Abstract
This study was carried out to understand the hydrogeochemical processes of groundwater quality and groundwater use in the Suyeong District of Busan city, Korea. Groundwater samples were collected from 40 wells in February, 2010. The abundance of major cations concentration in groundwater is Na+ > Ca2+ > Mg2+> K+, while that of anions is Cl− > HCO3 − > SO4 2− > NO3 − > F−. According to hydrogeochemical facies, Ca (HCO3)2, Ca Cl2 and NaCl are the dominant groundwater types in this study area. Mechanism controlling the water chemistry (Gibbs) indicates that most of groundwater samples fall at rock-weathering dominance zone. The geochemical processes and temporal variation in groundwater in this area are influenced by evaporation processes, ion exchange and dissolution of minerals. According to water quality index (WQI) of the study area exhibits 8 % of the groundwater samples fall at the unsuitable zone for drinking purpose. The spatial distribution map of WQI shows the poor quality of the water decrease toward the southern part of the study area. The results of SAR, Na%, PI, RSC and MH show that majority of groundwater samples are suitable for domestic and agricultural purposes. By the hydrogeochemical analysis, aquifer rock weathering, seawater intrusion, sewer leakage are the dominant factors that determine the major ionic composition. The proper management plan is necessary to preserve valuable groundwater resources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Groundwater is a natural resource, which is being renewed by different processes. Geochemical processes occurring within the groundwater and reactions with dissolved minerals have a profound effect on water quality. Groundwater quality may be affected by natural factors, such as geology and geochemical processes. Geogenic sources are one of the causes of the variation in chemical composition of groundwater, which changes with space and time (Madhavan and Subramanian 2006; Zahid et al. 2008; Vikas et al. 2009; Gunduz et al. 2009; Mamatha and Rao 2009; Brindha and Elango 2012; Kraiem et al. 2013). It depends on the parent rock, intensity of weathering, residence time and external factors, such as precipitation, temperature. Hydrogeochemical processes, such as weathering, dissolution, mixing and ion exchange. control the concentration of major and minor ions in groundwater (Rajmohan and Elango 2004; Liu et al. 2008; Singh et al. 2008; Zhu and Schwartz 2011; Rajesh et al. 2012). Hence, this various process is very essential to understand the geochemical sources of the groundwater. The geochemical properties of groundwater depend on the chemistry of water in recharge an area as well as on the different geological processes that take place in the subsurface. The groundwater chemically evolved due to the interaction with aquifer minerals or intermixing among different groundwater reservoirs along flow paths in the subsurface (Domenico 1972). Hydrogeochemical studies assist in taking proper measures to protect aquifers from groundwater contamination by natural phenomena or anthropogenic activities. Generally, the groundwater quality of a metropolitan city is affected by anthropogenic sources such as improperly treated industrial, municipal or domestic effluents. In case of metropolitan city near the sea, seawater intrusion is a main source of groundwater contamination (Shim et al. 2004; Kim and Chung 2011; Anithamary et al. 2012).
Water quality index (WQI) is an effective tool to assess the state of an ecosystem, and this method is based on a group of physico-chemical and biological characteristics of water samples (Namibian 2007; Simoes et al. 2008). Therefore, several researchers have developed various indices, technically referred to as WQI (Lermontov et al. 2009). Usually, WQI is a practical and comparatively simple approach of evaluating the composite influence of overall pollution and hardly provides evidences in pollution sources. The pollution indices were proposed to provide useful and comprehensible guiding tool for water quality executives, environmental managers, decision makers and potential users of a given water system. The chemical ions those are present in groundwater due to these reasons to determine its suitability for drinking, agriculture and industrial purposes. Assessment of water quality is of paramount importance, especially, in populated regions, which depend on groundwater. Water uses standards regulated by World Health Organization (WHO 2004), United States Environmental Protection Agency (USEPA 2003), Korea Ministry of Environment (KMOE 2012), etc. determine the usability of water for various purposes. Assessment of groundwater quality in populated regions, including large cities based on drinking water standards, have been carried out by several researchers (Ozcan et al. 2007; Umar et al. 2009; Gupta et al. 2009; Dar et al. 2011).
Busan is one of the metropolitan cities in Korea and has several industrial areas in its outskirts. Many residents of the city are using groundwater for their living, even though the surface water is the main source supplied by the city. The reason is because they believe that groundwater quality is better than surface water quality. By the way, groundwater is frequently contaminated by leaked sewage, seawater intrusion, industrial wastewater and fertilizer for grass plantation in the municipal area (Shim et al. 2002; Chung et al. 2012). Groundwater problems in Busan City have not been thoroughly studied yet. On the other hand, groundwater contamination (Kim 2004; Chae et al. 2008; Hosono et al. 2009) and heavy groundwater discharge (Kim et al. 2001; Kim et al. 2005) around the subway tunnel due to the domestic and industrial waste water contamination have been studied in Seoul, Korea. The objective of this study is to assess the present quality of groundwater and to determine the suitability of groundwater use for various purposes on the basis of various geochemical processes and WQI. Hence, this study will serve as a foundation to access the improvement of groundwater quality in future.
