Abstract
Aquatic organisms are considered excellent biomarkers of mercury (Hg) occurrence in the environment. Selenium (Se) acts in antagonism to this metal, stimulating its elimination, and reducing its toxicity. In this paper, tilapia (Oreochromis niloticus) were chronically acclimated in sub-lethal Hg2+, Hg2+ + Se4+ and Hg2+ + Se6+ concentrations. Distribution and bioaccumulation of both elements were evaluated in fish tissues. The kidney was the main target of the Hg and Se uptake, and the presence of Hg induced the Se hepatic elimination. The Hg bioaccumulation in the gill, spleen and heart were higher in the presence of Se6+ than in the presence of Se4+.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Mercury (Hg) is one of the most toxic chemical elements, arising in the environment from natural and anthropogenic sources, and accumulating in the biota (Akagi et al. 1995). As a consequence, aquatic organisms are considered important biomarkers of this metal fate. Selenium (Se) is an essential micronutrient for humans and animals (Beyrouty and Chan 2006). It interacts with toxic metals metabolism, especially with Hg, minimizing their toxic effects and stimulating it elimination and detoxification (Gailer 2007).
In vivo studies using creek chub (Semotilus antromaculatus) showed that Se is an effective antagonist to Hg (Kim et al. 1977). The presence of Se decreases the metal tissue retention, as already described by Cabañero et al. (2006), except in the liver, where the bioaccumulation of Hg was not affected (Hansen et al. 1981). Experiments conducted with blue mussels (Mytilis edulis) revealed that Se bioaccumulation increases in the presence of Hg. However, Hg retention was not affected by different concentrations and chemical forms of Se (Pelletier 1986). Bioassays with sea mollusks did not show protective effects of Se against the poisonous effects of Hg (Patel et al. 1988). This work evaluated the effects of selenite (Se4+) and selenate (Se6+) on mercury (Hg2+) uptake and distribution in young tilapia (Oreochromis niloticus) chronically exposed to these elements.
Materials and Methods
In vivo tests were carried out in agreement with the Standard Methods for the Examination of Water and Wastewater (APHA 1998). Young tilapia were acquired from a commercial fish farm, with an average weight of 32.04 g (±5.35) and an average length of 10.27 cm (±1.39). Fish were acclimated in chlorine free 250 L water tanks and fed with commercial feed for one week. Afterwards, they were transferred to 40 L aquaria and acclimated again for 48 h.
Physicochemical characteristics of the water such as temperature, dissolved oxygen, pH and electric conductivity were monitored during the entire experiment. Hardness was determined by EDTA volumetric method, alkalinity by titration and total ammonium by color form reagent, at the beginning and at the end of the experiment.
Aquaria (n = 8) were disposed with bioassays containing Hg2+, Hg2+ + Se4+ and Hg2+ + Se6+ at sub-lethal concentrations, as follow: Control group (n = 2), free of Hg and Se; 0.08 mg L−1 Hg2+ (n = 2); 0.08 mg L−1 Hg2+ + 0.4 mg L−1 Se4+ (n = 2); and 0.08 mg L−1 Hg2+ + 1.4 mg L−1 Se6+ (n = 2). Sodium selenite (Na2SeO3), sodium selenate (Na2SeO4) and mercury chloride (HgCl2) concentrations were established according to França et al. (2007). In all tests, the concentrations tested were NOEL (No Observed Effect Levels). The assays were carried out for 14 days, with 16 fish per aquarium. At the end of experiment, one fish was sampled per aquarium, resulting in two individuals per treatment. Fish were sacrificed by deep sedation with benzocaine. Samples of heart, liver, spleen, gill, brain, muscle and kidney were obtained from each individual, and were stored at −5°C until chemical analysis.
