Abstract
Trace metal pollution is a vital issue in ecological problems (air, soil, and water), and it threatens human health in many urban areas worldwide. The accumulation of heavy metals released from various sources can readily occur on plants and impairs their growth. Therefore, monitoring metal concentration is extremely important when released into the atmosphere from one place to another urban environment. Biomonitor is one of the passive methods used to track selected elements. Chromium (Cr) has adverse effects on plants when it is in high concentrations; therefore, the variation of its concentration in plants is important to be assessed. Another target element, zinc (Zn), has different essential metabolic functions in plants and is crucial in protein and carbohydrate synthesis. It directly affects the plant due to its protein and carbohydrate synthesis role. This study aimed to determine the variation of the Cr and Zn concentration ratio in the organs of Picea pungens Engelm. from Ankara, Türkiye. According to organ, age, washing status, and location, Picea pungens Engelm. showed significant differences (p < 0.05) for Cr and Zn pollution on the road shoulders. Their location on the tree can easily determine the age of the needles and branches. The total values of bark for Cr and Zn were calculated as 23,887 ppb and 672,012 ppb in barks in unwashed samples. The result of the Cr and Zn content was significantly evaluated using ANOVA and Duncan test. The P. pungens is an excellent passive sampler as a biomonitor for the Cr and Zn distribution in the local atmospheric environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Direct emissions of air pollutants gradually increase with anthropogenic activities' rapid support (Rovella et al., 2021). Uncontrolled industrialization and irregular urbanization lead to urban pollution (Kabir et al., 2021; Yilmaz & Isinkaralar, 2021a). The World Health Organization (WHO) estimates that about 7 million people die prematurely from air pollution exposure (WHO, 2016). Toxic metals are released from many factors such as excessive use of fossil fuels in cities and industries, uncontrolled emissions of exhaust gases, structures with high floors that can cut the prevailing winds of cities, etc. (Tarín-Carrasco et al., 2021). They are inorganic substances with a severe toxic effect on living organisms that accumulate in their bodies (Alengebawy et al., 2021). In addition, some metals are serious pollutants that are non-degradable in their form, making them a potential threat to the environment (Meena et al., 2021). Therefore, monitoring the concentrations of inorganic components and reducing their release in urban activities is a vital and complex process (Cai et al., 2021; Kabata-Pendias, 2004; Rajendran et al., 2021).
Air-monitoring stations in today's cities, which are constantly connected to electricity, are located in various places to monitor air quality (Levei et al., 2021; Sergeeva et al., 2021). However, the pollution type that follows them are limited in number, and there is almost no information about metals. Therefore, there is a need for something that can be passively monitored without the need for energy (Sablier & Garrigues, 2014; Isinkaralar et al., 2022). Numerous studies have shown that some species are suitable for biomonitors without energy (Markert et al., 1999; Petrova et al., 2012; Pignata et al., 2007). However, which living organisms are the most suitable biomonitors has not yet been determined. The lichens, mosses, landscape plants, and different species are used as bioaccumulator (Marié et al., 2016; Zinicovscaia et al., 2017). Recent studies have revealed tree species' hazardous and harmful metal absorption ability and organs commonly used in the urban environment (Lehndorff & Schwark, 2008; Yilmaz & Isinkaralar, 2021b). Biomonitoring studies supply direct proof accumulation of several elements in the background. Some tree species have been proposed as a biomonitor of environmental pollution. It could be said that trees' bark, leaves, and wood are likely to be suitable biomonitors more than lichens and mosses. The major problem with biomonitors is that they cannot be approximated for how long they have been exposed to metal pollution (Weinstein & Davison, 2003). Therefore, it will not be possible to know accurately how long the poisonous metal concentration obtained through research has been accumulated, and it casts doubt on the reliability of the data. They can endure for a long time without undergoing biodegradation and bioaccumulation in cells (Dixit et al., 2015). Roots took transfer characteristics to different organs of toxic metals carry the accumulation, and leaves (may vary according to the physicochemical properties of the species, meteorological factors including active/passive transfer processes, sequestration and speciation, redox states, the type of plant root system and the response of plants to elements with seasonal cycles) (Chojnacka et al., 2005; Shahid et al., 2017). Therefore, biomonitor species must possess certain qualities. The first of these features is that the selected organisms should have the ability to accumulate toxic metals. Still, they should not die immediately because of trace elements' low concentration values (Gall et al., 2015; Kabata-Pendias & Szteke, 2015). They should live stably in the region where they will be sampled, they should be found plentifully in the study area, and it should be possible to test them when desired at equilibrium conditions. They should be easy to obtain so species can be taken quickly from polluted areas. Thus, biomonitors can be revealed the difference between environmental areas. In addition, a sufficient amount of organs or tissues must be supplied to analyze the correlation between biomonitors and the environment (Chang et al., 2014; Wang et al., 2021).
Some elements have emerged with uptake via the soil or atmospheric (wet and dry) deposition over the past decades. Chromium (Cr) is one of them; air and soil pollution disrupts the ecosystem cycle and functioning mechanism (Yang et al., 2021). It is more common in urban areas than rural areas due to mining activities, tanneries, urban wastes, wastewater, exhaust gases of motor vehicles, and sewage wastes (Sharma et al., 2021). In addition to these specific central stones, mineral fertilizers and biocides are available sources of Cr deposition. It is generally found in Cr+6 (Group 1 carcinogenic for humans) and Cr+3 (Group 3 carcinogen for not humans) valences in environmental pollution by the International Agency for Research on Cancer (IARC, 2021; Yoshinaga et al., 2018). Although the Cr+3 (at < 1 mg/mL) is non-toxic, the Cr+6 (at > 0.2 mg/mL) has some toxic and adverse effects such as skin lesions, lung cancer, mutagenic and genotoxic, eardrum perforation, ulceration, and perforation of the nasal septum, decreased spermatogenesis (Bharagava & Mishra, 2018; Mishra & Bharagava, 2016; Novotnik et al., 2016). Zinc (Zn), the other research subject, water-soluble forms are nitrates, chlorates, sulfates, and chlorides. Relatively water-insoluble forms are in the form of oxides, carbonates, phosphates, and silicates (Jung & Thornton, 1996). The Zn is mainly used as alloy and metal coating. Also, the Zn is used frequently and is the fourth most used metal annually (Iwuozor et al., 2021). It is especially seen in silk yarn, fiber production, steel industry, and cooling systems applying cathode treatment and metal process wastewater (Amado Filho et al., 1999). In addition, the Zn is used in many areas such as cosmetics, varnish in the automobile industry, carbon papers, and dyestuffs (Pavithra & Jaikumar, 2019). In agriculture, it is also used as a nutritious fertilizer, insecticide, and wood preservative to be a vital micronutrient (Adisa et al., 2019). Although the accumulation, release, and movements of these elements are pretty similar, they can reveal the dimensions of the transport of air pollution.
