Abstract
The spatiotemporal distribution of potentially toxic species was studied in the topsoil of parks, playgrounds, and surrounding crop fields of Marcos Juarez City in Córdoba province, Argentina. The content of available metals and of some pesticides used in the region was determined. The mean values of available metal concentrations in all samples, expressed in mg kg−1, were 7.99 ± 6.58, 0.89 ± 0.71, 0.35 ± 0.26, and 1.50 ± 1.40 for Pb, Cr, Cd, and Ni, respectively. Pearson’s correlation coefficients, coefficient of variation (%), and principal component analysis were used to explore whether variations in metal content were associated with anthropogenic factors. Agrochemicals such as lindane, chlorobenzilate, endosulfan, endrin, permethrin, and chlorpyrifos were found in crop field soil samples. In turn, chlorothalonil, chlordanes, methoxychlor, DDT, permethrin, and chlorpyrifos were detected in park and playground soil samples. The degree of pollution with possible effects on environmental health was evaluated using the Nemerow integrated pollution index (NIPI) and the modified degree of contamination (mCd). In some campaigns, the values obtained from crop fields ranged from low to high pollution levels during periods of agrochemical application. Noteworthy, in periods of low agrochemical application in crop fields, a high level of pollution was observed in parks and playgrounds. For children, the hazard index (HI) values were higher than the threshold value of 1, suggesting a potential health risk. This study provides valuable information regarding land management practices and highlights the importance of monitoring and implementing policies to reduce human health risks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As a result of industrialization and the global increase in crop production, pollutant emissions have substantially increased in developing countries. In Argentina, the cultivated area for grain production has significantly increased in the last 40 years. For example, soybean fields expanded from 34,700 ha in 1969/1970 to about 18 million ha in 2011/2012 (Butinof et al., 2014). Consequently, there has been a growing interest in learning about the effect of agricultural practices on ecosystems. Moreover, different health and environmental risks are associated with this activity (Atabila et al., 2018; Primost et al., 2017; Yadav et al., 2016; Zeng et al., 2019). From an ecological perspective, there is concern about soil degradation, i.e., loss of organic matter and nutrients; erosion; and diversity decline, but the main problems related to agricultural activities are animal waste and the use of fertilizers and pesticides (Butinof et al., 2014; Chen et al., 2011; Rissato et al., 2006).
In the last 30 years, the use of pesticides has greatly contributed to the increase in food quantity and quality to meet the demands of the growing world population. Even so, pesticides are considered a hazardous group of contaminants for humans, fauna, and the environment (Iturburu et al., 2019). Most of such substances are applied directly to the soil or sprayed over crop fields and, hence, released directly to the environment. Heavy metals including copper, lead, cadmium, nickel, and chromium have been found in contaminated soils due to anthropogenic activities, such as vehicle use (leaded gasoline), petrochemicals, and agriculture (application of fertilizers, sewage sludge, and pesticides). Thus, metals can be considered potent tracers for monitoring the anthropogenic impact by studying their temporal and spatial variations due to natural changes in soils and anthropogenic activities (Hong-Gui et al., 2012; Li et al., 2012; Zhang et al., 2019).
The possible effects of these dangerous chemical species on human health, when found in the soil, have been widely discussed (National Research Council, 2003; Núñez-Gastélum et al., 2019; Pan et al., 2018; Plumlee et al., 2007). In the short or long term, these pollutants could impact both human and animal health due to different modes of exposure, such as passive ingestion of soil and/or dermal contact, with young children especially at risk (Frimpong & Koranteng, 2019; Kamunda et al., 2016; Madrid et al., 2008; Ryan et al., 2004; van Wijnen et al., 1990). In turn, suspended atmospheric particles, which are usually less than 63 μm in size, may be important in the transport of environmental contaminants, particularly those that remain attached to soil particles. Moreover, recent epidemiological studies have provided strong evidence of the association between the concentration of airborne particles and adverse health effects (Aparicio et al., 2018; Pope et al., 2002).
The impact of these potentially toxic species on human, plant, or animal life is quantitatively assessed via baseline measurements of total soil chemistry and an estimation of the available amounts of these contaminants to be absorbed by different organisms (external bioavailability) and incorporated into their metabolism. This means that the mass fraction of the pollutant in soil or sediment is readily available for receptors, including humans, through the gastrointestinal tract, lungs, or skin. One of the methods to evaluate external metal bioavailability is sequential extraction, which was originally developed on the basis of the SM&T protocol (Standard, Measurements and Testing, formerly known as BCR) in Europe and the SBRC (Solubility/Bioavailability Research Consortium) (Dean, 2007; McAllister et al., 2010).
The objectives of this study are (1) to provide information about the content and spatiotemporal distribution of agrochemicals and metals in typical crop agricultural land and its influence on urban soils, (2) to contribute to the knowledge of the actual pollution levels of the soils in the study area, and (3) to evaluate the possible health risk of metals and agrochemicals in soils. This is the first study of its kind in Argentina and may provide an insight into the state of the soil and associated human health problems. Agrochemicals may pose a risk to the world’s urban and agricultural soils, especially if there is a lack of regulation and proper land management.
Materials and methods
Study area
The study region includes the metropolitan area of Marcos Juarez City in southeast Córdoba province, central Argentina. This province, which covers a surface of 165,321 km2, has been strongly associated with agricultural activities for the last 30 years. The climate is temperate, continental, and subtropical, with a semiarid tendency, an average annual temperature of about 18 °C, and an average annual rainfall of 900–500 mm (Rodriguez et al., 2011).
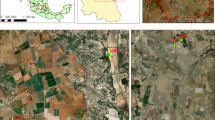
Marcos Juarez City is known for its sustained economic growth related to farming activities and agribusiness. Several sampling points were selected for this study, located in urban parks (site 39), playgrounds (sites 37, 38, and 40), and surrounding fields (sites 33, 34, 35, and 36). The location of each sample point was recorded using GPS (Fig. 1).
Sample collection and treatment
Crop cycles were taken into account when defining the seasons of pesticide application considered in this work. Topsoil samples were obtained from Marcos Juarez urban area and surrounding crop fields in December 2010 (campaign C1), April 2011 (campaign C2), September 2011 (campaign C3), March 2012 (campaign C4), September 2014 (campaign C6), February 2015 (campaign C7), May 2015 (campaign C8), December 2015 (campaign C9), and June 2016 (campaign C10). Sample names are hereafter followed by the campaign number (e.g. 39-C1 corresponds to a field sample taken from point 39 and collected in campaign C1).
For each sampling point, a composite topsoil sample was obtained consisting of approximately 2 kg of surface soil (0–5 cm) taken from 10 random subsamples. Samples were obtained using plastic tools, transported in polyethylene bags, and placed in a refrigerator. The samples were mixed in the lab, air-dried at room temperature, crushed, and sieved to obtain particle sizes of less than 63 μm. Finally, sieved samples were kept in a freezer until analysis.
