Abstract
The present investigation discusses the capacity of Cephalosporium species (sp.) and Mucor species (sp.) to degrade polystyrene. Fourier transform infrared (FTIR) spectroscopy, scanning electron microscopy (SEM), and thermogravimetric analysis (TGA) were carried out to analyze the degradation of polystyrene with fungal cultures. Shifting in the position of peaks after fungal treatment was observed by FTIR analysis. Changes in the surface texture from smooth to the rough surface were visualized by SEM analysis. The decrease in the thermal stability was showed by TGA when 95% weight reduction is chosen as a point of comparison. Further results were interpreted in terms of various parameters like pH, total dissolved solid (TDS), and conductivity. The pH of mineral salt media decreases while the value of TDS and conductivity increases after incubation with fungal cultures. Eight-week incubation of polystyrene with Cephalosporium sp. showed a weight loss of 2.17 ± 0.16% and 1.81 ± 0.13% with Mucor sp. Gas chromatography–mass spectrometer (GC–MS) and gel permeation chromatography analysis were carried out to determine the by-products and molecular weight of samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Polystyrene is an aromatic polymer synthesized from styrene monomer through the polymerization reaction. Low cost, lightweight, easy production, toughness, rigidity, and transparency are the major properties associated with huge production and diverse applications of polystyrene (Sheikh et al. 2013; Sowmya et al. 2015). Polystyrene is widely used as disposal cups, packaging, and construction applications (Krueger et al. 2017). Generation of municipal solid waste is the major problem associated with such packaging material (Aarnio and Hamalainen 2008). Lack of proper solid waste management promotes the generation of approximately 9.46 million tons per annum of plastic wastes in India, which constitutes to 25940 tons per day out of which polystyrene comprises around 4.77% (Central Pollution Control Board, India 2017).
Polystyrene is neither recyclable nor biodegradable (Tolinski 2012). Also, the cost of recycling is higher than the manufacturing cost which restricts the recycling of plastics (Ali and Ghaffar 2017). Therefore, a large amount of such plastics are utilized for one time and then discarded without any recycling. Lack of functional groups, hydrophobic nature, high molecular weight, and macromolecular nature of plastics are the main factors that inhibit degradation of polystyrene (Krueger et al. 2017; Schlemmer et al. 2009). As a result, plastics accumulated in the environment over a long duration causes environmental pollution, health problems, and destruction of our ecosystem (Ho et al. 2017). Two different approaches like landfilling and incineration are extensively used to remove the plastic wastes. Landfilling reduces the land fertility and its availability, as plastics remain inert for a longer period when buried under the soil. Plastic wastes are completely removed by incineration process, but the process generates a huge amount of toxic gases that lead to an increase in environmental pollution. Thus, there is a need of a process to degrade plastics in an eco-friendly way. Biodegradation is a natural decaying process in which microorganisms colonize with polymer surface, and then, the enzymes secreted from microorganisms convert the complex polymers into smaller molecules (oligomers, dimers, and monomers) and finally mineralized to CO2 and H2O (Gu 2003). Recalcitrant nature of polystyrene toward microorganisms makes polystyrene degradation process very slow in the environment (Tian et al. 2017). Still, fungi could be a feasible solution for the proper removal of plastic wastes from the environment due to their extracellular oxidation mechanisms (Kerem et al. 1999). Secretions of extracellular and intracellular enzymes from the microorganisms are responsible for the degradation of polymers. Production of CO2, water, and microbial biomass after degradation process would reveal the attainment of ultimate biodegradation.
Kaplan et al. (1979) reported 0.57% degradation of polystyrene when treated with the mixed microbial culture of activated sludge in 11 weeks. However, 1.9% weight loss was reported for the film of polystyrene buried under agricultural soil over a period of 6 months (Ali and Ghaffar 2017). Syranidou et al. (2017) reported a 4.7% reduction in weight loss when polystyrene samples treated with seawater microorganisms for a period of 6 months. Exceptionally, Yang et al. (2018) have reported the rapid biodegradation of polystyrene foams by a gut of yellow mealworms and larvae of Tenebrio molitor Linnaeus.
