Abstract
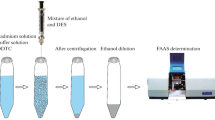
In this research, a new sample treatment technique termed solvent-assisted dispersive solid phase extraction (SADSPE) was developed. The new method was based on the dispersion of the sorbent into the sample to maximize the contact surface. In this approach, the dispersion of the sorbent at a very low milligram level was achieved by injecting a mixture solution of the sorbent and disperser solvent into the aqueous sample. Thereby, a cloudy solution formed. The cloudy solution resulted from the dispersion of the fine particles of the sorbent in the bulk aqueous sample. After extraction, the cloudy solution was centrifuged and the enriched analytes in the sediment phase dissolved in ethanol and determined by FAAS. Under the optimized conditions, the detection limit for silver ions was 0.8 μg L−1. The relative standard deviations for six separate extraction experiments for determination of 5 and 200 μg L−1 of silver ions was 3.4 and 3.1 %. The preconcentration factor was found to be 61.7. SADSPE was successfully applied for trace determination of silver ions in water and food samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The increasing use of silver compounds and silver-containing products in industry and medicine has resulted in an increased silver content of environmental samples (Kolthoff et al. 1966). Silver also enters the environment through industrial waters because it often occurs as an impurity in copper, zinc, arsenic, and antimony ores (Soager et al. 1984). Low-level exposure to silver compounds is widespread due to the use of soluble silver compounds to disinfect drinking water (Environmental Protection Agency EPA et al. 1980). On the other hand, recent information about the interaction of silver with essential nutrients, especially selenium, copper, Vitamins E, and B12, has focused attention on its potential toxicity (Environmental Protection Agency EPA et al. 1980).
Due to these important points, the accurate determinations of trace heavy metals are important part of the studies in analytical chemistry (Soylak and Cay 2007; Behbahani et al. 2013a, 2014a, b, c; Tuzen et al. 2013). Flame atomic absorption spectrometry (FAAS) is relatively simple and available technique in many laboratories for heavy metal determinations (Behbahani et al. 2014d, e, f, g). However, the conventional determinations of elements at microgram per liter range by flame atomic absorption spectrometry frequently are not possible. To solve this problem, preconcentration/separation procedures have been proposed. Preconcentration is a very important issue for achievement of low detection limits (Behbahani et al. 2014i, j; Salarian et al. 2014; Ebrahimzadeh and Behbahani 2013). Several procedures have been developed for these purposes such as liquid–liquid extraction (LLE) (Silvestre et al. 2009), liquid phase microextraction (LPME) (Behbahani et al. 2013b, 2014h; Hossien-poor-Zaryabi et al. 2014; Naeemullah et al. 2013, 2014; Unsal et al. 2014) co-precipitation (Saracoglu et al. 2003; Doner and Ege 2005), and solid phase extraction (SPE) (Bagheri et al. 2012a, b; Nabid et al. 2012, 2014; Ghorbani-Kalhor et al. 2014; Behbahani et al. 2015; Omidi et al. 2014a, b). However, some of these pretreatment methods need large amounts of sample and/or organic solvents, are time-consuming and expensive, and have unsatisfactory enrichment factors. These problems could be addressed by the development of modular and compact processes that provide adequate separation and preconcentration without complex processes. SPE is more flexible and efficient than LLE in terms of simplicity, low consumption of the organic solvents, flexibility in choosing the adsorbent, and high enrichment factor. Recently, SPE has been increasingly used for the preconcentration and separation of ultratrace amounts of inorganic and organic species from complex matrices (Bagheri et al. 2012c). Solid phase extraction techniques are surface-dependent processes since their efficiency directly depends on the particle size and the surface area of the sorbent (Abolhasani and Behbahani 2015). Dispersive solid phase extraction (DSPE) has risen as an alternative to conventional solid phase extraction. It was, for the first time, proposed by Anastassiades et al. (Anastassiades et al. 2003) and it can be considered as a quick, easy, cheap, effective, rugged, and safe (QuEChERS) sample treatment method. It is based on the SPE methodology, but the sorbent is added to the extract without conditioning, in small amounts (viz. 50 mg) and the dispersion is carried out assisted by an external energy (usually a vortex stirring). The phases are easily separated just by centrifugation. Sorbent dispersion leads to an increase of its active surface and, therefore, to an improvement in the extraction kinetics. Moreover, this enhancement allows the use of a smaller amount of sorbent compared to the conventional extraction approaches resulting in the saving of material. In order to obtain analytical information, the sorbent is then recovered after the extraction. The analytes can be directly monitorized on the sorbent surface by using a spectroscopic technique (Alcudia-Leon et al. 2009) or can be conveniently eluted/desorbed for the subsequent analysis of the eluted fraction (Drozdzynski and Kowalska 2009).
