Abstract
Chlorpyrifos (CPF) is an extensive environmental contaminant and disrupts the physiological status of living organisms. CPF is found to hinder the health of aquatic organisms and ecological function in aquatic systems. The current study aimed at evaluating the protective effects of vitamin C (VC) on the immune response, hematological parameters, and histopathological alterations in Nile tilapia exposed to CPF. Nile tilapia were exposed to waterborne CPF (15 μg/L) for 30 days. Fish were divided into control group: received basal diet; CPF group: received basal diet and exposed to waterborne CPF; VC group: received basal diet plus 0.8 mg VC/kg; and CPF/VC group: received basal diet plus 0.8 mg VC/kg and exposed to waterborne CPF. Blood samples were taken after 15 days and 30 days of the treatment. Liver, gills, and intestine tissues were collected on the 30th day of treatment. CPF showed a deleterious effect on fish’s growth performance; it decreased the weight gain by 6%, while VC increased it by 17–23% compared to the control group. CPF group recorded the lowest survival rate (83%), while VC achieved survivability of 96.7% and 93.3% in VC and CPF/VC groups, respectively. The blood picture revealed moderate changes in the CPF group, where the marked alteration was in the hemoglobin concentration and white blood cells. CPF disrupted the hepatic and renal function. Serum lysozyme activity, phagocytic activity, and phagocytic index displayed a dramatic decline in the CPF group but enhanced in VC and CPF/VC groups. An upregulation was observed in antioxidant genes (catalase and glutathione peroxidase), heat shock protein 70, caspase-3, and the cytokines interleukin 1β, interleukin 8, and interferon-gamma in the CPF group. Simultaneously, moderate or normal levels were shown in the VC and CPF/VC groups. CPF altered the histoarchitecture of gills, intestine, and hepatopancreas with apparent degenerative changes possibly resulted from the oxidative stress. At the same time, VC retained the normal structure of the studied tissues. This study raises concerns about the safety of CPF and its impact on the aquatic environment. VC has a high potential to restore the normal physiology of fish exposed to CPF.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The aquaculture industry has increasingly developed as a promising activity for seafood production and food security. Moreover, aquaculture contributes to the economic sector, particularly in low-income countries (FAO 2020). However, on the environmental level, the extremely high levels of toxicants and xenobiotics were found to affect the growth performance and digestive enzyme activity of aquatic species (Li et al. 2015). Low hygiene and environmental contamination of aquaculture could affect consumer health and aquaculture productivity (Eltholth et al. 2015).
Chlorpyrifos (CPF) is an organophosphorus insecticide, extensively used for pest control in the agriculture sector (Dawood et al. 2020; Racke 1993). The half-life of CPF in river water ranges from 24 to 126 days (Liu et al. 2001). CPF has reported a contaminating environment, causing neurodevelopmental and metabolism disorders (Gao et al. 2017; Greer et al. 2019; McClelland et al. 2018). For example, CPF alters lipid metabolites and serine lipase activity in the brain of rainbow trout (Oncorhynchus mykiss) (Greer et al. 2019). CPF disrupts the endocrine system, which alters thyroid hormones and the expression of their receptors (Holzer et al. 2017; Raibeemol and Chitra 2020). Also, CPF causes the degradation of extracellular DNA in aquatic organisms and impaired hemolymph biochemical parameters in the crayfish (Astacus leptodactylus) (Banaee et al. 2019; Jin et al. 2015; Yang et al. 2019). CPF has been found to disrupt the antioxidant systems and immune response genes in common carp (Cyprinus carpio L.) (Banaee et al. 2014; Hatami et al. 2019; Li et al. 2013; Xing et al. 2019; Zhang et al. 2017). In Nile tilapia (Oreochromis niloticus), CPF caused complex physiological changes that included cytokine expression, oxidative damage, and tissue alterations, and the reversibility of these changes was varied following recovery (De Anna et al. 2021; Zahran et al. 2018). There are trials to alleviate the toxicity of CPF (Abo-Al-Ela 2020); for example, Aswathi et al. (2019) investigated Pseudomonas nitroreducens AR-3 as a biological agent to degrade CPF in the environment. Other studies tried to use beneficial substances, such as mannanoligosaccharide, Spirulina platensis, and β-glucan to rescue the toxicity induced by CPF (Dawood et al. 2020; Mokhbatly et al. 2020).
Vitamin C (VC) is a powerful antioxidant that reduces genotoxicity caused by chemical agents (Abo-Al-Ela et al. 2017; Dawood et al. 2020; Guha and Khuda-Bukhsh 2002; Sharifinasab et al. 2016). VC is found to reverse the effect of herbicide-induced toxicity, such as oxidative damage in cell lines (Gehin et al. 2005). VC has a hepatoprotective effect by regulating the serum total cholesterol, aspartate aminotransferase (AST), alkaline phosphatase (ALP), alanine aminotransferase (ALT), triglyceride, high-density lipoprotein cholesterol, and total protein levels during herbicide intoxication (Otuechere et al. 2012). Dietary VC significantly improved the growth performance, survival, and metabolic enzyme activities (e.g., alanine aminotransferase in the liver and muscle and ALP activity in the liver) and bioaccumulation endosulfan in spotted murrel, Channa punctatus (Sarma et al. 2009). Markedly, dietary vitamin C relieved CPF-induced toxicity in Clarias batrachus by enhancing the growth rate, blood immune, and biochemical responses (Narra et al. 2015).
In the current study, we aimed to determine the effect of CPF on inflammation and immunomodulation processes and histoarchitecture of the liver, gills, and intestine tissues, as well as we hypothesized that VC could curb or mitigate the harmful effect of CPF, if any, using Nile tilapia as an aquatic animal model. We studied different physiological parameters, trying to obtain a clear vision for the involved processes.
Materials and methods
The experiments of the current study were approved by the Faculty of Agriculture, Kafrelsheikh University, Egypt.
Diet preparation
The basal diet was formulated to contain 30.9% crude protein and 7.1% ether extract. Two sets of diets were formulated by supplementing the basal diet with VC at 0.8 mg/kg of diet (l-ascorbic acid, Sigma-Aldrich) (Table 1). All ingredients were ground and mixed by the laboratory meat grinder, and finally, pellets with a plate of 2-mm die were produced. The formulated diets were dried in an oven at 50 °C until their moisture level reached less than 8%, then left to cool down, and preserved at 4 °C. The actual concentration of VC in the formulated diet was 0.62 mg/kg, which was in agreement with the recommendation of Barros et al. (2014) and Falcon et al. (2007). VC analysis was done using high-performance liquid chromatography (HPLC), according to AOAC (1998).