Cephalosporium species (sp.) and Mucor species (sp.) have the ability to utilize plastic materials like polyethylene and polystyrene as a source of carbon for their growth (Brown et al. 1974). Further, Chaudhary and Vijayakumar (2018) reported the capability of Cephalosporium sp. to degrade polyethylene. However, the ability of Cephalosporium sp. and Mucor sp. for the degradation of polystyrene under laboratory conditions is not reported in the literature. Studies on the biodegradability of polystyrene in the presence of two different pure fungi culture have been carried out in the present work.
2 Materials and methods
2.1 Preparation of polystyrene sample
Polystyrene foam was dissolved in benzene solution and poured into Petri dish. Then, the solution was kept at room temperature for 24 h for the removal of solvent by evaporation process. Further, these samples were kept in an oven at 70 °C for 8 h for complete removal of the solvent. Then, the plastic strips were cut into 4 × 4 cm size and washed with ethanol solution for further processing.
2.2 Source of biodegrading culture
Fungal culture of Cephalosporium sp. (NCIM 1251) and Mucor sp. (NCIM 881) was obtained from the National Collection of Industrial Microorganism (NCIM), NCL, Pune, India. Fungal cultures were preserved regularly on potato dextrose agar (PDA) at 28 °C and were stocked at 4 °C.
2.3 In vitro degradation study
K2HPO4, (NH4) 2SO4, NaCl, KH2PO4, MgSO4.7H2O, (NH4) 2SO4, CaCl2.2H2O, FeSO4 each of 0.5, 0.2, 0.1, 0.04, 0.02, 0.002, 0.001 g/L of distilled water were taken for the preparation of mineral salt media (MSM). In a 250 mL of conical flask, 100 mL of MSM was taken along with polystyrene strip. In vitro degradation studies of polystyrene were carried out in laminar air flow to make the environment clean and pathogen free with positive (mineral salt media + polystyrene + fungus) and negative (mineral salt media + polystyrene) controls, respectively. The flasks were then preserved in a biochemical oxygen demand (BOD) incubator at a shaking rate of 120 rpm for eight weeks at 28 °C.
2.4 Analysis of biodegradation
2.4.1 Weight reduction measurement
Weights of the samples were measured before and after the incubation period. Reduction in weight after the degradation was determined by using Eq. (1),
where Mi is the initial weight of polystyrene and Mf is the weight of polystyrene after an incubation period
2.4.2 Measurement of pH, TDS, and conductivity
WENSER LMMP-30 apparatus was used for the determination of pH, TDS, and conductivity of the mineral salt media. Two different electrodes were used to measure pH, TDS, and conductivity of mineral salt media. Ten milliliters of mineral salt media was taken in a beaker, and electrode was dipped into the test solution. The readings were noted after attaining the equilibrium condition.
2.4.3 Fourier transform infrared (FTIR) spectroscopy
FTIR analysis for the samples was performed by using FTIR spectroscopy (Shimadzu, FTIR-8400) in the frequency range of 400 cm−1 to 4000 cm−1.
2.4.4 Scanning Electron Microscopy (SEM)
Polystyrene samples after an incubation period of eight weeks with Cephalosporium sp. and Mucor sp. were washed with ethanol to remove the microorganisms attached on the surface and further washed with distilled water to clean the surface. Morphological analyses were carried out using scanning electron microscopy (SEM) (ZEISS, EVO 18) for pure polystyrene and polystyrene after inoculation with pure fungal cultures. A sample was adhered to SEM holder stub using carbon tape and also gold sputtering was carried out before the analysis.
2.4.5 Thermogravimetric analysis (TGA)–derivative thermogravimetry (DTG)
Thermal stability of the polystyrene samples before and after the incubation period was analyzed by using thermogravimetric analyzer (Shimadzu, TGA-50) in the temperature range of 25–800 °C. Samples were ignited under an inert nitrogen atmosphere (10 mL/min) at a heating rate of 10 °C/min in order to determine the reduction in weight.
2.4.6 Gas chromatography–mass spectrometer (GC–MS)
The by-products obtained after incubation period were analyzed by using gas chromatography–mass spectrometer (Agilent Technologies, GCMS_HSS). After incubation period, the polystyrene strips were separated and the fungal cultures were centrifuged at 6000 rpm for 15 min to remove the organic wastes. The compounds present in the supernatant were analyzed using GC–MS.