2 Study area
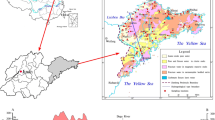
Busan Metropolitan city is the second populated city in Korea. Metropolitan area covers 770,000 km2 with a population of 3.7 million people. The weather is relatively mild during most of the time in a year. In summer, the temperature ranges from 23 to 31 °C, and in winter it varies from −1.5 to 10 °C. The average annual rainfall is about 1,560 mm. The city usually receives heavy rainfall through typhoon in August or September. The study area is the Suyeong District in Busan Metropolitan City (Fig. 1). Suyeong River is located at the eastern side of the study area, and its southern part is connected to the sea. Geology of the study area is composed of andesitic volcanic breccias, tuffaceous sedimentary rocks, rhyolitic rocks and intrusive granodiorites and granite porphyries (Chang et al. 1983; Son et al. 1978). The geologic time of the study area is the Cretaceous Period in the Mesozoic Era. Groundwater along the Suyeong Bay is relatively vulnerable to seawater intrusion, because the area is composed of sand beach and reclaimed land. In this study area, there are no irrigation activities, whereas grass plantation process is done around this area. There are 377 wells registered in the Suyeong District. Forty wells were developed in shallow aquifer of less than 50 m depth and the rest wells in deep aquifer of over 50 m. Groundwater level and quality of pH and electrical conductivity were measured for 119 wells in August, 2009 and February, 2010. The average groundwater level was 10.5 m and 9.83 m above mean sea level, respectively, and the variation in water level was 0.67 m. The average pH was 7.01 and 7.09, respectively, and the variation in pH was 0.08.
3 Materials and methods
3.1 Sample collection
Groundwater samples were collected in February, 2010 at 40 sampling wells, which were selected on the basis of detailed well inventory. The groundwater of borehole wells was directly sampled from water faucets after pumping for approximately 10 min to remove stagnant water. Groundwater was poured into high-quality polyethylene bottles through 0.45-µm cellulose nitric membrane filter to eliminate suspended materials. Groundwater samples for the analysis of metal components were acidified to pH < 2.0 in the field. All samples were stored in ice chests at 4 °C and transported directly to the laboratory where they were analyzed within 2 weeks.
3.2 Laboratory measurements
The samples were analyzed using standard analytical methods (APHA, 1995). pH was measured in the field by using Therm Orion 250A+, USA, and EC was also measured in the field by TOA CM-14P, Japan. Major cations were analyzed by Atomic Absorption Spectrometer (AAS, PerkinElmer 400). Major anions were determined by ion chromatography (IC, Water 431). The quality of the analysis was ensured by standardization using blank spike and also with duplicate samples. Further accuracy of the chemical analysis was verified by calculating ion balance error, which was generally within 5 %. All the concentrations were expressed in milligrams per liter (mg/L), except the pH and EC (μS/cm).
3.3 Data analysis
Geochemical facies type, Wilcox, Gibbs, Na%, RSC, SAR, TH, RSC, MH and PI were used to determine the various groundwater uses. Piper trilinear diagram and Wilcox plot were prepared by Aquachem (ver. 4), and other graphs were plotted in MS-Excel (ver. 2010). A spatial map of nitrate was plotted using Arc GIS (ver. 10.2), and saturation index (SI) of minerals was determined by WATEQ4F.
WQI is defined as a technique of rating that provides the composite influence of individual water quality parameters on the overall quality of water for human consumption (Mitra 1998). For this purpose, 10 water quality parameters were selected. Parameter consideration to develop a WQI depends on the purpose for which water is used. Parameters were selected according to the availability of data as well as their relative importance in defining water quality for human consumption. The standard set for this purpose follows the WHO guidelines (WHO 2004). WQI is calculated by assigning weights to the measured parameters based on their relative importance. The maximum weight 5 was assigned to parameters like sodium, chloride, TDS and sulfate because the components are very closely related with groundwater contamination. The minimum weight 1 is given to bicarbonate since it doesn’t contribute to groundwater contamination (Vasanthavigar et al. 2010; Sajil Kumar et al. 2013). In the second step, the relative weight (W i ) is computed with the following equation:
where, W i is the relative weight, w i is the weight of each parameter, n is the number of parameters.
In third step, a quality rating scale (qi) for each parameter is assigned by dividing its concentration in each water sample by its respective standard according to the guidelines laid down in the WHO (2004), and the result is multiplied by 100:
where, q i is quality rating, C i is concentration of each chemical parameter in milligrams per liter, S i is WHO standard for each chemical parameter in milligrams per liter according to the guidelines of WHO (2004).
