Abstract
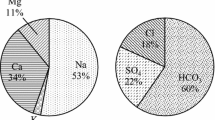
A study was conducted to evaluate the water quality of Kottur block, Thiruvarur district, Tamilnadu. Groundwater samples from hand pumps and tube wells of 16 stations were analyzed during postmonsoon and premonsoon (2008) with the help of standard methods of APHA (1995). Dominance of cations are in the following order Na>Ca>K>Mg and Cl>SO4>HCO3>NO3 by anions in both the seasons. The analytical results shows higher concentration of total dissolved solids, electrical conductivity, sodium, chloride, and sulfate which indicate signs of deterioration but values of pH, calcium, magnesium, and nitrate are within permissible limit as per World Health Organization standards. From the Piper trilinear diagram, it is observed that the majority of groundwater samples are Na-Cl and Ca-Mg-SO4 facies clearly indicates seawater incursion. In Wilcox diagram, most of the samples fall in low to very high sodium hazard and low to very high salinity hazard indicates moderately suitable for agricultural activities. Kelly’s ratio and magnesium ratio indicates most of the samples fall in suitable for irrigation purpose.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water is very vital for nature and can be a limiting resource to men and other living beings. Without a well-functioning water supply, it is difficult to imagine productive human activity be it agriculture or livestock. The quality of water is of almost the same importance to quantity in any water supply planning. Water quality is influenced by natural and anthropogenic effects including local climate, geology, and irrigation practices. The chemical character of any groundwater determines its quality and utilization. The quality is a function of the physical, chemical, and biological parameters, and could be subjective since it depends on a particular intended use. Various workers in our country have carried out extensive studies on water quality. In addition to the rising demands of potable water in households, the shallow aquifers have been the source of water for most irrigation schemes in Keta and surrounding areas. Whereas irrigation activities are very much encouraged as poverty reduction strategies, the use of high salinity water has the tendency to reduce soil and crop productivity in an area over time by destroying soil permeability and reducing the osmotic potential of crops. In light of this, one of the management strategies for groundwater resources especially in the coastal belt is to ensure that groundwater extraction does not lead to the intrusion of saline seawater into the aquifers. However, due to the relatively low elevations in the area and the lack of strict regulation, groundwater salinization continues to be a problem in some of the coastal areas (Bannerman 1994; Jorgensen and Banoeng-Yakubo 2001; Helstrup et al. 2007). On the other hand, this resource is the only source of water for agricultural activities in view of the erratic nature of rainfalls in recent times. The high salinities of groundwater from some of the wells in the area and the heavy reliance of the communities on groundwater for irrigation necessitate an overall assessment of the quality of the resource in the area for irrigation purposes (Yidana et al. 2010; Ramkumar et al. 2010; Venkatramanan et al. 2009). Laluraj et al. (2005) have studied groundwater chemistry of shallow aquifers in the coastal zones of Cochin and concluded that groundwater present in the shallow aquifers of some of the stations were poor in quality and beyond potable limit as per the standard set by World Health Organization and Bureau of Indian Standards. Rapid increase in urbanization and industrialization leads into deterioration in groundwater quality.
In coastal environments, the influence of saltwater intrusion is often significant. The specific objectives of this study are (1) the preliminary investigation and interpretation of the groundwater quality of Kottur block (2) find out the suitability of groundwater for irrigation and drinking purposes. This insight hopefully would aid in ensuring better water resource management in Kottur and its immediate environs. (3) This study proposes using spatial mapping to identify areas that are likely to be contaminated, and to use spatial variance of collected data from the field to identify areas that are not covered and lack information. With the help of GIS tools, this approach has been used to assess the sodium and chloride variation in Kottur block, India.
Description of the study area
The study area, Kottur block is situated in Tiruvarur district of Tamilnadu (Fig. 1). The study area lies between latitude 10° 41′45″ North and longitude 79° 25′00″ East in the survey of India toposheets number 58 N/7, N/10, and N/11. The area receives an annual average rainfall of 1,327.11 mm. The maximum and minimum temperature ranges between 28°C and 34°C in the months of January to May, respectively.