The present study has established a significant role in determining toxic metal concentrations in the recent past. The Picea pungens Engelm. is an evergreen tree belonging to the Pinaceae family's Picea genus. It was chosen to represent Cr and Zn deposition evidence because it is extensively used for landscaping such as highways, parks, and gardens. Also, it is an ornamental tree that grows horizontal branching with thick branches and a conical crown with a pyramidal appearance when it grows freely up to 50 m in height. Its needles are 2–3 cm long, four-cornered, slightly curved, spiky, penetrating, and have green, blue-green, and silvery colors. It is preferentially planted as a decorative in urban landscape areas because of its appropriate morphological-physicochemical structure, thick branches, and conical-topped pyramidal appearance. This study aimed to assess the recent past Cr and Zn concentrations in the needles of Picea pungens Engelm. in different locations in Ankara, Türkiye. Thus, it was attempted to detect whether P. pungens had the potential for being used in biomonitoring relevant elements for atmospheric pollution.
2 Materials and methods
2.1 Description of the study area
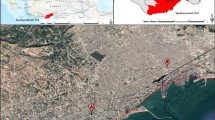
The study area was carried out in Ulus and Pursaklar, in Ankara, the capital of Türkiye. The two different regions were selected for the accumulation of trace metals. Two distinct areas were selected (Fig. 1): a park (number 1 in Ulus) and the main road from the center of the city (number 2 in Pursaklar); the traffic in both sites is generally heavy. Samples were obtained from 13 pieces in two directions: the exterior roadside (E-R: densely vehicle traffic) and the road interior side (I-R); exterior park (E-P) and an interior park (I-P) from the locations studied.
2.2 Plant sampling and collection
Sample collection procedures were carefully started with a collection without damage via pruning shears for analysis. They selected five locations at which intense three landscaped areas (road) with dense traffic (10 m from the main road) and one control site (15 km away from the unpolluted area). The samples were classified according to needle age classes and aimed toward involved of local point sources. The changes of Cr and Zn deposition levels in Picea pungens Engelm. were examined based on the organs (the direction of the prevailing wind was taken into account) over the years.
2.3 Metal analysis
All samples' needle, bark, and wood parts were separated as subsamples. Subsamples from the isolated organs were taken from the central axis part on the lateral branch. They were also divided into two groups washed (W) and unwashed (U-W) processes. First, the needles and barks were washed with distilled water of the group that would go through the washing process. Here, the aim was to clean entirely the particulate matters that had stuck to the organs over time. The samples of 7 age groups were taken as 4 positions, 2 directions, 2 washing, 3 organs, and 7 age groups. They were evaluated after the separation procedure at room temperature for 15 days. Then, they were ground into powder as weighed 0.5 g; 4 mL of 65% HNO3 was added to 50-mL borosilicate tubes for some initial oxidation at 24 h. Then digested samples were heated at 95 °C for 90 min in tubes until cool to room temperature. Four milliliters of H2O2 was added to all samples heated at 95 °C. After all, samples were washed with deionized water to remove any contaminants. They were filtered with Whatman 42 filters.
All chemicals were purchased from Merck and had high purity. The concentrations of the elements were determined after multiplying the obtained values with the dilution factor. The Cr and Zn concentrations in the samples were read in the inductively coupled plasma optical emission spectrometer (ICP-OES) from SpectroBlue, Spectro. All analytical methods were performed with standard protocols for environmentally relevant elements. Still, Cr and Zn values it was determined above the limit values, so we continued with these two elements. The digestion process used USEPA methods 3050 and 3051 for dissolved samples by ICP-OES (USEPA, 1996; USEPA, 1998). Replicate analyses of Cr and Zn elements were presented rare earth elements (REE) gave a S.D. < 10%.
2.4 Statistical analyses
Data were obtained from the analysis results evaluated with SPSS 23.0 software (IBM, Solutions Statistical Package for the Social Sciences, NY, USA) for Windows. The F value, error rate, and thus the difference of the factors were determined to be statistically significant at p < 0.01 by one-way analysis of variance (ANOVA). In addition, Duncan's test was used to determine the groups for homogeneity of variance that were statistically significant differences at the p < 0.01.
3 Results
The concentrations of all metals in the needles, barks and woods were analyzed, although Cr and Zn were significantly higher among the control site (Zn and Cu were higher than 2.85 and 5.18). The analysis did not include other elements because they were below the limit values. It was found a difference between the control site and the sites under traffic emissions. The Cr and Zn deposition between these groups depended on the organ age. The ANOVA results regarding Cr and Zn concentrations change in woods depending on organ age and location (position, direction, and washing) are examined. The changes based on age and location are statistically significant at a 99.9% confidence level. The mean values and homogeneous groups of Cr and Zn concentrations formed for organ age and location.
3.1 Concentrations in needles
The needles were determined for organ age and location (position, direction, and washed or unwashed) separately for the average values of the Cr concentrations in Table 1.
The lowest value of Cr concentration was obtained from 1-year-old needles in the I-P at 17.8 ppb, although the highest value was 4-year-old needles in the E-P at 2528.9 ppb from the U-W. It can be said that the highest values of Cr concentration are generally obtained in old needles and the lowest values in young needles. Three samples from the E-R out of 7, 6 samples from the I-R (samples in the same homogeneous groups as a result of Duncan's test were not included in the calculation), six samples from the E-R out of 7, and 4 samples from the I-P out of 7 have higher Cr concentrations compared to the unwashed ones in the washed samples. The lowest values according to the location were obtained needles from the I-P in 1-year-old needles (17.8 ppb) and the I-R (42.4 ppb) in 2-year-old needles from the U-W. The U-W needles are in the first homogeneous group due to Duncan's test in the I-R. On the other hand, the highest values were found in the E-R in 1-year-old needles (622.4 ppb) according to the location in the W, and the U-W needles in 2-year-old needles attained 830.8 ppb from the E-R, W needles from the E-P in 3-year-old needles as 581.2 ppb, U-W needles from the E-P in 4-year-old needles as 2528.9 ppb, U-W needles from the E-R in 5-year-old needles (977.7 ppb), W needles from the E-P in 6-year-old needles (1274.2 ppb), and W needles from the E-R in 7-year-old needles (1507.6 ppb). When they were compared in the same washing process, the concentrations from the interior side were higher only in 4 out of 28 samples (2-year-old and washed located in the road, 1-year-old and washed situated in the park, 7-year-old and washed and located in the park, and 7-year-old and unwashed located in the park). It is seen that the values of the needles obtained from the outer side are higher than others. The situation can be said that the concentration of Cr in the needles increases depending on traffic density, as supported by other studies (Karacocuk et al., 2021). Turkyilmaz et al. (2018) examined 3-year-old needles in four different conifer species and determined that element concentrations generally increased with needle age. It can be said that the Cr concentration in the needles increases about the traffic density and the needle's age, the Cr concentrations obtained from the washed specimen have higher levels, and the lowest values among all locations are obtained from the interior of the road. Wannaz et al. (2012) measured the amounts of Cu, Fe, Ni, Mn, Pb, and Zn by unwashed leaves of Tillandsia capillaris in the province of Córdoba, Argentina. The findings obtained from the study show that the Zn concentration is caused by industry and traffic. However, a significant increase in other heavy metals originates from mining, agriculture, etc. Alaqouri et al. (2020) were used as biomonitor Pinus sylvestris L. for Ba, Zn, Cd, K, and Na concentrations, and they showed that 2-year-old needles have higher levels than 1-year-old needles.
