Abstract
The current study aimed to evaluate the levels of some toxic and essential elements (Pb, Cd, Cu, Ti, Ni, Cr, Co, Fe, Ca, Hg, Mn, Se, and Zn) in the urine of opium-addicted compared to non-addicted cases. In this study, 126 participants were recruited and their fasting urine samples were collected (63 opium-addicted and 63 non-addicted subjects served as the reference group). ICP-MS was utilized to detect the concentration of trace elements. Results exhibited that the concentration of all elements than Ni, Cu, and Zn was markedly different between the addicted and non-addicted groups. Compared to controls, the Cd, Cr, Co, Hg, Mn, Pb, Se, and Ti levels were higher among opium-addicted cases (p < 0.05) whereas the Fe and Ca concentrations were higher among controls (p < 0.05). Robust regression analysis showed no statistically significant effect of gender on element levels. It revealed that age was associated with the levels of Ni and Cu only and also the route of administration was related to the urinary levels of Co, Cr, Hg, and Mn. In conclusion, results confirmed that it is opium consumption that affects the concentration levels of most elements.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the past decade, the consumption of illicit addictive drugs has been widespread throughout the world. The prevalence of illicit opioid use in Western Europe, Western and Central Europe, and North America has been estimated to be 0.85%, 0.37%, and 0.47% respectively (Peacock et al. 2018). Asia and specifically the Middle East are the main users of opioid material and its derivatives. Iran, a country in the heart of the Middle East, is known as the main route of the transition of opium from Afghanistan as the world’s largest opium producer to the western countries. Reports claim that more than half of the opium produced in Afghanistan is transported to Europe via Iran (Ansari-Moghaddam et al. 2012; Minozzi et al. 2014; Sadeghi et al. 2017). In the East, Iran shares about a 900 km border with Afghanistan (Rosen and Katzman 2014). Due to opium flow from Afghanistan, opium is readily available in Iran at a very low cost, putting various age groups at risk of drug-abusing. Overall, data suggest an 8% increase in the addiction rate in Iran annually. Studies estimate the overall prevalence of opiate usage among male youth by 6% and female youth by 2.0% reaching up to 22% in the rural parts (95% CI: 21.3–22.7) (Menati et al. 2017; Ansari-Moghaddam et al. 2016).
To increase its weight and gain more profit, smugglers process opium with various stuff which is susceptible to contamination to heavy metals (Alinejad et al. 2018; Ghane et al. 2018; Salehi et al. 2009). The presence of heavy metals in opioids is a major risk factor for disability and premature loss of life for opium users. Due to the high chemical stability, low degradation, and bioavailability of heavy metals in the body, they can gradually accumulate in some tissues such as the blood, liver, kidney, muscle, and bones and cause various disorders (Rajaei et al. 2012; Mansouri et al. 2012a, 2012b, 2012c; Rezaei et al. 2019). Some studies suggest an elevated risk of death, coronary artery diseases, laryngeal, bladder cancer, stomach cancer, pancreatic cancer, lung cancer, and esophageal cancer following long-term opium consumption among opium users compared to their controls (Hosseini et al. 2010; Malekzadeh et al. 2013; Masjedi et al. 2013; Nasrollahzadeh et al. 2008).
Toxicity studies show various side effects and symptoms for overexposure to heavy metals. The increased risks of lung, thyroid, and pancreatic cancers have been reported as the effects of inhalation of cadmium (Rezaei et al. 2019; Nozadi et al. 2021). Fatigue, bronchitis, hepatic necroinflammation, non-alcoholic fatty liver disease, hypothyroidism, hypertension and arteriosclerosis, diarrhea, bone fractures, infertility, central nervous system damage, immune system disorders, and mental disorders are other reported health effects following exposure to cadmium (García-Esquinas et al. 2014; Hyder et al. 2013; Jacobo-Estrada et al. 2017). Lead causes serious brain damage such as mental retardation, behavioral problems, memory disturbances, and mood changes. The most common complaints of lead poisoning are abdominal pain, myalgia, and arthralgia (Karri et al. 2008). Patients may use more opium for pain control, resulting in further lead exposure; thus, a vicious cycle is created. Symptoms of lead poisoning may be similar to withdrawal symptoms, which potentially increase opium use and subsequent lead uptake (Sadeghi et al. 2017; Sazegar and Ebrahimi 2012).
Thus, monitoring the levels of heavy metals in vulnerable groups is necessary to control their negative outcomes. Therefore, this study aimed to assess the concentration of essential and toxic metals (Pb, Cd, Cu, Ti, Ni, Cr, Co, Fe, Ca, Hg, Mn, Se, and Zn) in the urine of opium-dependents as one of the target populations at risk of heavy metal consequences compared to normal individuals.