Experimental design and procedure
Fish were collected from a commercial farm and transferred to the Fish Nutrition Laboratory, Baltim Research Station, National Institute of Oceanography and Fisheries (NIOF), Egypt. Following the acclimatization period (1 week, fed with basal diet), Nile tilapia (31.35 ± 0.25 g) were allocated into 12 glass aquaria (60 L) (10 fish per aquarium). The aquaria were distributed randomly into four groups (3 aquaria each) where the first and second groups were fed the basal diet without exposure to waterborne CPF (97% purity, Sigma-Aldrich), named control CPF, respectively, while the third and fourth groups were fed dietary VC without and with exposure to waterborne CPF, named VC and CPF/VC, respectively. The glass aquaria were continuously supplied with aeration, and fish were reared under natural day and dark cycle (approximately 12:12 h). The aquaria were siphoned daily to collect feces and remaining feed. The trial was done under laboratory conditions where the average values for water dissolved oxygen (6.5 ± 0.5 mg/L), temperature (25.1 ± 1.4 °C), total ammonia (0.23 ± 0.03 mg/L), and pH (7.1 ± 0.8) were registered and showed optimum levels for tilapia rearing.
For the waterborne CPF exposure, a stock of the CPF solution at a concentration of 15 μg/L was prepared and added to the CPF-exposed groups’ aquaria. The investigated level of CPF in the present study was determined by following Oruc (2012). The inclusion of CPF solution was added daily to the rearing water to keep the final concentration at 15 μg/L. In the beginning, the whole water in each aquarium was filled from the stock CPF solution (60 L/aquarium). Afterward, one-third of the aquarium water was replaced daily with the previously prepared CPF solution. Fish were fed with the prepared diets at a rate of 2% of their total biomass twice daily for 30 days. The concentration of CPF was regularly checked, which recorded a 14.58 ± 0.24 μg/L, according to Zalat et al. (2013). The fish were reared at 25.1 ± 1.4 °C, pH 7.1 ± 0.8, dissolved oxygen 6.5 ± 0.5 mg/L, and total ammonia 0.23 ± 0.03 mg/L.
Sampling
Considering the initial weight (IW) and the final weight (FW), the following equations were applied to calculate the growth performance of Nile tilapia after 30 days of CPF exposure:
Specific growth rate (SGR %) = 100 × (ln FW − ln IW)/30 days; Weight gain (%) = ((FW-IW)/IW)×100; Feed conversion ratio (FCR) = feed given/weight gain ; Survival rate (%) = (number at the end/number at the beginning) ×100.
Blood samples were collected after 15 and 30 days of CPF exposure for hematological and biochemical analysis. However, the liver, gills, and intestine tissues were collected at the end of the trial (30 days) for histological and gene expression tests. After 15 and 30 days, the fish were anaesthetized using 150 mg/L MS222 (Argent Laboratories, Redmond, Washington). Blood samples were collected from 3 fish per aquarium using EDTA-coated vials and non-coated vials for serum separation. Blood samples were left for 2 h to clot, then centrifuged at 3500×g for 15 min at 4 °C. The collected serum was kept at − 20 °C for further analysis. Liver, gills, and intestine tissues were collected from 3 fish per aquarium using Bouin’s solution for 18 h, then transferred to alcohol 70% for histopathological study. Besides, pieces of the liver tissue were preserved and immediately kept in liquid nitrogen then frozen at − 80 °C for gene expression analysis.
Blood analysis
Red blood cells (RBCs) and white blood cells (WBCs) were counted immediately with a hemocytometer using Natt and Herrick’s solution (Houston 1990). For differential leucocyte count, blood films were prepared and stained according to Lucky (1977), and cells were counted following the method by Jain (1986). The hemoglobin (Hb) concentration was determined using a spectrophotometer (Model RA 1000, Technicon Corporation, USA) at 540 nm (Blaxhall and Daisley 1973).
Serum total protein and albumin were determined according to Doumas et al. (1981) and Doumas et al. (1972). Globulin content was calculated mathematically. Activities of AST, ALP, and ALT were determined calorimetrically at wavelength 540 nm (Reitman and Frankel 1957). Serum creatinine, uric acid, urea, and bilirubin were determined following the protocol by Heinegård and Tiderström (1973) and Coulombe and Favreau (1963).
The phagocytic functional assay of leukocytes was analyzed according to Abo-Al-Ela et al. (2017) and Cai et al. (2004), which the phagocytic activity equals the percentages of leukocytes that engulfed bacteria, and phagocytic index equals the total number of bacterial cells phagocytized divided on the total number of phagocytic cells.
The lysozyme activity of sera was assayed, which the concentration of serum lysozyme (μg/mL) was obtained using a standard curve prepared from the lysozyme activity of a lyophilized hen egg-white according to Demers and Bayne (1997) and Abo-Al-Ela et al. (2017).
Gene expression
Total RNA was extracted from 50 mg of liver tissue using Trizol (iNtRON Biotechnology, Inc., Korea) according to the manufacturer’s manual. The total RNA of the samples was first extracted using Trizol (iNtRON Biotechnology). The quality and quantity of the extracted RNA were assessed by Nanodrop (Uv-Vis spectrophotometer Q5000/Quawell, USA). Then, complementary DNA (cDNA) was synthesized using the SensiFAST™ cDNA synthesis kit (Bioline, UK) according to the manufacturer’s protocol.
Gene-specific primer sequences were used for the antioxidant genes catalase (cat) and glutathione peroxidase (gpx), heat shock protein 70 (hsp70), caspase-3 (casp3), the immune-related genes: interleukin 1β (il1β), interleukin 8 (il8), and interferon-gamma (ifnγ) genes, and β-actin as a housekeeping gene (Table 2). The quantitative real-time PCR (Stratagene MX3000P) was used for gene expression.
SYBR green was used to quantify the gene expression using qRT-PCR (SensiFast SYBR Lo-Rox kit, Bioline). The thermocycling conditions for the reaction were 10 min at 95 °C, followed by 40 cycles of 15 s at 95 °C, 30 min at 60 °C, and finally 5 min at 85 °C (except for ifnγ, which was at 61 °C) for 1 min. Gene expression was corrected using the β-actin as a reference gene. After verification of PCR efficiency to be close to 100%, the gene expression data were calculated using the 2−ΔΔCt method according to Livak and Schmittgen (2001).
Histopathology
The histopathological examination was adopted according to Abumandour and Gewaily (2016). Nine fish were randomly selected from each treatment (3 fish per aquarium). After deep anesthesia using 40% ethyl alcohol, the abdomen was dissected to obtain samples from the gills, intestine, and liver. The collected tissues were cut into pieces of approximately 0.5 cm3 and fixed in Bouin’s solution for 18–24 h. The fixed samples were then dehydrated in ascending grades of alcohol (70%, 80%, 90%, and 100%, respectively), cleared with xylene, and embedded in paraffin wax. Sections with a thickness of 5 μm were obtained with Leica rotatory microtome (RM 20352035; Leica Microsystems, Wetzlar, Germany) and stained with hematoxylin and eosin. The tissue sections were examined using a BX50/BXFLA microscope (Olympus, Tokyo, Japan).
