Abstract
Numerous indicator models have been developed and utilized for the assessment of pollution levels in water resources. In the present study, modified water quality index (MWQI), integrated water quality index (IWQI), and entropy-weighted water quality index (EWQI) were integrated with statistical analysis for the assessment of drinking water quality in Umunya suburban district, Nigeria. There is no known study that has simultaneously compared their performances in water quality research. Overall, the results of this study showed that the water supplies are threatened by heavy metal pollution. The parametric quality rating analysis observed that Pb contamination has the most significant impact on the water supplies. Hierarchical cluster analysis was proved very efficient in the allotment of the possible sources of pollution in the study area. MWQI results classified the water supplies as “marginal”, signifying that they are frequently threatened. Based on the IWQI, 26.67% of the samples are suitable for drinking, 13.33% are acceptable for domestic uses, and 60% are unfit for drinking purposes. Similarly, the EWQI results showed that 60% of the samples are unfit for human consumption, whereas 40% are suitable. Investigation into the performance and sensitivity of the MWQI, IWQI and EWQI models in water quality assessment was analyzed and the results showed that they are all sensitive, efficient and effective tools. This study has indicated that the integration of the three models gives a better understanding of water quality. The excessive concentration of some potentially toxic heavy metals in the water supplies suggests that the contaminated water supplies should be treated before use.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
As much as three-quarters (about 71%) of the earth surface is covered by water, only a smaller amount is marked as “freshwater” and a more significant percentage as “saline water”. Factually, about 68% of the freshwaters are trapped in ice caps and glaciers (Li & Qian, 2018). The only accessible ones are the underground and surface waters (lakes, streams and springs). However, the quality of these available freshwaters has been threatened seasonally by several factors. The increased rate of urbanization and industrialization has remained a present-day challenge in demand and search for a quality water supply because the quality of available water keeps deteriorating by the day (Li et al., 2017). In many parts of the world, surface and groundwaters are inarguably the significant water sources for drinking, domestic, agricultural and industrial purposes. However, in recent times, their quality and suitability for specific purposes have remained questionable due to contaminations from both natural and anthropogenic activities (Gorgij et al. 2017; Barzegar et al. 2019; Egbueri 2018, 2019a, b, c, 2020a, b; Egbueri and Unigwe 2019; Mgbenu and Egbueri 2019; Sale et al. 2019). With the massive population explosion, increased socioeconomic activities, and unplanned development in many parts of the world, the demand for freshwater supply has risen. However, there is currently a high scarcity of fresh water in many regions across the globe. The latest report by the World Health Organization stated that by the year 2025, half of the world’s population would be living in water-stressed areas (Singh et al., 2019).
Nitrates and heavy metals are common water pollutants that have drawn the attention of many water quality researchers across the globe (Adimalla & Li, 2019; Adimalla et al., 2019; Li et al., 2019; Ma et al., 2018). Although they can occur in the water environment through some natural processes, anthropogenic activities due to agriculture, industrialization, poor waste disposal/management, and urbanization have significantly influenced their accumulation in recent times (Barzegar et al., 2019; Egbueri et al., 2019; Egbueri, 2018, 2020a; Ezugwu et al., 2019; Ukah et al., 2020). Some heavy metals, in small quantities, are essentially important in the human body for growth and development (Chowdhury & Chandra, 1987). However, the pollution of water systems has posed a significant threat to human and environmental health. In such a scenario, water reserved for drinking is thereby made unfit for human consumption. Some heavy metals are carcinogenic (cancer-inducing) in nature; however, some tend to cause other severe, chronic illnesses (Egbueri, 2020a; Mgbenu & Egbueri, 2019). In any attempts to protect and sustain human health and the environment, it is important to encourage and implement continuous monitoring and assessment of water quality for drinking purposes. Monitoring and evaluating water quality enable us to understand better how geogenic and anthropogenic activities could adversely affect the water systems and equally give insights on how to avoid or control the effects.
Over time, several numerical models have been proposed and developed by different researchers based on local and international standard limits, such as those of the Nigerian Industrial Standard (NIS, 2007), Bureau of Indian Standards (BIS, 2012), and World Health Organization (WHO, 2017). Such numerical models have been developed and used for summarizing the quality of drinking water. Water Quality Index (WQI), Pollution Index of Groundwater (PIG), Synthetic Pollution Index (SPI), Overall Index of Pollution (OIP), Modified Water Quality Index (MWQI), Integrated Water Quality Index (IWQI) and Entropy-weighted Water Quality Index (EWQI) are some of the index used for drinking water quality assessments. Several parameters are usually analyzed for water quality monitoring and projects assessment. Formulating numerical models for water quality evaluations has become very important and useful for streamlining the wide-range parametric data into whole numbers. This ensures that water quality reports are easily understood by experts, policymakers, and the public. These models are developed to serve as tools for (1) simple interpretation of water quality; and (2) planning, management and mitigation of water pollution. Integration of two or more numerical models in water quality assessment has been found useful as it tends to minimize the subjectivity related to using a single model (Egbueri & Unigwe, 2019). In recent times, several water quality assessments have been conducted using either MWQI, IWQI or EWQI. The EWQI was first proposed by Li et al. (2010). Nevertheless, the MWQI was proposed by Shankar and Sreevidya (2019), whereas the IWQI was developed by Mukate et al. (2019). Previous research has proven that these models have greatly improved water quality monitoring and assessment projects. However, there is no known study that has simultaneously compared their performances in water quality research.
