Abstract
By investigating three dominant mangrove species, namely Aegiceras corniculatum, Kandelia candel, Ceriops tagal and their rhizosediment in Mangrove wetlands in Hainan Island, this research analyzed absorption, concentration and distribution of heavy metals (Cr, Cu, Zn, As, Cd and Pb) in mangroves. The results found that the concentration of specific heavy metal differs in the different mangrove organs (leaf, stem and root). The content of heavy metals concentrated greatly in roots, but less in leaves and stems. The study also revealed that concentration capacity was weak in all three mangrove species (BCF0.02–0.91), with their organ ranking BCFroot > BCFstem > BCFleaf. Among three mangrove species, the transfer factors of leaves and stems in Ceriops tagal were highest, indicating a great distribution capability for heavy metals, followed by Kandelia candel. Transfer factors in Aegiceras corniculatum were the weakest. This ranking was opposite to bioconcentration factors of roots. This study can further reflect bioavailability of heavy metals in sediments, which provides scientific evidence on ecosystem protection and management in mangrove wetlands.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mangrove ecosystem is the unique coastal transition ecosystem between marine and terrestrial environment, widely distributed in tropical and subtropical estuary wetlands (Bayen 2012; Ji et al. 2015). Sediments are the important component of the mangrove ecosystem. Heavy metals are one of the most severe contaminants in the coastal environment. Mangrove wetland sediments have a great ability to retain pollutants from tidal seawater and surface runoff (Bastakoti et al. 2018). Therefore, sediments are considered to be the ultimate sink of heavy metals in offshore environment. Most heavy metals possess features such as high environmental stability, biotoxicity, non-biodegradability and bioaccumulation (Gopalakrishnan et al. 2019; Cai et al. 2019; Yi et al. 2019). Mangroves have the ability to absorb or utilize heavy metals, due to their inherent biological, physical and chemical properties (Nguyen et al. 2017; Ke and Tam 2012; Usman et al. 2013a, b; Naidoo et al. 2014). Many studies have proved that mangroves have the ability to accumulate heavy metals, for example, in Avicennia marina and Rhizophora sp. (Nguyen et al. 2017; Akhand et al. 2011). With rapid industrial and economic development, the occurrence of heavy metal contaminants has been increasing in adjacent environment (Wang et al. 2017a, b, c; Chetelat et al. 2008).According to relevant reports, roughly 35% of the world mangroves have been damaged by heavy metal contamination over the last 20 years (Feller et al. 2010). The remaining mangroves worldwide are often affected anthropogenic activities, such as industrial and agricultural waste discharge and urban-waste dumping. These activities threaten mangrove environment (Cabral et al. 2016). If heavy metals enter the food chain through mangrove plants, their features can seriously threaten aquatic organisms and human health via bioaccumulation and biomagnification (Radojevic and Brog 2007; Pan and Wang 2012). Therefore, it is necessary to investigate the accumulation and distribution characteristics of heavy metals in different components of mangrove ecosystem, so as to reveal the migration patterns of heavy metal pollutants and their biological accumulation in plants.
This study focuses on Hainan Island, which has the most abundant mangrove species in China. Mangrove forests are important in shoreline stabilization, biodiversity protection, sediment retention and storm protection (Acharya 2002; Zhang et al. 2017). Since the implementation of the international tourism island strategy and the construction of the national free trade area, the island has experienced industrial, agricultural and domestic runoff along with the rapid port activities, urban and tourism developments in recent years. Different contaminants are deposited in the surrounding areas, posing a serious threat to the local ecological environment. Several researchers have reported heavy metal contamination in surrounding areas. According to research reports of mangrove wetlands, contamination of the Hainan Island has been steadily increasing (Liu et al. 2019; Wang et al. 2017a, b, c). The extent of contamination of mangrove wetlands has seriously restricted sustainable social and economic developments of the surrounding areas. To mitigate this problem from the ecological perspective, phytoremediation could be considered as a good method and tool. Mangrove vegetation can absorb different types of contamination mainly from rhizosediment using its own characters. Limited research on the heavy metal accumulation, absorption and transfer capacity of mangrove plants in the Hainan Island of China has been conducted (Qiu et al. 2011a, b; Zhang et al. 2014). Therefore, the objectives of this study were to (1) assess the heavy metal concentration of individual organs (root, stem, leaf) in dominant mangrove species; (2) determine the concentration and transfer of heavy metals (Cr, Cu, Zn, As, Cd and Pb) from rhizosediment to mangrove organs; and (3) determine the possible sources of heavy metals using correlation analyss.