Materials and methods
Study population and sample collection
A total number of 126 subjects was recruited from July to November 2020 and organized in two groups: the opium-dependent group (n=63) and the non-opium-dependent group (n=63). Characteristics of participants including age, gender, route of administration, amount of opium use, duration of opium addiction, cigarette consumption, occupation, and education were recorded using a checklist. Case subjects were selected from opium-addicted individuals attending Imam Khomeini Hospital and Farabi Hospital in Kermanshah city, west of Iran. Inclusion criteria were to have a history of opium use, but individuals with kidney disease, cancer, cardiovascular disease, or a history of methadone use, or if he/she was under a specific treatment, were excluded from the study. The control was selected from individuals accompanied patient at the hospital or attended hospital for ordinary medical examinations. They did not suffer from any chronic disease with no history of opium use and smoking. For each participant, 10-ml urine samples were collected, capped, labeled, and kept in the refrigerator at −20 °C until analyses. Both groups entered this study with informed consent. This study had the consent of the Research and ethics committee of Kermanshah University of Medical Sciences (IR.KUMS.REC.1398.960).
Element analyses
In this study, urine samples were digested with the nitric acid and perchloric acid mixture (2:1v/v). For acid digestion, 5 cc of each urine sample was transferred into 25-ml glass test tubes. The amount of 2 cc of nitric acid (Merck, Germany) with a purity of 65% was added to each of the urine samples, and the mixture was kept at room temperature overnight for slow digestion. Then, 1 cc perchloric acid (72%, Merck, Germany) was added to the mixed specimens and placed in a hot water bath (Bain-Marie) for 4 h at 98 °C until complete digestion (Dos Santosa et al. 2018). After completing the digestion, the samples were cold at ambient temperature and the samples were diluted using 25 ml of deionized water. Finally, samples prepared for heavy metal readings were measured by an Agilent 7900 ICP-MS.
It is worth mentioning that all standard solutions used for the analysis of the metal were prepared from the Merck standard at a concentration of 1000 ppm. The concentration of heavy metals (Pb, Cd, Cu, Ti, Ni, Cr, Co, Fe, Ca, Hg, Mn, Se, and Zn) in this study was in micrograms per deciliter. The performance parameters of ICP-MS were as follows: radiofrequency power—1.5 kw; plasma gas flow rate—15 l per minute; carrier gas flow—1.01 l per minute; constituent gas—0.15 l per minute; sample absorption rate—1.7; sample depth—10 mm; detector mode—auto; scan type—peak hopping three sweeps per reading and three readings per repetition; and scan number—3.
Statistical evaluation
Results were reported as mean ± SD or median and interquartile range for numerical variables and number (percentage) for categorical variables. To assess the differences in subject characteristics, a t-test or chi-square test was utilized as appropriate. Normality assumption was assessed using the D’Agostino test. One-way ANOVA or Kruskal-Wallis omnibus test was used to make comparisons between urinary concentration levels across multiple groups such as administration routes. A follow-up univariate analysis using t-test or Wilcoxon rank-sum test (in the case of non-normal distribution) was performed to compare the concentration of metals between opium-addicted cases and their controls. Moreover, the rank-based robust regression analysis was used to assess the effect of multi-factor covariates, group, age, and gender, on metal concentration levels.
Results
Participants and heavy metals in both groups
Using the inclusion and exclusion criteria, 63 opium-addicted users were recruited and asked to attend a lab for collecting their first-morning urine samples. The main administration route of 34.92% of opium-addicted users was inhalation, 30.16% was oral, and the rest (34.92%) administered opium through both inhalation and oral route. The daily opium use was 5 g (± 2.31g) on average with 8.56 years (± 5.9) usage experience (ranged; 3 to 40 years). Opium users were mostly men (57%) with a mean age of 33 ± 10.75 (17 to 79 years). In terms of education, 11.1% of cases had an academic degree, 36.5% less than 5 years, and 52.4% completed high school. Compared to cases, controls were slightly older 34.06 ± 10.63 (18 to 61 years), but more likely to have a higher level of education (chi-square test, p=0.018, Table 1).
Results demonstrate differences in urinary trace element levels between opium-addicted and their controls (Table 2). Particularly, urinary Pb, Mn, and Cd levels in cases were 457.7% (19.5 vs 3.5; p < 0.001), 202.4% (6.35 vs 2.1; p < 0.001), and 175% (3.3 vs 1.2; p < 0.001) higher as compared to the controls, whereas the levels of Fe (2.7 vs 1.8; p < 0.001) and Ca (88 vs 83.5; p < 0.001) were higher in controls as compared to opium users. No significant group difference was observed in Cu and Ni concentration levels between controls and opium-addicted participants (p > 0.05).
Group, gender, and age effects
A rank-based regression analysis was employed to assess whether the concentrations of trace elements were affected by the sex, age, and opioid use habit of participants. The significance levels of these potential covariates are presented in Table 3. It turns out that the levels of trace elements were not affected by the sex of participants, but the age of participants was an influential factor to affect the levels of Ni (β=−0.01, p = 0.002) and Cu (β =0.003, p = 0.021) only. In other words, as individuals got older, the urinary levels of Ni tend to decline slowly whereas the urinary levels of Cu increased with age. Moreover, as confirmed by Table 2, the results of rank-based regression analysis showed that the levels of all elements than Ni, Cu, and Zn were markedly different between non-addicted and addicted cases (Table 3). To investigate this further, pairwise comparisons were made between two groups under each gender type (Table 4). Results confirmed that regardless of gender, it is opium consumption that affects the concentration levels of most elements.