For total mercury determinations, 100 mg of wet tissue were heated in a glass tube with an acid mixture of HNO3, HClO4 and H2SO4 (1.0:1.0:2.5 mL) up to 210°C (Akagi et al. 1995). The final volume was made with ultrapure water (18.2 M Ω cm−1) up to 25.0 mL. Total Se was determined by heating 7.5 mL of the former digested solution with 7.5 mL concentrated HCl to 100°C for ½ hour, reducing Se6+ to Se4+. The volume was made up to 25.0 mL, with a final acidity of 30% (v/v) HCl (Quevauviller et al. 1993). Blanks and certified reference material (CRM) were processed through an identical procedure. Both, Hg and Se detections were carried out by Atomic Fluorescence Spectrometry (AFS), PS Analytical Ltd. (Kent, UK), coupled to a Merlin and an Excalibur detectors respectively. Mercury was reduced by a 2.0% (m/v) SnCl2 solution and Se, by a 1.3% (m/v) NaBH4 solution.
Accuracy was assessed by using a CRM, DORM-2, constituted of lyophilized dogfish muscle tissue, produced by the National Research Council of Canada, with a certified Hg and Se concentrations of 4.64 ± 0.26 and 1.4 ± 0.09 mg kg−1, respectively.
Statistical evaluation of the data was performed using analysis of variance (ANOVA), Gauss and Markov model, with split plot design. The hypothesis tests were made by using Fischer and Snedecor F distributions. Tukey Multiple Comparison Test was applied (Montgomery 2001). Hg and Se concentrations were considered variables of response. In the mathematic-statistical model, the acclimations (Hg2+, Hg2+ + Se4+, Hg2+ + Se6+ and control) were considered primary treatments (located in the plots), and the organs as secondary treatments (sub-plot levels).
Results and Discussion
Physicochemical parameters of the water used in the bioassays were similar among treatments (p = 0.05), being in agreement with the limits recommended by APHA (1998) for fish maintenance in cultivation systems (Table 1). Fourteen days of sub-lethal exposure to either 0.08 mg L−1 Hg2+, 0.4 mg L−1 Se4+ or 1.4 mg L−1 Se6+ concentrations produced no mortalities and all fish appeared healthy, as already described (França et al. 2007).
Total mercury and selenium concentrations determined in the CRM DORM-2 were 4.72 ± 0.21 and 1.38 ± 0.16 mg kg−1. Recoveries of 101 and 99% were achieved for Hg and Se respectively and results were within the interval of the certified standard deviation values (Table 2).
High differences (≥99% of confidence) for Hg concentrations among the several organs were observed. According to the Tukey Test, these differences followed a standard behaviour for the four treatments, e.g. kidney always showed the highest Hg concentrations, while muscular tissues the lowest.
Total Hg concentrations (μg g−1) in organs of young tilapia submitted for 14 days to exposure to the elements and control are presented in Table 3. As expected the results indicated no significant differences between the Hg concentrations in all fish organs in the control group, at 95% of confidence. Although no significant differences (p > 0.05) for Hg concentrations in the treatments were identified, a great variation in magnitude in the arithmetic mean concentrations was observed when all seven organs were considered (Table 3). The three assays showed high Hg means in comparison to the control one, being around 20 times (19.6) for Hg2+, 15.8 for Hg + Se4+ and 14.6 for Hg + Se6+, indicating a decreasing Hg burden induced by the presence of Se (Gailer 2007; Cabañero et al. 2006).
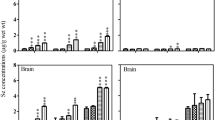
In comparison to the control, the other treatments (Hg2+, Hg2+ + Se4+, Hg2+ + Se6+) did not increase the Hg concentrations in the spleen, gill, heart, brain, liver and muscle (Table 3). As already described (Su et al. 2008 and Chen et al. 2006), the kidney of tilapia (Oreochromis niloticus) was the main target of the Hg accumulation, indicating a significant Hg uptake in the 3 accomplished treatments (Fig. 1). Kidney is involved in the self depuration of toxic metals, which implies that microsome takes part in the mercury metabolic process in the animal body (Chen et al. 2006). Furthermore, a previous study reported a copper and cadmium uptake in kidney of tilapias (Oreochromis mossambicus) chronically exposed to 0.1 mg L−1 Cu and 0.005 mg L−1 Cd (Pelgrom et al. 1995).