Materials and methods
Study area
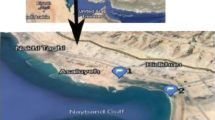
Hainan Island, the second largest island in China, is located in the north of South China Sea (118°37′–111°3′E, 18°10′–20°10′N) and has an area of about 33,920 km2 (Fig. 1).The major types of rocks are granite and basalt. Mangrove wetlands in Hainan Island are mainly concentrate in the northern regions. Hainan Island is located in the tropical zone, having various mangrove species and large preservation area, widely distributed in coastal areas of the island, namely Haikou and Wenchang in the north, Sanya and Lingshui in the south and Danzhou and Lingao in the west. This research focuses on northern Hainan Island mangrove distribution area (109.329°–110.804°E and 19.542–20.034°N), including mangrove wetland reserve in Dongzhai Harbor in eastern Haikou, mangrove wetland reserve in Qinglan Harbor in Wenchang, and mangrove wetland reserve in Xinying Harbor in Danzhou. The total research area comes to 9722.6 hm2, with mangrove area of 817.25 hm2.The mangrove in the researched area accounts for more than 85% of total mangrove area in Hainan Island and 12% of the total mangrove area in China. The distribution of mangrove in other areas of Hainan Island is relatively scattered. Mangrove wetland reserve in Dongzhai Harbor in eastern Haikou is the earliest, the largest coverage, the best conserved and the most iconic mangrove reserve in China. It is also China's first national nature reserve, enrolled in the list of international important wetland in 1992, with the mangrove area accounting for more than 50% of the existing mangrove area of Hainan Island. The mangrove wetland reserve in Qinglan Harbor in Wenchang is the second mangrove reserve established in China. It is known for the best growth, the tallest plants and well-preserved birds as a provincial natural reserve. It also is one of the key biodiversified areas in China. Its mangrove coverage accounts for 33% of the existing mangrove area of Hainan Island. The mangrove wetland reserve in Xinying Harbor at the border of Lingao County and Danzhou City is the only one national mangrove seashore wetland park in Hainan province, accounting for 6.5% of the existing mangrove area of Hainan Island.
The climate of Hainan Island is tropical oceanic monsoon, with average temperature 20–24 °C and average sunshine 2200 h annually. Due to distinct seasons between wet and dry, the rainfall mainly concentrates in summer (May to October), with annual precipitation 1500 mm. Surface sediments in the chosen area are acidic, pH 5–6, mainly consisting of silts, sands and fine grains, with shells and conch crusts. The area is between high and low tide lines. The banks are in ribbon or irregular shapes, with flat surface, and various precipitousness in front edges, some of which have steep ridges, leaning to the sea. Streams like Sanmen River, Yanfeng River, Wenjiao River and Wenchang River run through protected areas into the sea. Along the banks is abundant sludge, mainly struck by bays and estuary. Usually, there is little wind. Organic substance is plentiful, fit for the growth and reproduction of mangroves. A great variety of mangrove species are distributed widely in the area, including Aegiceras corniculatum, Kandelia candel, Ceriops tagal, Bruguiera sexangula, Sonneratia caseolaris, Rhizophora stylosa, Bruguiera gymnorrhiza, Avicennia marina, etc., making it one of the key biodiversity areas in China (Wang et al. 2017a, b, c, 2014; Ji et al. 2016). This area is also the one developed rapidly in Hainan in term of economic level and urbanization. Due to long-term irrational developments and utilization, poldering of seashore, excessive deforestation, aquaculture, the ecosystem of mangrove wetlands in Hainan has encountered severe destruction in recent 30 years. The areas of mangrove have contracted gradually, appearing secondary condition, with an increasing proportion of defect woods, and deterioration in ecological environment.
Hainan Island is the major part of China's Hainan Province, accounting for 95% of the total land area of Hainan Province, including 18 cities and counties (excluding Sansha City). According to Hainan Statistical Yearbook 2019, the GDP of the region in 2018 was 483.05 billion yuan, an increase of 5.8% over the previous year in terms of comparable prices. The per capita GDP of the province was 51,955 yuan, an increase of 4.8% over the previous year. Under the current background of Hainan's free trade port construction, taking ecological fragility and environmental constraints of the island into the consideration, Hainan will vigorously develop tourism, modern service industry and high-tech industry, continuously consolidate the foundation of the real economy and enhance the industrial competitiveness. Over the past 30 years since Hainan was established as a special economic zone, Hainan has made great progress in urbanization and rural development. Hainan has been committed to increasing the infrastructure construction of urbanization and the coverage of basic public services. It insists in planning as top priority, meanwhile increasing investment and driving industry, and has achieved remarkable results in those three decades. By the end of 2018, Hainan had a total population of 9.3432 million, with an urbanization rate of 59.06 percent, close to the national average of 59.58 percent. Rural residents' incomes have increased significantly. In 2018, the per capita disposable income of rural permanent residents in Hainan province was 13,989 yuan, up 8.4 percent year on year. The establishment of a pilot free trade zone and the exploration of a free trade port with Chinese characteristics on the whole island represent another historic opportunity for Hainan. As an important part of economic and social development, urbanization will undoubtedly be greatly influenced.