Administration route, daily opium intake, and consumption period
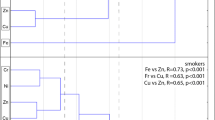
Since the statistical tests performed so far highlighted the effect of opium use on the urinary concentration levels of Ca, Cr, Mn, Co, Se, Cd, Ti, Hg, and Pb elements, we further investigated if the administration route, daily opium intake, and consumption period can explain the observed differences in concentration levels among opium users. The influence of administration routes on urinary element levels was assessed using ANOVA or Kruskal-Wallis test followed by t-test/Mann-Whitney pairwise comparison tests. Figure 1 demonstrates the results graphically. As it can be seen, the urinary levels of Ca, Cd, Cr, Pb, Se, and Ti did not differ significantly between various administration routes (ANOVA/Kruskal-Wallis test, p> 0.05). But the urinary levels were route dependent for Co (Mix < In & Oral), Hg (Mix < In & Oral), and Mn (Oral > In & Mix).
We further analyzed the association of urinary element concentration levels with daily opium intake (in gram) and duration of opium consumption (in years) using rank-based regression analysis. Table 5 summarizes the results. The urinary levels of Ca, Cd, Se, Ti, and Pb elements were found to be all uncorrelated with admiration route, daily opium intake, and opium consumption period (p > 0.05). Thus, to avoid a lengthy table, Table 5 only reports an element with at least one significant result. It appears that compared to parallel consumption of opium via inhalation and oral together, inhaler opium users faced elevated levels of Co (β = 0.032, p = 0.023) and Hg (β = 0.006, p = 0.041) trace elements and oral opium users revealed higher levels of Mn (β = 0.009, p = 0.003). Cr level was not associated with the administration route, but higher levels of Cr can be expected with an increase of daily opium intake and duration opioid consumption.
Discussion
The toxic metal contaminations in drug dependents are among the main health problems worldwide. The results of our study showed an elevated urinary level of Pb, Mn, Cd, Cr, Co, Se, Ti, and Hg and lowered levels of Fe and Ca in opium-dependent individuals. Previous literature has documented high blood lead concentrations in opium-dependents and its relation with some harmful effects of this heavy metal, including abdominal pain, anemia, induce folate, and vitamin B12 dysregulation (Amirabadizadeh et al. 2020; Domeneh et al. 2014; Ghaemi et al. 2017; Khatibi-Moghadam et al. 2016; Mohsen Masoodi et al. 2006). It seems that the elevated concentration of lead in addicted individuals may be a result of opium consumption. The presence of Pb toxic metal has been reported in opium samples and different varieties of the opium poppy (Aghababaei et al. 2018; Aghaee-Afshar et al. 2008; Ivan and Jozef 2011; Mudiam et al. 2005). For example, Aghababaei et al. (2018) evaluated contaminations of illegal opioid-like compounds. They reported elevated concentrations of heavy metals and bacterial contamination of some illicit drug samples. The highest level of lead was reported in opium samples compared to crack and heroin (Aghababaei et al. 2018).
The possible causes for Pb contamination of opium may be due to contamination of water and soil, increasing the weight of opium, improving the appearance of quality, the addition of Indian hair color to opium, and the use of unsuitable methods and equipment during opium production (Aghababaei et al. 2018; Karrari et al. 2012; Nakhaee and Mehrpour 2018). The World Health Organization reported the tolerable weekly intake (TWI) of 25 μg/kg (almost 1500 μg for a 60-kg adult) for lead. Assume that the opium sample contained approximately 138 μg/gram of Pb (Aghababaei et al. 2018; Kim et al. 2012). A person, who uses opium 3 to 5 gram/day, may consume 414–960 μg of Pb (2898–6720 μg in a week). Therefore, only through the use of opium, individuals may be exposed to more than acceptable daily amounts of lead (Nakhaee and Mehrpour 2019).
In addition to Pb contamination of opium, its transfer to the human body and heavy metal bioavailability should also be noted. Some variables, such as the Pb form, nutritional condition of opium-dependents, route of opium consumption, length, and method of opium use, can change the occurrence of Pb accumulation in the body among drug dependents (Nakhaee and Mehrpour 2019; Farnia et al. 2021). Alongside opium-induced lead poisoning, some researchers highlighted the effects of this toxic metal on opiate pharmacodynamics (Kupnicka et al. 2020). It has been proposed that lead can impair the functioning of neural pathways associated with the development of addiction. The lead can alter the metabolism of dopamine and the expression of dopamine receptors, and it can also cause neuro-inflammation and enhancement of morphine tolerance (Kupnicka et al. 2020).