Statistical analysis
Data were presented as means with the standard error of means, and they were analyzed by a one-way ANOVA method using SPSS 22.0 (SPSS version 22, SPSS Inc., Il, USA). Duncan’s multiple range test was used to determine differences among treatments with a significance set at P < 0.05.
Results
Growth performance and feed utilization
The final body weight (FBW), weight gain (WG), and specific growth rate (SGR) were significantly increased in the VC group compared to the CPF group (P<0.05) without significant differences with the other groups (P>0.05). CPF significantly decreased Nile tilapia’s growth performances, where the WG was decreased by approximately 6% compared to control (Table 3). However, VC and CPF/VC groups showed relatively increased WG by 11–16.5% and 17–23% compared to the control and CPF groups, respectively. It is noticed that the SGR recorded the highest values in VC and CPF/VC groups, while the lowest value was in the CPF group. Feed intake (FI) was significantly increased in all treated groups compared to control. However, the feed conversion ratio (FCR) of the CPF group achieved the highest significant record. CPF markedly decreased the survival rate of Nile tilapia by 10% than those in control. However, VC greatly rescued fish’s survivability, which achieved an increase of more than 3% in the VC group compared to control.
Blood parameters
The Hb concentration showed a remarkable decline in the CPF and CPF/VC groups, particularly after 30 days post-exposure (Table 4). However, the VC group recorded high values of Hb on the 15th and 30th days of treatment. There was no great change in RBC count, except for some increases in the VC group. The packed cell volume (PCV) displayed decreases in the CPF and CPF/VC groups but increased in the VC group. Slight differences were recorded in the mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC) among groups, except for the CPF/VC group, which showed decreased levels on the 15th day, but returned to normal levels on the 30th day (Table 4). WBCs were lower in CPF and CPF/VC groups than in the other treated groups, primarily due to decreases in lymphocyte and monocyte population.
The creatinine level was high in the CPF group, while it returned to a normal CPF/VC group (Table 5). In the VC group, creatinine was the lowest among other groups even than that of the control. Urea and bilirubin were high in the CPF group, but their levels were declined in the CPF/VC group and much lower values in the VC group. Globulin and albumin were higher in VC and CPF/VC groups than in CPF and control groups, reflected in the total protein levels. ALP, AST, and ALT showed substantial increases in the CPF group and the treatment duration; however, their levels were significantly improved in the CPF/VC group. ALP, AST, and ALT levels were low in the VC group but within the normal range.
VC induced increases in the lysozyme activity, while CPF reduced it (Table 6). However, VC succeeded in keeping lysozyme activity at a closer level of control. The same was observed in the phagocytic activity and phagocytic index, but phagocytic activity was low in the CPF/VC group on the 15th day of treatment; however, the activity was retained 30th day of treatment.
Gene expression
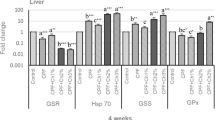
The expression of antioxidative genes (gpx and cat) in the liver of Nile tilapia was upregulated in the CPF, and CPF/VC groups, where the highest upregulation of gpx was in the VC group and cat was in the CPF/VC group (Fig. 1). In the CPF group, the expression of gpx was upregulated and the expression of cat was similar to the control.
Relative expression of antioxidative genes (catalase (cat) and glutathione peroxidase (gpx)) in the liver of Nile tilapia exposed to chlorpyrifos (CPF), supplemented with or without dietary vitamin C (VC) for 30 days. Values are expressed as mean ± SE from triplicate groups. Bars with different letters are significantly different from those of control group (P<0.05)
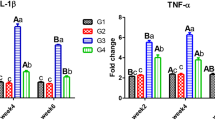
The highest expression of hsp70 and casp3 was recorded in the CPF group. However, the expression of these genes was kept at levels close to the control in the VC and CPF/VC groups (Fig. 2). Regarding the expression of immune-related genes (ifnγ, il8, and il1β), they were significantly upregulated in the liver of CPF treated fish; however, VC markedly reduced their expression in the CPF/VC group but remained at levels higher than that of the control (Fig. 3).
Relative expression of (A) heat shock protein 70 (hsp70) (B) caspase 3 (casp3) genes in the liver of Nile tilapia exposed to chlorpyrifos (CPF), supplemented with or without dietary vitamin C (VC) for 30 days. Values are expressed as mean ± SE from triplicate groups. Bars with different letters are significantly different from those of control group (P<0.05)
Relative expression of immune-related genes (ifnγ, il8, and il1β) in the liver of Nile tilapia exposed to chlorpyrifos (CPF), supplemented with or without dietary vitamin C (VC) for 30 days. Values are expressed as mean ± SE from triplicate groups. Bars with different letters are significantly different from those of control group (P<0.05)
Histopathology
Gills
In the control and VC-treated groups, the gills appeared normal. The lining epithelium of primary and secondary filaments was normal, with the presence of mucous cells (Fig. 4 A and C). CPF resulted in histopathological alterations, which displayed congestion of afferent blood vessels and the degeneration of the primary filaments’ epithelial lining. Telangiectasia (dilated apical end of secondary filaments) was also prominent in the CPF group (Fig. 4B). In the VC group, gills showed an improvement of the degenerative changes induced by CPF, and also, the degeneration and telangiectasia were not detected (Fig. 4D).
Histomicrograph of Nile tilapia gills shows the histological structure of the control group (A) and other treated groups, CPF (B), VC (C), and CPF/VC (D). In A and C, the gills show normal histological structures including primary filaments (PF), secondary filaments (SF), and mucous cells (black arrow head) between the secondary filaments. (B) Telangiectasia (black arrow) in the secondary filaments, and congestion of blood vessels of primary filaments (labeled with C) are observed. (D) the gills show a relatively normal structure in addition to leukocytic infiltration (white arrow head) (H&E; bar = 100 μm)
Intestine
The intestinal histology appeared normal, where the intestinal villi were lined by enterocytes (simple columnar epithelium), and submucosa, muscularis, and serosa showed a normal appearance in control (Fig. 5A) and CPF/VC (Fig. 5D) groups. The CPF group showed degeneration and sloughing of the apical part of the intestinal villi with lymphocytic infiltration (Fig. 5B). In the VC group, the intestinal villi were long, branched, and crowded (Fig. 5C).