Materials and methods
Groundwater samples from different hand pumps and tube wells of 16 sampling points were analyzed during post and premonsoon seasons during 2008. Samples were collected in good quality polyethylene bottles of 1-l capacity. Sampling was carried out without adding any preservatives in rinsed bottles directly for avoiding any contamination and brought to the laboratory. Monitoring was done during post and premonsoon (February 2008–September 2006). Only high pure (Analytical IR Grade) chemicals and double-distilled water was used for preparing solutions for analysis. Physical parameters like pH (eco-tester pH 1) and total dissolved solids (TDS tester 11) were determined at the site with the help of field kit. The samples collected were analyzed as per standard procedures (APHA 1995). The results of the physicochemical parameters of the samples are shown in Table 1.
Results and discussion
Major ion chemistry
The pH values of the groundwater varied from 7.6 to 8.4 (postmonsoon) with an average value 8.1, 7.7 (premonsoon) to 8.3 with an average value 8.0 which indicates that water is alkaline in nature. These variations are slightly higher than the permissible limit of drinking water standard. The general increase of pH in a sedimentary terrain relates to weathering of plagioclase feldspar in the sediments, aided by dissolved atmospheric carbon dioxide resulting in the release of sodium and calcium which progressively increases the pH and alkalinity of the water. The electrical conductivity average values were found between 2,992 (postmonsoon) and 2,894 (premonsoon) μS/cm at 25°C in the study area. The relatively higher values of electrical conductivity in the study area can be attributed to the higher amount of total dissolved salts in groundwater and the source for that may be the salts from the seawater This is similar to the observation of Tutmet et al. (2006) who made modeling studies on electrical conductivity of groundwater. The concentration of total dissolved solids average ranged from 2,018 (postmonsoon) to 1,997 (premonsoon) mg/l in the study area. Normally, total dissolved solids in water may originate from natural sources and sewage discharges. Calcium and magnesium ions present in the coastal groundwater is particularly derived from leaching of limestone, dolomites, gypsum, and anhydrites, whereas calcium ions has the possibility to derive from cation exchange process (Garrels 1976). Calcium concentration ranged from 40 to 280 and 60 to 280 mg/l in postmonsoon and premonsoon seasons, respectively. The sodium average concentration varies from 349 (postmonsoon) to 353 (premonsoon) mg/l in the study area. The high concentration of Na in the groundwater can be attributed to the cation exchange and to the human activities. High concentration of Na+ in irrigated area are also the result of the repeated use of water. The Na/Cl relationship has often been used to identify the mechanism for acquiring salinity and saline incursion in coastal regions. Bicarbonate ion varied from 122 to 274.5 and 91.5 to 268.4 mg/l in the groundwater samples during postmonsoon and premonsoon seasons. Chloride concentration of groundwater samples in the study average ranged between 611.4 (postmonsoon) and 639.5 (premonsoon) mg/l. Relatively, higher Cl concentration in groundwater in the coastal region is attributed to influence of seawater on the coastal aquifer which was highly visible during premonsoon due decline water table. Sulfate ion varied from 566 to 457 mg/l during the post and premonsoon seasons and nitrate ion varied from 1.9 to 4.07 mg/l in the study area. During the premonsoon season, most of the ion concentrations are high compared to the postmonsoon season which may be due to the dissolution of minerals (Navarro and Carnonal 2007; Anithamary 2008). The conspicuous variation observed for this parameter was mainly by the influence of agricultural activity and by the influence of seawater into the shallow aquifer system.