Cadmium (Cd) is another toxic metal that affects many biological processes in the body. It has a long half-life (about 20–30 years) and may result in chronic poisoning (Heshmati et al. 2017; Kupnicka et al. 2020). It has been documented a high tendency of the opium poppy to accumulate toxic metals, particularly Cd and Pb, and also semi-metal arsenic (Lachman et al. 2006). The previous findings suggest the existence of Cd in different parts of the opium poppy (Aghababaei et al. 2018; Ivan and Jozef 2011; Knapek et al. 2009; Knapek et al. 2011; Lachman et al. 2006). The tolerable weekly intake for cadmium has been established at 420 μg/kg (60 μg/day for a person with 60 kg body weight). The high cadmium contents in opium poppy may result in exceeding TWI (Aghababaei et al. 2018; Knapek et al. 2011). Cadmium exposure may be associated with neurotoxicity, nephrotoxicity, osteoporosis, carcinogens, genotoxicity, endocrine, and reproductive disorders (Knapek et al. 2011). Also, its effects on morphine metabolism have been proposed. It can decrease the synthesis of M3G (morphine-3-glucuronide) in vitro and in vivo studies (Antonilli et al. 2003; Lawrence et al. 1992). The antagonistic effects of cadmium on μ-opioid receptors (MORs) and inhibitory effects on dopamine release have been proposed. It results in the malfunctioning of pathways in the limbic system (Kupnicka et al. 2020; Lafuente et al. 2000; Smith et al. 2002). The reduced response to morphine has been observed in some experimental studies (Smith et al. 2002). This result also can be mediated by the interaction of cadmium with glutamate receptors, which also plays a significant role in the progress of dependence (Kupnicka et al. 2020).
Chromium (Cr), particularly hexavalent Cr compound, has been known as a carcinogen agent based on previous experimental and human studies (Park et al. 2004; Proctor et al. 2014; Thompson et al. 2014). In Aghababaei et al. (2018) study, the high Cr (447.38 ± 20.27 μg/g) concentrations were reported in opium samples that were greater than Reference limits for Cr (VI) (3 μg/kg/day) (Aghababaei et al. 2018). Experimental studies proposed that Ti dioxide may increase ROS and inflammatory cytokines. It could cause notable systemic inflammation, dysfunction of endothelium, and lipid metabolism (Huang et al. 2021). The results of Shadman et al. (2012) study showed that nail Ti concentrations were higher in opium-dependent subjects compared to the non-dependent subjects. The elevated urinary levels of Ti in opium-dependents can feature the existence of more toxic elements in the serum and tissues. Nevertheless, this has yet to be established and further investigated.
Manganese (Mn) and Iron (Fe) are among the essential elements. Mn is effective in the maintenance of neuron functions, mainly for the energy metabolism of the brain (O’Neal and Zheng 2015). In excess, however, it is highly toxic to nerve cells (Lucchini et al. 2007; Williams et al. 2010). Fe is an essential element for the body. However, it can generate highly reactive OH radicals, which are considered to be carcinogenic at high concentrations. The concentrations of Fe and Ca were reported to be elevated in nail samples of healthy participants compared to opium-addicted participants (Shadman et al. 2012). In the literature, there has been growing discussion about the interactions of toxic and essential elements in the human body. Excess content of Zn, Cd, Cu, and Mn elements can inhibit the absorption of Fe. This phenomenon is occurred by the competition for protein binding (Aksoy and Sözbilir 2015; Sarafanov et al. 2008) besides, Cd absorption is also related to Fe intake of diet (Aksoy and Sözbilir 2015). The lead absorption can be raised due to insufficient dietary intake of calcium and iron (Alinejad et al. 2018; Nakhaee and Mehrpour 2018).
Selenium (Se) is a required element for the development process. Se exists in the structure of many structural and enzymatic proteins. Glutathione peroxidase concentration, an antioxidant enzyme, in the body is directly associated with the Se concentration. This enzyme protects the membrane integrity (Aksoy and Sözbilir 2015; Bou-Resli et al. 2002). The results of the Aksoy and Sözbilir (2015) study showed that Se concentration was significantly higher (p < 0.05) in the kidney of rats fed with diesel derived from opium poppy than in the control group.
Our results showed that the age of participants was an influential factor affecting the urinary levels of Ni and Cu. The decrease/increase in urinary Cu/Ni levels with age is not well documented in the previous literature. A few studies reported the age effect on the absorption of Cu (August et al. 1989; Johnson et al. 1992). The relationship between plasma levels of Cu and age was proposed previously with contradictory results (Coudray et al. 2006a; Coudray et al. 2006b; Uchino et al. 1990). Coudray et al. (2006a) observed a reduction in absorption of Cu and a significant increase in plasma Cu concentrations with age in rats (Coudray et al. 2006a). Another experimental research showed that the plasma concentration of Cu increased with age, whereas its urinary excretion and its concentration in the liver and bone remained unchanged (Coudray et al. 2006b). The age-related increase in Cu concentration may be attributed to the inflammatory status that is generally observed with increasing age (Coudray et al. 2006b).