For computing WQI, SI is firstly determined for each chemical parameter with the following equation:
where, SI i is the subindex of ith parameter, q i is the rating based on concentration of ith parameter, n is the number of parameters.
A map of WQI was produced by computing the individual point data and then plotted using Arc GIS (Ver. 10.2) in this study.
4 Results and discussion
4.1 Hydrogeochemical processes
4.1.1 General water chemistry
The statistics of water chemistry is represented in Table 1. The quality of groundwater is very important because it is the main factor determining its suitability for drinking, domestic, agricultural and industrial purposes. pH values of groundwater ranged from 6.19 to 7.94 with an average value of 6.96. This shows that the groundwater of the study area is a little acidic to alkaline in nature. EC values are between 227.1 and 21,400 μS/cm. TDS values ranged from 154.3 to 15,000 mg/L. The chemical composition of the groundwater samples of the study is shown in Table 1. The general dominance of anion was in the order of Cl− > HCO3 − > SO4 2− > NO3 − > F−. The concentration of Cl− ranges from 15.88 to 24,525 mg/L in groundwater samples due to seawater intrusion, industrial and domestic activities (Ramkumar et al. 2011; Venkatramanan et al. 2012, 2013). SO4 2− ranged from 13.43 to 2,875 mg/L with the average of 167.3 mg/L. Most of groundwater samples have moderate sulfate concentrations, and some groundwater around the beach area are affected by seawater intrusion. However, sulfate might come from dissolution of sulfate minerals precipitated in the soil (Miller 1979; Craig and Anderson 1979). HCO3 − was from 70.8 to 878.4 mg/L with the average of 246.3 mg/L. Most of groundwater has moderate bicarbonate concentrations, and some groundwater have high concentrations. Bicarbonate is originated from the reaction of groundwater with CO2 in soil and air. NO3 − was from 0.31 to 126.9 mg/L with the average of 34.02 mg/L, and some groundwaters have high concentrations. Nitrate comes mainly from leached sewage and partly from fertilizers for plants in the municipal area.
F− concentrations ranged from 0.06 to 0.8 mg/L due to the interaction with fluoride rich minerals like apatite, fluorspar, fluorapatite and biotite from andesite, rhyolite and granodiorite rocks. Fluoride concentrations regularly are proportional to the degree of water–rock interaction because fluoride primarily originates from the geology. It is also reported that fluoride in groundwater is negatively related to anthropogenic contaminates that may infiltrate from the land surface (Brindha and Elango 2013a, b). General dominance of cations was in the order of Na+ > Ca2+ > Mg2+ > K+. For cations, Na+ was from 10.57 to 6,675 mg/L. High potassium was resulted from seawater intrusion, anthropogenic sources and the weathering of plagioclase-bearing rocks. K+ concentrations were between 0.4 and 439 mg/L due to seawater intrusion and the weathering of feldspar. Ca2+ ranged from 0.11 to 999.8 mg/L, with the average of 112 mg/L, and much amount of calcium came from seawater. Mg2+ was between 0.07 and 1,293 mg/L. Sewage and industrial wastes were the important sources of calcium and magnesium. Spatial distribution map of nitrate concentration (Fig. 2) showed that the NO3 − concentration was higher in southwestern part (station 31) of the study area. Medium concentration of nitrate levels was observed in central, northern and southern part. The higher concentration of NO3 − was originated mainly from leaked sewage and fertilizers for plants. The leakage from septic tanks may also increase nitrate concentration (Brindha and Elango 2013a, b).
4.1.2 Processes controlling groundwater chemistry
The groundwater geochemistry is influenced by geochemical reactions and mixing of neighboring samples. Groundwater evaluation was mainly dependent on the relationship between rock types and water composition. Hydrogeochemical facies interpretation is a useful tool for determining the flow pattern and origin of chemical histories of groundwater. Piper (1953) attempted to classify hydrogeochemical facies using a trilinear diagram. In most of the groundwater samples, there is mixing between Cl−, HCO3 − and SO4 2− (strong and weak acids; Fig. 3). The dominance of Na+ + K+ and Mg2+ cations trend is noted during the study period; K+ is much less significant. It indicates that an additional leachate (i.e., surface soil) contributes to the mixed trend. In general, samples those fall in Ca2+–HCO3 − facies in the diamond field of piper show hardness nature in the groundwater.
The reactions between groundwater and aquifer minerals have a significant role on groundwater quality (Cederstorm 1946; Gupta et al. 2008; Subramani et al. 2009). Gibbs diagram (Gibbs 1970) represented the ratios of Na+/(Na++Ca+) and Cl−/(Cl−+HCO3 −) as a function of TDS and are widely employed to evaluate the functional sources of dissolved chemical constituents, such as precipitation dominance, rock dominance and evaporation dominance. Most of samples fall in rock-weathering-dominated zone, which suggested the weathering of rocks primarily controls the major ion chemistry of groundwater in this region. Few samples fall away from this zone, showing the effects of salt precipitation and sewage waste (Fig. 4). The sources for these ions are secondary; hence, they fall away from the zone fixed by Gibbs. Thus, the study area experiences semi-dry condition, and evaporation and dissolution may also contribute to water chemistry in the study region.