The aim of this work was to combine solvent-assisted dispersive solid phase extraction (SADSPE) with FAAS to develop an improved procedure for the determination of silver ions at trace levels with respect to the advantages of SADSPE which is a fast and inexpensive method in comparison with other methods. In this method, the appropriate mixture of sorbent and disperser solvent was rapidly injected into the aqueous sample by syringe. Thereby, a cloudy solution formed. The cloudy state resulted from the dispersion of the fine particles of the sorbent in the bulk aqueous sample. Then, this cloudy solution was centrifuged, following which the fine particles sediment at the bottom of the conical test tube. Then the sediment phase was dissolved in ethanol and the analytes were determined using FAAS. In this extraction method, any component in the solution, directly or indirectly after previous derivatization reactions, interacts with the fine particles of the sorbent and, consequently, gets extracted from the initial solution. In this work, Eriochrome Cyanine R was used as a suitable chelating agent to form complex with silver ions and factors that would influence the efficiency of SADSPE were also investigated and optimized. Finally, the introduced sample treatment technique was used for preconcentration and traces determination of silver ions in different water samples.
Experimental
Apparatus
Silver concentration was determined by an AA-680 Shimadzu (Kyoto, Japan) flame atomic absorption spectrometer (FAAS) in an air-acetylene flame, according to the user’s manual, provided by the manufacturer. Silver hollow cathode lamps (HCL) were used as the radiation source with wavelengths of 328.1 nm. The pH was measured at 25 ± 1 °C with a digital WTW Metrohm 827 Ion analyzer (Herisau, Switzerland) equipped with a combined glass–calomel electrode.
Reagent and materials
All analytical grade reagents were purchased from Merck (Darmstadt, Germany, www.merck.de) and used without further purification. A stock solution (100.0 mg L−1) of silver ions was prepared by dissolving an appropriate amount of corresponding nitrate salts in double-distilled water. Working standard solutions were prepared daily through serial dilutions of the stock solution with deionized water prior to analysis. All standard solutions for FAAS instrument calibration were prepared in ethanol. A 100.0 mg L−1 solution of Eriochrome Cyanine R (Fluka (Buchs SG, Switzerland, www.sigmaaldrich.com)) in pure ethanol was prepared. Naphthalene, benzophenone, and 1,4-dichlorobenzene were purchased from Sigma-Aldrich (St. Louis, MO, USA). All glass vessels used for trace analysis were cleaned before use by soaking them in 10 % nitric acid solution for at least 24 h and then rinsed thoroughly with ultrapure water. Soil (NCS DC 73323) from Bulgaria was used as the reference materials for validation of the proposed technique.
Real sample pretreatment
Water sample pretreatment
The polyethylene bottles filled with the samples were cleaned with detergent, water, diluted nitric acid, and water in sequence. The samples were immediately filtered through a cellulose filter membrane (pore size 0.45 μm), and were acidified to pH of 2.0 for storage. Tap water samples (15.0 mL) were taken from our research laboratory without pretreatment (pH adjusted to 7.0). Before analysis, the water samples (15.0 mL) which were taken from Caspian Sea, river, and waste were adjusted to pH of 5.0 according to optimized experimental conditions.
Fruit samples (Citrus limetta, kiwi, and pomegranate)
Three types of fruits were chosen for analysis. These were C. limetta, Kiwi, and pomegranate, which were collected from the local supermarket. One gram of dried and grounded samples was put into burning cup with 15 mL of pure HNO3. The samples were incinerated in a MARS 5 microwave oven at 200 °C. After digestion treatment, the samples were filtrated through Whatman No. 42. After filtration, the obtained clear solution was diluted to 15 mL (pH of 5.0) for silver analysis.