The variation of Zn concentration depending on the position is examined in the needles in Table 2: five samples from in the inner road (I-R) position out of 7 samples, 3 of the samples in the outer road (E-R) position (the samples in the same homogeneous groups as a result of the Duncan test were not included in the calculation), out of 7 pieces in the park outer (E-P) position.
The Zn concentrations ensured in 4 W and 2 out of 7 samples in the -I-P were higher than those obtained in U-W. Depending on the place, the lowest values were obtained at 13.3 ppm at I-P-U-W for 1-year-old hands, 19 ppm at E-R-W for 2-year-old hands, 26 ppm at E-R-W for 3-year-old hands, 30.4 ppm at I-P-W for 4-year-old hands washed in the park inside position, 30.3 ppm at I-P-U-W for 5-year-old hands, 30.8 ppm at E-R-U-W for 6-year-old hands and 29.8 ppm at E-P-U-W for 7-year-old hands. The highest values were procured at 21.1 ppm at both washed and unwashed for 1-year-old hands, 62.1 ppm at E-R-U-W for 2-year-old hands, and 57.7 ppm at E-R-U-W for 3-year-old hands, 78.3 ppm at E-P-W for 4-year-old hands, 80 ppm at I-P-U-W for 5-year-old hands, 75.8 ppm at I-P-U-W for 6-year-old hands, and 74.8 ppm at I-P-W for 7-year-old hands. When the needles were compared, only 4 out of 28 samples (7-year-old washed and unwashed road, 6-year-old unwashed road, 5-year-old unwashed park) had higher concentrations on the inside, except that all needles values are higher. This situation can be interpreted as the Zn concentration increases depending on the needle traffic density. The Zn element increases depending on the traffic density and the needle age. Therefore, the Zn concentrations achieved in the washed samples are higher. The lowest values among all positions are received in the I-R in the needles.
3.2 Concentrations in barks
The change of Cr concentration changes through organ age, location, and washing criteria in the barks in Table 3.
The mean and homogeneous groups were examined as the lowest values obtained from 1-year-old barks in the I-P-U-W. One-year-old barks located in the I-R-U-W achieved 11.2 ppb. The highest value was acquired in 7-year-old barks situated in the I-R-U-W as 6299 ppb. Six of the lowest values were attained from 1-year-old and 1 of them from 2-year-old barks. On the other hand, 3 of the highest values were acquired from 7-year-old, 2 of them from 6-year-old, 2 of them from 5-year-old, and 1 of them from 4-year-old barks. Notably, the 5, 6, and 7-year-old samples with the highest values were U-W. Therefore, it can be said that the highest values of Cr concentration are generally gained from old barks and the lowest values from young barks; when the change of Cr concentration in the barks is examined according to their locations, five samples from the E-R out of 7, 2 samples from the I-R (the samples in the same homogeneous groups as a result of Duncan's test were not included in the calculation). Three samples from the E-P out of 7 and 4 selections from the I-P out of 7 have higher Cr concentrations in the W sample than the U-W ones. The lowest values were provided from the barks from the I-R-U-W and I-P-U-W in 1-year-old barks (11.2 ppb), barks from the W-I-R in 2-year-old barks as 131.2 ppb. The highest values are found in barks from the W-I-R in 6-year-old at 5336.8 ppb and barks from the U-W-I-R in 7-year-old at 6299 ppb. When the barks were compared with the washing process, the concentrations from the interior side were higher only in 4 out of 28 samples (6-year-old, washed, and located in the road; 7-year-old, unwashed, and located in the park, 7-year-old, washed and located in the park; and 5-year-old, unwashed, and located in the park). Except for these, the values of the barks obtained from the outer side are higher than others due to traffic density.
Many studies conducted with tree organs show that all elements' concentration in the needles increases with organ age (Cetin et al., 2020; Turkyilmaz et al., 2020). It is observed that there are significant differences between young and old needles. For example, while the values of Cr element in 1-year-old needles are calculated as 17.8 ppb, the values in 7-year-old needles are 1507 ppb. When the concentrations in the barks are examined, it is observed that the concentrations increase as the organ age increases though there are exceptions. It is observed that there are significant differences between the young and old needles as in the barks. The Cr concentrations have the highest difference between young and old individuals at 11.2 ppb in the 1-year-old bark. The value of the 7-year-old bark is calculated as 6299.1 ppb. Significant differences are observed between young and old woods as in the barks. While the value of Cr in 1-year-old wood was 4760.2 ppb, the value in 3-year-old wood was regarded as 7.3 ppb. Considering this situation parallels Cetin et al. (2021), it can be said that the Cr concentration generally increases between the traffic density and organs. Savas et al. (2021) studied Cr and Mn concentrations in Cedrus atlantica Manetti in Kastamonu, Turkey. They obtained barks that annual rings are more convenient biomonitors for atmospheric pollution control. Also, similarly to the needles, it increases in the direction facing the main road and the park's parking lot. In addition, the concentration of the Cr increases about the traffic density and the bark's age, and the Cr concentrations obtained from the W with a higher level. The lowest values among locations are attained from the I-P in the barks.
Table 4 shows that the lowest value was 7.6 ppm within 7-year-old barks in the I-P-W. The highest value was found in the 5-year-old barks in the I-R-U-W as 200.9 ppm. It is seen that 5 of the lowest values were obtained in 1-year-old, 1 in 2-year-old, 1 in 3-year-old, and 1 in 7-year-old barks.
On the other hand, the highest values were obtained at 1 in 7-year-old, 2 in 6-year-old, 4 in 5-year-old, and 1 in 4-year-old barks. Notably, the samples with the highest values obtained in 4-, 5-, 6-, and 7-year-old hands were U-W. Therefore, in general, it can be said that the highest values of Zn concentration were obtained in old barks, and the lowest values are obtained in young barks. When the variation of Zn concentration in the barks depending on the position was examined, 4 out of 7 samples in E-R and 4 out of 7 samples in I-R (samples in the same homogeneous groups as a result of the Duncan test were not included in the calculation), 4 out of 7 samples in E-P and I-P. The W concentrations in 4 out of 7 samples were higher than those obtained in U-W. Depending on the location, the lowest values were obtained in E-R-U-W (24.4 ppm) in 1-year-old skins, in I-R-W (17.9 ppm) in 2-year-old skins, in E-R-W (26.2 ppm) in 3-year-old skins, in E-R-W (46.9 ppm) in 4-year-old barks, E-R-U-W (42.7 ppm) in 5-year-old barks, E-R-U-W (52.3 ppm) in 6-year-old barks and in 7-year-old barks in barks at I-P -W (7.6 ppm). Depending on the location, the highest values were obtained in ER-W (52.4 ppm) in 1-year-old barks, in IR-UW (139.9 ppm) in 2-year-old barks, in I-R-W (68.9 ppm) in 3-year-old barks), in E-R-U-W (136.9 ppm) in 4-year-old barks, in I-P-U-W (200.9 ppm) in 5-year-old barks, in I-P-U-W (101.8 ppm) in 6-year-old barks and in 7-year-old barks was also obtained in E-P-W (101 ppm) barks. When compared to bark, only 5 out of 28 samples (2 years old U-W-R, five years old U-W-R, 6 years old U-W-R, five years U-W–P, and six years W–P) had higher concentrations in the interiors. Apart from this, it is seen that the values obtained on the outside of all barks are higher. This can be interpreted as the Zn concentration in the barks increases depending on the traffic density. The Zn element in the barks increases depending on the traffic density and the age of the bark. Therefore, the Zn concentrations obtained are higher, and the lowest values among all locations are accepted in the park's interior in the washed samples.