4.1.3 Evaporation
The process of evaporation is not only a common phenomenon in groundwater system. Na+/Cl− ratio was widely used to find out the evaporation process in groundwater. Evaporation will increase the concentration of TDS in summer, but the Na+/Cl− ratio remains the same. So, Na+/Cl− ratio was one of the good indicative factors of evaporation. Evaporation is an important process, which is understood from the plot between Na+ versus Cl− (Fig. 5). This figure shows that most of samples plot around the freshwater evaporation line, emphasizing that evaporation also plays a major role in decided the chemical composition of groundwater in this area. Direct evaporation of groundwater was possible as the region has many open wells where groundwater table occurs at shallow depths. Climatic condition influenced the rate of evapotranspiration, hence, the contribution of evaporation as an effective indicator of further enrichment of ions in the groundwater (Brindha et al. 2013).
4.1.4 Ion exchange
Schoeller (1965) proposed a measure called “Index of Base Exchange” (IBE) to describe reactions taking place in groundwater. Chloro-alkaline indices CAI1 and CAI2 are used to measure the extent of base exchange during rock–water interaction. It is calculated using the formulae:
and
where, all ions are expressed in meq/L.
Ca2+ or Mg2+ in groundwater was exchanged with Na+ and K+ in the aquifer material, both the above indices were negative, and if there was a reverse ion exchange, then both these indices would be positive (Schoeller 1965, 1967). Figure 6 of CAI I and CAI II represents that equal state ion exchange is the dominant process. There are few wells for ion exchange processes. In case of reverse ion exchange, the relation between (Ca2+ + Mg2+) and (SO4 2− + HCO3 −) will be close to 1:1 equiline, denoting dissolution of calcite, dolomite and gypsum. Reverse ion exchange can also be identified by the relationship between (Na+–Cl−) and Ca2+, (Mg2+–HCO3 −–SO4 2−). Fisher and Mullican (1997) put forth that such a relationship will be linear with a slope of −1. Groundwater samples of this study were plot in a linear fashion (Fig. 7), and the slope was −0.53. The groundwater samples are close to the 1:1 mineral dissolution lines with R 2 = 0.8811, indicating that dissolution of minerals in the groundwater is an important geochemical process controlling groundwater chemistry.
4.1.5 Saturation index (WATEQ4F)
The saturation index was calculated by WATEQ4F geochemical model for those minerals and other solids stored in the model data book, for which the dissolved constituents are reported in groundwater analyses. This determines the chemical equilibrium between minerals and waters, and saturation indices of carbonate minerals were calculated using the following equation (Lloyd and Heathcode 1985):
For thermodynamic equilibrium, log (IAP/KT) = 0; for over-saturated, log (IAP/KT) > 0; for under saturated, log (IAP/KT) < 0 with respect to certain solid phases (Trusdell and Jones 1973). The carbonate-calculated values of SI for aragonite, calcite, dolomite and magnesite revealed that all minerals fall near saturated to undersaturated state. All minerals do not reach saturation zone because of climatic condition. Hence, it is reasonable to assume that these minerals are reactive in groundwater environment, and they can control solution concentration (Prasanna et al. 2011, Rajesh et al. 2012), whereas the sulfate-calculated values of SI for anhydrite, epsomite and gypsum of the groundwater samples indicate that all the minerals fall undersaturated state. This supported major contribution of ions is from mineral dissolution and climatic condition (Fig. 8a, b).
4.2 Groundwater quality for drinking purposes
4.2.1 WQI
WQI values were ranged from 12.5 to 2,450.7. WQI range and type of the water can be classified as <50 (excellent water), 50–100 (good water), 100–200 (poor water), 200–300 (very poor water) and >300 (water unsuitable for drinking purpose). Relative weights of chemical parameters for WQI values are given in Table 2, and WQI classifications of groundwater samples are given in Table 3. A spatial distribution map of WQI of the groundwater sample is presented in Fig. 9. Higher values were recorded at Milak–dong area, and the values decrease toward the southern side of the study area. WQI model considers a group of water quality parameters and will be more accurate and representative of the groundwater quality. According to WQI classification, 86 % of the samples represent excellent water quality, 2 % good water, 4 % poor to very poor water and 8 % unsuitable for drinking. Water quality was unsuitable for drinking purpose sector in stations 23, 30 and 31. This suggests that the groundwater is influenced by seawater intrusion and by improperly treated effluents of municipal and domestic sectors.