Histomicrograph of Nile tilapia intestine shows the histological structure of the control group (A) and other treated groups, CPF (B), VC (C), and CPF/VC (D). In A and C, the intestinal villi (V), lamina propria sub mucosa (LP), tunica muscularis (M), and tunica serosa (S) show normal histological structures; in addition to an overcrowding and branching of the higher villi (C). (B) A degeneration and sloughing of the apical part of the intestinal villi epithelium (arrow) and leukocytic infiltration (arrow head) are observed. (D) The intestinal villi are intact and appeared normal (H&E; bar = 100 μm)
Liver
In the control group, the hepatopancreas showed a cord-like appearance of polyhedral hepatocytes and was separated by endothelial cell–lined blood sinusoids. The hepatopancreas contained a normal exocrine pancreatic tissue, and the afferent portal vein was surrounded by the exocrine pancreatic cover (Fig. 6 A and C). The CPF group displayed a hepatotoxic effect in the form of aneurysm and congestion of hepatic vein and degeneration and vacuolation of hepatocytes with pyknotic nuclei (Fig. 6B). VC restored the normal structure of the hepatopancreas (hepatic parenchyma and the pancreatic cells). The melanomacrophages were prominent at the periphery of pancreatic tissue (Fig. 6D).
Histomicrograph of Nile tilapia hepatopancreas shows the histological structure of the control group (A) and other treated groups, CPF (B), VC (C), and CPF/VC (D). In A and C, hepatopancreas shows cord-like appearance of polyhedral hepatocyte (H) and pancreatic cells that make pancreatic cover around afferent hepatic vein (P). (B) A degeneration of hepatocytes and pyknosis of their nuclei, aneurysm, and congestion of hepatic vein are observed. (D) Hepatopancreas retained its normal structure either in the hepatic (H) or pancreatic (P) parts (H&E; bar = 100 μm)
Discussion
The CPF group showed the highest FI and FCR, while the FBW, WG, and SGR recorded the lowest values among the other treated groups. FCR is usually low in young animals, while increases in older animals; however, young Nile tilapia were used in the current study. This may occur due to herbicides’ detrimental effect on the metabolism of nutrients (Dawood 2021). To illustrate, fish larvae exposed to CPF had underdeveloped guts and a reduced thyroid hormone level (T3) (Holzer et al. 2017). Other studies indicated that environmentally relevant CPF exposure modulated lipid metabolites and serine lipase activity in the trout brain (Greer et al. 2019). Besides, broken integrity of the gut barrier and altered microbiota was observed in CPF-exposed animals that led to the increased entry of lipopolysaccharide into the body, which ultimately resulted in low-grade inflammation and insulin resistance (Liang et al. 2019). The changes in gut microbiota composition included increases in opportunistic pathogens and short-chain fatty acid–producing bacteria (Fang et al. 2018), affecting health status and growth. In this study, VC significantly improved the growth performance (e.g., SGR and FBW) in both VC and CPF/VC groups with values more than that in control. This is consistent with the capacity of VC to increase SGR, WG, and feed efficiency in fish (El Basuini et al. 2017; Zhou et al. 2012). Similarly, Narra et al. (2015) stated that dietary vitamin C enhanced the growth performance of Clarias batrachus exposed to CPF toxicity.
The total leukocyte count and Hb concentration were low in CPF, and CPF/VC groups and CPF-exposed Labeo rohita (Ismail et al. 2018). The low Hb was attributed to the ability of CPF to decrease serum iron concentration (Goel et al. 2006). The elevated bilirubin in the CPF group likely resulted from Hb breakdown. Although the MCHC was not markedly changed among groups, there was a significant alteration between groups, indicating that VC and CPF do not affect the Hb inside cells. Thus, VC cannot restore the changes in blood cell as well as Hb levels; however, this might be explained by earlier reports indicating that VC could not modulate or has little effect on blood cell population and count (Abo-Al-Ela et al. 2017). However, pretreatment with VC could protect from the reduction in Hb in CPF-exposed animals (Goel et al. 2006). In the current study, the decrease in the total leukocytes was due to lower counts of the lymphocytes and monocytes. It has been found that CPF induces the overproduction of tumor necrosis factor-alpha (tnfα), which in turn mediates apoptosis of lymphocytes and monocytes (Dreschers et al. 2013; Navaei-Nigjeh et al. 2015). Another potential explanation might be that increased insulin resistance resulted in low glucose uptake by such cells’ deposit of sustainable high plasma glucose recorded during the CPF exposure (Narra et al. 2015), leading to their apoptosis (Liang et al. 2019; Ndonwi et al. 2020).
The high levels of creatinine and urea in the CPF group indicate impaired kidney function (e.g., low clearance) or liver oxidative stress because urea is formed by deamination of amino acids in the liver. Fish fed dietary VC showed improved or near-normal creatinine and urea levels, indicating the protective role of VC against CPF-induced renal damage.
ALP, AST, and ALT were increased in the CPF group, and this was observed all over the exposure (30 days). The liver enzymes (AST and ALT) are involved in the protein metabolism; their high levels may be associated with the availability of more amino acids (e.g., tissue breakdown) or inflammation or hepatocellular damage (Sarma et al. 2009). Oxidative stress is another possible cause of tissue damage during intoxication with CPF, indicated by low activities of catalase, glutathione peroxidase, and glutathione (Mokhbatly et al. 2020; Yonar 2018; Zahran et al. 2018). A high level of ALP suggests the stimulation of a metabolic reaction to neutralize the deleterious effects of CPF (El-Nahhal et al. 2020). Similarly, the pesticide endosulfan reduced the enzymatic activities of various metabolic pathways in the spotted murrel, Channa punctatus (Sarma et al. 2009). Our findings showed that the activities of these enzymes were restored to normal levels in fish fed with VC.
Gamma-globulins constitute the major fraction of the globulin of total serum protein. Immunoglobulins are the most significant gamma-globulins (Abdel-Mageid et al. 2020). Globulin fraction of the blood was high in VC and CPF/VC groups, indicating an active immune response. This supports the notion of VC acting on leukocyte function but not population (Abo-Al-Ela et al. 2017).
Lysozyme is an enzyme present in different body fluids, such as blood, mucus secretion and egg whites, and other parts of eukaryotic organisms. It is a hydrolytic enzyme that cleaves the cell wall of Gram-positive and some Gram-negative bacteria (McKenzie and White 1991). Phagocytosis, a critical biological activity, is modulated by changes in the physiological status. These changes are reflected in the phagocytic activity and phagocytic index of phagocytic cells (Platt and Fineran 2015). CPF reduced the capacity and ability of phagocytic cells to engulf foreign bodies, consistent with previous studies (Girón-Pérez et al. 2006), possibly because of its effect on cells’ sensitivity to recognize foreign materials. VC is a powerful molecule that can potentiate the immune system, including lysozyme and phagocytic activities (Abo-Al-Ela 2019; Abo-Al-Ela et al. 2017; Dawood et al. 2018). It was noted that VC could mitigate the harmful effects of CPF on the lysozyme activity and phagocytic function.
