the stress but start to decline when the concentrations of mercury become too high; that reduction account on liver necrosis (because of toxicant) that led to leakage from liver into the blood and/or tactual inhibition of liver enzymes (Haschek et al. 2010).
No effect has been associated to mercury exposure regarding plasma lipid parameters (total lipids, triglycerides, LHL, LDL); however, there were a nominal decrease in the exposed group and increased in carotene-supplemented group, indicating that fish were able to cope with the mercury potential to oxidative-degraded lipids, despite the detected breakdown of redox-defense system.
The data obtained regarding mercury accumulation in tissues showed that the mercury significantly rise in liver and white muscle. Hg levels showed lower affinity to white muscle compared to the accumulated level in liver. It was demonstrated that different metals show different affinities to fish tissues and mainly they accumulated in the liver, kidney, and gills (Huang et al. 2007; Fernandes et al. 2008). In addition, muscle tissues in fish usually have the lowest level of metals (Jezierska and Witeska 2007). Our results were similar to other studies examined the Hg accumulation in tissues; Elia et al. (2003) found that Ictalurus melas exposed to 35–140 μg L−1 of Hg for 10 days showed different pattern in Hg accumulation as gills > kidney > liver > muscle. In the same trend, Carvalho et al. (2009) showed that the highest Hg accumulation in Nile tilapia exposed to 0.85 mg L−1 of Hg+2 was in gills followed by heart, liver, and the least tissue was the muscle. This could be attributed to the organs nature and function; as the organs in the visceral region of the body which usually involved in absorption, distribution, and elimination considered a target for metals accumulation, similarly to our result in which liver as one of the visceral organs had a higher significant level of Hg accumulation than in muscle tissue (Giari et al. 2008; Rao et al. 2005). Similar to the present data Berntssen et al. (2004) detected higher Hg accumulation in liver than in muscles tissue in Atlantic salmon (Salmo salar). The significant reduction of Hg accumulation across the hepatic and muscular tissue strongly suggest that β-carotene may be extremely useful in detoxification and chelation and warrant further investigation to clarify mechanism protection. Therefore, it could be concluded that dietary β-carotene is efficient for reduction of HgCl2 toxicity due to its immune modulatory effect (Garewal et al. 1992). Quenching free radicals by β-carotene protect the cell membrane and receptors required for antigen recognition from damage by peroxidation of membrane lipid (Bendich 1991).
In the present study, dietary supplementation of β-carotene demonstrated a significant increase in liver enzymes (ALT and AST) and peroxide radicals (MDA) parallel to the time of pollution suggesting its function in improving liver protection. Our results are in accordance with Wang et al. (2006) in which dietary supplementation of high level of β-carotene to the ornamental fish, characins Hyphessobrycon callistus, lead to maximal increase in antioxidant capacity indices in form of serum antioxidant enzymes [superoxide dismutase (SOD), glutathione peroxidases (GPx)], and serum transaminases [alanine aminotransferase (ALT), aspartate aminotransferase (AST)]. Carotenoids suppress the respiratory burst in vivo as alternative way to protect host cells and tissues from excess free radicals, thereby stimulating the generation of specific immune responses (Chung and Secombes 1988).
Studies by others have also showed that carotenoids improve fish health (Torrissen and Christiansen 1995; Christiansen and Torrissen 1996). There wascorrelation between dietary astaxanthin concentration and antioxidant status in liver and muscle in Atlantic salmon (S. salar) (Christiansen et al. 1995). Moreover, dietary astaxanthin have been shown to improve the antioxidant status in penaeid postlarvae and enhance their resistance against various environmental stressors (Chien and Shiau 2005).