4.2.2 Total hardness (TH)
Total hardness is used to evaluate groundwater use for drinking or domestic purposes. It causes scaling of pots and boilers, closure to irrigation pipes and may cause health problems to humans, such as kidney failure. TH is calculated as follows (Todd 1980):
On the basis of hardness (Sawyer and McCarthy 1967), groundwater is classified into 0–75 (soft), 75–150 (moderate), 150–300 (high) and >300 (very hard). Total hardness varied between 0.04 and 540 mg/L with only 7 % of samples in the hard or very hard water category. The rest of them fall under moderate or soft water category. The origin of total hardness is mainly due to the dissolution of calcium and magnesium (Brindha et al. 2013).
4.3 Groundwater quality for irrigation purposes
4.3.1 SAR
SAR is a measurement of the ratio of sodium (Na+) ions to calcium (Ca2+) and magnesium (Mg2+) ions, expressed in meq/1. The following formula was used to evaluate SAR values:
The values of SAR in excess of 9 indicate that there is a medium or high sodium or low calcium plus magnesium content in the groundwater. If this kind of water is used in irrigation, it can cause the dispersion of soil colloids, destroying soil texture and permeability. This produces conditions similar to droughts. Prolonged exposure of soil to high-SAR groundwater can render large tracts of land not suitable for agriculture (Younger and Casey 2003). SAR values greater than 2 meq/L indicate groundwater is unsuitable for irrigation purposes. During the study period, SAR was ranged from 0.44 to 82.4 meq/L, indicating 30 % of samples are not suitable for irrigation purposes. Wilcox diagram (Wilcox 1955) is a useful representation of sodium alkalinity hazards. Based on this plot (Fig. 10), most of the samples are falling under C2S1 category, which indicates medium salinity to low sodium hazard. Higher demand for water in these regions results in over-exploitation and subsequent seawater intrusion. Thus, Na content was increased in groundwater of the study area (Chung et al. 2012).
4.3.2 Sodium percentage (Na%) and permeability index (PI)
Sodium percentage in soil is vital for determining groundwater suitability for irrigation purpose. Na reacts with soil, and soil permeability is reduced. This phenomenon is not helpful for plant growth. Sodium content is usually expressed in terms of percentage sodium calculated by:
In groundwater of high bicarbonate, there is a tendency for calcium and magnesium to precipitate as the water in the soil is more concentrated. As a result, the relative proportion of sodium in the water was increased in the form of sodium carbonate (Sadashivaiah et al. 2008). When the percentage of sodium exceeds the permissible limit, the permeability of water was reduced and causes damage to the crops. Based on the Na%, <35 meq/L in groundwater is suitable for irrigation purposes. In this study, Na% is ranged from 10.14 to 100 meq/L. 70 % of samples fall under permissible category, and 30 % of the samples were classified as unsuitable sector (Fig. 11). In addition to sodium percentage (Na%), Permeability Index (PI) was calculated for the groundwater. Ragunath (1987) defined a formula for the calculation of permeability index using Na+, Ca2+, Mg2+ and HCO3 −.
Class I and II groundwater samples were considered to be good and suitable for irrigation, while class III water is unsuitable for irrigation (Fig. 12). A slight deviation from the results of Na% was observed. This may be attributed to the incorporation of HCO3 − to the equation for PI (Srinivasamoorthy et al. 2011).
4.3.3 Residual sodium carbonate (RSC) and magnesium hazard (MH)
Residual sodium carbonate (RSC) method is used to determine irrigation water quality. It is calculated by the following formula
If the concentration of carbonate and bicarbonate is exceeded than the concentration of calcium and magnesium, it will be disturbance to the soil fertility and growth of plants (Brindha et al. 2013). Most of groundwater samples fall in >2.5 meq/L and are suitable for irrigation. Magnesium hazard (MH) is calculated using the following formula,
Most of the groundwater samples were suitable for agricultural purposes, because Magnesium hazard is less than 50 meq/L.
5 Conclusions
This research provides a framework of the geochemical processes controlling the groundwater chemistry and suitability of groundwater uses. The sequence of the abundance of major ions in the following order: Na+ > Ca2+ > Mg2+ > K+ for cations and Cl− > HCO3 − > SO4 2− > NO3 − > F− for anions. According to hydrogeochemical facies, the groundwater samples belong to Ca (HCO3)2, Ca Cl2 and NaCl types. Gibbs plot reveals that most of the groundwater samples fall in rock-weathering dominance zone. Evaporation and ion exchange were the dominant processes controlling the groundwater chemical composition. SI index of the carbonate and sulfate minerals fall saturated to undersaturated state, indicating the dissolution and ion exchange process. By WQI values, 12 % of the groundwater samples fall at poor to very poor quality in the southern part of the study area. This contrast in hydrochemistry is attributed to the heavy population in the southern side and to subsequent over-exploitation of groundwater. However, irrigation indices (SAR, Na%, PI. RSC and MH) represent that 70 % of groundwater samples were suitable for agricultural purpose. Wilcox plot indicates most of the samples fall in C2S1 sector. It is due to the long residence time of water, dissolution of minerals from lithological composition, seawater intrusion and domestic, industrial waste waters. This study results will be very helpful for sustainable management of groundwater resources, and it will enable planners and policymakers to evolve a strategy to solve similar problems elsewhere.