3.3 Concentrations in woods
Table 5 examines that the lowest value was obtained in 3-year-old woods on the I-R-U-W as 7.3 ppb, and the highest value in the 1-year-old woods in the E-R-U-W with 4760.2 ppb. It is seen that 3 of the lowest values were obtained from 3-year-old woods and one lowest value from 5-year-old woods. On the other hand, 1 of the highest importance was obtained from 1-year-old, one from 2-year-old, two from 5-year-old, and one from 6-year-old woods. The highest values were obtained from 1, 5, and 6-year-old woods. Therefore, it can be said that the highest Cr concentration values are generally obtained from old woods and the lowest values from the young ones.
The lowest values according to the location are obtained in woods from the I-R-U-W in 1-year-old woods as 85 ppb, in 2-year-old woods as 161.4 ppb, in 3-year-old woods 7.3 ppb, from the I-P in 4-year-old woods as 70.9 ppb, from E-R in 5-year-old woods as 120.3 ppb, from the I-P in 6-year-old woods as 103.7 ppb, and the I-P in 7-year-old woods as 86.9 ppb. On the other hand, the highest values according to the location are found in woods in 1-year-old woods as 4760.2 ppb, 2-year-old woods (338.4 ppb), and 3-year-old woods (378.1 ppb) from E-R-U-W, in 4-year-old woods as 562.2 ppb, in 5-year-old woods (1752.8 ppb), in 6-year-old woods as 1039.8 ppb from I-P-U-W, and in 7-year-old woods as 326.9 ppb from E-P-U-W. The concentrations from the interiors were higher only in 1 out of 28 samples (6-year-old and unwashed located in the road). It is seen that the values of the woods obtained from the exteriors are higher with traffic density and the bark age, and the lowest values were obtained from the I-P among all locations. Kardel et al. (2018) revealed that the deposition of many heavy metals was on unwashed and washed leaves of Chamaecyparis lawsoniana. Al, Fe, Ti, Co, Cr, Cu, Ni, Rb, Si, V, Zn, and Zr pollution occurred with traffic activities. Other studies have been carried out on heavy metal concentrations in plants (Baroudi et al., 2021; Mukhopadhyay et al., 2021; Turkyilmaz et al., 2019). Although these elements have been the subject of heavy metal concentration studies in general, these studies mainly concentrated on other details such as Pb, Ni, Cd, and Co (Jeddi et al., 2021). Koc (2021) analyzed annual rings of Cedrus atlantica for Ni and Co concentrations that found a relationship between traffic volume and organs in Kastamonu, Türkiye. Bozdogan et al. (2019) measured Al, Cd, Cr, Cu, Fe, Mn, Ni, Pb, and Zn in Rosmarinus officinalis L. (rosemary) on the highway connecting Adana with İskenderun (Hatay). They found accumulation on leaves and stem from traffic emissions. Oroian et al. (2012) showed five tree species: Pinus nigra, Aesculus hippocastanum, Betula pendula, Picea pungens var. glauca, and Tilia cordata in municipal areas where were recorded Cu, Pb, Cd, and Zn accumulation for heavy traffic area. The most suitable biomonitoring agent for Pb pollution is Aescullum hippocastanum and Betula pendula. Moreira et al. (2018) analyzed Tipuana tipu, Poincianella pluviosa, and Ligustrum sp. in São Paulo, Brazil. They have compared Al, Fe, Zn, Cu, Mn, Ba, and S in the bark with different levels of traffic intensity. Tipuana tipu was chosen appropriately for biomonitoring studies for heavy metals. Fasani et al. (2016) applied a study in areas with and without traffic in Seville city, Spain. They showed 16 polycyclic aromatic hydrocarbons (PAHs) concentrations with biomonitoring as Citrus aurantium from a traffic source.
Table 6 presents that the lowest value was obtained at 4.8 ppm in 1-year-old woods in E-R-U-W and the highest value in 5-year-old woods in the I-P-U-W with 68.7 ppm. Other lowest values are obtained in 1-year-old woods and 2 in 3-year-old woods. On the other hand, the highest values were obtained in woods of 1 age 4 and 3 woods five years old. The highest values were obtained in 4 and 5 years old woods. Therefore, in general, it can be said that the highest values of Zn concentration are obtained in old woods and the lowest ones in young woods.
Depending on the location, the lowest values are 4.8 ppm for 1-year-old woods in E-R-U-W, 18.2 ppm for 2-year-old, and 13.4 ppm for 3-year-old woods in I-P-U-W, 25.5 ppm for 4-year- old woods in I-P-U-W, 33.3 ppm for 5-year-old and 31.4 ppm for 6-year-old woods in E-P-U-W. Depending on the location, the highest values are 35.1 ppm for 1-year-old, 45.7 ppm for 2-year-old woods in I-P-U-W, 52.1 ppm for 3-year-old woods in E-R-U-W, 46.4 ppm for 4-year-old and 68.7 ppm for 5-year-old woods in I-P-U-W, 47.1 ppm for 6-year-old woods E-R-U-W, and 52.6 ppm for 7-year-old woods I-P-U-W. When compared to the woods subjected to the same washing process on the same tree, it is seen that the concentrations obtained on the inside are higher in only 1 of the 28 samples (unwashed park aged five years), except that the values obtained on the outside are higher for all woods. This can be interpreted as the Zn concentration in woods increases depending on the traffic density and the age of the bark, and the lowest values among all locations are obtained in the interior of the park.