The data pointed out that in the 0.08 mg L−1 Hg2+ acclimation, the concentration of Hg in the kidney was not statistically different from the gill, heart and liver, which were higher than in the spleen, brain and muscle (Table 3). In the presence of selenium, in both oxidation states, Se4+ and Se6+, a systematic trend for increasing Hg concentration in kidney were observed (Table 3). For Se6+, the significance of statistical analysis was between kidney and muscular tissue, liver and brain only. In the case of Se4+, differences were observed between kidney and all other organs. Therefore, this finding may indicate the importance of selenium speciation on mercury interaction with the biota (Gailer 2007).
When considering Se as variable of response, significant differences (95% of confidence) were observed for the treatment and organs factor, and for the treatment times organs interactions. In the Hg + Se6+ exposure, kidney showed the highest average Se concentrations, being different (95% of confidence) of all other organs (Table 4). When the treatment factor was analyzed for organs, significant changes for the liver and kidney were observed.
The results indicated no differences between total Se concentrations in tilapia organs acclimated to the Hg2+ and Hg2+ plus Se4+ treatments. The total Se concentrations found in muscle, gill, spleen, brain and heart of tilapia chronically exposed to both oxidation states of Se plus Hg were similar to the control (Table 4). A significant (95% of confidence) Se uptake was observed in the kidney, for fish exposed to Se6+. A mechanistic basis for this observation can be detailed (Gailer 2007; Cabañero et al. 2006; Chen et al. 2006). The 0.08 mg L−1 Hg2+ chronic exposures provided a decrease in the total Se accumulation in tilapia liver in comparison to the control (58.6% of control) (Fig. 1). In this sense, according to Gailer (2007), a long-term feeding study in which rats were exposed to mercury (10 mg L−1 HgCl2) in drinking water and given 10 mg L−1 Se4+ in the diet during 7 weeks, resulted in a decrease of Se concentration in the liver (87% of Se-only control).
The Hg detoxification process takes place in liver and erythrocytes, with subsequent accumulation of Hg2+ in the kidneys (Gailer 2007; Cabañero et al. 2006). The decreased concentration of Se in the liver of tilapia should be linked to this mechanism. Therefore, the detoxification process of Hg in liver and kidney could be attributed to the metal binding to metallothioneins or other thiol moieties, like glutathione and protein thiols (Gailer 2007; Chen et al. 2006). In this work, a greater Hg uptake in kidney was obtained in all tilapia chronically exposed to 0.08 mg L−1 Hg2+.
Experiments conducted by Pelletier (1986) with blue mussels (Mytilus edulis) demonstrated that Hg retention was not affected by different concentrations and chemical species of Se. On the other hand, Se4+ and Se6+ did not significantly affect the uptake of any mercury species by the diatoms (Thalassiosira pseudonana) and by the green mussels (Perna viridis) (Wang et al. 2004). In the present study, the data indicated that the Hg accumulation in the gill, spleen and heart were higher in the presence of Se+6 than in the presence of Se4+. Furthermore, when animals were pre-treated with 3.0 mg L−1 Se4+ the toxicity of Hg was decreased, pointing selenium dioxide as an effective antagonist to Hg2+ (Kim et al. 1977). In addition, rats fed with a diet containing 1.25 mg of methyl Hg/kg body weight/day plus 1.0 mg kg−1 Se resulted in an increase in post-natal survival when compared to methylmercury-treated group (Beyrouty and Chan 2006).
The chronically exposure of tilapia to 0.08 mg L−1 Hg2+ did not affect the Hg accumulation in brain (Table 3). It suggests that either a 14-day period of exposure, or the Hg2+ concentration used, were not enough to lead to a significant Hg biomagnification in the nervous system. The mercury accumulation in this organ could be affected in a longer exposure condition. The results indicated that the average Hg muscle concentrations were relatively lower than the other organs (Table 3). This may constitute an interesting finding in the public health and toxicology point of view, because muscular tissue generally constitutes the edible fish for human consumption. These results illustrate that understanding the previous fish acclimatation conditions is important not only for the metal of concern but also when other metals and metalloids are considered.
In general, the kidney of tilapia was the main target for both Hg and Se uptake. The data indicate that the Hg accumulation in liver, heart, spleen, and gill was higher in the presence of Se6+ than in the presence of Se4+. For all treatments, the experiments indicated that no changes in the Hg uptake were observed in the brain, and the average Hg muscle concentrations were relatively lower than other organs. It was verified that a decrease in the Se concentration in the liver after Hg exposures, corroborated with the mechanism of detoxification proposed by Gailer (2007).