References
Anithamary, I., Ramkumar, T., Venkatramanan, S. (2012). Application of statistical analysis for the hydrogeochemistry of saline groundwater in Kodiakarai, Tamilnadu, India. Journal of Coastal Research, 28, 89–98.
APHA. (1995). Standard methods for the examination of water and wastewater (19th ed.). New York: American Public Health Association.
Brindha, K., & Elango, L. (2012). Impact of tanning industries on groundwater quality near a metropolitan city in India. Water Resource Management, 26, 1747–1761.
Brindha, K., & Elango, L. (2013a). Soil and groundwater quality with reference to nitrate in a semiarid agricultural region. Arabian Journal of Geosciences. doi:10.1007/s12517-013-1100-5.
Brindha. K., & Elango, L. (2013b). Geochemistry of fluoride rich groundwater in a weathered granitic rock region, southern India. Water Quality, Exposure and Health. doi:10.1007/s12403-013-0096-0.
Brindha, K., Neena Vaman, K. V., Srinivasan, K, Sathis Babu, M., & Elango, L. (2013). Identification of surface water-groundwater interaction by hydrogeochemical indicators and assessing its suitability for drinking and irrigational purposes in Chennai, Southern India. Applied Water Science. doi:10.1007/s13201-013-0138-6.
Cederstorm, D. J. (1946). Genesis of groundwater in the coastal plain of Virginia. Environmental Geology, 41, 218–245.
Chae, G. T., Yun, S. T., Choi, B. Y., Yu, S. Y., Jo, H. Y., Mayer, B., et al. (2008). Hydrochemistry of urban groundwater, Seoul, Korea: The impact of subway tunnels on groundwater quality. Journal of Contaminated Hydrology, 101, 42–52.
Chang, T. W., Kang, P. C., Park, S. H., Hwang, S. K., & Lee, D. W. (1983). The geological map of Busan and Gadeog (1:50,000). Korea Institute of Energy and Resources. 22.
Chung, S. Y., Kim, T. H., & Park, N. (2012). The influence of the surrounding groundwater by groundwater discharge from the subway tunnel at Suyeong district, Busan city. Journal of Soil and Groundwater Environment, 17, 28–36.
Craig, E., & Anderson, M. P. (1979). The effects of urbanization of ground water quality. A case study of ground water ecosystems. Environmental Conservation, 30, 104–130.
Dar, I. A., Sankar, K., & Dar, M. A. (2011). Spatial assessment of groundwater quality in Mamundiyar basin, Tamil Nadu, India. Environmental Monitoring and Assessment, 178, 437–447.
Domenico, P. A. (1972). Concepts and models in groundwater hydrology. New York: McGraw-Hill.
Fisher, S. R., & Mullican, W. F. (1997). Hydrogeochemical evolution of sodium-sulfate and sodium-chloride groundwater beneath the northern Chihuahua desert, Trans-Pecos, Texas, USA. Journal of Hydrogeology, 5, 4–16.
Gibbs, R. J. (1970). Mechanisms controlling world water chemistry. Science, 170, 1088–1090.
Gunduz, O., Simsek, C., & Hasozbek, A. (2009). Arsenic pollution in the groundwater of Simav Plain, Turkey: its impact on water quality and human health. Water, Air, and Soil pollution, 205, 43–62.
Gupta, S., Dandele, P. S., Verma, M. B., & Maithani, P. B. (2009). Geochemical assessment of groundwater around Macherla-Karempudi Area, Guntur District, Andhra Pradesh. Journal Geological Society of India, 73, 202–212.
Gupta, S. K., Gupta, R. C., Chhabra, S. K., Eskiocak, S., Gupta, A. B., & Gupta, R. (2008). Health issues related to N pollution in water and air. Current Science, 94, 1469–1477.
Hosono, T., Ikawa, R., Shimada, J., Nakano, T., Saito, M., Onodera, S. I., et al. (2009). Human impacts on groundwater flow and contamination deduced by multiple isotopes in Seoul City, South Korea. Science of the Total Environments, 407, 3189–3197.
Kim, Y. Y. (2004). Analysis of hydrochemical processes controlling the urban groundwater system in Seoul area, Korea. Geoscience Journal, 8, 313–318.
Kim, H., & Chung, S. Y. (2011). Application of Multivariate statistical analysis for the evaluation of groundwater contamination characteristics at the Suyeong-gu of Busan city, Korea. Journal of the Geological Society of Korea, 47, 45–58.
Kim, S. J., Hyun, Y., & Lee, K. K. (2005). Time series modeling for evaluation of groundwater discharge rates into an urban subway system. Geoscience Journal, 9, 15–22.