4 Discussion
Urban air pollution is one of the most critical problems on a global scale, such as global climate change and urbanization. It has been conducted on the subject due to its direct impact on human health and natural habitats (Azadi et al., 2021; Isinkaralar, 2022a, 2022b). However, there are many components of aerial contamination with different exposure to road traffic, such as CO2, particulate matter, and heavy metal pollution (Ghoma et al., 2022; Turkyilmaz et al., 2020; Zsigmond et al., 2021). Therefore, heavy metal pollution is essential to air pollutants (Turkyilmaz et al., 2019; Isinkaralar et al., 2022). Biomonitors are used for atmospheric pollution; according to some reports, direct air pollution tracking is difficult and expensive. Air quality monitoring studies focus on different species of metal concentration as biomonitors, such as fungi, lichens, tree bark and leaves (Ayan et al., 2021; Eltier & Sivacioglu, 2021; Ishimaru et al., 2021). The use of tree organs as familiar and interconnected biomonitors is widely reported in atmospheric evaluation in research. Typical urban tree bark accumulates air pollutants via wet and dry deposition of airborne pollution for monitoring over a long period (Mandiwana et al., 2006). Supporting this hypothesis, investigations focused on biomonitoring studies with several plants with traffic density that some metals deposition increases in the Phoenix dactylifera L for atmospheric heavy metal pollution in Aqaba city, Jordan (Al-Khlaifat & Al-Khashman, 2007). Sawidis et al. (2011) investigated Cr, Cu, Fe, and Pb in Platanus orientalis L. and Pinus nigra Arn. as bioindicators due to abundant species in three sampling sites. Each sampling site selected areas with long-term air pollution contamination in the city center of Salzburg, Belgrade, and Thessaloniki. The leaves and bark of trees show that Hall Pioneer Park (Belgrade) has massive pollution for Cu, Pb, and Fe. However, Aristotelous Square (Thessaloniki) has revealed Cr pollution as 0.621 ± 0.153 μg/g by air pollution. It should be considered an effective and inexpensive way to monitor concentration, and studies should be carried out on the change of heavy metal concentrations accumulated by different origins of the same species. It is crucial to monitor the evolution of heavy metals and other heavy metal concentrations. However, there is insufficient information about the influential factors and mechanisms in the uptake of heavy metals into plants.
For this reason, studies on this subject should be diversified and continued. Gallego-Cartagena et al. (2021) studied six sampling locations in the Bilbao Metropolitan area, Spain, and the naturally growing Grimmia genus was founded in atmospheric heavy metal pollution. Studies are defined as the elements that are the basic building blocks in all plants and all living. Therefore, the subject elements exist as building blocks in the bodies of living things. It was observed that the heavy metal concentrations in the air increased due to the harm that this increase may cause to humans and other living things. Therefore, it becomes a subject that should be especially emphasized. Considering that people and other living things live intensely in urban areas, the studies to prevent possible damages are insufficient, so they need to be diversified and increased. The study results show that the concentrations of the studied elements differ significantly between the organs formed in different years on the same branch. This difference is seen in needle, bark, and wood parts formed in the same year and the same organs formed in successive years. This can be interpreted as the passage of the elements subject to the study between the organs is quite limited as a good biomonitor. Many factors affect the entry of heavy metals into the plant body, and these factors usually act simultaneously (Eid et al., 2021; Zhou et al., 2021).
In addition, it is recommended to conduct studies in controlled environments to obtain more detailed information such as the morphological, phenological, and anatomical features of different origins of some species. For this reason, it should not be ignored that there will be differences in the change in Cr and Zn concentration. These effects are a decrease in root and shoot growth, elongation and retardation in cell growth, fragmentation in cells' organelles, and decreased chlorophyll synthesis. This study examined the needle, bark, and wood for Cr and Zn concentration on P. pungens and obtained results for assessments tree. It has various responses in synthesizing proteins and carbohydrates to environmental pollution in plants. In addition, the activation of enzymes directly affects the quality and quantity of products by plants due to their effect on respiration, photosynthesis, and biological membrane stability to amounts of airborne pollution.
5 Conclusion
Passive sampling of air quality using P. pungens has effectively investigated the impacts of Cr and Zn pollution. However, it has not performed satisfactorily from some concerns in data interpretation about atmospheric deposition. It is impressive atmospheric trapping particles from root uptake and deposition on outer plant organs and ring-like foliage or bark for many years. This study determined that Cr and Zn pollution vary significantly based on the organs and location for many years. Furthermore, they differed considerably depending on the factors examined that can cause a change in the Cr and Zn concentrations, which is the most incredible amount of metals reasonably determined in the recent past. The effects of highways, factories, and similar places with heavy industrial activities can cause to release of the Cr and Zn into the air and soil because of their deposition and mobilities. This result indicated that P. pungens is a sustainable and widely distributed prosperous biomonitor of Cr and Zn pollution. Both elements have higher aerial deposit and accumulation levels on trees like a passive sampler that does not cause any vital damage to the plant and environment. It can be easily used and has significant positive correlations between organs. In addition, it is predicted that it can accumulate other metal types on tissue. For its applicability in the ecological system and dissemination as an indicator of urban air pollution, it can be easily used in landscape planting in cities and various areas. For future environmental impact research, P. pungens is preferred as a bioindicator of movement elements in the industry, traffic, urbanized, and park areas.
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Adisa, I. O., Pullagurala, V. L. R., Peralta-Videa, J. R., Dimkpa, C. O., Elmer, W. H., Gardea-Torresdey, J. L., & White, J. C. (2019). Recent advances in nano-enabled fertilizers and pesticides: A critical review of mechanisms of action. Environmental Science: Nano, 6(7), 2002–2030. https://doi.org/10.1039/C9EN00265K
Alaqouri, H. A. A., Ozer Genc, C., Aricak, B., Kuzmina, N., Menshikov, S., & Cetin, M. (2020). The possibility of using Scots pine (Pinus sylvestris L.) needles as biomonitor in the determination of heavy metal accumulation. Applied Ecology and Environmental Research, 18(2), 3713–3727. https://doi.org/10.15666/aeer/1802_37133727a
Alengebawy, A., Abdelkhalek, S. T., Qureshi, S. R., & Wang, M. Q. (2021). Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics, 9(3), 42. https://doi.org/10.3390/toxics9030042
Al-Khlaifat, A. L., & Al-Khashman, O. A. (2007). Atmospheric heavy metal pollution in Aqaba city, Jordan, using Phoenix dactylifera L. leaves. Atmospheric Environment, 41(39), 8891–8897. https://doi.org/10.1016/j.atmosenv.2007.08.028
Amado Filho, G. M., Andrade, L. R., Karez, C. S., Farina, M., & Pfeiffer, W. C. (1999). Brown algae species as biomonitors of Zn and Cd at Sepetiba Bay, Rio de Janeiro, Brazil. Marine Environmental Research, 48(3), 213–224. https://doi.org/10.1016/S0141-1136(99)00042-2
Ayan, S., Sarsekova, D., Kenesaryuly, G., Yilmaz, E., Gülseven, O., & Sahin, I. (2021). Accumulation of heavy metal pollution caused by traffic in forest trees in the park of Kerey and Janibek Khans of the city of Nur-Sultan, Kazakhstan. Journal of Forest Science, 67, 357–366. https://doi.org/10.17221/37/2021-JFS
Azadi, N., Nakhaee, S., Farnia, V., Pirsaheb, M., Mansouri, B., Ahmadi-Jouybari, T., & Khanegi, M. (2021). Multivariate statistical evaluation of heavy metals in the urine of opium individuals in comparison with healthy people in Western Iran. Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-021-16271-6
Baroudi, F., Al-Alam, J., Delhomme, O., Chimjarn, S., Fajloun, Z., & Millet, M. (2021). The use of Pinus nigra as a biomonitor of pesticides and polycyclic aromatic hydrocarbons in Lebanon. Environmental Science and Pollution Research, 28, 10283–10291. https://doi.org/10.1007/s11356-020-11954-y
Bharagava, R. N., & Mishra, S. (2018). Hexavalent chromium reduction potential of Cellulosimicrobium sp. isolated from common effluent treatment plant of tannery industries. Ecotoxicology and Environmental Safety, 147, 102–109. https://doi.org/10.1016/j.ecoenv.2017.08.040
Bozdogan, S. E., Turkmen, M., & Cetin, M. (2019). Heavy metal accumulation in rosemary leaves and stems exposed to traffic-related pollution near Adana-İskenderun Highway (Hatay, Turkey). Environmental Monitoring and Assessment, 191, 553. https://doi.org/10.1007/s10661-019-7714-7
Cai, A., Zhang, H., Wang, L., Wang, Q., & Wu, X. (2021). Source apportionment and health risk assessment of heavy metals in PM2.5 in Handan: A typical heavily polluted city in north China. Atmosphere, 12(10), 1232. https://doi.org/10.3390/atmos12101232
Cetin, M., Sevik, H., & Cobanoglu, O. (2020). Ca, Cu, and Li in washed and unwashed specimens of needles, bark, and branches of the blue spruce (Picea pungens) in the city of Ankara. Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-020-08687-3
Cetin, M., Sevik, H., Turkyılmaz, A., & Isinkaralar, K. (2021). Using Abies’s needles as biomonitors of recent heavy metal accumulation. Kastamonu University Journal of Engineering and Sciences, 7(1), 1–6.