CPF induces a pro-inflammatory response (Liang et al. 2019), oxidative stress (Dawood et al. 2020; Mokhbatly et al. 2020), and genotoxicity (Ali et al. 2008), and modulates the expression of the involved genes. Immunity and oxidative stress are closely linked and modulate each other (Abdel-Mageid et al. 2020). Our results indicated an upregulation of the antioxidative genes gpx and cat, the oxidative related genes hsp70 and casp3, and the immune-related genes ifnγ, il8, and il1β in the liver of Nile tilapia. CPF-induced reactive oxygen species regulate immune response by triggering the antigen-presenting ability of head kidney in common carp (Zhang et al. 2017). In a previous study, the changes in the expression of some of these genes are accompanied by an increase in the antioxidant enzyme activity (i.e., malondialdehyde and glutathione) and a significant decrease in catalase, glutathione S-transferase, and superoxide dismutase in the liver and gills of fish exposed to CPF for 14 or 15 days (Zahran et al. 2018; Zhang et al. 2017). Besides, continuous expression of cytokines has an immunotoxic effect and could badly affect the immune function (Abo-Al-Ela 2018). The microRNA machinery regulates the physiological response in any given organism (Abo-Al-Ela and Burgos-Aceves 2020). CPF might have an indirect effect on gene expression through modulating microRNA machinery, which induced oxidative stress and modulated the miR-19a-AMPK axis in common carp (Zhang et al. 2019). The induction of the antioxidative and detoxification systems may respond to detoxify CPF; however, VC as an antioxidative molecule showed relative expression values to the control (Tripathi and Shasmal 2010). In similar supporting findings, VC could adjust the expression of genes involved in the antioxidant function of Nile tilapia exposed to toxic concentrations of cadmium (El-Sayed et al. 2016). Of note, the survivability of fish was decreased in the CPF group, presumably because of its immunotoxic and oxidative stress that could decrease fish’s ability to cope with biotic and abiotic stress.
The histoarchitecture of gills, intestine, and hepatopancreas presented altered structures. The gill is an important organ for respiration and osmoregulation and allows the entrance of toxicants into the body (Zahran et al. 2018). Degenerative changes and telangiectasia were prominent in the CPF-exposed group that indicate severe irritation of pesticides, as evidenced in previous studies (Banaee et al. 2013; Zahran et al. 2018). This might be linked to a significant increase of acid DNase activity in gills of mussel Mytilus galloprovincialis exposed to high concentrations CPF (10 and 100 μg/L) (Kovačić and Medić 2016). These results indicate a high DNA metabolism rate to degrade the apoptotic and damaged DNA (Evans and Aguilera 2003). A similar alteration was detected in the intestine with lymphocytic infiltration, possibly because of the opportunistic microorganisms present in the intestine lumen. The sloughing of the apical part of the intestinal villi may be attributed to the irritation, and the inflammatory effect resulted from CPF (Dawood et al. 2020, Mokhbatly et al. 2020) or CPF-induced reactive oxygen species production (Zhang et al. 2017).
The liver is the primary detoxification organ and is rapidly affected by xenobiotics exposure (Banaee 2020; El-Sayed et al. 2016; Zahran et al. 2018). Aneurysm and congestion of the hepatic vein and degeneration, nuclear pyknosis, and vacuolation of the hepatocytes were observed in the CPF group, similar to observation by Zahran et al. (2018) in Nile tilapia exposed for 14 days. Generally, the destructive action or necrosis is markedly associated with oxidative stress (e.g., reactive oxygen species) where lipid peroxidation is the main source of membrane bilayer susceptibility (Avci et al. 2005; Li et al. 2000).
Overall, VC significantly improved the histoarchitecture of the studied tissues and increased the melanomacrophages centers in the hepatopancreas, which indicate enhanced immunity (Abo-Al-Ela 2019; Abo-Al-Ela et al. 2017). Similar findings indicated the protective role of VC in fish exposed to different pesticides (El-Sayed et al. 2016; Sarma et al. 2009).
The acute toxicity of CPF is usually considered moderate. Despite this, the environmental exposures, particularly continuously and at levels below that acute cause toxicity, could result in health concerns and developmental disorders (Gao et al. 2017; McClelland et al. 2018). Food additives are the best potential tool that promotes immunity and improves physiological function in aquaculture (Ahmadifar et al. 2020; El Basuini et al. 2021; Zaineldin et al. 2020), and sustainable supplementation of aquatic organisms with beneficial elements, particularly VC, would help such animals to cope with bad environmental conditions and contaminations (Dawood and Koshio 2018).
Conclusion
In conclusion, we found that chlorpyrifos causes immunotoxicity, hematological and biochemical disturbances, and damage to tissues such as gills, intestine, and liver, which makes fish exposed to chlorpyrifos have a low resistance to external biotic stresses, and could present a higher susceptibility to infection. These findings should be addressed concerning pesticide safety evaluations in future studies. Our results demonstrate that vitamin C treatment ameliorated the CPF-induced toxicity in Nile tilapia.
Availability of data and materials
Data and materials are available upon request.
References
Abdel-Mageid AD, Zaki AG, El Senosi YA, Fahmy HA, El Asely AM, Abo-Al-Ela HG, El-Kassas S (2020) Modulatory effect of lipopolysaccharide on immune-related gene expression and serum protein fractionation in grey mullet, Mugil cephalus. Aquacult Res 51:1643–1652
Abo-Al-Ela HG (2018) An introduction to selected innate immune-relevant genes in fish. Appl Ecol Env Res 16:955–976
Abo-Al-Ela HG (2019) Does vitamin C mitigate the detrimental effect of androgens on immunity? Res Vet Sci 125:43–44
Abo-Al-Ela HG (2020): Are pathogens completely harmful or useless? ACS Chem Neurosci
Abo-Al-Ela HG, Burgos-Aceves MA (2020) Exploring the role of microRNAs in axolotl regeneration. J Cell Physiol
Abo-Al-Ela HG, El-Nahas AF, Mahmoud S, Ibrahim EM (2017) Vitamin C modulates the immunotoxic effect of 17α-methyltestosterone in Nile tilapia. Biochemistry 56:2042–2050
Abumandour MMA, Gewaily MS (2016) Morphological studies on the gills of puffer fish (Lagocephalus sceleratus, Gmelin, 1789). Int J Morphol 34:817–829
Ahmadifar E, Dawood MAO, Moghadam MS, Shahrestanaki AH, Van Doan H, Saad AH, Aboubakr M, Abdelhiee EY, Fadl SE (2020) The effect of Pediococcus acidilactici MA 18/5 M on immune responses and mRNA levels of growth, antioxidant and immune-related genes in zebrafish (Danio rerio). Aquacult Rep 17:100374
Ali D, Nagpure NS, Kumar S, Kumar R, Kushwaha B (2008) Genotoxicity assessment of acute exposure of chlorpyrifos to freshwater fish Channa punctatus (Bloch) using micronucleus assay and alkaline single-cell gel electrophoresis. Chemosphere 71:1823–1831
AOAC (1998): Association of Official Analytical Chemists. Official methods of analysis of official analytical chemists international, 16th ed. Washington, DC.