While almost all hematological parameters measured significantly decreased in response to mercuric intoxication, dietary supplementation of β-carotene has improved all parameters significantly near to their control levels. Carotenoids have been implicated in enhancing the immune response such as proliferation, induction of specific effector cells as well as the secretion of cytokines (Bendich 1991). β-carotene levels up to 200 mg kg−1 diet enhanced some immune parameters like serum complement and total plasma immunoglobulin in the experimental rainbow trout (AMAR et al. 2000a). Moreover, immuno-enhancement of β-carotene involves the quenching of free radicals or the lowering of lipid peroxide levels and alterations in membrane fluidity (Bendich 1991), which account on increasing the levels of RBCs, Hb, and PCV upon its supplementation in diet to alleviate the toxic effect of mercury.
In the present study, dietary supplementation of β-carotene showed significant decrease in Hg accumulation in both liver and muscle tissue of Nile tilapia compared to the M group, which clues that addition of β-carotene remediated the effect of HgCl2 toxicity. Similar to the present data, Kaoud et al. (2012) showed that addition different concentration of Lemna gibba L-extract to water containing 0.06 mg L−1 doses of HgCl2 reduced significantly the Hg level in water and the metal uptake as compared to fish exposed to Hg alone. In the same event, polysaccharide from Acanthophyllum bracteatum 1 % weight/weight (w/w) was added to 60 mg L−1, tested its efficacy in reducing toxicity of HgCl2 in Atlantic salmon, and the Hg analysis showed that there was a significant depletion of Hg in blood, liver, and tissues of fish treated with polysaccharide (Jahanbin et al. 2012).
In conclusion, this study clearly demonstrated the effect of HgCl2 on the hematological, plasma, and lipid parameters in Nile tilapia, inducing significant variations, suggesting that it play a major role promoting the immune depressive and oxidative stress response. Correspondingly, our results further showed that β-carotene-supplemented diet stimulate the immune and antioxidative response. Thus, β-carotene-supplemented diet could be beneficial in toxicity reduction by modulating the immune and antioxidant functions that could be used to overcome the immune depressive stressful condition in farmed Nile tilapia that of a great significant when planning good management to protect farmed fish stocks.
References
Abu El-Ella SM (1996) Studies on the toxicity and bioconcentration of cadmium on grass carp, Ctenopharyngodon idella. MSc Thesis, Faculty of Science, Helwan University
Adel AK (2003) Heavy metal pollution and biomonitoring plants in lake Manzala, Egypt, Pakistan. J Biol Sci 6(13):1108–1117
Affonso EG, Polez VLP, Corrêa CF, Mazon AF, Araújo MRR, Moraes G, Rantin FT (2002) Blood parameters and metabolites in the teleost fish Colossoma macropomum exposed to sulfide or hypoxia. Comp Biochem Physiol C Toxicol Pharmacol 133(3):375–382. doi:10.1016/s1532-0456(02)00127-8
Ahmed AM, Hussein MM (2004) Residual levels of some heavy metals in fish flesh and water from El-Manzala Lake, Egypt. Journal of King Saud University Agricultural Sciences 16(2):187–196
Allen P (1994) Distribution of mercury in the soft tissues of the blue tilapia <i> Oreochromis aureus </i> (Steindachner) after acute exposure to mercury (II) chloride. Bulletin of Environmental Contamination and Toxicology 53(5):675–683. doi:10.1007/bf00196939