Collection and treatment of samples
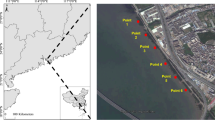
Field investigation and sample collection were conducted from July to August in 2019. With the help of GPS, 6 sites in the non-anthropogenic influencing area were selected as sampling spots from each estuary, covering the edge of mangrove forest, mudflat in low tide (10 m away from the forest) and inside of mangrove forest (10–15 m from the edge). Each 1 kg columnar sediments samples were, respectively, collected from surface depth 0–5 cm, 10–15 cm, 20–30 cm, 30–40 cm. Restricted to landform and time, sediments in partial mudflat and edge of forest were unable to be collected. Samples were sealed in bilayer polyvinyl plastic bags. Moreover, dominant mangrove species were selected near the sediment sampling sites, of which roots, stems and leaves were collected with 0.5 kg each. Animal remains and shells were removed from sediment samples. Plant samples were washed with ultra-pure water, air-dried at room temperature to a constant weight and stored in a dry place to be tested. A total of 192 sediment samples and 54 mangrove organ samples were collected and pretreated.
The study area in this paper is the most representative mangrove protection area in China. There are many mangrove species and large distribution area in this study area. There are 19 families and 36 species of mangrove plants distributed in this area, accounting for 97% of all mangrove plant species in China. By looking at the advantageous species of mangrove phytocenosis in the study area of the related research literature, the related research results show that by important value (IV) calculation method to select and distinguish the advantageous species. The important values of Aegiceras corniculatum, Kandelia candel, Ceriops tagal in the study area are relatively higher than others, with 6.85, 7.17, 7.26, respectively. These three species are advantageous over other dominant mangrove species, of which Kandelia candel belongs to tree, and Aegiceras corniculatum, Ceriops tagal belong to shrub.
Test and analysis of samples
This research tested the content of available heavy metals in sediment samples, and the content of heavy metals in various mangrove organs (roots, stems and leaves).Inductively coupled plasma mass spectrometry (ICP-MS) was used for measurements. According to classification method of Mao M. France and the size of each binding bioavailability, chemical forms were classified into three states, being available, potential available and non-available. Among them, available heavy metals were exchangeable and carbonated binding, which were most sensitive to the changes of water environment conditions, and prone to leaching and biological absorption. The potential available heavy metals were in Fe–Mn oxide binding and organic binding state. The non-available heavy metals were generally referred to residue, or mineral lattice binding state.
Based on single-solvent extraction approach, this research used ammonium salt solution of EDTA as extraction agent, to test bioavailability of heavy metals. EDTA, the chelating agent, not only time-efficient, but also good at data repeatability, could accurately extract the content of available heavy metals.
Microwave dissolution of sediment samples and plant samples
Each plant sample was decomposed with ETHOSONE microwave dissolution chemical system.
0.1 g dried sample (accurate to 0.0001) was accurately weighed and preliminarily dissolved with 9 ml of 6.0 ml HNO3 + 3.0 ml HF mixed acid. Placed in a special lining cup for dissolution, it was heated to 180 °C at first, cooled for 30 min and then quantitatively transferred into a beaker, where 0.5 ml H2O2 was added and the remaining acid was evaporated. Finally, the dissolved salt was washed with 0.02 mol HNO3, and the volume was fixed to 50 ml, then to be measured.
Extraction of available heavy metals from sediments.
The single extraction method was used to extract available heavy metals from sediment samples. The composition of extractive solution was 0.5 mol/L NH4Ac + 0.5 mol/L HAC + 0.02 mol/L Na2 EDTA. 1.000 g dried sample was weighed, and 10 mL extractive solution was added. Then, it was vibrated for 1 h and centrifugated for 25 min at the speed of 3500 r/min, then filtrated. Finally, 3 mL filtrate was taken, with a fixed volume of 10 mL ultrapure water, then to be measured.
Measurement of heavy metal content in samples
Use inductively coupled plasma mass spectrometry (ICP-MS) (Agilent 7700x) to draw standard curve. The linear correlation coefficient of standard curve was above 0.9995. Then measure the standard substance. The measured value of each element in the standard substance is consistent with the standard value. Finally determine the total of heavy metals and content of available heavy metals in the sediment samples, as well as the content of plant samples.
Bioconcentration factors and transfer factors
The concentration capacity of plants for heavy metals in sediments can be indicated by bioconcentration factor (BCF), defined as the ratio of concentration of chemical substance in biological organs to the concentration of chemical substance in sediments (Azibar et al. 2016; Phaenark et al. 2009).