Chang, C. Y., Yu, H. Y., Chen, J. J., Li, F. B., Zhang, H. H., & Liu, C. P. (2014). Accumulation of heavy metals in leaf vegetables from agricultural soils and associated potential health risks in the Pearl River Delta, South China. Environmental Monitoring and Assessment, 186(3), 1547–1560. https://doi.org/10.1007/s10661-013-3472-0
Chojnacka, K., Chojnacki, A., Gorecka, H., & Górecki, H. (2005). Bioavailability of heavy metals from polluted soils to plants. Science of the Total Environment, 337(1–3), 175–182. https://doi.org/10.1016/j.scitotenv.2004.06.009
Dixit, R., Malaviya, D., Pandiyan, K., Singh, U. B., Sahu, A., Shukla, R., Singh, B., Rai, J., Sharma, P., Lade, H., & Paul, D. (2015). Bioremediation of heavy metals from soil and aquatic environment: an overview of principles and criteria of fundamental processes. Sustainability, 7(2), 2189–2212. https://doi.org/10.3390/su7022189
Eid, E. M., Shaltout, K. H., Almuqrin, A. H., Aloraini, D. A., Khedher, K. M., Taher, M. A., & Barcelo, D. (2021). Uptake prediction of nine heavy metals by Eichhornia crassipes grown in irrigation canals: A biomonitoring approach. Science of the Total Environment, 782, 146887. https://doi.org/10.1016/j.scitotenv.2021.146887
Eltier, L. A. A., & Sivacioglu, A. (2021). Research Article determination of heavy metal accumulation in some coniferous species used in Kastamonu Urban Afforestation. Asian J. Biol. Sci, 14(2), 33–40.
Fasani, D., Fermo, P., Barroso, P. J., Martín, J., Santos, J. L., Aparicio, I., & Alonso, E. (2016). Analytical method for biomonitoring of PAH using leaves of bitter orange trees (Citrus aurantium): A case study in South Spain. Water, Air, & Soil Pollution, 227, 360. https://doi.org/10.1007/s11270-016-3056-z
Gall, J. E., Boyd, R. S., & Rajakaruna, N. (2015). Transfer of heavy metals through terrestrial food webs: A review. Environmental Monitoring and Assessment, 187(4), 1–21. https://doi.org/10.1007/s10661-015-4436-3
Gallego-Cartagena, E., Morillas, H., Carrero, J. A., Madariaga, J. M., & Maguregui, M. (2021). Naturally growing grimmiaceae family mosses as passive biomonitors of heavy metals pollution in urban-industrial atmospheres from the Bilbao Metropolitan area. Chemosphere, 263, 128190. https://doi.org/10.1016/j.chemosphere.2020.128190
Ghoma, W. E. O., Sevik, H., & Isinkaralar, K. (2022). Using indoor plants as biomonitors for detection of toxic metals by tobacco smoke. Air Quality, Atmosphere & Health, 15, 415–424. https://doi.org/10.1007/s11869-021-01146-z
IARC (International Agency for Rsesarch on Cancer) (2021). Agents Classified by the IARC Monographs, vols. 1–129. https://monographs.iarc.who.int/agents-classified-by-the-iarc/
Ishimaru, G., dos Santos, E. C., & Saiki, M. (2021). Evaluation of the spatial variability of the elements in tree barks used as biomonitors of atmospheric pollution. Brazilian Journal of Radiation Sciences, 9(11), 1. https://doi.org/10.15392/bjrs.v9i1A.1386
Isinkaralar, K. (2022a). The large-scale period of atmospheric trace metal deposition to urban landscape trees as a biomonitor. Biomass Conversion and Biorefinery. https://doi.org/10.1007/s13399-022-02796-4
Isinkaralar, K. (2022b). Atmospheric deposition of Pb and Cd in the Cedrus atlantica for environmental biomonitoring. Landscape and Ecological Engineering. https://doi.org/10.1007/s11355-022-00503-z
Isinkaralar, K., Koc, I., Erdem, R., & Sevik, H. (2022). Atmospheric Cd, Cr, and Zn deposition in several landscape plants in Mersin, Türkiye. Water, Air, & Soil Pollution, 233, 120. https://doi.org/10.1007/s11270-022-05607-8
Iwuozor, K. O., Oyekunle, I. P., Oladunjoye, I. O., Ibitogbe, E. M., & Olorunfemi, T. S. (2021). A review on the mitigation of heavy metals from aqueous solution using sugarcane bagasse. Sugar Tech. https://doi.org/10.1007/s12355-021-01051-w
Jeddi, K., Fatnassi, M., Chaieb, M., & Siddique, K. H. (2021). Tree species as a biomonitor of metal pollution in arid Mediterranean environments: Case for arid southern Tunisia. Environmental Science and Pollution Research, 28(22), 28598–28605. https://doi.org/10.1007/s11356-021-12788-y
Jung, M. C., & Thornton, I. (1996). Heavy metal contamination of soils and plants in the vicinity of a lead-zinc mine, Korea. Applied Geochemistry, 11(1–2), 53–59. https://doi.org/10.1016/0883-2927(95)00075-5
Kabata-Pendias, A. (2004). Soil–plant transfer of trace elements—An environmental issue. Geoderma, 122(2–4), 143–149. https://doi.org/10.1016/j.geoderma.2004.01.004
Kabata-Pendias, A., & Szteke, B. (2015). Trace elements in abiotic and biotic environments (p. 468). New York: Taylor & Francis.