Soil physicochemical parameters
The following soil physicochemical parameters were measured: grain size, humidity, pH, organic carbon (Corg), and inorganic carbon (Cinorg). The pH has a great influence on the solubility of many important elements in the soil and on the availability of plant nutrients (Harrison, 2007). Thus, soil pH was measured at a 1:2.5 soil/water ratio using a pH meter with a glass electrode.
The heavy metals present in the soil solution are positively charged inorganic ions that can be adsorbed to the organic matter surface (negatively charged) by electrostatic forces, forming complexes. Furthermore, the soil requires a certain amount of carbonates to maintain its structure, but not more than a certain amount, which reduces the soil’s ability to provide nutrients. The presence of carbonates guarantees high pH values that favor the precipitation of heavy metals (Alloway, 2010; Gustafsson et al., 2003).
All soil samples were pretreated according to the Handbook of Soil Analysis (Pansu & Gautheyrou, 2006). Briefly, 30 mL of HCl (1 mol L−1, J.T. Baker, USA) was added to 1 g of a previously dried and sieved sample (size < 63 μm) in order to eliminate carbonates. After that, 30 mL of 30% H2O2 was incorporated to remove the organic matter. Finally, 15% sodium hexametaphosphate ((NaPO3)6) was used as a dispersing solution. The samples were analyzed by LASER dispersion with a Horiba® LA-950 device in order to determine particle size.
Metal analysis
The availability of metals in soils is commonly evaluated using a set of standardized extraction methods (Dean, 2007; McAllister et al., 2010), which allow separation of the chemical species present in the soil into different fractions (exchangeable, reducible, oxidizable, and/or total) (Juhasz et al., 2009; Makris et al., 2008; Poggio et al., 2009; Rosende & Miró, 2013; Sialelli et al., 2011; Thums et al., 2008; Turner & Ip, 2007).
Reagents and solutions for metal determination
Different solutions were used for the experiments in applying the simBCR methodology described below: (1) Solution “A” (0.1 mol L−1 acetic acid): 25 mL of glacial acetic acid (Sigma-Aldrich, USA) was diluted with ultrapure water (MilliQ water) in a 1 L volumetric flask. Part of this solution (250 mL) was taken and diluted to 1 L in order to obtain an acetic acid solution of 0.11 mol L−1. (2) Solution “B”: 0.5 mol L−1 hydroxylamine hydrochloride was prepared by dissolving 3.475 g of the solute and making up to 1 L with ultrapure water. An appropriate amount of ultrapure nitric acid (2 mol L−1; Panreac, USA) was added in order to obtain a pH of 2.0.
Simplified sequential extractions (simBCR)
All the materials used in the experiments were carefully cleaned with a 5% HNO3 solution and rinsed with ultrapure water to avoid contamination. A two-stage sequential scheme employing the reagents of BCR steps (a) and (b) (termed simBCR hereafter) was used. The fractionation procedure involves four stages (Dean, 2007). Stage (a) exchangeable fraction: represents the bioavailable portion of metals released from sediments; stage (b) reducible fraction: metals like those bound to iron/manganese oxides are released; stage (c) oxidizable fraction; and stage (d) residual fraction. The present study attempted to simulate the natural (dynamic) processes of metal release into the environment (Jimoh et al., 2005). As a result, only stages (a) and (b) were applied because the oxidizable (stage c) and residual (stage d) fractions are considered not readily bioavailable and non-bioavailable, respectively (Dean, 2007). All the metals present in the first two fractions would be released in nature due to small changes in the redox-potential (Eh).
Following the simBCR protocol, in the first stage, the dried sample was weighed in a PTFE centrifuge tube, and 40 mL of solution “A” was added. The tube was shaken at 30 rpm at room temperature for 16 h in a mechanical horizontal shaker. Then, the mixture was centrifuged at 3000 rpm for 20 min. The supernatant was decanted and stored at − 20 °C in a polyethylene bottle until analysis. The solid residue was rinsed twice and shaken for 15 min with 20 mL of ultrapure water each time. In the following stage, solution “B” was added, and the procedure was repeated as described above. Each extraction batch was performed in triplicate and included a blank sample.
Instrumentation and operational conditions
A Buck 210 graphite furnace atomic absorption spectrometer (GFAAS) equipped with an autosampler was used. The traceability of the standard method for metal extraction and measurement was assessed using the BCR-701 certified reference material from the Community Bureau of Reference. Certified values were Pb, 318 ± 21; Cr, 226 ± 16; Cd, 734 ± 35; Zn, 205 ± 6; and Ni, 154 ± 9 mg kg−1. Results varied less than ± 6% from certified values. Total metal concentrations were analyzed by Actlabs in Canada. The alkaline fusion method (Li2B4O7, 1050 °C with HNO3 digestion) was used for sample treatment. In turn, inductively coupled plasma atomic emission spectrometry (ICP-AES; limit of detection: Cr and Ni 20 ppm; Pb 5 ppm; Cd 0.5 ppm) was used for sample analyses. The percentage relative standard deviation (%RSD) value was 3%. NIST 694, 696, and 1633b were used to validate the results.
Pesticide analysis
Reagents and solutions
Solvent and reagents: n-Hexane (98.5%, Sintorgan), acetone (99.5%, Sintorgan), and iso-octane (99.8%, JT Baker) were the pesticide grade solvents used. Florisil (60–100 mesh, Biopack) and anhydrous sodium sulfate (analytical reagent grade, Cicarelli) were pre-treated. Florisil was heated at 600 ± 5 °C for 120 min, while sodium sulfate was heated at 400 ± 5 °C for 6 h.
Pesticide standards: 1000 mg L−1 (Accu-Standard: M-508P-A and M-508P-B-R) in methyl tert-butyl ether was used.
P-A mixed solution: Aldrin (97% purity), α-BHC (99.5% purity), β-BHC (100% purity), γ-BHC (98.8% purity), δ-BHC (99.3% purity), p,p’DDD (98% purity), p,p’DDE (100% purity), p,p’DDT (99.7% purity), dieldrin (99.4% purity), endosulfan I (100% purity), endosulfan II (99.9% purity), endosulfan sulfate (97% purity), endrin (98% purity), endrin aldehyde (99.9% purity), heptachlor (99.8% purity), heptachlor epoxide (isomer B) (99.8% purity), methoxychlor (98.9% purity).
P-B-R mixed solution: α-chlordane (99.7% purity), γ-chlordane (99.3% purity), chlorbenzilate (100% purity), chloroneb (100% purity), chlorothalonil (98.9% purity), chlorpyrifos (100% purity), DCPA (100% purity), etridiazole (98.6% purity), hexachlorobenzene (100% purity), cis-permethrin (99.6% purity), trans-permethrin (99.6% purity), propachlor (99.8% purity), and trifluralin (98.5% purity).
Pesticide standard solution: 1 mg L−1 of P-A and P-B-R standard solutions were made up to 10 mL with isooctane to obtain the pesticide standard stock solution.
Pesticide working solutions: 4 to 100 μgL−1 were prepared by diluting an appropriate volume of pesticide standard solution to generate the calibration curve.