Amar EC, Kiron V, Satoh S, Okamoto N, Watanabe T (2000a) Effects of dietary b-carotene on the immune response of rainbow trout Oncorhynchus mykiss. Fish Sci 66:1068–1075
Amar EC, Kiron V, Satoh S, Okamoto N, Watanabe T (2000b) Effects of dietary β-carotene on the immune response of rainbow trout Oncorhynchus mykiss. Fish Sci 66(6):1068–1075. doi:10.1046/j.1444-2906.2000.00170.x
Amar EC, Kiron V, Satoh S, Watanabe T (2004) Enhancement of innate immunity in rainbow trout (Oncorhynchus mykiss Walbaum) associated with dietary intake of carotenoids from natural products. Fish Shellfish Immunol 16(4):527–537. doi:10.1016/j.fsi.2003.09.004
APHA (1985) Mercury determination by the cold vapor technique, standard method 303. In: Franson MAH (ed) Standard methods for examination of water and wastewater, 16th edn. American Public Health Association, Washington DC
Banerjee BD, Seth V, Bhattacharya A, Pasha ST, Chakraborty AK (1999) Biochemical effects of some pesticides on lipid peroxidation and free-radical scavengers. Toxicol Lett 107(1–3):33–47. doi:10.1016/s0378-4274(99)00029-6
Beena S, Viswaranjan S (1987) Effect of cadmium and mercury on the hematological parameters of the fish Cyprinus carpio. Environment and Ecology 5(4):726–732
Bendich A (1991) β-Carotene and the immune response. Proceedings of the Nutrition Society 50:263–274
Benfey TJ, Biron M (2000) Acute stress response in triploid rainbow trout (Oncorhynchus mykiss) and brook trout (Salvelinus fontinalis). Aquaculture 184(1–2):167–176. doi:10.1016/s0044-8486(99)00314-2
Berntssen MHG, Aatland A, Handy RD (2003) Chronic dietary mercury exposure causes oxidative stress, brain lesions, and altered behaviour in Atlantic salmon (Salmo salar) parr. Aquat Toxicol 65(1):55–72. doi:10.1016/s0166-445x(03)00104-8
Berntssen MHG, Hylland K, Julshamn K, Lundebye AK, Waagbø R (2004) Maximum limits of organic and inorganic mercury in fish feed. Aquac Nutr 10(2):83–97. doi:10.1046/j.1365-2095.2003.00282.x
Burtis CA, Ashwood ER (eds) (1999) Tietz textbook of clinical chemistry, 3rd edn. Saunders, Philadelphia
Burton GW, Ingold KU (1984) Beta-carotene: an unusual type of lipid antioxidant. Science 224(4649):569
Carvalho GGA, de França J, Dias D, Lombardi J, de Paiva MJR, Carvalho S, Sarriés G, Ferreira J (2009) Selenite and selenate effects on mercury (Hg 2+) uptake and distribution in Tilapia, Oreochromis niloticus L., assessed by chronic bioassay. Bull Environ Contam Toxicol 82(3):300–304
Chien YH, Shiau WC (2005) The effects of dietary supplementation of algae and synthetic astaxanthin on body astaxanthin, survival, growth, and low dissolved oxygen stress resistance of kuruma prawn, Marsupenaeus japonicus Bate. J Exp Mar Biol Ecol 318:201–211
Christiansen R, Torrissen OJ (1996) Growth and survival of Atlantic salmon, Salmo salar L. fed different dietary levels of astaxanthin. Juveniles Aquaculture Nutrition 2:55–62
Christiansen R, Glette J, Lie Ø, Torrissen OJ, Waagbø R (1995) Antioxidant status and immunity in Atlantic salmon, Salmo salar L., fed semi-purified diets with and without astaxanthin supplementation. J Fish Dis 18:317–328
Chung S, Secombes C (1988) Analysis of events occuring within teleost macrophages during the respiratory burst. Comp Biochem Physiol 89B:539–544
Cossu C, Doyotte A, Babut M, Exinger A, Vasseur P (2000) Antioxidant biomarkers in freshwater bivalves, unio tumidus, in response to different contamination profiles of aquatic sediments. Ecotoxicol Environ Saf 45(2):106–121. doi:10.1006/eesa.1999.1842