Fish samples
Four samples of fish samples (fish) from each supermarket were randomly selected, mixed, and transferred into an ice bag and transported to the laboratory and stored at −20 °C prior to analysis. The sampling was carried out in May 2014. The muscle tissues of the fishes were separated and freeze-dried in order to obtain a constant weight and were finely grounded. For metal analysis, approximately 1.0 g of the grounded samples was digested in 5 mL of concentrated nitric acid at 135 °C for 4 h; then, 1 mL of hydrogen peroxide (30 %) and 1 ml of concentrated perchloric acid were added, and the temperature maintained at 150 °C until the liquid was clear (Wua and Yang 2011). Then, the resulted solution was filtered and after the filtration, the clear solution was diluted to 15 mL and the pH was adjusted to 5.0 using NaOH or HNO3 for further analysis.
Certified reference materials
To digest the certified reference material, 10 mg from each of them was digested with 6 mL of HCl (37 %) and 2 mL of HNO3 (65 %) in a microwave digestion system. The digestion was carried out for 2 min at 250 W, 2 min at 0 W, 6 min at 250 W, 5 min at 400 W, 8 min at 550 W, and then venting for 8 min. Then, the resulted solution from digestion was diluted to 15 mL with deionized water before adjusting the pH to 5.0.
Solvent-assisted dispersive solid phase extraction procedure
A solution (15 mL) containing sample or standards of silver ions and Eriochrome Cyanine R (ECR) (with the concentration of eight times greater than silver ions) at pH of 5.0 was poured into a glass screw-cap conical-bottom centrifuge tube. Then, 500 μL of methanol solution (as disperser solvent) containing benzophenone (0.03 % (w/v)) (as sorbent) was rapidly injected into a sample solution by using 1.0-mL syringe. A cloudy solution formed in the test tube (the cloudy state was stable for a long time). This cloudy state resulted from the dispersion of fine particles of benzophenone in the bulk aqueous sample. Then, the mixture was centrifuged at 4500 rpm for 4 min. Accordingly, after centrifuge, the dispersed fine particles of benzophenone were sedimented in the bottom of the conical test tube. The aqueous phase was then separated completely by a syringe. Later, the sedimented phase was dissolved and made up to 0.5 mL by adding ethanol. The resultant solution was introduced into the flame by conventional aspiration.
Results and discussion
In this study, SADSPE was investigated for preconcentration of silver ions from aqueous samples followed by their analysis by flame atomic absorption spectrophotometry. The factors influencing the extraction procedure such as type and amount of sorbent, type and volume of dispersive solvent, pH and concentration of chelating reagent, salt effect, and centrifuge time were investigated and optimized. It is very important to optimize them in order to obtain good recovery and low limit of detection.
The effect of pH
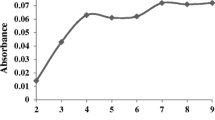
The separation of metal ions by solvent-assisted dispersive solid phase extraction involves prior formation of a complex with sufficient hydrophobicity interacting and adsorbing with the fine particles of the sorbent. Further, pH plays a unique role in metal–ligand chelate formation and its subsequent extraction because of the existing form of metal ions and ligand is pH dependent. The effect of pH on the silver complex extraction from water samples was studied in the range of 2–7 and results are listed in Fig. 1. As can be seen, the optimum pH for extraction of silver ions is 5.0. By decreasing the pH value of the solution, the extraction recovery of the method was also decreased due to the electrostatic repulsion of the protonated active sites on the chelating agent with the positively charged silver species. As shown in Fig. 1, extraction recovery was increased up to pH of 5 and it was constant in longer pH. According to the results, a pH value of 5.0 was chosen for further experiments. The pH of sample solution was adjusted by drop wise addition of 2 M-sodium hydroxide or nitric acid solutions.
The effect of Eriochrome Cyanine R concentration
The influence of ECR concentration on the SADSPE of silver ions was evaluated in the range of 1–15 times greater than silver ion concentration. The results were shown in Fig. 2. As it can be seen, the extraction recoveries of silver ions increased with the increase of ECR concentration up to eight times toward silver ion concentration. When the chelating reagent concentrations were greater than eight times toward silver ion concentration, a reduction in the extraction efficiency was observed because the small volume of the solvent extractor is easily saturated. Therefore, ECR with the concentration of eight times greater than silver ions was chosen as the optimum concentration for subsequent experiments.