Pesticide extraction procedure
The US-EPA Soxhlet extraction procedure (US-EPA, 1996) consisted of placing 10 g of anhydrous sodium sulfate and 10 g of soil sample in an extraction thimble. Next, the extraction solvent (300 mL of hexane/acetone 1:1 v/v) was placed in a 500-mL round-bottom flask containing one or two clean boiling chips. The flask was attached to the extractor, and the extraction was carried out at 5 cycles per hour for 16 h. The extract was reduced to 4 mL, after which a cleanup was performed prior to the analysis by gas chromatography coupled to an electron capture detector (GC-ECD).
Instrumentation and operational conditions
Organic pesticides (OPs) were identified and determined using a Thermo Finnigan Trace gas chromatograph (CG) equipped with an Electron Capture Detector (ECD), a Thermo auto-sampler, and a capillary column (HP-5 ms 50 m × 025 mm i.d., film thickness of 025 μm).Temperatures used for the injection port and the detector gas N2 were 250 °C and 290 °C, respectively. An injection volume of 1 μL was used in splitless operation mode.
Operational conditions: At the initial stage, a temperature of 100 °C was used for 1 min, which was then increased at a rate of 15 °C min−1 up to 200 °C and maintained for 1 min. After this, the temperature was raised from 200 to 250 °C at a 3 °C min−1 rate and kept for 1 min. Finally, the rate was incremented at a rate of 6 °C min−1 up to 280 °C and maintained for 4 min. Helium (99.999% purity) at a 2 mL min−1 flow rate was used as a carrier.
Individual pesticides were identified by comparing the retention times observed in both samples and the working solution. Pesticides were quantified using the five-point calibration method (from 4 to 100 μg L−1, r2 > 0.975). The limit of detection (LOD) and limit of quantification (LOQ) values ranged from 0.23 to 3.45 mg kg−1 and from 0.58 to 2.857 mg kg−1, respectively. OPs recovery varied from 48 to 106%. The identity of the studied pesticides was unequivocally confirmed using a Thermo Scientific Trace CG1300 system equipped with a GC–MS ISQ LT simple quadrupole mass spectrometer and an HP-5-MS column (60 m × 0.25 mm × 0.25 mm).
Indexes and statistical tools
The relationships among all the soil sample variables were analyzed using Pearson’s correlation coefficients. A principal component analysis (PCA) with Varimax normalized rotation was used. PCA reduced the dimensionality of the data set. In addition, this analysis allowed obtaining an appropriate visual representation of the data and provided information about potential sources of different chemicals that appeared in the samples (Massart et al., 1998; Miller & Miller, 2002).
The pollution index (PI) and the Nemerow integrated pollution index (NIPI) were applied to assess the single and combined pollution levels of metals in soils, respectively. The pollution index was calculated as follows (Du et al., 2019):
where Ci is the metal concentration in a sample, and Cb is the metal concentration of a pristine sample selected from a depth of 0.7 m that is mineralogically and texturally similar to the soils studied (Abrahim & Parker, 2007). The PI of each metal is categorized as non-pollution (PI ≤ 1), low level (1 < PI ≤ 2), moderate level (2 < PI ≤ 3), high level (3 < PI ≤ 5), and very strong level (PI > 5) of pollution. PI ≤ 1 indicates that the metal concentration was below the threshold concentration, but this does not necessarily imply that there was no effect on the sample from anthropogenic or other sources in comparison with background values.
In addition, the Nemerow Pollution index (NIPI) was calculated to estimate the combined pollution levels of metals (Jiang et al., 2014). NIPI values were calculated by the following Eq. (2):
where PIave is the mean of the PI of the set of elements studied, and PImax is the maximum value of the individual PI obtained from a single pollutant from among all those considered (Fei et al., 2019; Jiang et al., 2014; Zhao et al., 2020). NIPI values lower than 0.7 indicate no pollution, whereas a value of 0.7 < NIPI ≤ 1 represents the warning line of pollution. On the other hand, a value of 1 < NIPI ≤ 2 indicates a low level of pollution; 2 < NIPI ≤ 3 means a moderate level of pollution, and NIPI > 3 shows a high level of pollution (Jiang et al., 2014). PI and NIPI values are shown in Table 9 (see Supplementary Information).
Soil conditions during the study period were analyzed using a modified degree of contamination (mCd) (Abrahim & Parker, 2007). The mCd is also regarded as an appropriate measure to assess the effect of contamination on human health, calculated as follows:
where Cf is the ratio between the mean concentration of a pollutant i (metal or pesticide) in the soil sample and the mean background concentration in the study area (Siegel et al., 1994) and n is the number of potential contaminants analyzed. According to Abrahim and Parker (2007), the degrees of contamination based on mCd are classified as follows: very low (mCd < 1.5), low (1.5 ≤ mCd < 2), moderate (2 ≤ mCd < 4), high (4 ≤ mCd < 8), and very high (mCd > 8).
Human health risk assessment
Direct ingestion (ingest), inhalation (inh), and dermal (derm) absorption are all possible routes of exposure to soil contaminants (Liu et al., 2016). These exposure routes were considered for the non-carcinogenic effects of agrochemicals present in the topsoil in and around Marcos Juarez City. The exposure dose can be estimated by using the following equations:
where ADD is the average daily intake of an agrochemical or metal from oral, dermal, and inhalation absorption (mg kg−1 day−1); Cs is the concentration of a metal or pesticide in soil (mg kg−1); IRS is the ingestion rate of soil (mg kg−1); EF is exposure frequency (day year-1); ED is exposure duration (year); CF is a conversion factor (kg mg-1); BW is the body weight of the exposed individual (kg); AT is the time period over which the dose is averaged (day); AF is the adherence factor (mg cm−2 day−1); ABS is the dermal absorption factor (unitless); IhR is the inhalation rate (m3 day−1); PEF is the emission factor (m3 day−1); and SA is the exposed skin surface area (cm2). The values of parameters used in ADD are summarized in Table 6 (see Supplementary Information).
The hazard quotient (HQ) and hazard index (HI) were used to estimate the non-carcinogenic effects of metals and pesticides (US-EPA, 2002). The HQ for each metal and pesticide per site is calculated as follows:
where RfDk is the reference dose (mg kg−1 day−1) of a specific metal or pesticide for a single exposure pathway (k). The hazard index (HI) was calculated as the sum of HQk. The approach assumes that simultaneous subthreshold exposures to several chemicals could result in adverse health effects (US-EPA, 2002).
If HI is less than or equal to 1, little or no risk to human beings is expected, whereas if it is greater than 1, adverse effects on human health are likely (US-EPA, 2002).
Results and discussion
The concentration ranges for the different chemical species determined in the samples and their percentage coefficients of variation (CV%) are shown in Table 1.