BCF could be calculated by the following equation:
\({C}_{leaf}\), Cstem and \({C}_{\mathrm{root}}\) represented heavy metals in leaves, stems and roots. \({C}_{\mathrm{sediment}}\) represented heavy metals in rhizosediment.
The heavy metals in sediments were absorbed and accumulated by roots of mangrove, and transferred by xylem to other organs in form of dissociative metal ions. TF could estimate the migration capacity of heavy metals from roots to stems and leaves in plants. The formula was as follows:
\({C}_{\mathrm{leaf}}\), Cstem and \({C}_{\mathrm{root}}\) represented heavy metals in leaves, stems and roots (Usman et al. 2013a, b; Usman and Mohamed 2009).
The content of heavy metals in mangroves
According to results of analyzing heavy metal content in collected mangroves (Table 1), each metal content varied significantly in Aegiceras corniculatum. The average content of targeted heavy metals ranked: Zn(11.67 ng/g) > Cr(8.75 ng/g) > Cu(5.42 ng/g) > As(4.34 ng/g) > Pb(3.74 ng/g) > Cd(0.09 ng/g). The average content of targeted heavy metals in Kandelia candel was as follows: Zn(11.65 ng/g) > Cr(8.78 ng/g) > As(3.68 ng/g) > Pb(3.36 ng/g) > Cu(3.19 ng/g) > Cd (0.18 ng/g), while the average content of heavy metals in Ceriops tagal was shown as:Zn(8.92 ng/g) > Cr(4.96 ng/g) > Cu(3.67 ng/g) > As(3.14 ng/g) > Pb(2.87 ng/g) > Cd(0.05 ng/g).
It could be seen that the rankings of heavy metal content in three mangrove species were slightly different, among which Zn and Cr were relatively the highest, while Cd the lowest. The high content of Cr might be related to the high background value in the research area. Compared with the other six heavy metals, Zn represented higher concentration. It might be caused by the concentration of Zn in sediments, and as a necessary trace element for the synthesis of plant cellulose, it was often concentrated by plants proactively. The results of research from Ong and others also indicated that accumulation of Zn in mangrove roots was greater than that of Cu and Pb (Ong Che 1999; Ujwal et al.2019).
Figure 2 shows that the concentration of same metal in different mangrove organs (leaves, stems and roots) in this research also varied. In general, the targeted metals were all accumulated in roots, but the degrees of concentration in leaves and stems were low. Research has shown that heavy metals accumulated in roots in the largest proportion, indicating most of the absorbed metals were confined to the outer cortex of roots (Zheng and Lin 1996; Macfararlane 2002). Normally, heavy metals accumulated in roots could bind to cell walls or other large molecules. In this way, they were prevented from transferring to other organs above the ground (Tam and Wong 2000).
Compared with the content of heavy metals in mangroves in other regions, the data in this research were relatively low. Previous studies indicated that the average content of heavy metals in organs of nine mangrove species in Hainan Island was Cu(2.8 ng/g), Zn(8.7 ng/g), Cd(0.03 ng/g), Pb(1.4 ng/g), Cr(1.1 ng/g) and As(0.2 ng/g), which were all lower than the results of this research(Qiu et al. 2011a, b; Usman et al. 2013a, b; Yu et al. 2007). The results of studies by Usman and others revealed that in Red Sea coast, the average content of heavy metals in leaves of Avicennia marina was Cd(1.04 ng/g), Cr(9.30 ng/g), Cu(356.6 ng/g) and Zn(29.5 ng/g).Except lower content of Ni, the others were higher than those in this research. The Cu(270.5 ng/g) and Zn(36.8 ng/g) in roots of Avicennia marina were far higher than data in this research. The concentrations of metals in mangrove stems in Leizhou peninsula in China were Cd(0.15 ng/g), Cr(26.64 ng/g), Cu(10.89 ng/g), Pb(0.51 ng/g) and Zn(14.73 ng/g), far higher than the results of this research. Moreover, heavy metals of Cu, Pb and Zn in leaves and roots of mangroves have also been studies in other parts of southern China (Macfarlane et al. 2007). In Ting Kok, Hongkong, the content of Cu in mangrove leaves and roots was 11 and 12 ng/g, while in freshwater estuary in Taiwan, Cu content was 35–165 and 4.5–55 ng/g, respectively. Pb content in mangrove leaves and roots in above two regions was 11 and 28 ng/g, 1.0–5.0 and 16–77 ng/g, while Zn was 23 and 29 ng/g, 15–20 and 49–340 ng/g. The above data were significantly higher than that of this research. It indicated that mangroves in northern part of Hainan Island were still relatively clean and less polluted by heavy metals.