The present work was conducted in Umunya area, southeastern Nigeria. Previous studies (Egbunike, 2018; Mgbenu & Egbueri, 2019) on the water quality in this suburban district revealed that the drinking suitability of some of the available water resources utilized by the public (inhabitants) is very questionable. Therefore, with respect to nitrate and selected heavy metals (Fe, Zn, Mn, Pb, Cr, and Ni) concentrations, this study aims to assess the quality of the public water resources using a joint statistical and numerical modeling approach. The specific objectives are to (1) examine the suitability of the public water supplies for human consumption using MWQI, IWQI and EWQI; (2) identify essential pollutants, their parametric associations and possible sources using Hierarchical Cluster Analysis (HCA), and (3) highlight on the performance and sensitivity relationships between the three indexical models. To the best of the authors’ knowledge, this paper is the first to simultaneously use the MWQI, IWQI and EWQI in water quality assessment. It is also the first of its kind to compare the performances of these models. Although these models utilize different classification schemes and there may be some uncertainties associated with variances in their methods of calculation, the reasons behind the testing and analysis of their efficiencies (sensitivities) include: (1) There is a similarity in the presumptions and assumptions that founded their development. (2) The models are all used for drinking water quality assessment. (3) They are not region-specific. (4) The EWQI and IWQI do not require parameter weight assignment, which may introduce bias. (5) The same standard limits could be used in the calculation of all the models, as this would ensure fairness and uniform calculation conditions. (6) All analyzed water parameters could be considered in the quality evaluations, in order to further ensure that the same calculation conditions are maintained. And, (7) The limitations of these models are not well-known. Although the comparison of the sensitivities of these models in detecting water quality may not provide exact information, it can provide us with general insight regarding the agreements and trends between them. It is hoped that this study will provide insights to local and international water quality researchers regarding the water quality of the fast-growing suburb and the validation and sensitivity of the selected models’ performance in water quality assessment.
2 Background information of the study area
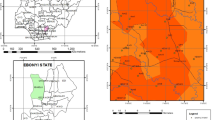
The area under study lies within latitudes 6° 10′ N to 6° 15′ N and longitudes 6° 54′ E to 7° 00′ E in Anambra State, southeastern Nigeria (Fig. 1). Umunya is situated at the heart of the two major cities (Awka and Onitsha). A polyvinyl chloride (PVC) industry, an abattoir servicing the surrounding communities, a National Youth Service Commission (NYSC) camp, and Tansian University are located in Umunya suburban district. Generally, these influence the population of this suburb. Moreover, despite increasing commercial and industrial activities in this area, small to large scale farming activities are a means of livelihood for many of its inhabitants. However, due to the unplanned population growth and increasing human activities, waste management programs in this area is still poorly executed. Wastes are indiscriminately disposed into roadway drainage channels, surface water bodies, and open dumpsites. Usually, such activities are known to release potentially toxic metals into water bodies. The study region experiences two distinct seasons, rainy and dry seasons. The rainy season lasts from April to October with an average annual rainfall of 1400–2500 mm, whereas the dry season starts from November and extends up to March.
From the geologic point of view (Fig. 1), the study area is underlain by the Eocene Nanka Formation of the Ameki Group (Mgbenu & Egbueri, 2019; Nwajide, 1979). The lithology is composed of very friable, flaser-bedded units of fine-medium-grained sands, with intervals of light gray mudrocks and ironstones (Nwajide, 2006, 2013). The main aquifer in the area is the Nanka Formation (comprising over 60 m sandstone interval), while the underlying older Imo Formation, mostly composed of mudrocks, acts as the aquitard (Mgbenu & Egbueri, 2019). The Nanka Formation, characterized by high porosity and permeability (Egbueri & Igwe, 2021), forms the aquifer in this area (with over 60 m sandstone interval). Nfor et al. (2007) reported that a major ridge system in the area acts as a water divider. This ridge system creates groundwater flow patterns running southwards and eastwards away from the divide (Mgbenu & Egbueri, 2019; Nfor et al., 2007). Groundwater depth in the Nanka Formation has been reported to be at varying depths, while Nfor et al. (2007) reported a depth of ≥ 20 m, Okoro et al. (2010) reported a depth of ≥ 7 m. However, the study by Egbueri and Igwe (2021) reported the water table in some parts of the Nanka Formation to have a depth of ≥ 9.6 m. It is believed that several streams and river systems in and around the study area serve as recharge systems. However, there is limited literature on groundwater properties such as flow direction, which should be of significant focus for future research.
3 Materials and methods
3.1 Sampling and analysis
Both surface and groundwater samples were collected from water supplies within the study area. In total, fifteen samples were collected from available water sources in prewashed and sterilized 1 L polyethene containers and labeled accordingly from WS01–WS15 (Fig. 1). Furthermore, samples were preserved in an ice-crested cooler before laboratory analysis. The pH readings of the fifteen water samples were measured in the field. In the laboratory, the nitrate (NO3–) was determined using the titrimetric method. In contrast, the metals such as iron (Fe), zinc (Zn), manganese (Mn), lead (Pb), chromium (Cr), and nickel (Ni) were analyzed using atomic absorption spectrophotometer (AAS). It is pertinent to state that all the performed analyses followed the American Public Health Association (APHA, 2005) recommendation. Figure 2 is a pictorial summary of the steps taken in this water quality assessment.
3.2 Modified water quality index (MWQI)
Proposed and developed by Shankar and Sreevidya (2019), the MWQI considers an overall quality of water with respective background values. The IWQI was developed as a modification of the commonly used Canadian WQI (CWQI). This model allows its users to assign certain weight factors to input parameters. This is done by either considering the parameter with a higher threat to human health or done by considering parameters with higher concentration, depending on the selected area of study. Also, the MWQI considers parameters excursing benchmarks, measurements excursing benchmarks and the amount of excursion from standards in failed measures. According to Shankar and Sreevidya (2019), the MWQI explains “the complete picture of water quality in a simple and extremely reliable manner and hence shows enormous promise for its application and large suitability across the globe.” The MWQI evaluation involves three basic steps:
3.2.1 Selection of water quality parameters and corresponding benchmarks
This study identified eight water quality parameters with their sub-indices, as shown in Table 1. The WHO (2017) and the NIS (2007) standard limits were selected as the corresponding benchmarks.
3.2.2 Appropriate weight factors for key input parameters
At this stage, significance were placed on the parameters with higher health threat to humans (Shankar & Sreevidya, 2019). Pb was given the highest weight factor of four (4) dues to its health threat and concentration in the study area. Next, Ni and Cr with a weight factor of three (3). Mn was apportioned two (2), then pH, Fe, Zn and NO3 were given one (1) (Table 1).