The mean concentrations of available metal fractions followed this order: Pb > Ni > Cr > Cd. The CV% of metals in topsoil decreased in the following order: Cd (121%) > Ni (94%) > Pb (82%) > Cr (81%). These results showed an important variation (i.e., high CV%) in metal concentrations, which might be attributed to an anthropogenic origin (Huang et al., 2009; Karimi Nezhad et al., 2015). There are some extreme outliers that indicate potentially polluted spots with Pb (sample 33-C4), Ni (sample 33-C9), and OPs (samples 34-C9 and 39-C8). All pesticide concentrations varied among campaigns. In the first campaigns (C1-C4), crop field samples contained HCH, chlorobenzilate, endosulfan, and endrin, while cis/trans-permethrin, endosulfan, chlorpyrifos, chlorothalonil, and DCPA were found in the last four campaings (C6, C7, C8, and C10). On the other hand, chlorothalonil, α and γ chlordane, methoxychlor, and chlorpyrifos were found in parks and playgrounds in campaigns C4 to C6. From campaign C7 onwards, DDT, DDD, DDE, and endosulfan were detected in parks and playgrounds as well as permethrin and chlorpyrifos also found in crop fields. In campaigns C9 and C10, in particular, endosulfan, chlorpyrifos, permethrin, and chlorothalonil were found both in field samples 33 and 34 and in the nearby parks and playgrounds (39 and 40). Furthermore, these results might suggest that agricultural pesticides are reaching surrounding cities.
The coefficients of variation of OPs ranged from 13 to 277%, showing the variable management of agrochemical application (Zhang et al., 2017). The analyzed data also warns about an increase in the use of chlorpyrifos and permethrin in the last sampling campaigns. A possible explanation for this fact is the use of alternative agrochemicals for pest control due to the resistance to chemical products developed by some pests as a result of the intensive use of pesticides, as reported by the Chamber of Agricultural Safety and Fertilizers (CASAFE, 2016).
Given the current legislation, some agrochemicals tested should not have been detected in these campaigns. Therefore, the time of their application was analyzed. The ratio of pesticides and their degradation products (e.g., DDT/[DDD + DDE]) > 1 implies an ongoing use, while values < 1 entail a historical usage (Jiang et al., 2009; Liu et al., 2012; Sánchez-Palencia et al., 2015; Xiao et al., 2017; Pegoraro & Wannaz, 2019). The results of crop field samples revealed that DDT and lindane residues originated from historical application, but endosulfan was recently introduced at some sampling sites. However, in parks and playground samples, the results showed that DDT, heptachlor, and endosulfan (banned since July 2013) residues originated from fresh applications. Some authors suggest that DDT residues found in the last few years could be due to the use of dicofol acaricides (which contain traces of DDT as impurities in the manufacturing process) or to the application of this pesticide in Córdoba province to control the dengue-carrying mosquito during the sampling period (Pegoraro & Wannaz, 2019).
Physicochemical parameters
The accumulation and impact of potential contaminants depends on the physicochemical characteristics of the soil. Here, soil physicochemical parameters were obtained from sieved soil samples (Table 2) to explore possible changes in the medium throughout the study period.
In crop field samples, the pH ranged between 4.4 and 7.1, with a mean value of 6.3. This value was slightly lower than that obtained for parks and playgrounds, which varied between 6.1 and 8.7, with a mean of 7.01 (neutral). Organic matter (Corg) content in crop field samples ranged from 1.81 to 6.90. In general, the samples belonging to campaign 4 had a slightly higher content of carbonates (CInorg) than samples from the other campaigns.
Data treatment and statistical analysis
Data were classified according to the land use soil types in order to provide better information about the spatiotemporal distribution and environmental behavior of agrochemicals in a typical agricultural region. Soil types were classified as “urban soil” (public playgrounds and parks) and “agricultural soil” (crop fields). The statistics software IBM SPSS Statistics 24.0 (free trial) and InfoStat (2012) were employed to perform statistical analyses. Atypical values were detected by using the interquartile range test (Massart et al., 1998).
Pearson’s correlation analysis
Pearson’s correlation analysis was carried out considering the Cf values of different chemical species determined in soil samples (results are shown in Tables 3 and 4). The corresponding Cf values of pesticides were summed (mCdpest) instead of considering pesticide concentrations. Only values statistically significant above 0.05 and 0.01 were considered (confidence intervals at 95% and 99%). According to the correlation matrix (Tables 3 and 4), none of the physicochemical parameters had a significant correlation with the Cf of metals or pesticides.
The significantly positive metal–metal/metal-pesticide correlation could indicate a possible similar contribution source for these chemicals (Devi et al., 2013). In crop fields, a positive correlation was observed between Cr and Pb (38%) (see Table 3), whereas no significant correlation was found with the remaining metals. Nevertheless, some agrochemicals not studied here could be contributing sources of heavy metal contamination. In this sense, some authors have linked Cd, Pb, and Ni content to the use of fertilizers (Cai et al., 2012; Cheraghi et al., 2012; Giuffré de López Carnelo et al., 1997; Liu et al., 2015; Milinovic et al., 2008). Significant correlations were found between CfΣpermethrin and etridiazole (50%), CfΣpermetrin and chlorpyrifos (37%), ΣCfDDTs and chlorothalonil (95%), methoxychlor and CfΣpermethrins (83%), and endrin and chlorobenzilate (56%). Similar results were also found for Cr and heptachlor epoxide (47%), indicating that part of the Cr content could have been contributed by the formulation of this agrochemical. The significant positive correlations found between the selected pesticides indicate that pesticide sources in the soil are similar. This is not necessarily true in all the samples, due to the fact that pesticides are applied to the crops at different stages of crop cycles. Thus, these pesticides enter the soil at different periods of time. On the other hand, the values of Pearson’s correlation coefficient for urban samples (parks and playgrounds, Table 4) showed a significantly positive correlation between Cr and Ni (58%), while Cd showed a negative correlation with Cr (46%). The Cr and Ni correlation has been pointed out in soil studies carried out in agricultural regions by other authors (Cai et al., 2012). Apparently, more persistent compounds like DDT and endosulfan were found to be correlated with Ni and Cr. Moreover, CfΣDDTs correlate with CfΣendosulfans (78%). It was found that chlorpyrifos was positively correlated with Cr (50%), Pb with CfΣEndrin (63%), and Cd with chlordanes (47%). CfDCPA showed a significant correlation with chlorpyrifos (70%) and chlorothalonil (68%). Moreover, Cr, Ni, Pb, and Cd correlated with some of the pesticides analyzed, indicating that the agrochemicals used could be important contributing sources of heavy metal pollution (e.g., Cr-Chlorpyrifos [Pérez et al., 2018]). However, the metals analyzed might also have been contributed by industrial establishments located in Marcos Juarez City.
Principal component analysis
Principal component analysis (PCA) was performed considering the following variables: CfPb (Pb), CfCr (Cr), CfCd (Cd), CfNi (Ni), and mCdpest and was conducted by the method of Varimax rotation with Kaiser normalization of variables.