The results of the current study showed urinary levels of Ca, Cd, Se, Ti, and Pb elements were found to be all uncorrelated with administration route, daily opium intake, and the years of opium consumption. There is limited information about the relationship of different trace elements with reported variables in opium-dependents. Our results are similar to the previously published results in which blood lead concentration was not significantly influenced by the substance type, route of exposure, duration of opium use, and daily amount of substance (Ghaemi et al. 2017; Hayatbakhsh et al. 2017; Salehi et al. 2009; Sazegar and Ebrahimi 2012). In many cases, the exact daily amount of opium use is not provided by opium-dependents and a non-significant relationship between the amounts of consumption with the concentration of heavy metals may be attributed to unreliable responses.
The absorption of trace elements by the body has complexity. The transition of metals during inhalation or ingestion of opium could be a subject in future researches. Some factors can affect the transfer of elements to the body, for example, complete volatilization of lead dependents on the temperature applied to it; the temperature generated on the opium does not completely release lead vapor or some part of the vapor may run away without being smoked reducing exposure through inhalation of opium (Alinejad et al. 2018; Nakhaee and Mehrpour 2019). For the inhalation route, the bioavailability of lead relates to the airway geometry, airflow velocity, depth and duration of inhalation, vital capacity, smoking instruments, and size of particles (Abadin et al. 2019; Nakhaee and Mehrpour 2019).
Absorption following the oral route may be influenced by nutritional condition, pH of the gastrointestinal system, and transit through the digestive system. Opium reduces the motility of the gastrointestinal system; therefore, constipation may lead to more absorption of lead into the bloodstream via prolonged intestinal exposure (Hayatbakhsh et al. 2017; Nakhaee and Mehrpour 2019). Opium-dependents also mostly consumed oral opium in the fasting state to increase the absorption of opium. It has been suggested that 35% of Pb can be absorbed in the fasting state, whereas if opium is consumed with food, less amount of Pb (8.2%) is absorbed (Alinejad et al. 2018; Domeneh et al. 2014). As insufficient toxicological information is available, it is necessary to assess the health hazards and bioavailability of inhaled and ingested opium at the various length of exposure (Nakhaee and Mehrpour 2019). Our study has some limitations; first, in the current study, there was no assessment for lead content of opium due to problems in preparing opium samples from the participants; this should be investigated in future works. Further, we did not have complete information of participants’ nutrition habits or the effects of dietary intake on trace element status.
Conclusion
The results of our study showed an elevated urinary level of Pb, Mn, and Cd and lowered levels of Fe and Ca in opium-dependent individuals compared to non-dependents. Age affected the levels of Ni and Cu only. Opium consumption, regardless of gender, affects the concentration levels of most elements. The urinary levels of Ca, Cd, Se, Ti, and Pb elements were found to be all uncorrelated with admiration route, daily opium intake, and the years of opium consumption; the urinary levels were route-dependent for Co, Hg, and Mn. It is recommended that screening tests be performed to determine the level of different metals for each opium-dependent patient.
Data availability
The datasets used and analyzed during the current research are available from the corresponding author on request.
References
Abadin H, Taylor J, Buser MC, Scinicariello F, Przybyla J, Klotzbach JM, Diamond GL, Chappell LL, McIlroy LA (2019): Toxicological profile for lead: draft for public comment. available at: https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=96&tid=22.
Aghababaei R, Javadi I, Nili-Ahmadabadi A, Parsafar S, Ahmadimoghaddam D (2018) Occurrence of bacterial and toxic metals contamination in illegal opioid-like drugs in Iran: a significant health challenge in drug abusers. DARU J Pharm Sci 26:77–83
Aghaee-Afshar M, Khazaeli P, Behnam B, Rezazadehkermani M, Ashraf-Ganjooei N (2008) Presence of lead in opium. Arch Iran Med 11:553–554
Aksoy L, Sözbilir NB (2015) Trace and major element levels in rats after oral administration of diesel and biodiesel derived from opium poppy (Papaver somniferum L.) seeds. Toxicol Indust Health 31:890–897