Aswathi A, Pandey A, Sukumaran RK (2019) Rapid degradation of the organophosphate pesticide – chlorpyrifos by a novel strain of Pseudomonas nitroreducens AR-3. Bioresour Technol 292:122025
Avci A, Kaçmaz M, Durak İ (2005) Peroxidation in muscle and liver tissues from fish in a contaminated river due to a petroleum refinery industry. Ecotoxicol Environ Saf 60:101–105
Banaee M (2020) Alkaline phosphatase activity as a biochemical biomarker in aqua-toxicological studies. Int J Aquat Biol 8:143–147
Banaee M, Akhlaghi M, Soltanian S, Gholamhosseini A, Heidarieh H, Fereidouni MS (2019) Acute exposure to chlorpyrifos and glyphosate induces changes in hemolymph biochemical parameters in the crayfish, Astacus leptodactylus (Eschscholtz, 1823). Comp Biochem Physiol Part C: Toxicol Pharmacol 222:145–155
Banaee M, Haghi BN, Ibrahim ATA (2014) Sub-lethal toxicity of chlorpyrifos on common carp, Cyprinus carpio (Linnaeus, 1758): biochemical response. Int J Aquat Biol 1:281–288
Banaee M, Sureda A, Mirvaghefi AR, Ahmadi K (2013) Biochemical and histological changes in the liver tissue of rainbow trout (Oncorhynchus mykiss) exposed to sub-lethal concentrations of diazinon. Fish Physiol Biochem 39:489–501
Barros MM, Falcon DR, de Oliveira OR, Pezzato LE, Fernandes AC, Guimarães IG, Fernandes A, Padovani CR, Sartori MMP (2014) Non-specific immune parameters and physiological response of Nile tilapia fed β-glucan and vitamin C for different periods and submitted to stress and bacterial challenge. Fish Shellfish Immunol 39:188–195
Blaxhall PC, Daisley KW (1973) Routine haematological methods for use with fish blood. J Fish Biol 5:771–781
Cai W-q, Li S-f, Ma J-y (2004) Diseases resistance of Nile tilapia (Oreochromis niloticus), blue tilapia (Oreochromis aureus) and their hybrid (female Nile tilapia×male blue tilapia) to Aeromonas sobria. Aquaculture 229:79–87
Coulombe JJ, Favreau L (1963) A new simple semimicro method for colorimetric determination of urea. Clin Chem 9:102–108
Dawood MAO (2021) Nutritional immunity of fish intestines: important insights for sustainable aquaculture. Rev Aquacult 13:642–663
Dawood MA, Abdel-Razik NI, Gewaily MS, Sewilam H, Paray BA, Soliman AA, Abdelhiee EY, Aboubakr M, Van Doan H, El-Sabagh M (2020) β-Glucan improved the immunity, hepato-renal, and histopathology disorders induced by chlorpyrifos in Nile tilapia. Aquacult Rep 18:100549
Dawood MAO, El-Shamaa IS, Abdel-Razik NI, Elkomy AH, Gewaily MS, Abdo SE, Soliman AA, Paray BA, Abdelkhalek N (2020) The effect of mannanoligosaccharide on the growth performance, histopathology, and the expression of immune and antioxidative related genes in Nile tilapia reared under chlorpyrifos ambient toxicity. Fish Shellfish Immunol 103:421–429
Dawood MAO, Koshio S (2018) Vitamin C supplementation to optimize growth, health and stress resistance in aquatic animals. Rev Aquacult 10:334–350
Dawood MAO, Koshio S, Esteban MÁ (2018) Beneficial roles of feed additives as immunostimulants in aquaculture: a review. Rev Aquacult 10:950–974
Dawood MA, Zommara M, Eweedah NM, Helal AI, Aboel-Darag MA (2020) The potential role of nano-selenium and vitamin C on the performances of Nile tilapia (Oreochromis niloticus). Environ Sci Pollut Res:1–10
De Anna JS, Castro JM, Darraz LA, Elías FD, Cárcamo JG, Luquet CM (2021) Exposure to hydrocarbons and chlorpyrifos alters the expression of nuclear receptors and antioxidant, detoxifying, and immune response proteins in the liver of the rainbow trout, Oncorhynchus mykiss. Ecotoxicol Environ Saf 208:111394
Demers NE, Bayne CJ (1997) The immediate effects of stress on hormones and plasma lysozyme in rainbow trout. Develop Comp Immunol 21:363–373
Doumas BT, Bayse DD, Carter RJ, Peters T Jr, Schaffer R (1981) A candidate reference method for determination of total protein in serum. I Dev Valid. Clin Chem 27:1642–1650
Doumas BT, Biggs HG, Arends RL, Pinto PVC (1972) Determination of serum albumin. In: Cooper GR (ed) Standard Methods of Clinical Chemistry. Academic Press, New York, pp 175–188
Dreschers S, Gille C, Haas M, Grosse-Ophoff J, Schneider M, Leiber A, Bühring H-J, Orlikowsky TW (2013) Infection–induced bystander-apoptosis of monocytes is TNF-alpha-mediated. PLoS One 8:e53589
El Basuini MF, El-Hais AM, Dawood MAO, Abou-Zeid AE-S, SZ EL-D, MME-S K, Koshio S, Ishikawa M, Dossou S (2017) Effects of dietary copper nanoparticles and vitamin C supplementations on growth performance, immune response and stress resistance of red sea bream, Pagrus major. Aquacult Nutr 23:1329–1340
El Basuini MF, Shahin SA, Teiba II, Zaki MAA, El-Hais AM, Sewilam H, Almeer R, Abdelkhalek N, Dawood MAO (2021) The influence of dietary coenzyme Q10 and vitamin C on the growth rate, immunity, oxidative-related genes, and the resistance against Streptococcus agalactiae of Nile tilapia (Oreochromis niloticus). Aquaculture 531:735862
El-Nahhal Y, Lubbad R, Al-Agha MR (2020) Toxicity evaluation of chlorpyrifos and diuron below maximum residue limits in rabbits. Toxicol Environ Health Sci 12:177–190
El-Sayed YS, El-Gazzar AM, El-Nahas AF, Ashry KM (2016) Vitamin C modulates cadmium-induced hepatic antioxidants’ gene transcripts and toxicopathic changes in Nile tilapia, Oreochromis niloticus. Environ Sci Pollut Res 23:1664–1670
Eltholth M, Fornace K, Grace D, Rushton J, Hasler B (2015) Characterisation of production, marketing and consumption patterns of farmed tilapia in the Nile Delta of Egypt. Food Policy 51:131–143