2.4.7 Gel permeation chromatography (GPC)
Determination of weight-average molecular weight (Mw) and number-average molecular weight (Mn) of polystyrene samples before and after microbial exposure over a period of 8 weeks was carried out by gel permeation chromatography (GPC) (PerkinElmer, Series 200). The polydispersity index (Mw/Mn) of the samples was calculated by using the molecular weight data.
3 Results and discussions
The study on degradation of polystyrene by pure fungal cultures in the mineral salt media was performed. The mean of three experiments is considered to analyze the results.
3.1 Weight loss measurement
Weight loss is the primary method to detect degradation in polymers. Microorganisms attach to the surface of polymers and initiate the degradation process. Microorganisms secrete enzymes and disrupt the integrity of polymers which leads to the weight loss. A weight loss of 2.17 ± 0.16% is observed when polystyrene samples are incubated with Cephalosporium sp. for a period of 8 weeks, whereas 1.81 ± 0.13% weight loss is observed in PS inoculated with Mucor sp. The weight loss of polystyrene films after inoculation with fungal cultures attributes to the loss of carbon content due to the utilization of polystyrene films by the microorganisms (Chaudhary and Vijayakumar 2018). No weight loss is obtained in PS sample inoculated in the negative control flask (without microorganisms). Reduction in weight of samples reveals that the Cephalosporium sp. and Mucor sp. have the ability to degrade PS sample and utilized PS as a source of carbon for their metabolic activities. Cephalosporium sp. is found to be more efficient than Mucor sp. in degradation of PS as revealed by weight loss measurements. Thus, the weight loss analysis interprets that the fungal cultures have the potential to degrade polystyrene films and confirms the potential of fungal cultures to survive on the surface of polystyrene (Brown et al. 1974). Similar observations have been reported based on the gravimetric analysis (Ali and Ghaffar 2017; Kaplan et al. 1979; Syranidou et al. 2017).
3.2 Measurement of pH, TDS, and conductivity of mineral salt media
The variations observed in the values of pH, TDS, and conductivity are shown in Table 1. The degradation of plastics depends on pH of the mineral salt media, which is responsible for the increase in cell numbers. Initial pH of the mineral salt media is 7.01 ± 0.01. pH of mineral salt media decreases after incubation with fungal cultures. The value reduced to 5.91 ± 0.05 and 5.21 ± 0.08 after an incubation period of eight weeks with Cephalosporium sp. and Mucor sp., respectively. Lowering in the value of pH is resulted due to secretion of enzymes and acids from the microorganisms (Gu 2003). The changes in the value of pH confirm the ability of fungal cultures to utilize PS as a source of carbon. Similar results were reported where the presence of microorganisms decreases the pH value of mineral salt media (Awasthi et al. 2017).
The initial value of TDS and conductivity is 0.546 ± 0.006 ppm and 0.452 ± 0.018 µS, respectively. The value increased to 1.615 ± 0.045 ppm and 3.141 ± 0.072 µS when inoculated with Cephalosporium sp. On the other hand, the value becomes 1.465 ± 0.091 ppm and 2.814 ± 0.047 µS when inoculated with Mucor sp. for a period of eight weeks. Again, the secretions of enzymes and acids are responsible for the increase in the value of TDS and conductivity (Gu 2003; Chaudhary and Vijayakumar 2018). Increase in the value of TDS and conductivity confirms the effectiveness of fungal cultures in the degradation of polystyrene. Cassidy et al. (2001) reported similar observations where the value of TDS and conductivity increases after inoculation with microorganisms.