Kabir, M. H., Kormoker, T., Islam, M. S., Khan, R., Shammi, R. S., Tusher, T. R., Proshad, R., Islam, M. S., & Idris, A. M. (2021). Potentially toxic elements in street dust from an urban city of a developing country: Ecological and probabilistic health risks assessment. Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-021-14581-3
Karacocuk, T., Sevik, H., Isinkaralar, K., Turkyilmaz, A., & Cetin, M. (2021). The change of Cr and Mn concentrations in selected plants in Samsun city center depending on traffic density. Landscape and Ecological Engineering, 18, 75–83. https://doi.org/10.1007/s11355-021-00483-6
Kardel, F., Wuyts, K., De Wael, K., & Samson, R. (2018). Biomonitoring of atmospheric particulate pollution via chemical composition and magnetic properties of roadside tree leaves. Environmental Science and Pollution Research, 25, 25994–26004. https://doi.org/10.1007/s11356-018-2592-z
Koc, I. (2021). Using Cedrus atlantica’s annual rings as a biomonitor in observing the changes of Ni and Co concentrations in the atmosphere. Environmental Science and Pollution Research, 28, 35880–35886. https://doi.org/10.1007/s11356-021-13272-3
Lehndorff, E., & Schwark, L. (2008). Accumulation histories of major and trace elements on pine needles in the Cologne Conurbation as function of air quality. Atmospheric Environment, 42(5), 833–845. https://doi.org/10.1016/j.atmosenv.2007.10.025
Levei, L., Cadar, O., Babalau-Fuss, V., Kovacs, E., Torok, A. I., Levei, E. A., & Ozunu, A. (2021). Use of black poplar leaves for the biomonitoring of air pollution in an urban agglomeration. Plants, 10(3), 548. https://doi.org/10.3390/plants10030548
Mandiwana, K. L., Resane, T., Panichev, N., & Ngobeni, P. (2006). The application of tree bark as bio-indicator for the assessment of Cr (VI) in air pollution. Journal of Hazardous Materials, 137(2), 1241–1245. https://doi.org/10.1016/j.jhazmat.2006.04.015
Marié, D. C., Chaparro, M. A., Irurzun, M. A., Lavornia, J. M., Marinelli, C., Cepeda, R., Böhnel, H. N., Miranda, A. G. C., & Sinito, A. M. (2016). Magnetic mapping of air pollution in Tandil city (Argentina) using the lichen Parmotrema pilosum as biomonitor. Atmospheric Pollution Research, 7(3), 513–520. https://doi.org/10.1016/j.apr.2015.12.005
Markert, B., Wappelhorst, O., Weckert, V., Herpin, U., Siewers, U., Friese, K., & Breulmann, G. (1999). The use of bioindicators for monitoring the heavy-metal status of the environment. Journal of Radioanalytical and Nuclear Chemistry, 240(2), 425–429. https://doi.org/10.1007/BF02349387
Meena, M., Sonigra, P., & Yadav, G. (2021). Biological-based methods for the removal of volatile organic compounds (VOCs) and heavy metals. Environmental Science and Pollution Research, 28(3), 2485–2508. https://doi.org/10.1007/s11356-020-11112-4
Mishra, S., & Bharagava, R. N. (2016). Toxic and genotoxic effects of hexavalent chromium in environment and its bioremediation strategies. Journal of Environmental Science and Health, Part C, 34, 1–32. https://doi.org/10.1080/10590501.2015.1096883
Moreira, T. C., Amato-Lourenco, L. F., da Silva, G. T., Saldiva de Andre, C. D., de Andre, P. A., Barrozo, L. V., Singer, J. M., Saldiva, P. H. N., Saiki, M., & Locosselli, G. M. (2018). The use of tree barks to monitor traffic-related air pollution: A case study in São Paulo-Brazil. Frontiers in Environmental Science, 6, 72. https://doi.org/10.3389/fenvs.2018.00072
Mukhopadhyay, S., Dutta, R., & Dhara, A. (2021). Assessment of air pollution tolerance index of Murraya paniculata (L.) Jack in Kolkata metro city, West Bengal, India. Urban Climate, 39, 100977. https://doi.org/10.1016/j.uclim.2021.100977
Novotnik, B., Ščančar, J., Milačič, R., Filipič, M., & Žegura, B. (2016). Cytotoxic and genotoxic potential of Cr (VI), Cr (III)-nitrate and Cr (III)-EDTA complex in human hepatoma (HepG2) cells. Chemosphere, 154, 124–131. https://doi.org/10.1016/j.chemosphere.2016.03.118
Oroian, I., Covrig, I., Viman, O., Odagiu, A., Burduhos, P., Milasan, A., & Șulea, C. (2012). Testing biomonitoring capacity of trees from urban areas. A case study: Cu, Cd, Pb, Zn pollution in Cluj-Napoca, reflected by foliar accumulation of five species located within intense traffic area. Note 1. Results recorded in 2010. ProEnvironment Promediu, 5(12), 195–199.
Pavithra, K. G., & Jaikumar, V. (2019). Removal of colorants from wastewater: A review on sources and treatment strategies. Journal of Industrial and Engineering Chemistry, 75, 1–19. https://doi.org/10.1016/j.jiec.2019.02.011
Petrova, S., Yurukova, L., & Velcheva, I. (2012). Horse chestnut (Aesculus hippocastanum L.) as a biomonitor of air pollution in the town of Plovdiv (Bulgaria). Journal of BioScience & Biotechnology, 1(3), 241–247.
Pignata, M. L., Plá, R. R., Jasan, R. C., Martínez, M. S., Rodriguez, J. H., Wannaz, E. D., & Gonzalez, C. M. (2007). Distribution of atmospheric trace elements and assesment of air quality in Argentina employing the lichen, Ramalina celastri, as a passive biomonitor: Detection of air pollution emission sources. International Journal of Environment and Health, 1(1), 29–46.
Rajendran, S., Priya, T. A. K., Khoo, K. S., Hoang, T. K., Ng, H. S., Munawaroh, H. S. H., & Show, P. L. (2021). A critical review on various remediation approaches for heavy metal contaminants removal from contaminated soils. Chemosphere. https://doi.org/10.1016/j.chemosphere.2021.132369
Rovella, N., Aly, N., Comite, V., Randazzo, L., Fermo, P., Barca, D., & La Russa, M. F. (2021). The environmental impact of air pollution on the built heritage of historic Cairo (Egypt). Science of the Total Environment, 764, 142905. https://doi.org/10.1016/j.scitotenv.2020.142905
Sablier, M., & Garrigues, P. (2014). Cultural heritage and its environment: An issue of interest for environmental science and pollution research. Environmental Science and Pollution Research, 21(9), 5769–5773.