Evans CJ, Aguilera RJ (2003) DNase II: genes, enzymes and function. Gene 322:1–15
Falcon DR, Barros MM, Pezzato LE, Sampaio FG, Hisano H (2007) Physiological responses of Nile tilapia, Oreochromis niloticus, fed vitamin C- and lipid-supplemented diets and submitted to low-temperature stress. J World Aquacult Soc 38:287–295
Fang B, Li JW, Zhang M, Ren FZ, Pang GF (2018) Chronic chlorpyrifos exposure elicits diet-specific effects on metabolism and the gut microbiome in rats. Food Chem Toxicol 111:144–152
FAO (2020) The State of World Fisheries and Aquaculture 2020. Sustainability in action, Rome, Italy
Gao J, Naughton SX, Beck WD, Hernandez CM, Wu G, Wei Z, Yang X, Bartlett MG, Terry AV (2017) Chlorpyrifos and chlorpyrifos oxon impair the transport of membrane bound organelles in rat cortical axons. NeuroToxicology 62:111–123
Gehin A, Guillaume YC, Millet J, Guyon C, Nicod L (2005) Vitamins C and E reverse effect of herbicide-induced toxicity on human epidermal cells HaCaT: a biochemometric approach. Int J Pharm 288:219–226
Girón-Pérez MI, Barcelós-García R, Vidal-Chavez ZG, Romero-Bañuelos CA, Robledo-Marenco ML (2006) Effect of chlorpyrifos on the hematology and phagocytic activity of Nile tilapia cells (Oreochromis niloticus). Toxicol Mech Methods 16:495–499
Goel A, Dani V, Dhawan DK (2006) Role of zinc in mitigating the toxic effects of chlorpyrifos on hematological alterations and electron microscopic observations in rat blood. Biometals 19:483–492
Greer JB, Magnuson JT, Hester K, Giroux M, Pope C, Anderson T, Liu J, Dang V, Denslow ND, Schlenk D (2019) Effects of chlorpyrifos on cholinesterase and serine lipase activities and lipid metabolism in brains of rainbow trout (Oncorhynchus mykiss). Toxicol Sci 172:146–154
Guha B, Khuda-Bukhsh AR (2002) Efficacy of vitamin-C (L-ascorbic acid) in reducing genotoxicity in fish (Oreochromis mossambicus) induced by ethyl methane sulphonate. Chemosphere 47:49–56
Hatami M, Banaee M, Nematdoost Haghi B (2019) Sub-lethal toxicity of chlorpyrifos alone and in combination with polyethylene glycol to common carp (Cyprinus carpio). Chemosphere 219:981–988
Heinegård D, Tiderström G (1973) Determination of serum creatinine by a direct colorimetric method. Clinica Chimica Acta 43:305–310
Holzer G, Besson M, Lambert A, François L, Barth P, Gillet B, Hughes S, Piganeau G, Leulier F, Viriot L, Lecchini D, Laudet V (2017) Fish larval recruitment to reefs is a thyroid hormone-mediated metamorphosis sensitive to the pesticide chlorpyrifos. eLife 6:e27595
Houston AH (1990) Blood and circulation. In: Moyle PB (ed) Schreck CB. Methods for fish biology. American Fisheries Society, Bethesda, MD, pp 273–334
Ismail M, Ali R, Shahid M, Khan MA, Zubair M, Ali T, Mahmood Khan Q (2018) Genotoxic and hematological effects of chlorpyrifos exposure on freshwater fish Labeo rohita. Drug Chem Toxicol 41:22–26
Jain NC (1986): Schalm’s veterinary hematology. Lea & Febiger, Philadelphia, ix + 1221 pp. pp
Jin Y, Liu Z, Peng T, Fu Z (2015) The toxicity of chlorpyrifos on the early life stage of zebrafish: A survey on the endpoints at development, locomotor behavior, oxidative stress and immunotoxicity. Fish Shellfish Immunol 43:405–414
Kovačić I, Medić N (2016) The effect of chlorpyrifos on protein content and acid DNase activity in the mussel, Mytilus galloprovincialis. Mar Freshw Behav Physiol 49:265–275
Li X, Liu L, Zhang Y, Fang Q, Li Y, Li Y (2013) Toxic effects of chlorpyrifos on lysozyme activities, the contents of complement C3 and IgM, and IgM and complement C3 expressions in common carp (Cyprinus carpio L.). Chemosphere 93:428–433
Li Y, Ru B, Liu X, Miao W, Zhang K, Han L, Ni H, Wu H (2015) Effects of extremely low frequency alternating-current magnetic fields on the growth performance and digestive enzyme activity of tilapia Oreochromis niloticus. Environ Biol Fish 98:337–343
Li Q-T, Yeo MH, Tan BK (2000) Lipid peroxidation in small and large phospholipid unilamellar vesicles induced by water-soluble free radical sources. Biochem Biophys Res Commun 273:72–76
Liang Y, Zhan J, Liu D, Luo M, Han J, Liu X, Liu C, Cheng Z, Zhou Z, Wang P (2019) Organophosphorus pesticide chlorpyrifos intake promotes obesity and insulin resistance through impacting gut and gut microbiota. Microbiome 7:19
Liu B, McConnell LL, Torrents A (2001) Hydrolysis of chlorpyrifos in natural waters of the Chesapeake Bay. Chemosphere 44:1315–1323
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Lucky Z (1977) Methods for the diagnosis of fish diseases. Amerind Publishing Co. PVT. Ltd., New Delhi, Bombay, India
McClelland SJ, Bendis RJ, Relyea RA, Woodley SK (2018) Insecticide-induced changes in amphibian brains: how sublethal concentrations of chlorpyrifos directly affect neurodevelopment. Environ Toxicol Chem 37:2692–2698
McKenzie HA, White FH (1991) Lysozyme and α-lactalbumin: structure, function, and interrelationships. In: Richards FM, Edsall JT, Eisenberg DS (eds) Anfinsen CB. Academic Press, Advances in Protein Chemistry, pp 173–315
Mokhbatly A-AA, Assar DH, Ghazy EW, Elbialy Z, Rizk SA, Omar AA, Gaafar AY, Dawood MAO (2020) The protective role of spirulina and β-glucan in African catfish (Clarias gariepinus) against chronic toxicity of chlorpyrifos: hemato-biochemistry, histopathology, and oxidative stress traits. Environ Sci Pollut Res 27:31636–31651
Narra MR, Rajender K, Rudra Reddy R, Rao JV, Begum G (2015) The role of vitamin C as antioxidant in protection of biochemical and haematological stress induced by chlorpyrifos in freshwater fish Clarias batrachus. Chemosphere 132:172–178