3.3 Fourier transform infrared (FTIR) spectroscopy
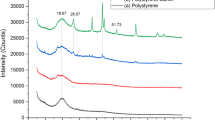
The characteristic bands at 3553, 3448, 3051, 2881, 2650, 2333, 1925, 1757, 1480 and 726 wave numbers are seen in PS sample (Fig. 1a). The interpretation of characteristic wave numbers is given in Table 2. Characteristics bands of pure PS samples are shifted to 3635, 3429, 3032, 2902, 2644, 2330, 1905, 1766 and 746 after an incubation period of 8 weeks with Cephalosporium sp. (Figure 1b). The FTIR spectrum of polystyrene after 8 weeks of incubation with Mucor sp. is presented in Fig. 1c. Similar shifting of the bands is observed when PS samples are treated with Mucor sp. (Figure 1c). Characteristics bands of pure PS samples are shifted to 3651, 3450, 3036, 2891, 2636, 2328, 1907, 1778 and 748 after an incubation period of 8 weeks with Mucor sp. (Figure 1c). Ojha et al. (2017) reported a similar shift in the peaks during degradation of a polymer sample by the potential fungal strains. Further, some of the characteristic peaks shifted to higher values after the degradation, which is due to the weakening of bonds in the polystyrene sample (Pushpadass et al. 2010). Shifting in peaks after microbial exposure confirms the degradation of polystyrene samples. The decrease in the intensity of bands is also observed for the PS samples exposed to microorganisms. The decrease in the intensity of bands indicates that the degradation of polystyrene samples takes place due to secretion of enzymes from the microorganisms, which leads to disintegrations of polymer samples (Shimpi et al. 2012). Syranidou et al. (2017) reported a similar decrease in the intensity of peaks of polystyrene after treatment with a fungal culture.
3.4 Scanning electron microscopy (SEM)
Polystyrene sample has a smooth, uniform and homogeneous surface as analyzed by SEM (Fig. 2a). Morphological changes in the PS surface are seen for the samples after 8 weeks of the microbial attack. Incubation of PS samples with Cephalosporium sp. and Mucor sp. changes the surface texture and leads to the creation of cracks, erosion, and holes, which indicate an attack on polystyrene film (Fig. 2c and d). Further, the generation of cracks, erosion, and holes after the degradation process confirms the ability of Cephalosporium sp. and Mucor sp. to utilize polystyrene as a source of energy in the form of carbon. No morphological changes are observed when polystyrene samples were inoculated in the negative control (without microorganisms) (Fig. 2b). Treatment of polystyrene with Mucor sp. created less deformation than that of Cephalosporium sp. treated sample as shown in SEM analysis. These results are in agreement with the results obtained in weight loss measurement where weight loss was lower in Mucor sp. treated PS than that of Cephalosporium sp. treated PS. Morphological changes occurred after an incubation period indicates the extent of polymer degradation (Ali and Ghaffar 2017). Similar observations of morphological changes in polystyrene samples were reported when polystyrene samples were subjected to microbial treatment (Syranidou et al. 2017; Sekhar et al. 2016). Thus, the results showed the potential capacity of Cephalosporium sp. and Mucor sp. to degrade polystyrene sample.
3.5 Thermogravimetric analysis (TGA)–derivative thermogravimetry (DTG)
Thermogravimetric analysis of polystyrene samples was studied under a nitrogen atmosphere. The weight loss occurred at the temperature range of 215–450 °C in polystyrene is due to backbone thermal decomposition (Ali and Ghaffar 2017). All the polystyrene samples were completely decomposed in the temperature range of 450 to 600 °C (Fig. 3). Zhao et al. (2018) reported similar observations for the decomposition of polystyrene. TGA result shows the shifting of degradation temperature of polystyrene to lower side after incubation with microorganisms. This reveals that the thermal stability of PS sample decreases after an attack with microorganisms. Jeyakumar et al. (2013) reported similar observations in shifting of degradation temperature to a lower side for the polypropylenes treated with fungal cultures. TGA showed a weight loss of 95% at 437 °C for pure polystyrene sample. The onset of degradation reduced to 417 °C and 408 °C for a 95% reduction in weight for polystyrene samples treated with Mucor sp. and Cephalosporium sp., respectively. The decrease in the degradation temperature is related to the decrease in the thermal stability of polystyrene samples. Reduction in thermal stability indicates the changes in crystallinity, molecular weight, and polymer chain length (Sudhakar et al. 2008). Therefore, the reduction in thermal stability confirms the utilization of PS samples by the microorganisms.