Kim, Y. Y., Lee, K. K., & Sung, I. H. (2001). Urbanization and the groundwater budget, metropolitan Seoul area, Korea. Hydrogeology Journal, 9, 401–412.
Kraiem, Z., Zouari, K., Chkir, N., & Agoune, A. (2013). Geochemical characteristics of arid shallow aquifers in Chott Djerid, south-western Tunisia. Journal of Hydro-environment Research, doi:10.1016/j.jher.2013.06.002.
Lermontov, A., Yokoyama, L., Lermontov, M., & Machado, M. A. S. (2009). River quality analysis using fuzzy water quality index: Ribeira do Iguape river watershed, Brazil. Ecological Indicators, 9, 1188–1197.
Liu, C. W., Jang, C. S., Chen, C. P., Lin, C. N., & Lou, K. L. (2008). Characterization of groundwater quality in Kinmen Island using multivariate analysis and geochemical modelling. Hydrological Processes, 22, 376–383.
Lloyd, J. W., & Heathcode, J. A. (1985). Nature inorganic hydrochemistry in relation to groundwater. New York: Oxford University Press.
Madhavan, N., & Subramanian, V. (2006). Environmental impact assessment including evolution of fluoride and arsenic contamination process in groundwater and remediation of contaminated groundwater system. In: M. Thangarajan (Ed.), Sustainable development and mangement of groundwater reserve (pp. 128–155). New Delhi: Capital Publishing Company.
Mamatha, P., & Rao, S. M. (2009). Geochemistry of fluoride rich ground-water in Kolar and Tumkur Districts of Karnataka. Environmental Earth Sciences, 61, 131–142.
Miller, G. T. (1979). Living in the environment. Belmond, CA: Wadsworth Publishing Company.
Mitra, B. K. (1998). Spatial and temporal variation of ground water quality in sand dune area of aomori prefecture in Japan. Paper number 062023, 2006 ASAE Annual Meeting.
Namibian, M. (2007). A new Water Quality Index for environmental contamination contributed by mineral processing: a case study of Amang (tin tailing) processing activity. Journal of Applied Sciences, 7, 2977–2987.
Ozcan, H., Ekinci, H., Baba, A., Kavdır, Y., Yuksel, O., & Yigini, Y. (2007). Assessment of the water quality of Troia for the multipurpose usages. Environmental Monitoring and Assessment, 130, 389–402.
Piper, A. M., (1953). Agraphic procedure I the geo-chemical interpretation of water analysis. USGS Groundwater Note no. 12.
Prasanna, M. V., Chidambaram, S., Senthil Kumar, G., Ramanathan, A. L., & Nainwal, H. C. (2011). Hydrogeochemical assessment of groundwater in Neyveli Basin, Cuddalore district, South India. Arabian Journal of Geosciences, 4, 319–330.
Ragunath, H. M. (1987). Groundwater (p. 563). New Delhi: Wiley Eastern Ltd.
Rajesh, R., Brindha, K., Murugan, R., & Elango, L. (2012). Influence of hydrogeochemical processes on temporal changes in groundwater quality in a part of Nalgonda district, Andhra Pradesh, India. Environmental Earth Sciences, 65, 1203–1213.
Rajmohan, N., & Elango, L. (2004). Identification and evolution of hydrogeochemical processes in the groundwater environment in an area of the Palar and Cheyyar River Basins, Southern India. Environmental Geology, 46, 47–61.
Ramkumar, T., Venkatramanan, S., Anithamary, I., & Ibrahim, S. (2011). Evaluation of hydrogeochemical parameters and quality assessment of the groundwater in Kottur blocks, Tiruvarur district, Tamilnadu, India. Arabian Journal of Geosciences, 6, 101–108.
Sadashivaiah, C., Ramakrishnaiah, C. R., & Ranganna, G. (2008). Hydrochemical analysis and evaluation of groundwater quality in Tumkur Taluk, Karnataka State, India. International Journal of Environmental Research and Public Health, 5, 158–164.
Sajil Kumar, P. J., Elango, L., James, E. J. (2013). Assessment of hydrochemistry and groundwater quality in the coastal area of South Chennai, India. Arabian Journal of Geosciences. doi:10.1007/s12517-013-0940-3.
Sawyer, G. N., & McCarthy, D. L. (1967). Chemistry of sanitary engineers (2nd ed., p. 518). New York: McGraw Hill.
Schoeller, H., (1965). Qualitative evaluation of groundwater resources. In: Methods and techniques of groundwater investigations and development. UNESCO, pp. 54–83.
Schoeller, H. (1967). Geochemistry of groundwater-an international guide for research and practice, Chapter 15 (pp. 1–18). Paris: UNESCO.
Shim, B. Y., Chung, S. Y., Kim, H. J., & Sung, I. H. (2004). Intrinsic random function of order k-kriging of electrical resistivity data for estimating the extent of saltwater intrusion in a coastal aquifer system. Environmental Geology, 46, 533–541.