Spatial distribution pattern
The spatial distribution pattern of electric conductivity and total dissolved solids during the study period is shown in Figs. 2a, b and 3a, b. The exception is during the postmonsoon season when a higher concentration of TDS and higher electrical conductivity (EC) were observed in the northeast direction. This spatial pattern clearly indicates that large values of TDS and EC in groundwater correspond to the influence of seawater in some coastal wells. This phenomenon is widely observed throughout the world in freshwater aquifers lying close to the sea coast. In premonsoon season, EC and TDS were observed in the southern part of the study area indicating the influence of agricultural activity. The spatial distribution pattern of sodium during the postmonsoon season (Fig. 4a, b) shows a higher concentration on the north and south sides. These well lie near the coastal area, and seawater influences the distribution pattern. The spatial distribution of chloride during the study period is shown in Fig. 5a, b and is similar to the spatial distribution of sodium. During the postmonsoon season, higher concentrations of chloride are observed on the northeastern coastal side. During the premonsoon seasons, high levels of chloride are observed on the southwest side. On the southern and northern sides, the spatial distribution of sodium and chloride is determined by seawater intrusion; irrigation return flow from agricultural activity also plays a significant role in determining the sodium and chloride content.
Hydrogeochemical nature
Hydrogeochemical facies are distinct zones that possess cation and anion concentration categories (Freeze and Cherry 1979). The interpretation of distinct facies from the 0% to 10% and 90% to 100% domains on the diamond-shaped cation and anion graph is more helpful than using equal 25% increment. This is useful to understand the total chemical character of water samples in terms of cation–anion pairs. The percentage-reacting values at the three cation groups Ca, Mg, and (Na + K) are plotted as a single point in the left triangular field and the three anion groups—(HCO3 + CO3), SO4, and Cl similar on the right triangular field. Piper (1953) classification is used to express similarity and dissimilarity in the chemistry of different water samples based on the dominant cations and anions.
Major cations and anions such as Ca2+, Mg2+, Na+, K+, HCO −3 , SO 2−4 , and Cl− in meq/l were plotted in Piper’s trilinear diagram to evaluate the hydrochemistry of groundwater of Kottur City with the help of Aquachem 4.1 software (Fig. 6a, b). The plot shows that most of the groundwater samples fall in the field of Na–Cl facies. The remaining samples fall in Ca Mg SO4 facies, it clearly indicates the presence of seawater incursion and hard water in the study area.
Kelley’s ratio
The level of sodium measured against calcium and magnesium is known as Kelley’s (1997) ratio index. Kelley’s ratio is used to find whether the groundwater is suitable for irrigation or not. All concentration values are expressed in equivalents per million. Kelly’s ratio is calculated as follows:
In the study area, majority of the samples in all seasons found to be suitable for irrigations with respect to Kelley’s ratio.
Magnesium ratio
Magnesium ratio is the excess amount of magnesium over calcium and magnesium amount. Magnesium present in water would adversely affect soil quality, rendering it alkaline; thus, resulting in decreased crop yields for magnesium ratio with more than 50% can poison the water. All concentrations are epm. Magnesium ratio is calculated as follows:
During postmonsoon, it varied from 16.25 to 61.8 with mean of 47.1 and Premonsoon, it varied from 26.10 to 66.42. Based on magnesium ratio, the most of the samples found to be unfit for irrigations with respect to magnesium ratio (Table 2).
Wilcox classification
Wilcox (1955) classification, the groundwaters in the study area are ranging between good to permissible for irrigation uses. The primary effect of high EC reduces the osmotic activity of plants and thus interferes with the absorption of water and nutrients from the soil.
Throughout the study period, Wilcox classification indicates that groundwater is low to very high sodium hazard and low to very high salinity hazard, and was observed to be moderately suitable for agricultural activities (Fig. 7a, b)
Conclusion
Interpretation of hydrogeochemical analysis reveals that the groundwater of study area is slightly saline and alkaline in nature. In postmonsoon, the higher concentration of ions was observed in northeast side indicates saline water incursion near the coastal area. During premonsoon, the higher concentration was observed in southern part indicates irrigation return flow into the coastal aquifer. The trilinear diagram shows that most of the groundwater samples fall in the field of Na-Cl and Ca-Mg-SO4 facies. Kelly’s ratio indicates that most of the samples fall in suitable category and unfit zone in Mg ratio. The Wilcox diagram illustrates that most of the groundwater samples fall in the field of C3S1, indicating low to very high sodium hazard and low to very high salinity hazard which can be used for irrigation on almost all type of soil with little danger of exchangeable sodium. The result obtained indicates that there is a greater thread of seawater incursion to the coastal groundwater aquifer system. Hence, the study has helped to improve understanding of hydrogeochemical characteristics of the area for effective management and proper utilization of groundwater resources for better living conditions of the people. A continuous monitoring program of the water quality will avoid further deterioration of the water quality in the coastal region.