Derivative thermogravimetry (DTG) curve shows a major peak at 422.83 °C for pure polystyrene sample (Fig. 4). DTG curves shifted to 398.26 °C and 388.33 °C after microbial treatment with Mucor sp. and Cephalosporium sp., respectively. This shifting of temperature implies that the thermal stability of polystyrene sample decreases after fungal treatment. The decrease in thermal stability of polymer occurs due to changes and breaking of bonds caused by the enzymes secreted from microorganisms. Soni et al. (2009) reported similar observations for the low-density polyethylene (LDPE) samples after treatment with the microbial consortium.
3.6 Gas chromatography–mass spectrometer (GC–MS)
Supernatant samples were subjected to GC–MS analysis to analyze the by-products produced after incubation with pure fungal cultures. Figure 5a and b shows the chromatogram for the supernatant obtained on polystyrene degradation by the fungal cultures Cephalosporium sp. and Mucor sp., respectively. The presence of alkanes, alkenes, acids, alcohols, antioxidants, and aromatic hydrocarbon compounds are identified by GC–MS. The by-products formed due to activity of fungal culture during the degradation process using Cephalosporium sp. and Mucor sp. are shown in Tables 3 and 4, respectively. Similar by-products such as alkanes, alkenes, acids, alcohols, antioxidants, and aromatic hydrocarbons have been formed on the degradation of polyethylene (Muenmee et al. 2016; Shahnawaz et al. 2016; Awasthi et al. 2017).
3.7 Gel permeation chromatography (GPC)
The weight-average molecular weight (Mw) and number-average molecular weight (Mn) of the polystyrene sample before and after fungal treatment are shown in Table 5. Mn of the polystyrene sample decreased by 4.69% and 4.56% after treatment with Cephalosporium sp. and Mucor sp., respectively. Also, Mw of polystyrene samples decreased by 4.96% and 4.29% after 8 weeks of incubation with Cephalosporium sp. and Mucor sp., respectively. The decrease in the value of Mn and Mw suggests that the scissioning of polymer chains occurred due to the impact of microorganisms and leads to the formation of shorter fragments. Syranidou et al. 2017 reported similar decrease in the Mn and Mw of polystyrene samples after treatment with marine microorganisms. Value of polydispersity is almost constant for all the polystyrene samples which indicates the heterogeneous distribution of molecules in the polymer matrix.
4 Conclusions
The drastic increase in applications of polystyrene significantly increases the generation of plastic wastes. Biodegradation is one of the promising approaches for degradation by using microorganisms to overcome the plastic waste problem. The process is safe and eco-friendly as it releases a negligible amount of green house gases. The present work deals with the study on degradation of polystyrene by the Cephalosporium sp. and Mucor sp. in eight weeks of duration. FTIR, SEM, and TGA studies confirmed the utilization of polystyrene by pure fungal cultures. These findings could be useful for the safer removal of polystyrene in the presence of microorganisms and could be a viable solution to deal with plastic wastes problem prevailing in the society.
References
Aarnio, T., & Hämäläinen, A. (2008). Challenges in packaging waste management in the fast food industry. Resources, Conservation and Recycling,52(4), 612–621. https://doi.org/10.1016/j.resconrec.2007.08.002.
Ali, H. E., & Ghaffar, A. M. A. (2017). Preparation and Effect of Gamma Radiation on The Properties and Biodegradability of Poly (Styrene/Starch) Blends. Radiation Physics and Chemistry,130, 411–420. https://doi.org/10.1016/j.radphyschem.2016.09.006.
Assessment & Quantification of Plastics Waste Generation in Major Cities, (2017).—A report by Central pollution control board (CPCB), India.
Awasthi, S., Srivastava, P., Singh, P., Tiwary, D., & Mishra, P. K. (2017). Biodegradation of thermally treated high-density polyethylene (HDPE) by Klebsiella pneumoniae CH001. 3 Biotech,7(5), 1–10. https://doi.org/10.1007/s13205-017-0959-3.
Brown, S. B., Millls, J., & Husle, M. J. (1974). Chemical and biological degradation of waste plastics. Nature,250, 161–163.
Cassidy, D. P., Werkema, D. D., Sauck, W. A., Atekwana, E. A., Rossbach, S., & Duris, J. (2001). The effects of LNAPL biodegradation products on electrical conductivity measurements. Journal of Environmental and Engineering Geophysics,6(1), 47–52. https://doi.org/10.4133/JEEG6.1.47.