Savas, D. S., Sevik, H., Isinkaralar, K., Turkyilmaz, A., & Cetin, M. (2021). The potential of using Cedrus atlantica as a biomonitor in the concentrations of Cr and Mn. Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-021-14826-1
Sergeeva, A., Zinicovscaia, I., Vergel, K., Yushin, N., & Urošević, M. A. (2021). The effect of heavy industry on air pollution studied by active moss biomonitoring in Donetsk Region (Ukraine). Archives of Environmental Contamination and Toxicology, 80(3), 546–557. https://doi.org/10.1007/s00244-021-00834-2
Shahid, M., Dumat, C., Khalid, S., Schreck, E., Xiong, T., & Niazi, N. K. (2017). Foliar heavy metal uptake, toxicity and detoxification in plants: A comparison of foliar and root metal uptake. Journal of Hazardous Materials, 325, 36–58. https://doi.org/10.1016/j.jhazmat.2016.11.063
Sharma, N., Sodhi, K. K., Kumar, M., & Singh, D. K. (2021). Heavy metal pollution: Insights into chromium eco-toxicity and recent advancement in its remediation. Environmental Nanotechnology, Monitoring & Management, 15, 100388. https://doi.org/10.1016/j.enmm.2020.100388
Sawidis, T., Breuste, J., Mitrovic, M., Pavlovic, P., & Tsigaridas, K. (2011). Trees as bioindicator of heavy metal pollution in three European cities. Environmental Pollution, 159(12), 3560–3570. https://doi.org/10.1016/j.envpol.2011.08.008
Tarín-Carrasco, P., Im, U., Geels, C., Palacios-Peña, L., & Jiménez-Guerrero, P. (2021). Contribution of fine particulate matter to present and future premature mortality over Europe: A non-linear response. Environment International, 153, 106517. https://doi.org/10.1016/j.envint.2021.106517
Turkyilmaz, A., Cetin, M., Sevik, H., Isinkaralar, K., & Saleh, E. A. A. (2020). Variation of heavy metal accumulation in certain landscaping plants due to traffic density. Environment, Development and Sustainability, 22(3), 2385–2398. https://doi.org/10.1007/s10668-018-0296-7
Turkyilmaz, A., Sevik, H., Isinkaralar, K., & Cetin, M. (2018). Using Acer platanoides annual rings to monitor the amount 520 of heavy metals accumulated in air. Environmental Monitoring and Assessment, 190, 578. https://doi.org/10.1007/s10661-018-5216956-0
Turkyilmaz, A., Sevik, H., Isinkaralar, K., & Cetin, M. (2019). Use of tree rings as a bioindicator to observe atmospheric heavy metal deposition. Environmental Science and Pollution Research, 26(5), 5122–5130. https://doi.org/10.1007/s11356-018-3962-2
US Environmental Protection Agency. (1996). Soil screening guidance: Technical background document. USEPA Rep.540/R-95/128. US Gov. Print. Office, Washington, DC.
US Environmental Protection Agency. (1998). Method 3051a—Microwave assisted acid digestion of sediments, sludges, soils, and oils.
Wang, J., Wang, P., Zhao, Z., & Huo, Y. (2021). Uptake and concentration of heavy metals in dominant mangrove species from Hainan Island, South China. Environmental Geochemistry and Health, 43(4), 1703–1714. https://doi.org/10.1007/s10653-020-00717-w
Wannaz, E. D., Carreras, H. A., Rodriguez, J. H., & Pignata, M. L. (2012). Use of biomonitors for the identification of heavy metals emission sources. Ecological Indicators, 20, 163–169. https://doi.org/10.1016/j.ecolind.2012.02.022
Weinstein, L. H., & Davison, A. W. (2003). Native plant species suitable as bioindicators and biomonitors for airborne fluoride. Environmental Pollution, 125(1), 3–11. https://doi.org/10.1016/S0269-7491(03)00090-3
World Health Organization. (2016). Ambient air pollution: A global assessment of exposure and burden of disease. https://apps.who.int/iris/handle/10665/250141 Accessed 2022, January 11.
Yang, Z., Zhang, X., Jiang, Z., Li, Q., Huang, P., Zheng, C., & Yang, W. (2021). Reductive materials for remediation of hexavalent chromium contaminated soil–A review. Science of the Total Environment. https://doi.org/10.1016/j.scitotenv.2021.145654
Yoshinaga, M., Ninomiya, H., Al Hossain, M. A., Sudo, M., Akhand, A. A., Ahsan, N., Alim, AMd., Khalequzzaman, Md., Lida, M., Yajima, I., Ohgami, N., & Kato, M. (2018). A comprehensive study including monitoring, assessment of health effects and development of a remediation method for chromium pollution. Chemosphere, 201, 667–675. https://doi.org/10.1016/j.chemosphere.2018.03.026
Zhou, X., Hu, R., & Fang, Y. (2021). Source and spatial distribution of airborne heavy metal deposition studied using mosses as biomonitors in Yancheng, China. Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-021-12814-z
Zinicovscaia, I., Hramco, C., Duliu, O. G., Vergel, K., Culicov, O. A., Frontasyeva, M. V., & Duca, G. (2017). Air pollution study in the Republic of Moldova using moss biomonitoring technique. Bulletin of Environmental Contamination and Toxicology, 98(2), 262–269. https://doi.org/10.1007/s00128-016-1989-y
Zsigmond, A. R., Száraz, A., & Urák, I. (2021). Macro and trace elements in the black pine needles as inorganic indicators of urban traffic emissions. Environmental Pollution, 291, 118228. https://doi.org/10.1016/j.envpol.2021.118228
Yilmaz, D., & Isinkaralar, O. (2021a). How can natural environment scoring tool (nest) be adapted for urban parks? Kastamonu University Journal of Engineering and Sciences, 7(2), 127–139.
Yilmaz, D., & Isinkaralar, O. (2021b). Climate action plans under climate-resilient urban policies. Kastamonu University Journal of Engineering and Sciences, 7(2), 140–147.
Acknowledgements
This study is produced from the MSc thesis titled "Determining the recent changes of some heavy metal concentrations from traffic with the help of Blue spruce (Picea pungens Engelm)" conducted at Kastamonu University, Graduate School of Natural and Applied Sciences, Sustainable Agriculture and Natural Plant Resources Program.
Author information
Authors and Affiliations
Contributions
Omer Faruk Sulhan contributed to the raw material collection, processing analysis, and interpretation. Hakan Sevik contributed to the thesis supervisor, processing analysis, and interpretation. Kaan Isinkaralar helped in the conceptualization, software, writing—original draft, data curation, formal analysis, and review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sulhan, O.F., Sevik, H. & Isinkaralar, K. Assessment of Cr and Zn deposition on Picea pungens Engelm. in urban air of Ankara, Türkiye. Environ Dev Sustain 25, 4365–4384 (2023). https://doi.org/10.1007/s10668-022-02647-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10668-022-02647-2