Crop field soil samples: The PCA results are shown in Fig. 2 and in Table 8 (see Supplementary Information). According to Kaiser’s criterion (Yeomans & Golder, 1982), the first three components with eigenvalues higher than 1.0 have dominant influences. Components I (PC1) and II (PC2) explain 55.4% of the total variance (Fig. 2), achieving 76.8% with component III (PC3). PC1 was strongly related to Pb (77%) and Cr (87%), whereas PC2 was tightly linked with Cd (76%) and Ni (87%). PC3 was most dependent on mCdpest (88%), Cd (41%) and, to a lesser extent, Pb (34%).
The scores (soil sampling sites) and loading vectors (CfPb, CfCr, CfCd, CfNi, and mCdpest) are shown in bi-plots of the rotated principal components plane (Fig. 2a, b). As can be noted, there is a certain tendency for the samples to be grouped by campaigns. The grouping is mainly due to metal content and, to a lesser degree, to pesticides. The bi-plot based on the first two components depicts samples from campaign C1 (December 2010, pesticide application period) grouped in quadrant II due to the high content of Cd (CfCd 1.1–2.3) and Ni (CfNi 1.3–1.5) (see Pearson correlation matrix in Table 3). A high Cd concentration was particularly detected in sample 33-C1 (CfCd = 2.3). Samples from campaign C2 (April 2011, post-harvest period) were grouped in quadrant I, mainly due to Cr (Cf range 2.84–4.27) and Ni (Cf range 1.75–2.05) content compared to C1. Samples from campaigns C3 and C8 (September 2011 and May 2015, pre-sowing periods) appear in quadrant III due to an important decrease in metal content. Samples from C10 (June 2016, pesticide application period for winter crop development) are clustered together in quadrant IV due to high Cd (CfCd 4.1–6.2) and Pb (CfPb 2.6–3.6) levels and their low Ni content (CfNi 0.3–0.7).
Figure 2b shows the biplot PC1 and PC3, which revealed that samples from campaign C1 (December 2010) were grouped in quadrant II due to the high content of Cd (CfCd 1.1–2.3) and mCdpest (mCdPest 11.3–18.2), which was expected since this campaign coincides with the period of pesticide application in the spring/summer. Samples from campaign C10 (June 2016, pesticide application period in winter) were grouped in quadrant I, mainly due to their Cr, Pb, and pesticide (mCdpest 5.4–28.2) content. Samples from campaigns C3 and C8 (September 2011 and May 2015, low pesticide application periods) appear in quadrant III due to an important decrease in metals and pesticide content. Meanwhile, most of the samples from C2 and C4 (April 2011 and March 2012) were grouped in quadrant IV, mainly due to an increase in Cr, Ni, and Pb.
Park and playground soil samples
The PCA results for park and playground samples are shown in Fig. 3 and in Table 8 (Supplementary Information). The first three components account for 82.1% of the total explained variance (PC1, 34.5%; PC2, 27.7%; and PC3: 20.9%). PC1 was strongly related to Pb (69%) and Cr (57%) and had a negative contribution of Cd (84%), whereas PC2 was tightly linked with Cd (74%) and Ni (94%). PC3, on the other hand, was the most dependent on mCdpest (91%).
a Principal component analysis biplot of variables (loadings) and parks and playgrounds sampled (scores) for the first two meaningful principal components (PC1-PC2). b The biplot of the principal component analysis (PCA) shows scores (parks and playground sites) on the PC1-PC3 plane. Vectors represent the loadings (variables)
In this case, the PCA also grouped samples mostly according to campaigns. The grouping is mainly due to metal content and, to a lesser extent, to pesticides. Figure 3a depicts the biplot obtained by PCA showing the distribution of soil samples and variables for the first two meaningful principal components. Samples from campaign C4 (March 2012, post-harvest period in field samples) were grouped in quadrants II and III, mainly due to Cd (CfCd 3.8–9.8) and Ni (CfNi 1.0–3.6). Figure 3a shows that samples belonging to C7 (February 2015) are detached from the rest of the samples (except 38-C8) due to their high PC2 values, which indicate high concentrations of Cr (CfCr 3.8–13.8) and Ni (CfNi 11–4.3). This is also reflected in the correlation coefficients reported in Table 4. Samples from C6 and C10 (September 2014 and June 2016, respectively) were mainly grouped in quadrant IV due, on one hand, to their high pesticide content (ΣCfpest range 93.16–228.87) and, on the other, to their Pb, Cr, and Cd concentrations. In addition, samples of parks and playgrounds from campaign C9 (December 2015) were grouped in quadrant III due to their low Cd concentration (CfCd 0.8–2.3).
According to the PC1-PC3 biplot (Fig. 3b), samples from campaign C7 (pesticide application period) were grouped in quadrant I due to their pesticide (mCdpest 7.5–24) and Cr content. Samples from C4 (March 2012) appear separate from the others in the biplot due to their Cd and Ni values. Most of the samples from campaign C9 (December 2015) were grouped in quadrants III and IV because of their moderate concentrations of Cd, Pb, and low pesticide content. On the contrary, sample 39-C9 exhibited high positive values for PC2, which could be associated with a clear permethrin and chlorpyrifos (mCdpest 109.3) burden as revealed in campaign C9 in field samples.
Degree of contamination and metal pollution level
In order to assess the spatial and temporal levels of potential contaminants and their possible effect on human and environmental health, PI, NIPI (Fig. 4 and Table 9, Supplementary Information), and mCd values (Fig. 5 and Table 10, Supplementary Material) were obtained.
Mean PI values of Pb, Cr, Cd, and Ni ranged from 0.16 to 1.36 in field soils and from 0.32 to 1.93 in parks and playground soils, indicating that the samples had a low pollution level. However, this is not entirely true, taking into account the standard deviation from the mean. Therefore, the median values of PI indicated that 50% of soils ranged from moderate to highly polluted with Pb and Cr. The PI indices denoted the absence of or low pollution level with Cd and Ni for both field and urban soils. However, regarding the combined pollution effects of the set of elements studied, the NIPI indicates high pollution levels for the crop fields studied. In turn, parks and playgrounds exhibited NIPI values that ranged from 0.57 to 10.38 (see Fig. 4), with a mean of 4.24, denoting that urban topsoils were highly polluted as well as crop field topsoils. These results show a high level of pollution, mainly due to the Pb, Cr, and Ni content. Parks and playgrounds from campaign C7 (pesticide application period) showed the highest level of metal pollution.