The concentration and transfer capacities of mangroves to heavy metals
Heavy metals in sediments and pore water were not only highly toxic to local communities, but also difficult to be fixed naturally. In the process of adapting to the living environment, wetland organisms took initiative to absorb the heavy metals through roots in the concentrated sedimentary environment, so as to achieve the aim to reduce content of heavy metals in sediments (Li et al. 2000). As perennial trees in intertidal zones, mangroves were not only able to absorb pollutants from sediments, but also reflected the bioutilization of heavy metals in sedimentary environment, showing bioavailability.
Bioconcentration factors of heavy metals
The bioconcentration factors (BCF) of heavy metals in different organs of three mangrove species, Aegiceras corniculatum, Kandelia candel, Ceriops tagal in the research area, are shown in Table 2 and Fig. 3.
Heavy metals BCF could not only reflect the accumulation characteristics of heavy metals in different organs of three mangrove species, but also indicate the difficulties of migration for heavy metals in sedimentary environment and in botanical system (Li et al. 2000). As seen from Table 2 and Fig. 3, compared with plants highly concentrated by heavy metals (the content of heavy metals in plant organs exceeds 1000 ng/g), the capacity of accumulating heavy metals for three mangrove species was small (BCF ranged from 0.02 to 0.91), and BCF of different plant organs ranked BCFroot > BCFstem > BCFleaf. The data suggested the capacity of heavy metal migration was weak in stems and leaves, but strong in roots. The distributions of heavy metals in plants mainly followed the rule to concentrate in metabolically active organs such as roots, rather than distributing in nutrient-storing organs such as stems and leaves.
Different mangroves had different capacities to accumulate heavy metals. As for BCF in roots of Aegiceras corniculatum, Zn, As and Pb were all greater than 0.7, especially Zn, reaching 0.87. Cu was 0.65, respectively. Cr and Cd were relatively small, below 0.4. The ranking order of BCF in stems was the same as that of roots. BCF in leaves was slightly different, among which As and Pb were the largest, and Cd and Cr were the smallest. The data suggested that Aegiceras corniculatum had the strongest capacity to concentrate Zn and As, but the weakest capacity to accumulate and migrate Cd and Cr. In regard to the roots of Kandelia candel, the highest BCF values in roots were As and Pb, both above 0.8, and As reached 0.92. The values of Cd, Zn lay in the middle, ranging from 0.5 to 0.7. The values of Cu and Cr were the lowest, merely about 0.4. The BCF values in stems and leaves were in a similar order. The only difference was that the BCF value of Cd in stems was the lowest, while that of Cr in leaves the lowest. In could be considered that As and Pb were the most easily absorbed and accumulated by roots, while Cu, Cr and Cd were relatively weak in migration, making Kandelia candel difficult to concentrate those heavy metals. As to the roots of Ceriops tagal, the BCF value of As was the highest, up to 0.74, while values of Zn, Cu, Pb and Cd were relatively lower, ranging from 0.2 to 0.4. The values of Cr and Ni were the lowest, below 0.2. The BCF values in stems and leaves were exactly in the same order, with Cu the highest, followed by As, Zn, Pb, Cr and Cd. It suggested that As and Zn easily migrated from sediments to Ceriops tagal, while Cr and Ni were poorly absorbed, thus difficult to accumulate in roots.
In addition, the concentration capacity of three mangrove species to the same heavy metal was different. With regard to Cr, As, Cd and Pb, Kandelia candel had strongest capacity to absorb them, followed by Aegiceras corniculatum. Ceriops tagal was the weakest. While as to Cu and Zn, Kandelia candel lay in the middle position, and Aegiceras corniculatum was the strongest. Ceriops tagal was the weakest. In general, Kandelia candel had strongest capacity to concentrate 6 heavy metals and tended to accumulate Cr, As, Cd as well as Pb. Aegiceras corniculatum was on the second position, mainly concentrating on essential elements for plant survival, such as Cu and Zn. Ceriops tagal was the weakest in concentrating any heavy metal.
Transfer factors of heavy metals
The heavy metal transfer factors of three mangrove species, Aegiceras corniculatum, Kandelia candel and Ceriops tagal in the research area, are shown in Table 3 and Fig. 4.
As seen from the graph, heavy metals Pb and As had relatively greater TF values in stems and leaves of Aegiceras corniculatum, which suggested Aegiceras corniculatum was able to transfer these two heavy metals from roots effectively. The TF value of Cr was the smallest in stems and leaves, making it difficult to transfer from roots to leaves and stems. Among TF values in stems and leaves of Kandelia candel, Cu and Zn were greatest, because they were essential for plant growth. TF values in stems and leaves of Ceriops tagal were similar to those of Kandelia candel. It could be found that TF values of Cd were low in all three mangrove species, mainly because the Cd in roots of mangroves existed in insoluble form, making it difficult to transfer from roots as well as accumulate in roots.