The effect of the extraction time
In SADSPE, the extraction time is defined as the time interval between the injection of the mixture of disperser solvent and sorbent in the sample and the beginning of centrifugation. This parameter was evaluated in the range of 10 s to 2 min, while all the experimental conditions were kept constant. No significant difference in the analytical response to each element was observed by varying the extraction time from 10 s to 2 min. This result is due to the increased contact area between the aqueous phase and the sorbent extractor with the formation of dispersed particles in aqueous solution after the turbidity. Thus, the passage of the metal-ECR complexes from the aqueous phase to the extraction sorbent is fast.
Selection of sorbent and its amount
Careful attention must be paid to in the selection of the sorbent, and it should have extraction capability for concerning compounds, high solubility in disperser solvent, and low solubility in water. Naphthalene, benzophenone, and 1,4-dichlorobenzene were evaluated in the extraction of silver-ECR complex. A series of sample solutions was studied using 500-μL methanol containing 0.03 % (w/v%) of the sorbent. As Fig. 3 shows, the highest extraction recovery was obtained with benzophenone. The similarity of the structure of benzophenone and the complex of ECR with silver ions and their proper interaction could thus explain the higher extraction recovery of benzophenone. Therefore, benzophenone was selected as the sorbent.
To examine the effect of the sorbent amount, a series of sample solutions was studied using 500-μL methanol containing different amounts (0.01– 0.05 % (w/v%)) of benzophenone. As Fig. 4 shows, the extraction recovery increased as the amount of benzophenone increased from 0.01 to 0.03 %, as was expected. A further increase of the amount of benzophenone to 0.05 % did not have any effect on extraction recovery. Therefore, 0.03 % of the benzophenone was selected as the optimum amount of the sorbent for further studies.
The effect of the type and volume of the disperser solvent
The disperser solvent plays a key role in the extraction procedure since it permits the appropriate dispersing of the sorbent into the sample, generating a cloud of fine particles.
Essentially, solvent must fulfill the following requirements: (a) it should be miscible with the sample matrix in order to release the sorbent easily, (b) it should be able to dissolve the sorbent, and (c) it should not interfere in the extraction of the target analytes. Therefore, different solvents (acetone, tetrahydrofuran, methanol, and ethanol) were evaluated. A series of sample solutions was studied using 500 μL of each disperser solvent containing 0.03 % of benzophenone. As can be seen in Fig. 5, the best extraction recovery for target ions was obtained with methanol as the dispersing solvent. Therefore, methanol was selected as the disperser solvent for subsequent experiments.
The effects of the volumes of methanol on the extraction efficiency were investigated in detail. In the range of 250–1000 μL along with 0.03 % of benzophenone as the extracting sorbent, the volume of methanol on the extraction recovery was tested. As shown in Fig. 6, the extraction recovery in 500 μL of methanol was quantitative. The extraction efficiency decreased slightly when the volume of methanol exceeded 500 μL. Clearly, an increase in the volume of methanol results in an increase in the solubility of the complex. Therefore, the extraction recovery decreases. Thus, 500 μL of methanol was selected as the optimum volume.
Effect of centrifugation time
Centrifugation time was another parameter that influenced the separation of dispersed particles from water phase. The centrifugation time was evaluated between 1 and 10 min. The results exhibited that when the centrifugation time was up to 4 min, silver ions was enriched completely. When the centrifugation time was shorter or longer, the recoveries were both lower than 90 %. The short centrifugation time could not insure the preferable phase separation, and longer centrifugation time would generate heat, which may enhance the dissolving of silver complex into the aqueous phase, which also resulted in the decrease of dissolved silver complex, and all these would lead to the decrease of the sedimented amount of silver complex. So, 4 min was selected for further experiments.
The effect of ionic strength
When the ionic strength of solution was increased, several effects were observed. The presence of dissolved salt in water can change the physical properties of the Nernst diffusion film and reduce the rate of diffusion of the target analytes into the sorbent. On the other hand, salting-out effect causes an increase on the extraction efficiency (Sobhi et al. 2008). Furthermore, the presence of salt could change the viscosity of aqueous solution; thus, reducing the rate of diffusion of the analyte into the sorbent (Rezaei et al. 2013). Therefore, for investigating the influence of ionic strength on performance of SADSPE, various experiments were performed by adding varying NaCl amounts from 0 to 5 % (w/v). Other experimental conditions were kept constant. According to the obtained experimental results, salt addition has no significant effect on extraction recovery. Therefore, all the extraction experiments were carried out without adding salt.