3.2.3 Calculation of the MWQI
This was calculated by considering three key factors, scope factor (FS), frequency factor (FF), and amplitude factor (FA) (Shankar & Sreevidya, 2019). The FS signifies the number of parameters that excurse benchmarks, FF signifies the number of measurements that excurse benchmarks, and the FA represents the amount of excursion from benchmarks in the failed measurements (Shankar & Sreevidya, 2019). The FS and FF were calculated using Eqs. (1 and 2), respectively.
where Wqi represents the weight factor of an input parameter; Wqj represents the weight factor of a violator parameter; Nqi represents the number of input parameter measurements; Nqj represents the number of violator parameter measurements; m is the number of input parameters and n is the number of violator parameters.
For FA determination, the excursion amount of violator parameters (Eqj) was first calculated (Eq. 3), and then the Normalized Sum of Excursions (NSE) (Eq. 4).
where Cqi represents the violator parameter's concentration of the violator parameter; SVqi represents standard value; and Eqj, excursion amount of violator parameter.
The FA, and the MWQI are calculated using equations Eqs. (5 and 6), respectively.
3.3 Integrated water quality index (IWQI)
The IWQI was developed by Mukate et al. (2019) and have been found helpful for water quality assessment (Egbueri, 2019b). With the IWQI, water quality parameters below their desirable limits and above permissible limits are described as deficient and excessive, respectively. Both scenarios are considered to have associated health problems. Just as the names imply, “deficient” indicates that something is lacking, and “excessive” suggests that something is beyond its standard limit. By considering both the deficiencies and excessiveness of parameters, a better understanding of water quality could be established. The primary focus of the IWQI is assessing the mineral or ion balance of water resources to avoid health implications due to excesses or deficiencies (Mukate et al., 2019). Targeted at improving the conventional WQI model, the IWQI was designed to exclude the problems of ambiguity, eclipsing, and aggregation observed when using the WQI model. According to Mukate et al. (2019), “the IWQI is flexible, unbiased, easy to calculate and time-saving, and provides useful information to prioritize and maintain the water quality of potable sources and reduce human health impacts from using poor-quality water resources.” Summarily put, the IWQI is described as a comprehensive and intensive water quality index that considers both the desirable and permissible parameters. The IWQI has a different step mechanism from MWQI which has been shown as follows:
3.3.1 Calculation of range
The NIS (2007) standard limits were utilized in the range calculation as shown in Table 2. The range was calculated using Eq. (7).
3.3.2 Calculation of modified permissible limits (MPL)
The NIS (2007) was also considered in determining the MPL (Eq. 8) and is presented in Table 2.
In IWQI calculation, it is believed that the concentration of any parameter below the Desirable limit (DL) or above Permissible Limit (PL) is not suitable for drinking. Also, the concentrations that are less than the minimum require more concentration and above MPL, which will affect the water quality.
3.3.3 Determination of the subindex (SI)
Three different parameters are considered while calculating for the subindex. They are as follows.
If the ith parameter (Pi) ’s observed value is > DL but < MPL, then SI1 will be expressed as zero.
Equation (10) is considered when the value of ith parameter is less than or equal to the DL; i.e., Pi ≤ DL.
Equation (11) is considered when Pi is greater than or equal to the MPL; i.e., Pi ≥ MPL.
The final IWQI is then evaluated using Eq. (12);
where SIij is the sub-index value of ith sample and jth water quality parameter (Egbueri, 2021; Mukate et al., 2019).
3.4 Entropy-weighted water quality index (EWQI)
Among the three models utilized in this work, the EWQI was first developed by Li et al. (2010). This model was developed to improve the conventional WQI model, which requires an assignment of specific weights to water quality parameters regarding their relative importance to human health. In EWQI, information entropy (ej) is used instead to get the uncertainty of the stochastic event, rather than assigning relative weights to parameters, as done with the conventional WQI model. The EWQI uses a discrete entropy equation to determine the weights of parameters. Also, EWQI helps identify the parameter that has the most significant impact on water quality without bias. This is obtained by determining the parameter with the lowest information entropy (ej) and the highest entropy weight (wj). Over time, the EWQI has been used by several researchers from different parts of the world and has been described as a model that provides unbiased, justifiable, accurate and reliable water quality analysis (Adimalla et al., 2020; Amiri et al., 2014; Feng et al., 2019; Li et al., 2010; Singh et al., 2019; Ukah et al., 2020; Wu et al., 2011). It also provides an overall understanding of different sets of water samples (Adimalla et al., 2020; Ukah et al., 2020). In this study, EWQI was employed to help assess the water quality and confirm other water quality tools used in the study area. The EWQI computation steps are as follows (Li et al., 2018; Ukah et al., 2020; Wang et al., 2020):
The evaluation matrix “X” is computed as shown in Eq. (13)
where “m” signifies numbers of samples; “n” represents the number of analyzed parameters.
The standardization process for “yij” and “Y” is computed using Eqs. (14 and 15), respectively.
where xij represents the initial matrix; (xij)min and (xij)max signify the minimum and maximum values of the analyzed parameters of the samples (Adimalla et al., 2020).
The first step in EWQI, is to determine the information entropy (ej) for each chemical parameter as shown in Eq. (16).
where Pij denotes the probability of occurrence of the normalized value of the parameter j expressed in Eq. (17).
Next is the entropy weight (wj) calculation for each parameter as expressed in Eq. (18).
Third step involves the calculation of the quality rating scale (qj) for each parameter.
where Cj is the concentration of parameters (mg/L); Sj is the standard permissible limit (mg/L) of the NIS (2007).
The final stage is the calculation of the EWQI as shown in Eq. (20).