The concentration capacities of heavy metals in the stems and leaves of different mangroves were also different. As seen from Fig. 4, among three mangrove species, TF values in stems and leaves for Ceriops tagal were the largest, which meant the strongest migration capacity to heavy metals. The TF values of Aegiceras corniculatum followed, and those of Kandelia candel was the weakest. This order was contrary to BCF values in mangrove roots. It suggested that having transferred from roots of Ceriops tagal, heavy metals would concentrate in stems and leaves for a second time, which could be a mechanism for heavy metal accumulation.
Correlation analysis between heavy metals in roots of mangroves and available heavy metals in sediments
The process of migrating and concentrating heavy metals from sediments by mangroves was mainly as follows: firstly, heavy metals were released in the sedimentary environment, and mangroves absorbed heavy metals from sediments by roots. Secondly, absorbed heavy metals partially concentrated in roots, they would further transfer to stems and leaves in order to accumulate in plant organs again. Furthermore, roots could only absorb certain or some available heavy metals in sedimentary environment (in this research, it could be considered that mangroves mainly absorbed available heavy metals as previously mentioned).
Root as an important link in the process of migrating and concentrating heavy metals,it not only decides to absorb the content of heavy metals from sediments, but also restricts heavy metals transferring to other organs of mangrove. Therefore, this paper explored the correlation between content of heavy metals in mangrove roots and content of available heavy metals in sediments. The results of correlation analysis are listed in Table 4.
Obviously in Table 4, there was a significant positive correlation between Cu and Zn in roots of three mangrove species and the content of available heavy metals in sediments, with the correlation coefficient above 0.9 and 0.8 (P < 0.05). It indicated that the roots of three mangrove species had a strong absorptive capacity for available content of Cu and Zn. The Cr content in roots of Ceriops tagal and Ni in roots of Kandelia candel had significant correlations with the content of available metals in rhizosediment (P < 0.05), while As, Cd and Pb in roots of three mangrove species had no significant correlation with content of available heavy metals in sediments. It was worth mentioning that As in roots of Aegiceras corniculatum and Kandelia candel, as well as Pb in roots of all mangroves had negative correlations with content of available heavy metals in sediments. This might be caused by characteristics of As and Pb themselves. Both had toxic effects on most plants and could arouse counter-reaction by plants.
Conclusions
By investigating (1) the content of heavy metals in organs of three typical mangrove species in the research area, (2) concentration and transfer capacities of mangroves to heavy metals and (3) their correlations with available heavy metals in rhizosediment, the following conclusions were obtained:
-
(1)
The content of Zn and Cr was relatively the highest, while that of Cd the lowest. Generally, the concentrations of same heavy metal in different mangrove organs (leaves, stems and roots) varied. Heavy metals mainly accumulated in roots, while concentration in leaves and stems was low. In addition, compared with the content of heavy metals in mangroves in other regions, the content of heavy metals in mangrove organs was relatively low in this research.
-
(2)
The capacity of three mangrove species to concentrate heavy metals was small (BCF ranging from 0.02 to 0.91). The values of BCF for different mangrove organs were BCFroot > BCFstem > BCFleaf. Heavy metals in plants mainly focused on the organs with strong metabolism such as roots, while only small amount distributed in nutrition-storing organs such as stems and leaves. Mangroves not only had different concentration capacities for different heavy metals, but also different accumulation capacities for the same heavy metal. As for Cr, As, Cd and Pb, the concentration capacities of mangroves ranked as Kandelia candel > Aegiceras corniculatum > Ceriops tagal, while for Cu and Zn, the ranking was Aegiceras corniculatum > Kandelia candel > Ceriops tagal. In general, Kandelia candel had the strongest concentration capacity for 7 heavy metals and tended to accumulate Cr, As, Cd and Pb, followed by Aegiceras corniculatum, mainly concentrated essential metals for plant survival such as Cu and Zn. Ceriops tagal had the weakest concentration capacity for any heavy metal.
-
(3)
Based on the analysis of heavy metal transfer factors (TF) in leaves and stems of mangroves, the TF values of Cu and Zn were high, while Cd existed in plant roots was in an insoluble form, making it difficult to transfer to other organs, so that it had the lowest TF value. Among three mangrove species, the TF value of Ceriops tagal was relatively the highest, indicating a strong transferring capacity of heavy metals, followed by Aegiceras corniculatum, and Kandelia candel was the least.
-
(4)
Cu and Zn had an obvious correlation with content of available heavy metals in sediments, indicating that the roots of mangroves had strong absorption capacity to availability of Cu and Zn, while As and Pb had toxic effects on most plants, which could cause counter-reaction by plants.
References
Akhand, A., Chanda, A., Dutta, S., Hazra, S., & Sanyal, P. (2011). Comparative study of heavy metals in selected mangroves of Sundarban ecosystem. Journal of Environmental Biology, 33, 1045–1049.