Alinejad S, Aaseth J, Abdollahi M, Hassanian-Moghaddam H, Mehrpour O (2018) Clinical aspects of opium adulterated with lead in Iran: a review. Basic Clin Pharmacol Toxicol 122:56–64
Amirabadizadeh A, Nakhaee S, Mehrpour O (2020): Risk assessment of elevated blood lead concentrations in the adult population using a decision tree approach. Drug Chem Toxicol, 1-8
Ansari-Moghaddam A, Habybabady RH, Shakiba M, Mirzaei R, Shahriyari F, Aghaei S (2012) Predictors of initiation, continuation and transition of drug use in south-eastern Iran. J Pak Med Assoc 62:698–703
Ansari-Moghaddam A, Rakhshani F, Shahraki-Sanavi F, Mohammadi M, Miri-Bonjar M, Bakhshani NM (2016) Prevalence and patterns of tobacco, alcohol, and drug use among Iranian adolescents: A meta-analysis of 58 studies. Child Youth Serv Rev 60:68–79
Antonilli L, Suriano C, Paolone G, Badiani A, Nencini P (2003) Repeated exposures to heroin and/or cadmium alter the rate of formation of morphine glucuronides in the rat. J Pharmacol Exp Ther 307:651–660
August D, Janghorbani M, Young V (1989) Determination of zinc and copper absorption at three dietary Zn-Cu ratios by using stable isotope methods in young adult and elderly subjects. Am J Clin Nutri 50:1457–1463
Bou-Resli M, Mathew T, Dashti H, Al-Zaid N (2002) Brain selenium accumulation in rat pups of selenium supplemented mothers. Anatomia, Histologia, Embryologia 31:228–231
Coudray C, Feillet-Coudray C, Gueux E, Mazur A, Rayssiguier Y (2006a) Dietary inulin intake and age can affect intestinal absorption of zinc and copper in rats. J Nutri 136:117–122
Coudray C, Feillet-Coudray C, Rambeau M, Tressol JC, Gueux E, Mazur A, Rayssiguier Y (2006b) The effect of aging on intestinal absorption and status of calcium, magnesium, zinc, and copper in rats: a stable isotope study. J Trace Elem Med Biol 20:73–81
Domeneh BH, Tavakoli N, Jafari N (2014) Blood lead level in opium dependents and its association with anemia: a cross-sectional study from the capital of Iran. J Res Med Sci Official J Isfahan Univ Med Sci 19:939
Dos Santosa M, Soaresa MCF, Baischa PRM, Baischa ALM, Silva RMR (2018) Biomonitoring of trace elements in urine samples of children from a coal-mining region. Chemosphere 197:622–626
Farnia V, Pirsaheb M, Mansouri B, Azadi NA, Radmehr F (2021) Blood lead concentration among oral/inhaled opium users: systematic review and meta-analysis. Crit Rev Toxicol 51:24–35
García-Esquinas E, Pollan M, Tellez-Plaza M, Francesconi KA, Goessler W, Guallar E, Umans JG, Yeh J, Best LG, Navas-Acien A (2014) Cadmium exposure and cancer mortality in a prospective cohort: the strong heart study. Environ Health Pers 122:363–370
Ghaemi K, Ghoreishi A, Rabiee N, Alinejad S, Farzaneh E, Zadeh AA, Abdollahi M, Mehrpour O (2017): Blood lead levels in asymptomatic opium addict patients; a case control study. Emergency 5
Ghane T, Zamani N, Hassanian-Moghaddam H, Beyrami A, Noroozi A (2018) Lead poisoning outbreak among opium users in the Islamic Republic of Iran, 2016–2017. Bull World Health Org 96:165–172
Hayatbakhsh MM, Oghabian Z, Conlon E, Nakhaee S, Amirabadizadeh AR, Zahedi MJ, Moghadam SD, Ahmadi B, Soroush S, Aaseth J (2017) Lead poisoning among opium users in Iran: an emerging health hazard. Subs Abuse Treat Prevent Policy 12:1–8
Heshmati A, Karami-Momtaz J, Nili-Ahmadabadi A, Ghadimi S (2017) Dietary exposure to toxic and essential trace elements by consumption of wild and farmed carp (Cyprinus carpio) and Caspian kutum (Rutilus frisii kutum) in Iran. Chemosphere 173:207–215
Hosseini SY, Safarinejad MR, Amini E, Hooshyar H (2010): Opium consumption and risk of bladder cancer: a case-control analysis, Urologic Oncology: Seminars and Original Investigations. Elsevier, pp. 610-616
Huang M, Chen J, Yan G, Yang Y, Luo D, Chen X, He M, Yuan H, Huang Z, Lu Y (2021) Plasma titanium level is positively associated with metabolic syndrome: a survey in China’s heavy metal polluted regions. Ecotoxicol Environ Safety 208:111435
Hyder O, Chung M, Cosgrove D, Herman JM, Li Z, Firoozmand A, Gurakar A, Koteish A, Pawlik TM (2013) Cadmium exposure and liver disease among US adults. J Gastrointest Surg 17:1265–1273
Ivan S, Jozef F (2011): Content of heavy metals in poppy seeds (Papaver somniferum L.). Adv Environ Biol, 496-501
Jacobo-Estrada T, Santoyo-Sánchez M, Thévenod F, Barbier O (2017) Cadmium handling, toxicity and molecular targets involved during pregnancy: lessons from experimental models. Intl J Mol Sci 18:1590
Johnson PE, Milne DB, Lykken GI (1992) Effects of age and sex on copper absorption, biological half-life, and status in humans. Am J Clin Nutri 56:917–925