3.5 Statistical analysis
In this study, Stepwise Regression Analysis (SRA) and Hierarchical Cluster Analysis (HCA) were performed using SPSS (v. 22). These analyses show and understand the spatio-temporal distribution of water quality and the relationship between the models. The SRA and the HCA were both utilized for the analysis of the sensitivities and agreement between the EWQI and IWQI models. The possible sources of water contaminants and drinking water quality demarcations (classification) of the samples were analyzed using HCA (Egbueri, 2020b; Wu et al., 2020). For the water quality demarcation, the HCA was performed using the IWQI and EWQI scores. Moreover, the Ward’s linkage method (with squared Euclidean distance) was used for the HCA. The data were normalized using z-score standardization to remove any bias due to variances in the obtained scores.
4 Results and discussion
4.1 General characteristics of the water resources
Table 3 presents the analyzed results for the physicochemical parameters, while the univariate statistical summary of all parameters is also presented in Table 3. Results were compared to the WHO (2017) and NIS (2007) drinking water standards. In this study, the water samples are acidic, with pH values ranging from 4.61 to 6.53 and an average of 5.561 (Table 3). The chemical parameters follow the trend of NO3 > Pb > Zn > Fe > Ni > Mn > Cr. Nitrate (NO3) can contribute to lowering pH in water supplies through oxidation and hydration processes (Collin et al., 2018). NO3 concentration in the study swerves from 0 to 21.1 mg/L with a mean of 6.417 mg/L (Table 3). NO3 were below the permissible limit in all water samples. However, 20% recorded no NO3 enrichment (Table 3). The source of NO3 in the study area is attributable to the use of fertilizers, domestic waste, agricultural waste, leachates from landfills and sewage leakages (Egbueri et al., 2019; Ezugwu et al., 2019; Mgbenu & Egbueri, 2019). About 26.6% of the water samples did not contain iron (Fe). However, 20% of the water samples exceeded the permissible limit (Table 3). Fe concentration in the water samples swerves from 0 to 0.54 mg/L, the mean of 0.127 mg/L (Table 3). Although Fe is an essential element in the human body, “red hot disease” is associated with drinking Fe-laden water. The possible source of Fe in the water supplies may include domestic sewages and coating of pipes (Wagh et al., 2018). Manganese (Mn) in this study is not widely distributed. About 60% of the water samples have no Mn enrichment, while 40% have Mn scores below the permissible limit (Table 3). Excess Mn's associated health effects include retarded mental development and damage of the central and peripheral nervous systems (SON, 2015; Ukah et al., 2019). Zinc (Zn) is essential in human, animal and plant growth (Hotz and Brown, 2004). Zn was present in all water samples with concentrations ranging from 0.001 to 0.54 mg/L and a mean of 0.127 mg/L (Table 3).
A report had shown that Lead (Pb) is a very toxic heavy metal that enters both the tissues of plants and the skeletal framework of man through ingestion by water, inhalation through soil and consumption of crops (WHO, 2017). Its accumulation gives rise to different health-related problems like mental disorders, cancer development and failure of the hematologic and renal system (Adimalla & Wang, 2018; Egbueri, 2020a). In this study, about 33% of the samples had no Pb enrichment, 46.6% had scores above the acceptable limit for drinking water, and however, 13.3% had scores below the permissible limit (Table 3). Overall, Pb concentration ranges from 0 to 3.087 mg/L with an average of 0.825 mg/L (Table 3). The sources of Pb in the water supplies are attributed to the use of chemical anti-killing agents (insecticides and pesticides), onsite sewages, faucets, leaded plumbing fittings, urban wastes and dumpsites (Egbueri, 2018, 2020a, 2020b; Wagh et al., 2018). Chromium (Cr) had a very low concentration which swerves from 0 and 0.01 mg/L with an average of 0.002 mg/L (Table 3). In this study, about 46.6% of the water samples had no Cr enrichment; 53% were enriched but below the permissible drinking water limit. Nickel (Ni) is associated with different health-related problems, including dermatitis, lung fibrous, cardiovascular and kidney disease (Egbueri, 2020a; SON, 2015; WHO, 2017). About 40% of the samples had no Ni enrichment, while 13.3% had low Ni enrichment. On the contrary, 33.3% were above the permissible limit for drinking (Table 3). Ni concentration in this study ranges from 0 to 0.34 mg/L with a mean of 0.084 mg/L (Table 3). Ni contamination in this study is attributed to poor waste disposal, sewage sludge and application of chemical fertilizers (Adimalla & Wang, 2018).
4.2 Modified water quality index (MWQI)
The MWQI classification scheme is given in Table 4. In this study, the violator parameters affecting the quality of the water supplies include Pb, Cr, and Ni. The MWQI was calculated for the 15 water samples and eight physicochemical parameters. The results for MWQI are presented in Table 5. The results showed that the number of parameters that excurse benchmarks (FS) is 0.781. However, the FF been the number of measurements that excurse benchmarks is 0.11, while 9.79 is the amount of excursion from benchmarks in the failed measurements (FA). The final MWQI was observed as 44.95. Based on the classification criteria for MWQI, the water supplies fall within the “Marginal” rank, which signifies that the water quality is frequently threatened at a desirable level. The study area is a fast-developing area with increasing developmental, agricultural and improper waste management (open waste dumping) activities. It is believed that the high threat to these water supplies is due to the waste generation and management method practised in the study area, as reported by Egbunike (2018), Mgbenu and Egbueri (2019), Egbueri and Unigwe (2020).
4.3 Integrated water quality index (IWQI)
The classification scheme for IWQI subdivides water quality into five distinct groups: IWQI < 1 (Excellent water for drinking); IWQI 1–2 (Good water for drinking); IWQI 2–3 (Acceptable water for domestic); IWQI 3–4 (Poor water for drinking); IWQI > 5 (Unacceptable water) (Egbueri, 2021; Mukate et al., 2019). After due computation, formulation and evaluation of the IWQI, the summary results were presented in Table 6. The observed values ranged from − 9.11 to 401.8 with a mean of 106.8, which shows that most of the water samples are polluted. Following the classification criteria by Mukate et al., 2019, any IWQI value above 3 is classified as poor, whereas any above 5 is unfit for human consumption. Based on the information provided in Table 6, about 20% of the water samples are excellent for drinking, 6.67% is of good quality, 13.33% are acceptable for domestic purposes, and 60% are unsuitable for human consumption. However, water samples 2, 5, 6, 9, 11 and 14, which are believed to have the strongest anthropogenic influence, show very high pollution levels, far more than other water samples. Similar research findings have been reported (Egbunike, 2018; Mgbenu & Egbueri, 2019), whereby some water samples were found to have higher heavy metal pollution than others.