Acharya, G., (2002). Life at the margins: the social, economic and ecological importance of mangroves. Madera y Bosques Número especial, pp 53–60.
Bayen S., (2012). Occurrence, bioavailability and toxic effects of trace metals and organic contaminants in mangrove ecosystems: a review. Environment International, 84–101.
Bastakoti, U., Robertson, J., & Alfaro, A. C. (2018). Spatial variation of heavy metals in sediments within a temperate mangrove ecosystem in northern New Zealand. Marine Pollution Bulletin, 135, 790–800.
Chetelat, B., Liu, C. Q., Zhao, Z. Q., Wang, Q. L., Li, S. L., Li, J., et al. (2008). Geochemistry of the dissolved load of the Chang jiang Basin rivers: anthropogenic impacts and chemical weathering. Geochimica et Cosmochimica Acta, 72, 4254–4277.
Cai, L. M., Wang, Q. S., Luo, J., Chen, L. G., Zhu, R. L., Wang, S., et al. (2019). Heavy metal contamination and health risk assessment for children near a large Cu-smelter in central China. Science of the Total Environment, 650, 725–733.
Cabral, L., Júnior, L., Vieira, G., de Sousa, S., Pereira, T., et al. (2016). Anthropogenic impact on mangrove sediments triggers differential responses in the heavy metals and antibiotic resistomes of microbial communities. Environmental Pollution, 216, 460–469.
Feller, I. C., Lovelock, C. E., Berger, U., Mckee, K. L., Joye, S. B., & Ball, M. C. (2010). Biocomplexity in mangrove ecosystems. Ann. Rev. Mar. Sci. The Annual Review of Marine Science, 2, 395–417.
Gopalakrishnan ,G., Wang, S. G., Mo, L. P. (2019). Distribution determination, risk assessment, and source identification of heavy metals in mangrove wetland sediments from Qi'ao Island, South China. Regional Studies in Marine Science, 33, 1–10.
Ji, Y. N., Zhao, Z. Z., & Wu, D. (2015). Accumulation and distribution of heavy metals of mangrove wetland and kandelia candel in Dong zhai Harbor. Safety and Environmental Engineering., 2, 66–73.
Ji, Y. N., Zhao, Z. Z., Wu, D., et al. (2016). Distribution and bioavailability of heavy metals in mangrove deposits in dongzhai harbor, Hainan. Journal of Applied Ecology 27, 593–600.
Ke, L., Tam, N.F. (2012). Phytoremediation Using Constructed Mangrove Wetlands: Mechanisms and Application Potential. New York: Nova Science Publishers, Inc.
Liu, Y. Y., Ji, C. Y., Fu, B., He, L. S., Fu, P. P., Shen, M. C., et al. (2019). Factors influencing the accumulation of Pd in mangrove wetland sediments in Dongzhai Harbor, Hainan, China. The Journal of Coastal Conservation 23, 1039–1045.
Li, H. H., Liu, J. W., Li, S. R. (2000). The present progness of research on heavy metal pollution and plant enrichment in soil-plant system. Journal of Henan Agricultural University, 34, 30–34.
Macfarlane, G. R. (2002). Leaf biochemical parameters in Avicennia marina (Forsk.) Vierh as potential biomarkers of heavy metal stress in estuarine ecosystems. Marine Pollution Bulletin, pp 244–256.
Macfarlane, G. R., Koller, C. E., & Blomberg, S. P. (2007). Accumulation and partitioning of heavy metals in mangroves: a synthesis of field-based studies. Chemosphere, 69, 1454–1464.
Naidoo, G., Hiralal, T., & Naidoo, Y. (2014). Ecophysiological responses of the mangrove Avicennia marina to trace metal contamination. Flora, 209, 63–72.
Nguyen, K. L., Nguyen, H. A., Richter, O., Pham, M. T., & Nguyen, V. P. (2017). Ecophysiological responses of young mangrove species Rhizophora apiculata (Blume) to different chromium contaminated environments. Science of the Total Environment, 574, 369–380.
Ong Che, R. G. (1999). Concentration of 7 Heavy Metals in Sediments and Mangrove Root Samples from Mai Po, Hong Kong. Marine Pollution Bulletin, 39(1–12), 269–279.
Phaenark, C., Pokethitiyook, P., Kruatrachue, M., & Ngernsansaruay, C. (2009). Cd and Zn accumulation in plants from the Padaeng zinc mine area. International Journal of Phytoremediation, 11, 479–495.
Pan, K., & Wang, W. (2012). Trace metal contamination in estuarine and coastal environments in China. Science of the Total Environment, 421, 3–16.
Qiu, Y. W., Yu, K. F., Zhang, G., et al. (2011). Accumulation and partitioning of seven trace metals in mangroves and sediment cores from three estuarine wetlands of Hainan Island, China. Journal of Hazardous Materials, 190(1–3),631–638.