References
Akagi H, Malm O, Branches FJP (1995) Human exposure to mercury due to gold mining in the Tapajós river basin, Amazon, Brazil: speciation of mercury in human hair, blood and urine. Water, Air and Soil Poll 80:85–94. doi:10.1007/BF01189656
APHA (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Helath Association, Washington, DC
Beyrouty P, Chan HM (2006) Co-consumption of selenium and vitamin E altered the reproductive and developmental toxicity of methylmercury in rats. Neurotoxicol Teratol 28:49–58. doi:10.1016/j.ntt.2005.11.002
Cabañero AI, Madrid Y, Cámara C (2006) Selenium long-term administration and its effect on mercury toxicity. J Agr Food Chem 54:4461–4468. doi:10.1021/jf0603230
Chen C, Qu L, Zhao J, Liu S, Deng G, Li B, Zhang P, Chai Z (2006) Accumulation of mercury, selenium and their binding proteins in porcine kidney and liver from mercury-exposed areas with the investigation of their redox responses. Sci Total Environ 366:627–637. doi:10.1016/j.scitotenv.2005.12.021
França JG, Paiva MJTR, Lombardi JV, Carvalho S, Seriani R (2007) Chronic mercury chloride toxicity combined with selenium, by means of hematological study in tilapia Oreochromis niloticus. Bioikos 21:11–19
Gailer J (2007) Arsenic-selenium and mercury-selenium bonds in biology. Coordin Chem Rev 251:234–254. doi:10.1016/j.ccr.2006.07.018
Hansen JC, Kristensen P, Al-Masri SN (1981) Mercury/selenium interaction. Nord Vet Med 33:57–64
Kim JH, Birks E, Heisinger JF (1977) Protective action of selenium against mercury in northern creek chubs. Bull Environ Contam Toxicol 17:132–136. doi:10.1007/BF01685539
Montgomery DC (2001) Design and analysis of experiments, 5th edn. John Wiley, New York
Patel B, Chandy JP, Patel S (1988) Do selenium and glutathione inhibit the toxic effects of mercury in marine lamellibranchs? Sci Total Environ 76:147–165. doi:10.1016/0048-9697(88)90104-0
Pelgrom MGJ, Lamers LPM, Lock RAC, Balm PHM (1995) Interactions between copper and cadmium modify metal organ distribution in mature tilapia, Oreochromis mossambicus. Environ Pollut 90:415–423. doi:10.1016/0269-7491(95)00022-J
Pelletier E (1986) Modification de la bioaccumulation du selenium chez Mytilus edulis en presence du mercure organique et inorganique. Can J Fish Aquat Sci 43:203–210
Quevauviller P, Vercoutere K, Muntau H, Griepink B (1993) Certified reference material (CRM414) for the quality control of trace element analysis in plankton. Fresen J Anal Chem 345:12–17. doi:10.1007/BF00323319
Su L, Wang M, Yin A, Wang H, Chen L, Sun L, Ruan D (2008) The interaction of selenium and mercury in the accumulations and oxidative stress of rat tissues. Ecotox Environ Safe 70:483–489. doi:10.1016/j.ecoenv.2007.05.018
Wang W, Wong RSK, Wang J, Yen Y (2004) Influences of different selenium species on the uptake and assimilation of Hg(II) and methylmercury by diatoms and green mussels. Aquat Toxicol 68:39–50. doi:10.1016/j.aquatox.2004.02.003
Acknowledgments
Our gratitude to FAPESP (Fundação de Amparo à Pesquisa do estado de São Paulo), by the grant obtained in the Project no: 00/14460-3 and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for the scholarship to Gabriel G. A. Carvalho (Process no: 110751/2005-1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carvalho, G.G.A., de França, J.G., Dias, D.C. et al. Selenite and Selenate Effects on Mercury (Hg2+) Uptake and Distribution in Tilapia, Oreochromis niloticus L., Assessed by Chronic Bioassay. Bull Environ Contam Toxicol 82, 300–304 (2009). https://doi.org/10.1007/s00128-008-9617-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-008-9617-0