4.4 Entropy-weighted water quality index (EWQI)
The EWQI classification scheme for water quality is as follows: “Rank 1” < 50 (excellent water quality); “Rank 2” 50-100 (good water quality); “Rank 3” 100–150 (average water quality); “Rank 4” 150–200 (poor water quality); “Rank 5” > 200 (Extremely poor water quality) (Adimalla et al., 2020; Amiri et al., 2014; Feng et al., 2019; Li et al., 2010; Singh et al., 2019; Ukah et al., 2020; Wu et al., 2011). The information entropy (ej) and entropy weight (wj) results of all analyzed water quality parameters are presented in Table 7. However, Table 8 contains the summarized EWQI results. In this study, Pb was observed to have the highest effect on the water quality. This assertion was made following Gorgij et al. (2017) report that the parameter with the highest wj and lowest ej will have the highest effect on water quality (Table 7). The summary results for the EWQI shown in Table 8, swerves from 84.02 to 21,430 with an average of 5866. Furthermore, any EWQI value that exceeds Rank 3 (i.e., above 150) is believed to be unsuitable for drinking purpose.
Based on the information presented in Table 8, it was realized that about 26.6% of the total water samples are of good quality, 13.3% are of average quality, and however, 60% of the water samples are extremely poor in quality. Meanwhile, 13.3% of the water categorized as average water quality could only be used for domestic purposes. However, 60% of the water, categorized as extremely poor water quality, are unfit for human consumption. Furthermore, water samples 2, 5, 6, 9, 11 and 14 show extreme high values of EWQI ranging from 7527 to 21,430 and an average of 14,116. It is further confirmed that the high pollution in these samples is due to the high impact from anthropogenic activities, which is often common in fast-developing suburbs. Thus, it is suggested that any pollution remediation measures proposed for the study area should consider these six extremely polluted water stations first before the others marked to have better water quality.
Figure 2 presents the quality rating (qj) for the six water samples collected within the zone of high waste accumulation. From this figure, Pb (in green) has the highest values in the six water samples, followed by Mn (in yellow). NO3 (in orange) shows a high presence in WS6 and WS14. Ni (in purple) was relatively low, except for WS2 and WS11. Fe (in black) showed a very high presence in WS11. However, Mn (in red) and Cr (in gray) were relatively low in all water samples. The pH in all waters were relatively low, indicating their acidic nature. Figure 2 was created in order to establish a better understanding of the significant elements contributing to the quality deterioration of the water supplies within the points of interest and also to prove that the parameter with the highest wj and lowest ej has the highest impact on the water supplies (Gorgij et al., 2017). Summarily, with the quality rating (qj) of the six water samples shown in Fig. 2, Pb was observed as the major heavy metal deteriorating the water supplies. This may be attributed to the nature of the anthropogenic activities taking place in the study area. However, inhbaitants who make use of these water supplies (WS2, WS5, WS6, WS9, WS11 and WS14) may be exposed to cancerous and chronic illnesses due to the high concentration of Pb.
4.5 Water quality classification/demarcation using HCA
As can be observed from Tables 7 and 9, the differences in classification schemes of the IWQI and EWQI introduced some kind of bias in deciding which sample is suitable or not. The samples identified as excellent by the IWQI were placed in the ‘good’ category by the EWQI. Hence, there was need to utilize HCA to show the quality groupings of the analyzed water samples. Some water quality studies (Egbueri & Unigwe, 2019; Egbueri et al., 2019; Egbueri, 2018, 2019a, 2020a, 2020b) have shown that HCA is a suitable tool for classifying water samples into various classes based on their quality and chemical genetics.
The IWQI and EWQI scores were integrated to produce a dendrogram (Fig. 3) for the water quality classes. Cluster 1 comprises of those samples generally classified as lowly-marginally contaminated. However, Cluster 2 is dominantly comprised of those samples that are highly polluted with heavy metals. Compared with those of Cluster 1, it was observed that the samples in Cluster 2 have extremely high IWQI and EWQI scores. By this demarcation, it can be generally stated that the samples in Cluster 1 (making about 60% of the total) have low to high pollution, while those in Cluster 2 (making about 40%) have very high to extreme heavy metals pollutions. Following the quality classes given by the IWQI and EWQI, the result of the MWQI (Table 5), which highlighted that the overall water quality of the study area is frequently threatened, is confirmed.
In this study, it was also noticed that the surface waters, which because of their clean appearances, are often judged by the local consumers as suitable drinking water supplies, have very questionable drinking quality. The spring samples were identified by both IWQI and EWQI as extremely polluted and unsuitable drinking waters. On the other hand, the only stream water supply was marked by both indices to be of marginal or average drinking quality. The pollution of these surface waters could be attributed to anthropogenic inputs related to indiscriminate waste disposal. However, some boreholes were highly polluted. This could be indicating that they are shallow aquifers prone to easy surface contaminations. Additionally, because the Nanka Formation is mainly composed of loose, porous and permeable materials (Egbueri and Igwe 2020), the contamination of its shallow groundwater would be much easier.
4.6 Water contaminant source allotment using HCA
The discussion so far has indicated that the quality of some water supplies is questionable due to pollution. Therefore, it was thought to analyze the possible source of pollution further using the HCA. The application of HCA for water pollutant source apportionment is well demonstrated in the current study. A dendrogram (Fig. 4) was produced using the Ward’s linkage method and z-score standardization. From Fig. 4, it can be seen that the analyzed parameters formed two major cluster groups, both of which have their separate sub-clusters.