The modified degree of contamination can provide an integrated assessment of the overall contamination impact of both metals and pesticides (see Table 10 in Supplementary Material). In field samples, the results obtained for the mCdtot ranged from 0.8 to 98.5 with a median of 2.8, indicating a moderate degree of contamination. Parks and playgrounds showed mCdtot values that ranged from 1.28 to 1534.2 with a median of 4.5, indicating a high pollution level. Given these findings, parks and playgrounds were more polluted than fields, and pesticide pollution was greater than metal pollution (see Table 10 in Supplementary Material). In general, the highest pollution was found in campaign C1 (mCdtot values were higher than 6.73, mainly due to pesticides) and also from campaign C6 onwards (see Fig. 5). Parks and playgrounds showed the most damaging contamination levels, especially from campaign C6 onwards (see Fig. 5). Noteworthy, playgrounds showed a low to moderate degree of pollution during campaign C4 (period of low agrochemical application in crop fields), except for sample 38-C4 (mCdtot: 5.9), which qualifies as having a high degree of pollution. These findings could be attributed to urban pesticide application management (38-C4 showed mCdpest 9.95, see Table 10 in Supplementary Material). However, a high level of pollution was not observed in field samples collected during the same campaign. In addition, given that campaign C8 (May 2015) was a period of low pesticide application, the mCd values for fields, parks, and playgrounds ranged from moderate to very high. This could be related to the changes in pesticide application management during 2014–2015 caused by intense rain, waterlogging, and/or hail events (the accumulated rainfall from January to May was 542 mm; data obtained from the Córdoba Grain Exchange Bureau [BCCBA]).
Human health risk assessments
The non-cancer risks from OPs and metals to local villagers via inhalation, soil ingestion, and dermal contact exposure routes are outlined in Table 5. For the agricultural worker population, calculated values of HI were lower than 1. This means that there are no adverse health effects on agricultural workers. Several of the HI values in parks and playgrounds for children under the age of six were greater than 1, owing primarily to metals > pesticides. Finally, the integrated effects of pesticides, and mainly metals, due to exposure to agricultural soils, may pose non-carcinogenic health risks to children. The low body weight of children results in a higher exposure dose to pollutants than for adults. Therefore, children have a higher hazard of non-carcinogenic pollutant exposure than adults under similar conditions.
Conclusion
The results of this study provide basic information that contributes to the knowledge of soil management in a typical agricultural area. The contribution of 28 agrochemicals and available heavy metals to the soils of Marcos Juarez City and surrounding fields throughout the 2010–2016 period was assessed. The significant variability of available heavy metals may be attributed to some anthropogenic sources. The highest metal pollution levels were obtained during the pesticide application periods, not only in crop fields, but also in parks and playgrounds. In addition, the %CV pesticide values might show a disorderly and variable pesticide management. The residues of some banned OPs in topsoil samples originated from historical application, but there is recent introduction of DDT and endosulfan at some sampling sites in Marcos Juarez. The mCd index values indicate a high degree of pollution mainly from pesticides in the study area. Because of the pollutant spread processes, crop fields and urban sites in the region might constitute a source of exposure of the population to pesticides and metals from the fine fraction of topsoil.
The results suggest that pesticide and metal levels in Marcos Juarez topsoils are an example of how agrochemicals can represent a hazard to the environment and pose obvious non-carcinogenic risks to local children. Thus, proper topsoil guidelines together with studies involving the analysis of other commonly used pesticides and a continuous monitoring program are all actions needed to safeguard the health of the population and the environment. Furthermore, future research is required to study how climate factors can contribute to spreading a broader range of active substances used in current agricultural practices nationwide in non-target areas, particularly along field edges.
Availability of data and materials
Not applicable.
Code availability
Not applicable.
References
Abrahim, G. M. S., & Parker, R. J. (2007). Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Tamaki Estuary, Auckland, New Zealand. Environmental Monitoring and Assessment, 136(1–3), 227–238. https://doi.org/10.1007/s10661-007-9678-2
Alloway, B. J. (2013). Heavy Metals in Soils. In B. J. Alloway (Ed.), (3rd ed., Vol. 22). Springer Netherlands. https://doi.org/10.1007/978-94-007-4470-7
Aparicio, V. C., Aimar, S., De Gerónimo, E., et al. (2018). Glyphosate and AMPA concentrations in wind-blown material under field conditions. Land Degradation and Development, 29, 1317–1326. https://doi.org/10.1002/ldr.2920.
Atabila, A., Phung, D. T., Hogarh, J. N., et al. (2018). Health risk assessment of dermal exposure to chlorpyrifos among applicators on rice farms in Ghana. Chemosphere, 203, 83–89. https://doi.org/10.1016/j.chemosphere.2018.03.121
Butinof, M., Fernndez, R., Lantieri, M. J., Stimolo, M. I., Blanco, M., L. Machado, A., Franchini, G., Gieco, M., Portilla, M., Eandi, M., Sastre, A., & Diaz, M. P. (2014). Pesticides and Agricultural Work Environments in Argentina. Pesticides - Toxic Aspects (Vol. 2, pp. 64). https://doi.org/10.5772/57178
Cai, L., Xu, Z., Ren, M., et al. (2012). Source identification of eight hazardous heavy metals in agricultural soils of Huizhou, Guangdong Province, China. Ecotoxicology and Environmental Safety, 78, 2–8. https://doi.org/10.1016/j.ecoenv.2011.07.004
CASAFE. (2016). Informe Mercado de Fitosanitarios. Cámara de Sanidad Agropecuaria y Fertilizantes/ Datos del Mercado Argentino de fitosanitarios. Retrieved January, 2017, from https://www.casafe.org/publicaciones/datos-del-mercado-argentino-defitosanitarios/
Chen, W., Jing, M., Bu, J., et al. (2011). Organochlorine pesticides in the surface water and sediments from the Peacock River Drainage Basin in Xinjiang, China: A study of an arid zone in Central Asia. Environmental Monitoring and Assessment, 177, 1–21. https://doi.org/10.1007/s10661-010-1613-2
Cheraghi, M., Lorestani, B., & Merrikhpour, H. (2012). Investigation of the effects of phosphate fertilizer application on the heavy metal content in agricultural soils with different cultivation patterns. Biological Trace Element Research, 145, 87–92. https://doi.org/10.1007/s12011-011-9161-3
Dean, J. R. (2007). Bioavailability, bioaccessibility and mobility of environmental contaminants. John Wiley & Sons, Ltd.
Devi, N. L., Chakraborty, P., Shihua, Q., & Zhang, G. (2013). Selected organochlorine pesticides (OCPs) in surface soils from three major states from the northeastern part of India. Environmental Monitoring and Assessment, 185, 6667–6676. https://doi.org/10.1007/s10661-012-3055-5
Du, Y., Chen, L., Ding, P., et al. (2019). Different exposure profile of heavy metal and health risk between residents near a Pb-Zn mine and a Mn mine in Huayuan county, South China. Chemosphere, 216, 352–364. https://doi.org/10.1016/j.chemosphere.2018.10.142
Fei, X., Xiao, R., Christakos, G., et al. (2019). Comprehensive assessment and source apportionment of heavy metals in Shanghai agricultural soils with different fertility levels. Ecological Indicators, 106, 105508. https://doi.org/10.1016/j.ecolind.2019.105508
Frimpong, S. K., & Koranteng, S. S. (2019). Levels and human health risk assessment of heavy metals in surface soil of public parks in Southern Ghana. Environmental Monitoring and Assessment, 191, 588. https://doi.org/10.1007/s10661-019-7745-0
de López, G., Carnelo, L., de Miguez, S. R., & Marbán, L. (1997). Heavy metals input with phosphate fertilizers used in Argentina. Science of the Total Environment, 204, 245–250. https://doi.org/10.1016/S0048-9697(97)00187-3
Gustafsson, J. P., Pechová, P., & Berggren, D. (2003). Modeling metal binding to soils: The role of natural organic matter. Environmental Science and Technology, 37, 2767–2774.