de Oliveira Ribeiro CA, Belger L, Pelletier É, Rouleau C (2002) Histopathological evidence of inorganic mercury and methyl mercury toxicity in the arctic charr (Salvelinus alpinus). Environ Res 90(3):217–225. doi:10.1016/s0013-9351(02)00025-7
Drabkin D (1964) Spectrophotometric studies. XIV. The crystallographic and optical properties of man Hbs in comparison with those of other species. Zh Biol Chim 164:702–723
Elia AC, Galarini R, Taticchi MI, Dörr AJM, Mantilacci L (2003) Antioxidant responses and bioaccumulation in Ictalurus melas under mercury exposure. Ecotoxicol Environ Saf 55(2):162–167. doi:10.1016/s0147-6513(02)00123-9
Fernandes D, Bebianno MJ, Porte C (2008) Hepatic levels of metal and metallothioneins in two commercial fish species of the Northern Iberian shelf. Sci Total Environ 391(1):159–167. doi:10.1016/j.scitotenv.2007.10.057
Fletcher TC, White A (1986) Nephrotoxic and haematological effects of mercuric chloride in the plaice (Pleuronectes platessa L.). Aquat Toxicol 8(2):77–84. doi:10.1016/0166-445x(86)90054-8
Fossati P, Principle L (1982) Estimation of the concentration of triglyceride in plasma and liver. Clin Chem 28:2077–2081
Garewal HS, Ampel NM, Watson RR, Prabhala RH, Dols CL (1992) A preliminary trial of beta-carotene in subjects infected with the human immunodeficiency virus. J Nutr 122:728–732
Ghorpade N, Mehta V, Khare M, Sinkar P, Krishnan S, Rao CV (2002) Toxicity study of diethyl phthalate on freshwater fish cirrhina mrigala. Ecotoxicol Environ Saf 53(2):255–258. doi:10.1006/eesa.2002.2212
Giari L, Simoni E, Manera M, Dezfuli BS (2008) Histo-cytological responses of Dicentrarchus labrax (L.) following mercury exposure. Ecotoxicol Environ Saf 70(3):400–410. doi:10.1016/j.ecoenv.2007.08.013
Gill T, Pant J (1985) Mercury-induced blood anomalies in the freshwater teleost. Water Air Soil Pollut 24(2):165–171. doi:10.1007/bf00285441
Gill TS, Pande J, Tewari HM (1991) Effects of endosulfan on the blood and organ chemistry of fresh water fish, Barbus conchonius Hamilton. Ecotoxicol Environ Saf 21:80–91
Haschek WM, Walling MA, Rousseaux C (2010) Fundamental of toxicologic pathology, 2nd edn. Academies Press, p 286
Huang ZY, Zhang Q, Chen J, Zhuang ZX, Wang XR (2007) Bioaccumulation of metals and induction of metallo-thioneins in selected tissues of common carp (Cyprinus carpio L.) co-exposed to cadmium, mercury and lead. Appl Organomet Chem 21(2):101–107
Ishikawa NM, Ranzani-Paiva MJT, Lombardi JV, Ferreira CM (2007) Hematological parameters in Nile Tilápia, Oreochromis niloticus exposed to sub-letal concentrations of mercury. Brazilian Archives of Biology and Technology 50:619–626
Jahanbin K, Hedayati A, Moini S, Gohari AR, Emam-Djomeh Z, Esposito A, Bagheri T (2012) The first application of a new polysaccharide from Acanthophyllum bracteatum for the health improvement of Atlantic Salmon exposed to mercury chloride. Toxicol Industrial Health 28(4):377–384
Jain NC (1986) Schalman’s veterinary haematology, 4th edn. Lea and Babings, USA
Jezierska B, Witeska M (2007) The metal uptake and accumulation in fish living in polluted waters. Soil and water pollution monitoring. Prot Rem 69:107–114
Juneja CJ, Mahajan CL (1983) Hematological and haemopoietic changes in fish Channa punctatus due to mercury pollution in water. Indian J Anim Res 17(2):63–71
Kaoud HA, Mahran KMA, Rezk A, Khalf MA (2012) Bioremediation the toxic effect of mercury on liver histopathology, some hematological parameters and enzymatic activity in Nile tilapia, Oreochromis niloticus. Researcher 4(1):60–69