Chaudhary, A. K., & Vijayakumar, R. P. (2018). Effect of chemical treatment on biological degradation of high-density polyethylene (HDPE). Environment, Development and Sustainability. https://doi.org/10.1007/s10668-018-0236-6.
Gómez, S., Rendtorff, N. M., Aglietti, E. F., Sakka, Y., & Suarez, G. (2017). Intensity of sulfonitric treatment on multiwall carbon nanotubes. Chemical Physics Letters,689, 135–141. https://doi.org/10.1016/j.cplett.2017.10.020.
Gu, J. D. (2003). Microbiological deterioration and degradation of synthetic polymeric materials: Recent research advances. International Biodeterioration and Biodegradation,52(2), 69–91. https://doi.org/10.1016/S0964-8305(02)00177-4.
Ho, B. T., Roberts, T. K., & Lucas, S. (2017). An overview on biodegradation of polystyrene and modified polystyrene: The microbial approach. Critical Reviews in Biotechnology,8551(August), 1–13. https://doi.org/10.1080/07388551.2017.1355293.
Jeyakumar, D., Chirsteen, J., & Doble, M. (2013). Synergistic effects of pretreatment and blending on fungi mediated biodegradation of polypropylenes. Bioresource Technology,148, 78–85. https://doi.org/10.1016/j.biortech.2013.08.074.
Kaplan, D. L., Hartenstein, R., & Sutter, J. (1979). Biodegradation of polystyrene, poly (metnyl methacrylate), and phenol formaldehyde. Applied and Environmental Microbiology,38(3), 551–553.
Kerem, Z., Jensen, K. A., & Hammel, K. E. (1999). Biodegradative mechanism of the brown rot basidiomycete Gloeophyllum trabeum: Evidence for an extracellular hydroquinone-driven fenton reaction. FEBS Letters,446(1), 49–54. https://doi.org/10.1016/S0014-5793(99)00180-5.
Krueger, M. C., Seiwert, B., Prager, A., Zhang, S., Abel, B., Harms, H., et al. (2017). Degradation of polystyrene and selected analogues by biological Fenton chemistry approaches: Opportunities and limitations. Chemosphere,173, 520–528. https://doi.org/10.1016/j.chemosphere.2017.01.089.
Mohan, A. J., Sekhar, V. C., Bhaskar, T., & Nampoothiri, K. M. (2016). Microbial assisted high impact polystyrene (HIPS) degradation. Bioresource Technology,213, 204–207. https://doi.org/10.1016/j.biortech.2016.03.021.
Muenmee, S., Chiemchaisri, W., & Chiemchaisri, C. (2016). Enhancement of biodegradation of plastic wastes via methane oxidation in semi-aerobic landfill. International Biodeterioration and Biodegradation,113, 244–255. https://doi.org/10.1016/j.ibiod.2016.03.016.
Nikolic, V., Velickovic, S., & Popovic, A. (2014). Biodegradation of polystyrene-graft-starch copolymers in three different types of soil. Environmental Science and Pollution Research,21(16), 9877–9886. https://doi.org/10.1007/s11356-014-2946-0.
Ojha, N., Pradhan, N., Singh, S., Barla, A., Shrivastava, A., Khatua, P., et al. (2017). Evaluation of HDPE and LDPE degradation by fungus, implemented by statistical optimization. Scientific Reports,7, 39515. https://doi.org/10.1038/srep39515.
Pushpadass, H. A., Weber, R. W., Dumais, J. J., & Hanna, M. A. (2010). Biodegradation characteristics of starch-polystyrene loose-fill foams in a composting medium. Bioresource Technology,101(19), 7258–7264. https://doi.org/10.1016/j.biortech.2010.04.039.
Sarmiento, A. M., Guzmán, H. L., Morales, G., Romero, D. E., & Pataquiva-Mateus, A. Y. (2016). Expanded polystyrene (EPS) and waste cooking oil (WCO): From urban wastes to potential material of construction. Waste and Biomass Valorization,7(5), 1245–1254. https://doi.org/10.1007/s12649-016-9511-7.
Schlemmer, D., Sales, M. J. A., & Resck, I. S. (2009). Degradation of different polystyrene/thermoplastic starch blends buried in soil. Carbohydrate Polymers,75(1), 58–62. https://doi.org/10.1016/j.carbpol.2008.06.010.