References
Anithamary, I (2008) Hydrogeochemical and environmental geochemistry of water in Kodiakarai region—coastal zone of Tamilnadu. Published M. Phil thesis. Annamalai University. Annamalainagar, Chidambaram 38
APHA (1995) Standard methods for the examination of water and waste water, 19th edn. NewYork, USA
Bannerman RR (1994) Appraisal of the limestone aquifer of the Keta Basin, Ghana. In: Soveri J, Suokko T (eds) Future groundwater resources at risk. Publ. 222. International Association for Hydrological Sciences, Wallingford, pp 315–321
Bureau of Indian Standards (BIS) (1991) Indian standard specification for drinking water, IS 10500. 24
Freeze RA, Cherry JA (1979) Groundwater. Prentice Hall Inc, New Jersey, p 604
Garrels A (1976) Survey of low temperature water mineral relations. In: Interpretation of environmental isotope and hydrogeochemical data in groundwater hydrology, pp. 65–84. International Atomic Energy Agency, Vienna
Helstrup T, Jorgensen NO, Banoeng-Yakubo B (2007) Investigation of hydrochemical characteristics of groundwater from the Cretaceous–Eocene limestone in southern Ghana and southern Togo using hierarchical cluster analysis. Hydrogeology 15:977–989
Jorgensen NO, Banoeng-Yakubo BK (2001) Environmental isotopes (18O, 2 H, and 87Sr/86Sr) as a tool in groundwater investigations in the Keta Basin, Ghana. Hydrogeology 9:190–201
Kelley (1997) Heterogeneities in groundwater geochemistry of in a sand aquifer beneath an irrigated field. J Hydrol 198:254
Laluraj, Gopinath, Dineshkumar (2005) Groundwater chemistry of shallow aquifers in the coastal zones of Cochin, India. Appl Ecol Environ Res 3:133–139
Navarro A, Carnonal ME (2007) Evaluation of groundwater contamination beneath an urban environment: the Beso’s river basin (Barcelona, Spain). J Environ Manage 85:259–269
Piper (1953) A graphic procedure in the geochemical interpretation of water analyses. Trans. US Geol Survey. Groundwater Notes, 12
Ramkumar T, Venkatramanan S, Anita Mary I, Tamil Selvi M, Ramesh G (2010) hydrogeochemical quality of groundwater in Vedaraniyam town, Tamilnadu, India. Res J Environ Earth Sciences 2:44–48
Tutmet, Hatipoglu, Kaymak (2006) Modeling electrical conductivity of groundwater using an adaptive neuro-fuzzy inference system. Comput Geosci 32:421–433
Venkatramanan S, Ramkumar T, Vasudevan S, Ramesh G, Anitha Mary I, Vijayakumar V (2009) Assessment of Hydrogeochemistry of groundwater in Muthupet coastal region, Tamilnadu, India. Int J Appl Environ Sci 4:169–176
Wilcox (1955) Classification and use of irrigation waters. US Department of Agriculture, Washington, p 969
World Health Organization (WHO) (1984) Guidelines for drinking water 1: 52–82
Yidana SM, Banoeng-Yakubo B, Akabzaa TM (2010) Analysis of groundwater quality using multivariate and spatial analyses in the Keta basin, Ghana. J Afr Earth Sci 58:220–234
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramkumar, T., Venkatramanan, S., Anithamary, I. et al. Evaluation of hydrogeochemical parameters and quality assessment of the groundwater in Kottur blocks, Tiruvarur district, Tamilnadu, India. Arab J Geosci 6, 101–108 (2013). https://doi.org/10.1007/s12517-011-0327-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12517-011-0327-2