Shim, B. Y., Chung, S. Y., Kim, H. J., Sung, I. H., & Kim, B. W. (2002). Characteristics of Sea water intrusion using geostatistical analysis of geophysical surveys at the southeastern coastal area of Busan, Korea. Journal of Soil and Groundwater Environment, 7, 3–17.
Simoes, F. S., Moreira, A. B., Bisinoti, M. C., Gimenez, S. M. N., & Yabe, M. J. S. (2008). Water Quality Index as a simple indicator of aquaculture effects on aquatic bodies. Ecological Indicators, 8, 476–484.
Singh, A. K., Mondal, G. C., Kumar, S., Singh, T. B., Tewary, B. K., & Sinha, A. (2008). Major ion chemistry, weathering processes and water quality assessment in upper catchment of Damodar River basin, India. Environmental Geology, 54, 745–758.
Son, C. M, Lee, S. M., Kim, Y. K., Kim, S. W., Kim, H. S. (1978). The Geological Map of Dongrae and Weolnae (1:50,000). Korea Research Institute of Geoscience and Mineral Resources.
Srinivasamoorthy, K., Vasanthavigar, M., Vijayaraghavan, K., Sarathidasan, R., & Gopinath, S. (2011). Hydrochemistry of groundwater in a coastal region of Cuddalore district, Tamilnadu, India: Implication for quality assessment. Arabian Journal of Geosciences,. doi:10.1007/s12517-011-0351-2.
Subramani, T., Rajmohan, N., & Elango, L. (2009). Groundwater geochemistry and identification of hydrogeochemical processes in a hard rock region, Southern India. Environmental Monitoring and Assessment doi:10.1007/s10661-009-0781-4.
Todd, D. K. (1980). Ground water hydrology (p. 535). New York: Wiley.
Trusdell, A. H., & Jones, B. F. (1973). Wateq: A computer program for calculating chemical equilibria of natural waters. National Technical Information Service, VA, USA.
Umar, R., Ahmed, I., Alam, F., & Khan, M. M. (2009). Hydrochemical characteristics and seasonal variations in groundwater quality of an alluvial aquifer in parts of Central Ganga Plain, Western Uttar Pradesh, India. Environmental Geology, 58, 1295–1300.
USEPA. (2003). Human health toxicity values in super- fund risk assessments. OSWER Directive 9285.753. December 5, 2003.
Vasanthavigar, M., Srinivasamoorthy, K., Vijayaragavan, K., Rajiv Ganthi, R., Chidambaram, S., Anandhan, P., et al. (2010). Application of water quality index for groundwater quality assessment: Thirumanimuttar sub-basin, Tamilnadu, India. Environmental Monitoring and Assessment, 171, 595–609.
Venkatramanan, S., Chung, S. Y., Ramkumar, T., Gnanachandrasamy, G., & Vasudevan, S. (2013). A multivariate statistical approaches on physicochemical characteristics of groundwater in and around Nagapatttinam district, Cauvery deltaic region of Tamil Nadu, India. Earth Science Research Journal, 17, 97–103.
Venkatramanan, S., Ramkumar, T., & Anithamary, I. (2012). A statistical approach on hydrogeochemistry of groundwater in Muthupet coastal region, Tamilnadu, India. Carpathian Journal of Earth and Environmental Sciences, 7, 47–54.
Vikas, C., Kushwaha, R. K., & Pandit, M. K. (2009). Hydrochemical status of groundwater in district Ajmer (NW India) with reference to fluoride distribution. Journal Geological Society of India, 73, 773–784.
WHO. (2004). Guidelines for drinking water quality V.1 Recommendations (p. 130). Switzerland: Geneva.
Wilcox, L. V. (1955). Classification and use of irrigation water. US Geol Dep Agri Arc, 969, 19.
Younger, P., & Casey, V. (2003). A simple method for determining the suit- ability of brackish groundwaters for irrigation. Waterlines, 22, 11–13.
Zahid, A., Hassan, M. Q., Balke, K. D., Flegr, M., & Clark, D. W. (2008). Groundwater chemistry and occurrence of arsenic in the Meghna floodplain aquifer, southeastern Bangladesh. Environmental Geology, 54, 1247–1260.
Zhu, C., & Schwartz, W. (2011). Hydrogeochemical processes and controls on water quality and water management. Elements, 7, 169–174.
Acknowledgments
This work was supported by a Research Grant of Pukyong National University (2014 Year). The manuscript was greatly benefited from the constructive comments of an anonymous reviewer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chung, S.Y., Venkatramanan, S., Kim, T.H. et al. Influence of hydrogeochemical processes and assessment of suitability for groundwater uses in Busan City, Korea. Environ Dev Sustain 17, 423–441 (2015). https://doi.org/10.1007/s10668-014-9552-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10668-014-9552-7