The first cluster comprises parameters (Zn, Pb, Ni, pH, and Cr) that are more typical of origins due to anthropogenic influences. It was observed that these parameters in this cluster class greatly influenced the water quality of the study area more than other parameters. Cluster 1 has two sub-clusters. In the first sub-cluster Zn, Pb, and Ni were grouped. This points that similar processes, such as leaching from batteries, tires and heavy chemical wastes in dumpsites, could be influencing their enrichment in the water supplies (Egbueri, 2018, 2020a; Mgbenu & Egbueri, 2019). Because the waters are generally acidic, they have the potential to leach Pb from water distribution pipes (Egbueri, 2021). The association between pH and Cr could suggest that the acidity of the water may be influencing the enrichment of Cr. However, the variation in their Euclidean distances indicates that this association is not so strong. Other factors that may add Cr to water include metallurgical and heavy chemical wastes (Egbueri, 2018, 2020a; Mgbenu & Egbueri, 2019).
However, Cluster 2 is comprised of such parameters (Fe, Mn, and NO3) that are typical of both natural and anthropogenic origins. Mgbenu and Egbueri (2019) had attributed the presence of nitrate in the waters from the Umunya district to anthropogenic activities. For this area, such anthropogenic activities that could influence nitrate enrichment in the water include poor disposal mechanics for organic wastes such as vegetables and sewage (Egbueri et al., 2019). However, Barzegar et al. (2019) reported that NO3 in water could also be due to some geogenic processes like denitrification, redox reactions, and anoxic conditions of the aquifer. The use of metallic pipes for water distribution and metallic wastes may be responsible for the Fe contents of the waters. Nevertheless, geogenic processes like weathering ironstones and ferromagnesian minerals could also be responsible for the Fe in the waters. Mn is commonly associated with NO3. However, the higher concentrations of NO3 more than Mn (and Fe) could explain their varied Euclidean distances in Fig. 4. Moreover, their separation into different sub-clusters could be suggesting a higher impact of anthropogenic activities in the addition of NO3 more than the other duo. For the study area, the presence of Mn could be due to human inputs such as sewage, fertilizers, and fossil fuel combustion (Egbueri, 2018, 2020a). Natural factors like redox reactions and anoxic and flow conditions may influence the concentration of Mn in water (Liao et al., 2018).
4.7 Establishing the relationships between the indexical models
The observations made so far have shown that there is some kind of similarity between the results of these models in this study. The MWQI did not give the quality description for individual samples; however, it did present the general overview of the quality of the public water supplies. The result it showed was in line with those given by the IWQI and EWQI. However, much of our comparative analysis of the models’ sensitivities will focus on IWQI and EWQI.
An SRA was performed on the IWQI and EWQI scores. It was observed that a strong relationship exists between the two models (Fig. 5), indicating that they followed a similar trend in identifying the water quality of the study area. In other words, the values of IWQI and EWQI increased accordingly in the same direction across the water samples. Additionally, Pearson’s correlation analysis was performed between the parameters’ concentrations and the IWQI and EWQI scores. Usually, correlation coefficients < 0.5, 0.5 < r < 0.7, and > 0.7 are, respectively, marked as weak, moderate, and strong. The correlation results obtained (Table 9) showed that both models sensitively identified Pb, Zn, and Ni as the most important pollutants that influenced the IWQI and EWQI scores. But, the identification of Zn as one of the important pollutants does not make it a significant pollutant in the study area, as all the Zn concentrations in all the samples were well below the maximum allowable limit of 3 mg/L.
5 Conclusion
The three indexical models (MWQI, IWQI and EWQI) utilized in this current study have proven to be sensitive, efficient and effective in water quality assessment. This study has indicated that the integration of the three models gives a better understanding of the water quality in the area. Moreover, the HCA proved very efficient in the allotment of the possible sources of pollution in the study area. Overall, the results of this study confirmed that the water supplies are threatened by heavy metals pollution. Based on the indexical approach employed to investigate the quality of the water supplies, it has been proven that the water supplies are relatively lowly to very highly polluted. In this study area, about 40% of the analyzed water samples are suitable for both drinking and domestic purposes, whereas the majority (60%) are unfit for human consumption. Investigation into the performance and sensitivity of the MWQI, IWQI and EWQI models in water quality assessment was analyzed and the results showed that they are all sensitive, efficient and effective tools. This study has indicated that the integration of the three models gives a better understanding of water quality. Therefore, adequate measures should be taken to prevent further deterioration of the water quality. Moreover, the contaminated water should be adequately treated, either by boiling activated carbon filtration, or advanced technology, before human consumption and used for domestic purposes such as cooking.
References
Adimalla, N., & Li, P. (2019). Occurrence, health risks, and geochemical mechanisms of fluoride and nitrate in ground-water of the rock dominant semi-arid region, Telangana State, India. Human Ecological Risk Assessment, 25(1–2), 81–103. https://doi.org/10.1080/10807039.2018.1480353
Adimalla, N., Li, P., & Qian, H. (2019). Evaluation of groundwater contamination for fluoride and nitrate in semi-arid region of Nirmal Province, South India: A special emphasis on human health risk assessment (HHRA). Human and Ecological Risk Assessment, 25(5), 1107–1124. https://doi.org/10.1080/10807039.2018.1460579
Adimalla, N., Qian, H., & Li, P. (2020). Entropy water quality index and probabilistic health risk assessment from geochemistry of groundwaters in hard rock terrain of Nanganur County, South India. Geochemistry, 80(4), 125544. https://doi.org/10.1016/j.chemer.2019.125544
Adimalla, N., & Wang, H. (2018). Distribution, contamination, and health risk assessment of heavy metals in surface soils from northern Telangana, India. Arabian Journal of Geosciences, 11, 684. https://doi.org/10.1007/s12517-018-4028-y
Amiri, V., Rezaei, M., & Sohrabi, N. (2014). Groundwater quality assessment using entropy weighted water quality index (EWQI) in Lenjanat, Iran. Environmental Earth Sciences. https://doi.org/10.1007/s12665-014-3255-0
APHA. (2005). Standard methods for the examination of water and wastewater (21st ed.). Washington DC: American Public Health Association.