Harrison, R. M. (2007). Principles of environmental chemistry. Royal Society of Chemistry.
Hong-Gui, D., Teng-Feng, G., Ming-Hui, L., & Xu, D. (2012). Comprehensive assessment model on heavy metal pollution in soil. International Journal of Electrochemical Science, 7, 5286–5296.
Huang, S., Tu, J., Liu, H., et al. (2009). Multivariate analysis of trace element concentrations in atmospheric deposition in the Yangtze River Delta, East China. Atmospheric Environment, 43, 5781–5790. https://doi.org/10.1016/j.atmosenv.2009.07.055
Iturburu, F. G., Calderon, G., Amé, M. V., & Menone, M. L. (2019). Ecological Risk Assessment (ERA) of pesticides from freshwater ecosystems in the Pampas region of Argentina: Legacy and current use chemicals contribution. Science of the Total Environment, 691, 476–482. https://doi.org/10.1016/j.scitotenv.2019.07.044
Jiang, X., Lu, W. X., Zhao, H. Q., et al. (2014). Potential ecological risk assessment and prediction of soil heavy-metal pollution around a coal gangue dump. Natural Hazards and Earth Systems Sciences, 14, 1599–1610. https://doi.org/10.5194/nhess-14-1599-2014
Jiang, Y.-F., Wang, X.-T., Jia, Y., et al. (2009). Occurrence, distribution and possible sources of organochlorine pesticides in agricultural soil of Shanghai, China. Journal of Hazardous Materials, 170, 989–997. https://doi.org/10.1016/j.jhazmat.2009.05.082
Jimoh, M., Frenzel, W., & Müller, V. (2005). Microanalytical flow-through method for assessment of the bioavailability of toxic metals in environmental samples. Analytical and Bioanalytical Chemistry, 381, 438–444. https://doi.org/10.1007/s00216-004-2899-0
Juhasz, A. L., Weber, J., Smith, E., et al. (2009). Assessment of four commonly employed in vitro arsenic bioaccessibility assays for predicting in vivo relative arsenic bioavailability in contaminated soils. Environmental Science and Technology, 43, 9487–9494. https://doi.org/10.1021/es902427y
Kamunda, C., Mathuthu, M., & Madhuku, M. (2016). Health risk assessment of heavy metals in soils from Witwatersrand Gold Mining Basin, South Africa. International Journal of Environmental Research and Public Health, 13, 663. https://doi.org/10.3390/ijerph13070663
Karimi Nezhad, M. T., Tabatabaii, S. M., & Gholami, A. (2015). Geochemical assessment of steel smelter-impacted urban soils, Ahvaz, Iran. Journal of geochemical exploration, 152, 91–109. https://doi.org/10.1016/j.gexplo.2015.02.005
Li, X., Liu, L., Wang, Y., et al. (2012). Integrated assessment of heavy metal contamination in sediments from a coastal industrial basin, NE China. PLoS ONE, 7, 1–10. https://doi.org/10.1371/journal.pone.0039690
Liu, C., Lu, L., Huang, T., et al. (2016). The distribution and health risk assessment of metals in soils in the vicinity of industrial sites in Dongguan, China. International Journal of Environmental Research and Public Health, 13, 832. https://doi.org/10.3390/ijerph13080832
Liu, W.-X., He, W., Qin, N., et al. (2012). Residues, distributions, sources, and ecological risks of OCPs in the water from Lake Chaohu, China. The Scientific World Journal, 2012, 1–16. https://doi.org/10.1100/2012/897697
Liu, Y., Bralts, V. F., & Engel, B. A. (2015). Evaluating the effectiveness of management practices on hydrology and water quality at watershed scale with a rainfall-runoff model. Science of the Total Environment, 511, 298–308. https://doi.org/10.1016/j.scitotenv.2014.12.077
Madrid, F., Biasioli, M., & Ajmone-Marsan, F. (2008). Availability and bioaccessibility of metals in fine particles of some urban soils. Archives of Environmental Contamination and Toxicology, 55, 21–32. https://doi.org/10.1007/s00244-007-9086-1
Makris, K. C., Quazi, S., Nagar, R., et al. (2008). In vitro model improves the prediction of soil arsenic bioavailability: Worst-case scenario. Environmental Science and Technology, 42, 6278–6284. https://doi.org/10.1021/es800476p
Massart, D. L., Vandeginste, B. G. M., Buydens, L. M. C., De Jong, S., & Lewi, P. J. (1998). Handbook of Chemometrics and Qualimetrics: Part A. Data Handling in Science and Technology Volume 20A.
McAllister, L., Dean, J. R., Sipos, R., et al. (2010). Bioremediation methods and protocols. Humana Press.
Milinovic, J., Lukic, V., Nikolic-Mandic, S., & Stojanovic, D. (2008). Concentrations of heavy metals in NPK fertilizers imported in Serbia. Pestic i Fitomedicina, 23, 195–200. https://doi.org/10.2298/PIF0803195M
Miller, N., Miller. J. C. (2002). Estadística y Quimiometría para química Analítica. Pearson Educación.
National Research Council. (2003). Bioavailability of contaminants in soils and sediments: Processes, tools, and applications. The National Academies Press.
Núñez-Gastélum, J. A., Hernández-Carreón, S., Delgado-Ríos, M., et al. (2019). Study of organochlorine pesticides and heavy metals in soils of the Juarez valley: An important agricultural region between Mexico and the USA. Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-019-06724-4
Pan, L., Sun, J., Li, Z., et al. (2018). Organophosphate pesticide in agricultural soils from the Yangtze River Delta of China: Concentration, distribution, and risk assessment. Environmental Science and Pollution Research, 25, 4–11. https://doi.org/10.1007/s11356-016-7664-3
Pansu, M., Gautheyrou, J. (2006). Handbook of soil analysis mineralogical, organic and inorganic methods. Berlin, Netherlands.