Karrari P, Mehrpour O, Abdollahi M (2012) A systematic review on status of lead pollution and toxicity in Iran; guidance for preventive measures. DARU J Pharm Sci 20:1–17
Karri SK, Saper RB, Kales SN (2008) Lead encephalopathy due to traditional medicines. Curr Drug Safety 3:54–59
Khatibi-Moghadam H, Khadem-Rezaiyan M, Afshari R (2016) Comparison of serum and urine lead levels in opium addicts with healthy control group. Human Exp Toxicol 35:861–865
Kim JA, Lee SH, Choi SH, Jung KK, Park MS, Jeong JY, Hwang MS, Yoon HJ, Choi DW (2012) Heavy metal risk management: case analysis. Toxicol Res 28:143–149
Knapek J, Buchtova R, Vosmerova D (2011): Content of cadmium in poppy seeds and poppy seeds containing products marketed in Czech Republic. J Environ Sci Eng5
Knapek J, Buchtova R. Vosmerova D (2009): Determination of cadmium in poppy seeds and in poppy seeds containing products. Book of abstract. 4th international symposium on recent advances in food analysis. 226–26
Kupnicka P, Kojder K, Metryka E, Kapczuk P, Jeżewski D, Gutowska I, Goschorska M, Chlubek D, Baranowska-Bosiacka I (2020) Morphine-element interactions–the influence of selected chemical elements on neural pathways associated with addiction. J Trace Elem Med Biol 60:126495
Lachman J, Hejtmankova A, Miholova D, Kolihova D, Tluka P (2006) Relations among alkaloids, cadmium and zinc contents in opium poppy (Papaver somniferum L.). Plant Soil Environ 52:282
Lafuente A, Márquez N, Pazo D, Esquifino AI (2000) Effects of subchronic alternating cadmium exposure on dopamine turnover and plasma levels of prolactin, GH and ACTH. Biometals 13:47–55
Lawrence A, Michalkiewicz A, Morley J, MacKinnon K, Billington D (1992) Differential inhibition of hepatic morphine UDP-glucuronosyltransferases by metal ions. Biochem Pharmacol 43:2335–2340
Lucchini RG, Albini E, Benedetti L, Borghesi S, Coccaglio R, Malara EC, Parrinello G, Garattini S, Resola S, Alessio L (2007) High prevalence of Parkinsonian disorders associated to manganese exposure in the vicinities of ferroalloy industries. Am J Indust Med 50:788–800
Malekzadeh MM, Khademi H, Pourshams A, Etemadi A, Poustchi H, Bagheri M, Khoshnia M, Sohrabpour AA, Aliasgari A, Jafari E (2013) Opium use and risk of mortality from digestive diseases--a prospective cohort study. Am J Gastroenterol 108:1757–1765
Mansouri B, Ebrahimpour M, Babaei H (2012a) Bioaccumulation and elimination of nickel in the organs of black fish (Capoeta fusca). Toxicol Ind Health 28:361–368
Mansouri B, Baramaki R, Ebrahimpour M (2012b) Acute toxicity bioassay of mercury and silver on Capoeta fusca. Toxicol Ind Health 28(5):393–398
Mansouri B, Salehi J, Etebari B, Kardan Moghaddam H (2012c) Metal concentrations in the groundwater in Birjand Flood Plain, Iran. Bull Environ Contam Toxicol 89:138–142
Masjedi MR, Naghan PA, Taslimi S, Yousefifard M, Ebrahimi SM, Khosravi A, Karimi S, Hosseini M, Mortaz E (2013) Opium could be considered an independent risk factor for lung cancer: a case-control study. Respiration 85:112–118
Minozzi S, Amato L, Bellisario C, Davoli M (2014) Maintenance treatments for opiate-dependent adolescents. Cochrane Database Syst Rev
Menati W, Valizadeh R, Menati R, Niazi M, Nazarzadeh M, Bidel Z (2017) Determination of opium abuse prevalence in Iranian young people: a systematic review and meta-analysis. J Subst Use 22(1):3–10
Mohsen Masoodi M, Mohammad-Reza Zali M, Mohammad-Javad Ehsani-Ardakani M, Mohammad-Alizadeh A-H, Kazem Aiassofi M, Rahim Aghazadeh M, Ahmad Shavakhi M, Mohammad-Hossein Somi M, Mohammad-Hossein Antikchi M, Yazdani S (2006) Abdominal pain due to lead-contaminated opium: a new source of inorganic lead poisoning in Iran. Arch Iran Med 9:72–75
Mudiam MR, Kumar SA, Mahadevan S, Ghosh P, Sarin RK, Beedu SR (2005) Quantitative evaluation of 28 mineral elements by inductively coupled plasma/mass spectrometry and its application in source identification of Indian opium. J AOAC Intl 88:1469–1484
Nakhaee S, Mehrpour O (2018) Opium addiction as new source of lead poisoning: an emerging epidemic in Iran. EXCLI J 17:513
Nakhaee S, Mehrpour O (2019) The transition of lead and microbial contamination from adulterated opium to the human body. Expert Opinion Drug Metab Toxicol 15:259–260
Nasrollahzadeh D, Kamangar F, Aghcheli K, Sotoudeh M, Islami F, Abnet C, Shakeri R, Pourshams A, Marjani H, Nouraie M (2008) Opium, tobacco, and alcohol use in relation to oesophageal squamous cell carcinoma in a high-risk area of Iran. British J Cancer 98:1857–1863