Kehrer JP (1993) Free radicals as mediators of tissue injury and disease. Crit Rev Toxicol 23(1):21–48. doi:10.3109/10408449309104073
Kumar M, Sharma MK, Kumar A (2005) Spirulina fusiformis: a food supplement against mercury induced hepatic toxicity. J Health Sci 51(4):424–430
Larose C, Canuel R, Lucotte M, Di Giulio RT (2008) Toxicological effects of methylmercury on walleye (Sander vitreus) and perch (Perca flavescens) from lakes of the boreal forest. Comp Biochem Physiol C Toxicol Pharmacol 147(2):139–149. doi:10.1016/j.cbpc.2007.09.002
Low KW, Sin YM (1998) Effects of mercuric chloride and sodium selenite on some immune responses of blue gourami, Trichogaster trichopterus (Pallus). Sci Total Environ 214(1–3):153–164. doi:10.1016/s0048-9697(98)00061-8
Lushchak VI (2011) Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101(1):13–30. doi:10.1016/j.aquatox.2010.10.006
Milaeva ER (2006) The role of radical reactions in organomercurials impact on lipid peroxidation. J Inorg Biochem 100(5–6):905–915. doi:10.1016/j.jinorgbio.2006.02.014
Misra SK, Behera SC (1992) Evaluation of toxic effects of mercuric chloride on hematological parameters of a freshwater fish Channa punctatus (Bloch). Environ Ecol 10(2):394–396
Monteiro D, Rantin F, Kalinin A (2010) Inorganic mercury exposure: toxicological effects, oxidative stress biomarkers and bioaccumulation in the tropical freshwater fish matrinxã, Brycon amazonicus (Spix and Agassiz, 1829). Ecotoxicology 19(1):105–123. doi:10.1007/s10646-009-0395-1
Moore JW, Ramamoorthy S (1984) Heavy metals in natural waters: applied monitoring and impact assessment. Related information: springer series on environmental management
National Research Council (US). Committee on Animal Nutrition. (1993). Nutrient requirements of fish. National Academies Press
O’Connor DV, Fromm PO (1975) The effect of methyl mercury on gill metabolism and blood parameters of rainbow trout. Bull Environ Contam Toxicol 13(4):406–411. doi:10.1007/bf01721843
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358. doi:10.1016/0003-2697(79)90738-3
Oliveira Ribeiro CA, Pelletier E, Pfeiffer WC, Rouleau C (2000) Comparative uptake, bioaccumulation, and gill damages of inorganic mercury in tropical and Nordic freshwater fish. Environ Res 83(3):286–292. doi:10.1006/enrs.2000.4056
Rana SVS, Singh R, Verma S (1995) Mercury-induced lipid peroxidation in the liver, kidney, brain and gills of a fresh water fish Channa punctatus. Jpn J Ichthyol 42:255–259
Rao J, Begum G, Pallela R, Usman P, Rao R (2005) Changes in behavior and brain acetylcholinesterase activity in mosquito fish, Gambusia affinis in response to the sub-lethal exposure to chlorpyrifos. Int J Environ Res Public Health 2(3):478–483
Rodríguez-Ariza A, Peinado J, Pueyo C, López-Barea J (1993) Biochemical indicators of oxidative stress in fish from polluted littoral areas. Can J Fish Aquat Sci 50(12):2568–2573. doi:10.1139/f93-280
Sánchez-Chardi A, López-Fuster MJ, Nadal J (2007) Bioaccumulation of lead, mercury, and cadmium in the greater white-toothed shrew, Crocidura russula, from the Ebro Delta (NE Spain): sex- and age-dependent variation. Environ Pollut 145(1):7–14. doi:10.1016/j.envpol.2006.02.033
Shakoori AR, Iqbal MJ, Mughal AL, Ali S (1994) Biochemical changes induced by inorganic mercury on the lood, liver and muscles of freshwater Chinese grass carp, Ctenopharyngodon idella. J Ecotoxicol Environ Monit 4(2):81–92
Shalaby AME (2000) Sublethal of heavy metals copper, cadmium and zinc alone or in combinations on enzymes activities of common carp (Cyprinus carpio L.). Egypt J Aquat Biol Fish 4(2):229–246