Barzegar, R., Moghaddam, A. A., Soltani, S., Baomid, N., Tziritis, E., Adamowski, J., & Inam, A. (2019). Natural and anthropogenic origins of selected trace elements in the surface waters of Tabriz area, Iran. Environmental Earth Sciences, 78, 254. https://doi.org/10.1007/s12665-019-8250-z
BIS. (2012). Bureau of Indian Standards (BIS 10500). Guidelines for drinking water quality standards.
Chowdhury, B. A., & Chandra, R. K. (1987). Biological and health implications of toxic heavy metal and essential trace element interactions. Progress in Food Nutrition Science, 11(1), 55–113.
Collin, J. W., Jennifer, N. P., John, M., Christopher, M. C., Scott, G., & Lynch, J. A. (2018). Estimating base cation weathering rates in USA: Challenges of uncertain soil mineralogy and specific surface area with the application of PROFILE model. Water, Air, and Soil Pollution, 222(3), 61–90.
Egbueri, J. C. (2018). Assessment of the quality of groundwaters proximal to dumpsites in Awka and Nnewi metropolises: A comparative approach. International Journal of Energy and Water Resources, 2(1–4), 33–48. https://doi.org/10.1007/s42108-018-0004-1
Egbueri, J. C. (2019a). Water quality appraisal of selected farm provinces using integrated hydrogeochemical, multi-variate statistical, and microbiological technique. Modeling Earth Systems and Environment, 5(3), 997–1013. https://doi.org/10.1007/s40808-019-00585-z
Egbueri, J. C. (2019b). Evaluation and characterization of the groundwater quality and hydrogeochemistry of Ogbaru farming district in southeastern Nigeria. SN Applied Sciences, 1(8), 851. https://doi.org/10.1007/s42452-019-0853-1
Egbueri, J. C. (2020a). Heavy metals pollution source identification and probabilistic health risk assessment of shallow groundwater in Onitsha, Nigeria. Analytical Letters, 53(10), 1620–1638. https://doi.org/10.1080/00032719.2020.1712606
Egbueri, J. C. (2020b). Groundwater quality assessment using pollution index of groundwater (PIG), ecological risk index (ERI) and hierarchical cluster analysis (HCA): A case study. Groundwater for Sustainable Development, 10, 100292. https://doi.org/10.1016/j.gsd.2019.100292
Egbueri, J. C. (2021). Signatures of contamination, corrosivity and scaling in natural waters from a fast-developing suburb (Nigeria): Insights into their suitability for industrial purposes. Environment, Development and Sustainability, 23(1), 591–609. https://doi.org/10.1007/s10668-020-00597-1
Egbueri, J. C., & Igwe, O. (2021). The impact of hydrogeomorphological characteristics on gullying processes in erosion-prone geological units in parts of southeast Nigeria. Geology, Ecology, and Landscapes, 5(3), 227–240. https://doi.org/10.1080/24749508.2020.1711637
Egbueri, J. C., Mgbenu, C. N., & Chukwu, C. N. (2019). Investigating the hydrogeochemical processes and quality of water resources in Ojoto and environs using integrated classical methods. Modeling Earth Systems and Environment, 5(4), 1443–1461. https://doi.org/10.1007/s40808-019-00613-y
Egbueri, J. C., & Unigwe, C. O. (2019). An integrated indexical investigation of selected heavy metals in drinking water resources from a coastal plain aquifer in Nigeria. SN Applied Sciences, 1(11), 1422. https://doi.org/10.1007/s42452-019-1489-x
Egbueri, J. C., & Unigwe, C. O. (2020). Understanding the extent of heavy metal pollution in drinking water supplies from Umunya, Nigeria: An indexical and statistical assessment. Analytical Letters, 53(13), 2122–2144. https://doi.org/10.1080/00032719.2020.1731521
Egbunike, M. E. (2018). Hydrogeochemical investigation of groundwater resources in Umunya and environs of the Anambra Basin, Nigeria. Pacific Journal of Science and Technology, 19(1), 351–366.
Ezugwu, C. K., Onwuka, O. S., Egbueri, J. C., Unigwe, C. O., & Ayejoto, D. A. (2019). Multi-criteria approach to water quality and health risk assessments in a rural agricultural province, southeast Nigeria. HydroResearch, 2, 40–48. https://doi.org/10.1016/j.hydres.2019.11.005
Feng, Y., Fanghui, Y., & Li, C. (2019). Improved Entropy Weighting Model in Water Quality Evaluation. Water Resources Management, 33(6), 2049–2056.
Gorgij, A. D., Kisi, O., Moghaddam, A. A., & Taghipour, A. (2017). Groundwater quality ranking for drinking purposes, using the entropy method and the spatial autocorrelation index. Environmental Earth Sciences, 76(7), 269. https://doi.org/10.1007/s12665-017-6589-6
Hortz, C., & Brown, K. H. (2004). International zinc nutrition consultative group (IZiNCG) technical document No. 1. Assessment of the risk of zinc deficiency in populations and options for its control. Food and Nutrition Bulletin., 25, S94–S203.
Li, P., He, X., & Guo, W. (2019). Spatial groundwater quality and potential health risks due to nitrate ingestion through drinking water: A case study in Yan’an City on the Loess Plateau of northwest China. Human and Ecological Risk Assessment, 25(1–2), 11–31. https://doi.org/10.1080/10807039.2018.1553612
Li, P., & Qian, H. (2018). Water resources research to support a sustainable China. International Journal of Water Resources Development, 34(3), 327–336. https://doi.org/10.1080/07900627.2018.1452723
Li, P., Qian, H., & Wu, J. (2010). Groundwater quality assessment based on improved water quality index in Pengyang County, Ningxia, northwest China. E-Journal of Chemistry, 7, 209–216.