Navaei-Nigjeh M, Asadi HR, Baeeri M, Pedram S, Rezvanfar MA, Mohammadirad A, Abdollahi M (2015) In vitro protection of human lymphocytes from toxic effects of chlorpyrifos by selenium-enriched medicines. Iran J Basic Med Sci 18:284–291
Ndonwi EN, Atogho-Tiedeu B, Lontchi-Yimagou E, Shinkafi TS, Nanfa D, Balti EV, Katte JC, Mbanya A, Matsha T, Mbanya JC, Shakir A, Sobngwi E (2020): Metabolic effects of exposure to pesticides during gestation in female Wistar rats and their offspring: a risk factor for diabetes? Toxicol Res
Oruc E (2012) Oxidative stress responses and recovery patterns in the liver of Oreochromis niloticus exposed to chlorpyrifos-ethyl. Bull Environ Contam Toxicol 88:678–684
Otuechere CA, Abarikwu SO, Rufai MA, Ohiozoje AE, Martins E, Farombi EO (2012) Protective effects of vitamin C against propanil-induced hepatotoxicity in wistar rats. Asian Pac J Trop Dis 2:S212–S217
Platt N, Fineran P (2015) Chapter 14 - Measuring the phagocytic activity of cells. In: Platt N (ed) Platt F. Academic Press, Methods in Cell Biology, pp 287–304
Racke KD (1993) Environmental Fate of Chlorpyrifos. Reviews of Environmental Contamination and Toxicology. Springer New York, New York, NY, pp 1–150
Raibeemol KP, Chitra KC (2020) Induction of immunological, hormonal and histological alterations after sublethal exposure of chlorpyrifos in the freshwater fish, Pseudetroplus maculatus (Bloch, 1795). Fish Shellfish Immunol 102:1–12
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28:56–63
Sarma K, Pal AK, Sahu NP, Ayyappan S, Baruah K (2009) Dietary high protein and vitamin C mitigates endosulfan toxicity in the spotted murrel, Channa punctatus (Bloch, 1793). Sci Total Environ 407:3668–3673
Sharifinasab Z, Banaee M, Mohiseni M, Noori A (2016) The protective role of vitamin C and chitosan against paraquat-induced oxidative stress in muscles of common carp (Cyprinus carpio). Croatian J Fish 74:149–158
Tripathi G, Shasmal J (2010) Reparation of chlorpyrifos-induced impairment by thyroxine and vitamin C in fish. Ecotoxicol Environ Saf 73:1397–1401
Xing H, Chen J, Peng M, Wang Z, Liu F, Li S, Teng X (2019) Identification of signal pathways for immunotoxicity in the spleen of common carp exposed to chlorpyrifos. Ecotoxicol Environ Saf 182:109464
Yang B, Qin C, Hu X, Xia K, Lu C, Gudda FO, Ma Z, Gao Y (2019) Enzymatic degradation of extracellular DNA exposed to chlorpyrifos and chlorpyrifos-methyl in an aqueous system. Environ Int 132:105087
Yonar ME (2018) Chlorpyrifos-induced biochemical changes in Cyprinus carpio: Ameliorative effect of curcumin. Ecotoxicol Environ Saf 151:49–54
Zahran E, Risha E, Awadin W, Palić D (2018) Acute exposure to chlorpyrifos induces reversible changes in health parameters of Nile tilapia (Oreochromis niloticus). Aquatic Toxicol 197:47–59
Zaineldin AI, Hegazi S, Koshio S, Ishikawa M, Dawood MA, Dossou S, Yukun Z, Mzengereza K (2020): Singular effects of Bacillus subtilis C-3102 or Saccharomyces cerevisiae type 1 on the growth, gut morphology, immunity, and stress resistance of red sea bream (Pagrus major). Annals of Animal Science 1
Zalat OA, Elsayed MA, Fayed MS, Abd El Megid MK (2013) Validation of UV spectrophotometric and HPLC methods for quantitative determination of chlorpyrifos. Int Lett Chem Phys Astron 21:58–63
Zhang Z, Liu Q, Cai J, Yang J, Shen Q, Xu S (2017) Chlorpyrifos exposure in common carp (Cyprinus carpio L.) leads to oxidative stress and immune responses. Fish Shellfish Immunol 67:604–611
Zhang Q, Zheng S, Wang S, Wang W, Xing H, Xu S (2019) Chlorpyrifos induced oxidative stress to promote apoptosis and autophagy through the regulation of miR-19a-AMPK axis in common carp. Fish Shellfish Immunol 93:1093–1099
Zhou Q, Wang L, Wang H, Xie F, Wang T (2012) Effect of dietary vitamin C on the growth performance and innate immunity of juvenile cobia (Rachycentron canadum). Fish Shellfish Immunol 32:969–975
Acknowledgements
The authors would like to thank the Fish Nutrition Laboratory staff, Baltim Research Station, National Institute of Oceanography and Fisheries (NIOF), Egypt, for their assistance during the study.
Author information
Authors and Affiliations
Contributions
Conceptualization, Azza H. Elkomy, Mahmoud A.O. Dawood; formal analysis, Safaa E. Abdo, Mahmoud S. Gewaily, Ali A. Soliman, Azza H. Elkomy, Mahmoud A.O. Dawood; funding acquisition, Safaa E. Abdo, Mahmoud S. Gewaily, Rafa Almeer, Ali A. Soliman, Azza H. Elkomy, Mahmoud A.O. Dawood; investigation, Safaa E. Abdo, Mahmoud S. Gewaily, Ali A. Soliman, Azza H. Elkomy, Mahmoud A.O. Dawood; methodology, Safaa E. Abdo, Mahmoud S. Gewaily, Ali A. Soliman, Azza H. Elkomy, Mahmoud A.O. Dawood; project administration, Mahmoud A.O. Dawood; writing—original draft, Haitham G. Abo-Al-Ela, Mahmoud A.O. Dawood.
Corresponding author
Ethics declarations
Ethical approval
The experiments of the current study were approved by the Faculty of Agriculture, Kafrelsheikh University, Egypt.
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Bruno Nunes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdo, S.E., Gewaily, M.S., Abo-Al-Ela, H.G. et al. Vitamin C rescues inflammation, immunosuppression, and histopathological alterations induced by chlorpyrifos in Nile tilapia. Environ Sci Pollut Res 28, 28750–28763 (2021). https://doi.org/10.1007/s11356-021-12711-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-12711-5