Nozadi F, Azadi N, Mansouri B, Tavakoli T, Mehrpour O (2021) Association between trace element concentrations in cancerous and non-cancerous tissues with the risk of gastrointestinal cancers in Eastern Iran. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-021-15224-3
O’Neal SL, Zheng W (2015) Manganese toxicity upon overexposure: a decade in review. Curr Environ Health Rep 2:315–328
Park RM, Bena JF, Stayner LT, Smith RJ, Gibb HJ, Lees PS (2004) Hexavalent chromium and lung cancer in the chromate industry: a quantitative risk assessment. Risk Anal Intl J 24:1099–1108
Peacock A, Leung J, Larney S, Colledge S, Hickman M, Rehm J, Giovino GA, West R, Hall W, Griffiths P (2018) Global statistics on alcohol, tobacco and illicit drug use: 2017 status report. Addiction 113:1905–1926
Proctor DM, Suh M, Campleman SL, Thompson CM (2014) Assessment of the mode of action for hexavalent chromium-induced lung cancer following inhalation exposures. Toxicology 325:160–179
Rajaei G, Mansouri B, Jahantigh H, Hamidian AH (2012) (2012) Metal concentrations in the water of Chah Nimeh Reservoirs in Zabol, Iran. Bull Environ Contam Toxicol 89:495–500
Rezaei M, Javadmoosavi SY, Mansouri B, Azadi NA, Mehrpour O, Nakhaee S (2019) Thyroid dysfunction: how concentration of toxic and essential elements contribute to risk of hypothyroidism, hyperthyroidism, and thyroid cancer. Environ Sci Pollut Res 26:35787–35796
Rosen L, Katzman K (2014): Afghanistan: drug trafficking and the 2014 Transition Washington: The Congressional Research Service, 2014. Available on: https://fas. org/sgp/crs/row 43540
Sadeghi A, Soleimani H, Nasseri-Moghadam S, Radmard AR (2017): Lead contaminated opium as unusual cause of abdominal pain-case series. Iranian Journal of Radiology
Salehi H, Sayadi AAR, Tashakori M, Yazdan DR, Soltanpour N, Sadeghi H, Aghaei AM (2009): Comparison of serum lead level in oral opium addicts with healthy control group.
Sarafanov AG, Todorov TI, Kajdacsy-Balla A, Gray MA, Macias V, Centeno JA (2008) Analysis of iron, zinc, selenium and cadmium in paraffin-embedded prostate tissue specimens using inductively coupled plasma mass-spectrometry. J Trace Elem Med Biol 22:305–314
Sazegar S, Ebrahimi F (2012) Oral opium: an unusual cause of lead poisoning. Singapore Med J 53:395–397
Shadman S, Bahreini M, Tavassoli S (2012) Comparison between elemental composition of human fingernails of healthy and opium-addicted subjects by laser-induced breakdown spectroscopy. Appl Opt 51:2004–2011
Smith KR, Nation JR, Bratton GR (2002) The effects of developmental cadmium exposure on morphine sensitization and challenge with selective D1 and D2 antagonists. Pharmacol Biochem Behav 72:581–590
Thompson CM, Kirman CR, Proctor DM, Haws LC, Suh M, Hays SM, Hixon JG, Harris MA (2014) A chronic oral reference dose for hexavalent chromium-induced intestinal cancer. J Appl Toxicol 34:525–536
Uchino E, Tsuzuki T, Inoue K (1990) The effects of age and sex on seven elements in Sprague-Dawley rat organs. Labor Animals 24:253–264
Williams BB, Kwakye GF, Wegrzynowicz M, Li D, Aschner M, Erikson KM, Bowman AB (2010) Altered manganese homeostasis and manganese toxicity in a Huntington’s disease striatal cell model are not explained by defects in the iron transport system. Toxicol Sci 117:169–179
Acknowledgements
The authors would like to acknowledge the Imam Khomeini Hospital in Kermanshah city for their assistance. We thank Mr. Nemati for his help in collecting samples and completing the checklist of patients. We are also very grateful to all of the patients who participated in this project.
Funding
This project was generously supported financially by the Kermanshah University of Medical Sciences (grant number: 1398/3008728).
Author information
Authors and Affiliations
Contributions
N. A., S. N., V. F., M. P., T. J., B. M., and M. K. contributed to the design of the study, the interpretation of the results, and the drafting of the manuscript. B. M. and M. K. conducted the collection of the data. N. A. and B. M. conducted the statistical analyses. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted by the World Medical Association Declaration of Helsinki. This study was approved by the Research and Ethics Committee of Kermanshah University of Medical Sciences (IR.KUMS.REC.1398.960).
Consent for publication
Not applicable
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Azadi, ., Nakhaee, S., Farnia, V. et al. Multivariate statistical evaluation of heavy metals in the urine of opium individuals in comparison with healthy people in Western Iran. Environ Sci Pollut Res 29, 8232–8241 (2022). https://doi.org/10.1007/s11356-021-16271-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-16271-6