Li, P., Tian, R., Xue, C., & Wu, J. (2017). Progress, opportunities and key fields for groundwater quality research under the impacts of human activities in China with a special focus on western China. Environmental Science and Pollution Research, 24(15), 13224–13234. https://doi.org/10.1007/s11356-017-8753-7
Li, P., Wu, J., Tian, R., He, S., He, X., Xue, C., & Zhang, K. (2018). Geochemistry, hydraulic connectivity and quality appraisal of multilayered groundwater in the Hongdunzi Coal Mine, northwest China. Mine Water and the Environment, 37(2), 222–237. https://doi.org/10.1007/s10230-017-0507-8
Liao, F., Wang, G., Shi, Z., Huang, X., Xu, F., Xu, Q., & Guo, L. (2018). Distributions, sources, and species of heavy metals/trace elements in shallow groundwater around the Poyang Lake, East China. Exposure and Health, 10(4), 211–227. https://doi.org/10.1007/s12403-017-0256-8
Ma, L., Hu, L., Feng, X., & Wang, S. (2018). Nitrate and nitrite in health and disease. US National Library of Medicine National Institute of Health., 9(5), 938–945.
Mgbenu, C. N., & Egbueri, J. C. (2019). The hydrogeochemical signatures, quality indices and health risk assessment of water resources in Umunya district, southeast Nigeria. Applied Water Science, 9(1), 22. https://doi.org/10.1007/s13201-019-0900-5
Mukate, S., Wagh, V., Panaskar, D., Jacobs, J. A., & Sawant, A. (2019). Development of new integrated water quality index (IWQI) model to evaluate the drinking suitability of water. Ecological Indictors, 101, 348–354. https://doi.org/10.1016/j.ecolind.2019.01.034
Nfor, B. N., Olobaniyi, S. B., & Ogala, J. E. (2007). Extent and distribution of groundwater resources in parts of Anambra State, Southeastern Nigeria. Journal of Applied Science and Environmental Management, 11(2), 215–221.
NIS. (2007). Nigerian standard for drinking water quality. Nigerian Industrial Standard, 554, 13–14.
Nwajide, C. S. (1979). A lithostratigraphic analysis of the Nanka Sand, Southeast Nigeria. Niger J Min Geol, 16, 103–109.
Nwajide, C. S. (2006). Outcrop analogues as a learning facility for sub-surface practitioner: The value of geological field trips. Pet Train J, 3, 58–68.
Nwajide, C. S. (2013). Geology of Nigeria’s sedimentary basins. CSS Press.
Okoro, E. I., Egboka, B. C. E., & Onwuemesi, A. G. (2010). Evaluation of the aquifer characteristics of the Nanka sand using hydrogeological method in combination with vertical electric sounding (VES). Journal of Applied Science and Environmental Management, 14(2), 5–9.
Sale, J., Yahaya, A., Ejim, C., & Okpe, I. (2019). Physicochemical assessment of water quality in selected borehole450 in Anyigba Town, Kogi State, Nigeria. Journal of Applied Sciences and Environmental Management, 23(4), 711–714.
Shankar, B. S., & Sreevidya, R. (2019). A novel approach for the formulation of modified water quality index and its application for groundwater quality appraisal and grading. Human and Ecological Risk Assessment. https://doi.org/10.1080/10807039.2019.1688638
Singh, K. R., Dutta, R., Kalamdhad, A. S., & Kumar, B. (2019). Review of existing heavy metal contamination indices and development of an entropy-based improved indexing approach. Environment, Development and Sustainability. https://doi.org/10.1007/s10668-019-00549-4
Standards Organization of Nigeria (SON). (2015). Nigerian standard for drinking water quality (pp. 15–16). SON Publication.
Ukah, B. U., Ameh, P. D., Egbueri, J. C., Unigwe, C. O., & Ubido, O. E. (2020). Impact of effluent-derived heavy metals on the groundwater quality in Ajao industrial area, Nigeria: An assessment using entropy water quality index (EWQI). International Journal of Energy and Water Resources, 4(3), 231–244. https://doi.org/10.1007/s42108-020-00058-5
Ukah, B. U., Egbueri, J. C., Unigwe, C. O., & Ubido, O. E. (2019). Extent of heavy metals pollution and health risk assessment of groundwater in a densely populated industrial area, Lagos, Nigeria. International Journal of Energy and Water Resources, 3(4), 291–303. https://doi.org/10.1007/s42108-019-00039-3
Wagh, V. M., Panaskar, D. B., Mukate, S. V., Gaikwad, S. K., Muley, A. A., & Varade, A. M. (2018). Health risk assessment of heavy metal contamination in groundwater of Kadava River Basin, Nashik, India. Modeling Earth Systems and Environment. https://doi.org/10.1007/s40808-018-0496-z
Wang, D., Wu, J., Wang, Y., & Ji, Y. (2020). Finding high-quality groundwater resources to reduce the hydatidosis incidence in the Shiqu county of Sichuan Province, China: Analysis, assessment, and management. Exposure and Health, 12(2), 307–322. https://doi.org/10.1007/s12403-019-00314-y
WHO. (2017). Guidelines for drinking water quality (3rd ed.). World Health Organization.
Wu, J., Li, P., & Qian, H. (2011). Groundwater quality in Jingyuan county, a semi-humid area in Northwest China. E-Journal of Chemistry, 8(2), 787–793.
Wu, J., Li, P., Wang, D., Ren, X., & Wei, M. (2020). Statistical and multivariate statistical techniques to trace the sources and affecting factors of groundwater pollution in a rapidly growing city on the Chinese Loess Plateau. Human and Ecological Risk Assessment, 26(6), 1603–1621. https://doi.org/10.1080/10807039.2019.1594156
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors hereby declare that there is no conflict of interest regarding this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Unigwe, C.O., Egbueri, J.C. Drinking water quality assessment based on statistical analysis and three water quality indices (MWQI, IWQI and EWQI): a case study. Environ Dev Sustain 25, 686–707 (2023). https://doi.org/10.1007/s10668-021-02076-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10668-021-02076-7