Singer C, Zimmermann S, Sures B (2005) Induction of heat shock proteins (hsp70) in the zebra mussel (Dreissena polymorpha) following exposure to platinum group metals (platinum, palladium and rhodium): comparison with lead and cadmium exposures. Aquat Toxicol 75(1):65–75. doi:10.1016/j.aquatox.2005.07.004
Slaninova A, Smutna M, Modra H, Svobodova Z (2009) A review: oxidative stress in fish induced by pesticides. Neuro Endocrinol Lett 30:2–12
Tachibana K, Yagi M, Hara K, Mishima T, Tsuchimoto M (1997) Effects of feeding of β-carotene-supplemented rotifers on survival and lymphocyte proliferation reaction of fish larvae Japanese parrotfish (Oplegnathus fasciatus) and Spotted parrotfish (Oplegnathus punctatus): preliminary trials. Hydrobiologia 358(1):313–316. doi:10.1023/a:1003189020623
Tacon AGJ (1981) Speculative review of possible carotenoid function in fish. Prog Fish-Culturist 43(4):205–208. http://dx.doi.org/10.1577/1548-8659(1981)43[205:SROPCF]2.0.CO;2
Torres DP, Vieira MA, Ribeiro AS, Curtius AJ (2005) Determination of inorganic and total mercury in biological samples treated with tetramethylammonium hydroxide by cold vapor atomic absorption spectrometry using different temperatures in the quartz cell. J Anal At Spectrom 20(4):289–294
Torrissen OJ (1984) Pigmentation of salmonids: effects of carotenoids in eggs and start-feeding diet on survival and growth rate. Aquaculture 43:185–193
Torrissen OJ, Christiansen R (1995) Requirements for carotenoids in fish diets. J Appl Ichthyol 11:225–230
Veena KB, Radhakrishnan CK, Chacko J (1997) Heavy metal induced biochemical effects in an estuarine teleost, vol 26, vol 1. Council of Scientific and Industrial Research, New Delhi
Verakunpiriya V, Watanabe K, Mushiake K, Kawano K, Kobayashi T, Hasegawa I (1997) Effect of krill meal supplementation in soft-dry pellets on spawning and quality of egg of yellowtail. Fish Sci 63:433–439
Verlecar XN, Jena KB, Chainy GBN (2007) Biochemical markers of oxidative stress in Perna viridis exposed to mercury and temperature. Chem Biol Interact 167(3):219–226. doi:10.1016/j.cbi.2007.01.018
Verlecar XN, Jena KB, Chainy GBN (2008) Modulation of antioxidant defences in digestive gland of Perna viridis (L.), on mercury exposures. Chemosphere 71(10):1977–1985
Wang Y-J, Chien Y-H, Pan C-H (2006) Effects of dietary supplementation of carotenoids on survival, growth, pigmentation, and antioxidant capacity of characins, Hyphessobrycon callistus. Aquaculture 261(2):641–648. doi:10.1016/j.aquaculture.2006.08.040
Watanabe T, Lee MJ, Mizutani J, Yamada T, Satoh S, Takeuchi T (1991) Effective component of cuttlefish meal and raw krill for improvement of quality of red sea bream Pagrus major eggs. Nippon Suisan Gakkaishi 57:681–694
Winston GW, Di Giulio RT (1991) Prooxidant and antioxidant mechanisms in aquatic organisms. Aquat Toxicol 19(2):137–161. doi:10.1016/0166-445x(91)90033-6
Acknowledgments
This work was supported by department of physiology and department of Internal medicine, infectious and fish diseases, Faculty of veterinary medicine, Mansoura University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elseady, Y., Zahran, E. Ameliorating effect of β-carotene on antioxidant response and hematological parameters of mercuric chloride toxicity in Nile tilapia (Oreochromis niloticus). Fish Physiol Biochem 39, 1031–1041 (2013). https://doi.org/10.1007/s10695-012-9760-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-012-9760-8