Qiu, Y. W., Yu, K. F., Zhang, G., & Wang, W. X. (2011). Accumulation and partitioning of seven trace metals in mangroves and sediment cores from three estuarine wetlands of Hainan Island, China. Journal of Hazardous Materials, 190(1–3), 15.
Radojevic, M., & Brog, J. (2007). Factor-cluster analysis and enrichment study of mangrove sediments-an example from Mengkabong, Sabah. The Malaysian Journal of Analytical Sciences, 11, 421–430.
Tam, N. F. Y., Wong, Y. S. (2000). Spatial variation of heavy metals in surface sediments of Hong Kong mangrove swamps. Environmental Pollution, 110, 195–205.
Usman, R. A., Alkredaa, R. S., & Al-Wabel, M. I. (2013). Heavy metal contamination in sediments and mangroves from the coast of Red Sea: Avicennia marina as potential metal bio-accumulator. Ecotoxicology and Environmental Safety, 97, 263–270.
Usman, A. R. A., & Mohamed, H. M. (2009). Effect of microbial inoculation and EDTA on the uptake and translocation of heavy metals by corn and sunflower. Chemosphere, 76, 893–899.
Usman, A. R. A., Alkreda, R. S., & Al-Wabel, M. I. (2013). Heavy metal contamination in sediments and mangroves from the coast of Red Sea: Avicennia marina as potential metal bioaccumulator. Ecotoxicology and Environmental Safety, 97, 263–270.
Ujwal, B. K., John, R., Carine, B., et al. (2019). Temporal variations of trace metals and a metalloid in temperate estuarine mangrove sediments. Environmental Monitoring and Assessment, 191, 780.
Usman, A. R. A., Alkredaa, R. S. (2013) Heavy metal contamination in sediments and mangroves from the coast of Red Sea: Avicennia marina as potential metal bioaccumulator. Ecotoxicology and Environmental Safety, 97, 263–270.
Wang, J., Ye, S. Y., Laws, E. A., Yuan, H. M., Ding, X. G., & Zhao, G. M. (2017). Surface sediment properties and heavy metal pollution assessment in the Shallow Sea Wetland of the Liaodong Bay. Marine Pollution Bulletin, 120, 347–354.
Wang, P., Zhao, Z. Z., Ma, R. L., et al. (2014). Accumulation characteristics of heavy metals in mangroves of intertidal zone in northern Hainan Island. Journal of Ecology and Environment, 5, 842–846.
Wang, J. G., Wang, P., Zhao, Z. Z., et al. (2017). Distribution characteristics of available Cd, Zn, As, Pb in Sediments of Mangrove Wetland and Their Correlation. Environmental Pollution Control, 39, 1289–1293.
Wang, J. G., Wang, P., Fu X. N., et al. (2017). Speciation and Bioavailability of Heavy Metals in Sediments of Mangrove Wetland in Qing lan Harbor, Hainan Island, Southwest China. Southwest China. J Agr Sci Journal of Agricultural Science, 32, 2425–2431.
Yi, X., Zhang, C., & Liu, H. (2019). Occurrence and distribution of insecticides in surface water and sediment of the Guangzhou section of the Pearl River, South China. Environmental Pollution, 251, 892–900.
Yu, K. F., Kamber, B. S., Lawrence, M. G. (2007) High-precision analysis on annual variations of heavy metals, lead isotopes and rare earth elements in mangrove tree rings by inductively coupled plasma mass spectrometry. Nuclear Instruments and Methods in Physics Research Section B, 255, 399–408.
Zhang, D. L., Liu, N., Zhu, J. F., Lin, X. H., Jiang, X. J., & Meng, X. W. (2017). Characterization, sources and ecological risk assessment of polycyclic aromatic hydrocarbons in surface sediments from the mangroves of China. Wetl Ecol Manag, 25, 105–117.
Zhang, Z. W., Xu, X. R., Sun, X. Y., & Yu, S. (2014). Heavy metal and organic contaminants in mangrove ecosystems of China: a review. Environmental Science and Pollution, 21, 11938–11950.
Zheng, W. J., Lin, P. (1996). Accumulation and distribution of Cu, Pb, Zn and Cd in avicennia marina mangrove community of Futian in Shenzhen. Oceanologia Et Limnologia Sinica, 27, 386–393.
Acknowledgements
This research was supported by Hainan National Science Foundation (419MS049) and the Science Research Program of Hainan Province Institutions of Higher Education (Hnky2019-34).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, J., Wang, P., Zhao, Z. et al. Uptake and concentration of heavy metals in dominant mangrove species from Hainan Island, South China. Environ Geochem Health 43, 1703–1714 (2021). https://doi.org/10.1007/s10653-020-00717-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-020-00717-w