Sekhar, V. C., Nampoothiri, K. M., Mohan, A. J., Nair, N. R., Bhaskar, T., & Pandey, A. (2016). Microbial degradation of high impact polystyrene (HIPS), an e-plastic with decabromodiphenyl oxide and antimony trioxide. Journal of Hazardous Materials,318, 347–354. https://doi.org/10.1016/j.jhazmat.2016.07.008.
Shahnawaz, M., Sangale, M. K., & Ade, A. B. (2016). Bacteria-based polythene degradation products: GC-MS analysis and toxicity testing. Environmental Science and Pollution Research,23(11), 10733–10741. https://doi.org/10.1007/s11356-016-6246-8.
Sheikh, N., Akhavan, A., & Ataeivarjovi, E. (2013). Radiation grafting of styrene on starch with high efficiency. Radiation Physics and Chemistry,85, 189–192. https://doi.org/10.1016/j.radphyschem.2012.10.005.
Shimpi, N., Borane, M., Mishra, S., & Kadam, M. (2012). Biodegradation of polystyrene (PS)-poly(lactic acid) (PLA) nanocomposites using Pseudomonas aeruginosa. Macromolecular Research,20(2), 181–187. https://doi.org/10.1007/s13233-012-0026-1.
Soni, R., Kapri, A., Zaidi, M. G. H., & Goel, R. (2009). Comparative biodegradation studies of non-poronized and poronized LDPE using indigenous microbial consortium. Journal of Polymers and the Environment,17(4), 233–239. https://doi.org/10.1007/s10924-009-0143-x.
Sowmya, H. V., Ramalingappa, K. M., & Thippeswamy, B. (2015). Degradation of polyethylene by Penicillium simplicissimum isolated from local dumpsite of Shivamogga district. Environment, Development and Sustainability,17(4), 731–745. https://doi.org/10.1007/s10668-014-9571-4.
Sudhakar, M., Doble, M., Murthy, P. S., & Venkatesan, R. (2008). Marine microbe-mediated biodegradation of low- and high-density polyethylenes. International Biodeterioration and Biodegradation,61(3), 203–213. https://doi.org/10.1016/j.ibiod.2007.07.011.
Syranidou, E., Karkanorachaki, K., Amorotti, F., Franchini, M., Repouskou, E., Kaliva, M., et al. (2017). Biodegradation of weathered polystyrene films in seawater microcosms. Scientific Reports,7(1), 1–12. https://doi.org/10.1038/s41598-017-18366-y.
Tian, L., Kolvenbach, B., Corvini, N., Wang, S., Tavanaie, N., Wang, L., et al. (2017). Mineralisation of 14C-labelled polystyrene plastics by Penicillium variabile after ozonation pre-treatment. New Biotechnology,38, 101–105. https://doi.org/10.1016/j.nbt.2016.07.008.
Tolinski, M. (2012). Plastics and Sustainability. Plastics and Sustainability: Towards a Peaceful Coexistence between Bio-based and Fossil Fuel-based Plastics. https://doi.org/10.1002/9781118217849.
Yang, S. S., Wu, W.-M., Brandon, A. M., Fan, H.-Q., Receveur, J. P., Li, Y., et al. (2018). Ubiquity of polystyrene digestion and biodegradation within yellow mealworms, larvae of Tenebrio molitor Linnaeus (Coleoptera: Tenebrionidae). Chemosphere,212, 262–271. https://doi.org/10.1016/j.chemosphere.2018.08.078.
Zhao, H., Nam, P. K., Richards, V. L., & Lekakh, S. N. (2018). Thermal decomposition studies of EPS foam, polyurethane foam, and epoxy resin (SLA) as patterns for investment casting; Analysis of hydrogen cyanide (HCN) from thermal degradation of polyurethane foam. International Journal of Metalcasting. https://doi.org/10.1007/s40962-018-0240-5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chaudhary, A.K., Vijayakumar, R.P. Studies on biological degradation of polystyrene by pure fungal cultures. Environ Dev Sustain 22, 4495–4508 (2020). https://doi.org/10.1007/s10668-019-00394-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10668-019-00394-5