Pegoraro, C. N., Wannaz, E. D. (2019). Occurrence of persistent organic pollutants in air at different sites in the province of Córdoba, Argentina. Environmental Science and Pollution Research, 26, 18379–18391. https://doi.org/10.1007/s11356-019-05088-z
Pérez, D. J., Harguinteguy, C. A., Okada, E., & Menone, M. L. (2018). IMPACTO DE LA AGRICULTURA EN CURSOS DE AGUA SUPERFICIALES: PLANTAS ACUÁTICAS COMO ORGANISMOS INDICADORES DE CONTAMINACIÓN. INTA/Artículos de divulgación. https://inta.gob.ar/documentos/impacto-de-la-agricultura-en-cursos-de-agua-superficiales-plantasacuaticas-como-organismos-indicadores-de-contaminacion
Plumlee, G. S., & Ziegler, T. L. (2007). The Medical Geochemistry of Dusts, Soils, and Other Earth Materials. In D. Heinrich, & K. Turekian (Eds.), Treatise on Geochemistry (vol 9, pp. 1–61). https://doi.org/10.1016/B0-08-043751-6/09050-2
Poggio, L., Vrščaj, B., Schulin, R., et al. (2009). Metals pollution and human bioaccessibility of topsoils in Grugliasco (Italy). Environmental Pollution, 157, 680–689. https://doi.org/10.1016/j.envpol.2008.08.009
Pope, A., Burnett, R., Thun, M., et al. (2002). Long-term exposure to fine particulate air pollution. JAMA, 287, 1192. https://doi.org/10.1001/jama.287.9.1132
Primost, J. E., Marino, D. J. G., Aparicio, V. C., et al. (2017). Glyphosate and AMPA, “pseudo-persistent” pollutants under real-world agricultural management practices in the Mesopotamic Pampas agroecosystem, Argentina. Environmental Pollution, 229, 771–779. https://doi.org/10.1016/j.envpol.2017.06.006
Rissato, S. R., Galhiane, M. S., Ximenes, V. F., et al. (2006). Organochlorine pesticides and polychlorinated biphenyls in soil and water samples in the Northeastern part of São Paulo State, Brazil. Chemosphere, 65, 1949–1958. https://doi.org/10.1016/j.chemosphere.2006.07.011
Rodriguez, J. H., Weller, S. B., Wannaz, E. D., et al. (2011). Air quality biomonitoring in agricultural areas nearby to urban and industrial emission sources in Córdoba province, Argentina, employing the bioindicator Tillandsia capillaris. Ecological Indicators, 11, 1673–1680. https://doi.org/10.1016/j.ecolind.2011.04.015
Rosende, M., & Miró, M. (2013). Recent trends in automatic dynamic leaching tests for assessing bioaccessible forms of trace elements in solid substrates. TrAC, Trends in Analytical Chemistry, 45, 67–78. https://doi.org/10.1016/j.trac.2012.12.016
Ryan, J. A., Scheckel, K. G., Berti, W. R., et al. (2004). Reducing children’s risk from lead in soil. Environmental Science and Technology, 38, 18A-24A. https://doi.org/10.1021/es040337r
Sánchez-Palencia, Y., Ortiz, J., & Torres, T. (2015). Origen y distribución de pesticidas organoclorados (OCPs) en sedimentos actuales de la Laguna de El Hito (Cuenca, España Central). Geogaceta, 58, 119–122.
Sialelli, J., Davidson, C. M., Hursthouse, A. S., & Ajmone-Marsan, F. (2011). Human bioaccessibility of Cr, Cu, Ni, Pb and Zn in urban soils from the city of Torino, Italy. Environmental Chemistry Letters, 9, 197–202. https://doi.org/10.1007/s10311-009-0263-5
Siegel, F. R., Slaboda, M. L., & Stanley, D. J. (1994). Metal pollution loading, Manzalah lagoon, Nile delta, Egypt: Implications for aquaculture. Environmental Geology, 23, 89–98. https://doi.org/10.1007/BF00766981
Thums, C. R., Farago, M. E., & Thornton, I. (2008). Bioavailability of trace metals in brownfield soils in an urban area in the UK. Environmental Geochemistry and Health, 30, 549–563. https://doi.org/10.1007/s10653-008-9185-6
Turner, A., & Ip, K.-H. (2007). Bioaccessibility of metals in dust from the indoor environment: Application of a physiologically based extraction test. Environmental Science and Technology, 41, 7851–7856. https://doi.org/10.1021/es071194m
US EPA. (1996). Method 3540c soxhlet extraction. Environmental protection agency of United Satates of América, Collection of Methods. Retrived 2017 from https://www.epa.gov/measurements-modeling/collection-method
van Wijnen, J. H., Clausing, P., & Brunekreef, B. (1990). Estimated soil ingestion by children. Environmental Research, 51, 147–162. https://doi.org/10.1016/S0013-9351(05)80085-4
Xiao, R., Wang, S., Li, R., et al. (2017). Soil heavy metal contamination and health risks associated with artisanal gold mining in Tongguan, Shaanxi China. Ecotoxicology and Environmental Safety, 141. https://doi.org/10.1016/j.ecoenv.2017.03.002
Yadav, I. C., Devi, N. L., Li, J., et al. (2016). Occurrence, profile and spatial distribution of organochlorines pesticides in soil of Nepal: Implication for source apportionment and health risk assessment. Science of the Total Environment, 573, 1598–1606. https://doi.org/10.1016/j.scitotenv.2016.09.133
Yeomans, K. A., & Golder, P. A. (1982). The Guttman-Kaiser criterion as a predictor of the number of common factors. Stat, 31, 221. https://doi.org/10.2307/2987988
Zeng, S., Ma, J., Yang, Y., et al. (2019). Spatial assessment of farmland soil pollution and its potential human health risks in China. Science of the Total Environment, 687, 642–653. https://doi.org/10.1016/j.scitotenv.2019.05.291
Zhang, J., Wang, Y., & Hua, D. (2017). Occurrence, distribution and possible sources of organochlorine pesticides in peri-urban vegetable soils of Changchun, Northeast China. Human and Ecological Risk Assessment, 23, 2033–2045. https://doi.org/10.1080/10807039.2017.1362545
Zhang, X., Ding, X., & Lu, X. (2019). Analyzing the possible sources of heavy metals in farmland soil around Weihe coal-fired plant based on multivariate statistical analysis. IOP Conference Series: Earth and Environmental Science, 346, 012–060. https://doi.org/10.1088/1755-1315/346/1/012060
Zhao, X., Shen, J.-P., Zhang, L.-M., et al. (2020). Arsenic and cadmium as predominant factors shaping the distribution patterns of antibiotic resistance genes in polluted paddy soils. Journal of Hazardous Materials, 389, 121838. https://doi.org/10.1016/j.jhazmat.2019.121838
Acknowledgements
The authors thank the Universidad Nacional de Córdoba, Facultad de Ciencias Exactas, Físicas y Naturales (FCEFyN-UNC) and Centro de Investigaciones de Ciencias de la Tierra (CICTERRA) of Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET-UNC), and Centro de Química Aplicada (CEQUIMAP) of Facultad de Ciencias Químicas (FCQ-UNC).
Funding
This work was supported by (CONICET) Consejo Nacional de Investigaciones Científicas y Técnicas, (SeCyT-UNC) Secretaría de Ciencia y Tecnología de la Universidad Nacional de Córdoba, and (MINCyT Córdoba) Ministerio de Ciencia y Tecnología de Córdoba, Argentina.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Avendaño, M.C., Palomeque, M.E., Roqué, P. et al. Spatiotemporal distribution and human health risk assessment of potential toxic species in soils of urban and surrounding crop fields from an agricultural area, Córdoba, Argentina. Environ Monit Assess 193, 661 (2021). https://doi.org/10.1007/s10661-021-09358-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-021-09358-7