The effect of potentially interfering ions
The effects of common potentially interfering ions in real samples on the recovery of silver ions were also studied. In these experiments, 15 mL of solutions containing 50.0 μg L−1 of silver and various amounts of interfering ions were treated according to the recommended procedure. Table 1 shows the maximum tolerance of the cations and anions investigated. According to the results, the major ions in the real samples have no obvious influence on the Ag(I) extraction under the selected conditions.
Analytical performance
Under the optimized conditions, calibration curves were sketched for the determination of silver ions according to the general procedure. Linearity was maintained 2–450 μg L−1 for silver in initial solution. The coefficient of determination (r 2) was 0.99. The limit of quantification was 2 μg L−1 and limit of detection which is defined as C LOD = 3Sb/m, where Sb is the standard deviation of six replicate blank signals, and m is the slope of the calibration curve after preconcentration, for a sample volume of 15 mL, was found to be 0.8 μg L−1. Also, the preconcentration factors calculated as the ratio between the slopes of the calibration curves acquired by the proposed method (Y = 1.79 X − 0.001) and through the direct analysis of silver ions by FAAS (Y = 0.029 X − 0.007) was found to be 61.7 (1.79/0.029 = 61.7). The relative standard deviations for six separate extraction experiments for determination of 5 and 200 μg L−1 of silver ions was 3.4 and 3.1 %. Table 2 provides a comparison between the proposed method and previously published works (Fathi et al. 2009; Shamsipur et al. 2002; Afzali et al. 2011; Mohammadi et al. 2009).
Real samples analysis
Since different water samples have complex matrices, non-specific background absorption was caused by interfering species of the sample matrix. To reduce this undesirable effect, sample treatment technique (SADSPE) was applied for extraction of silver ions in pH of 5.0. Table 3 shows the silver ions recoveries in different water and food samples.
Validation of the method
The concentration of silver ions obtained by SADSPE procedure was compared to the standard materials. For this reason, the concentration of the silver ions was determined at optimum conditions in standard reference material. As it can be seen in Table 4, a good correlation was achieved between estimated content by the present method and reference materials (the recovery for this experiment was 97.7 %). Therefore, SADSPE can be used as a reliable sample treatment technique for extraction and determination of silver ions in real samples.
Conclusion
The proposed solvent-assisted dispersive solid phase extraction was successfully used for preconcentration of silver ions in various water and food samples. The SADSPE allows the rapid extraction of silver ions using a low amount of sorbent material (0.03 % (w/v%)). The introduced method was based on the dispersion of the sorbent into the sample to maximize the contact surface. In this approach, the dispersion of the sorbent at a very low milligram level was achieved by injecting a mixture solution of the sorbent and disperser solvent into the aqueous sample. Thereby, a cloudy solution formed. The cloudy solution resulted from the dispersion of the fine particles of the sorbent in the bulk aqueous sample. After extraction, the cloudy solution was centrifuged and the enriched analytes in the sediment phase dissolved in ethanol and determined by FAAS. The obtained LOD (0.8 μg L−1) for analysis of silver ions is satisfactory. This method is environment friendly and robust against very high contents of salt. The other benefits of the method are its simplicity, ease of operation, good accuracy and precision, short extraction time, low cost, and a good enrichment factor. Although the obtained results in this work are related to silver determination, the system could be readily applied for the determination of other metals using various ligands.
References
Abolhasani, J., & Behbahani, M. (2015). Application of 1-(2-pyridylazo)-2-naphthol-modified nanoporous silica as a technique in simultaneous trace monitoring and removal of toxic heavy metals in food and water samples. Environmental Monitoring and Assessment, 187, 1–12.
Afzali, D., Mohadesi, A. R., Bahadori Jahromi, B., & Falahnejad, M. (2011). Separation of trace amount of silver using dispersive liquid–liquid based on solidification of floating organic drop microextraction. Analytica Chimica Acta, 684, 54–58.
Alcudia-Leon, M. C., Lucena, R., Cardenas, S., & Valcarcel, M. (2009). Dispersive solid phase extraction for in-sorbent surface attenuated total reflection infrared detection. Analytical Chemistry, 81, 1184–1190.
Anastassiades, M., Lehotay, S. J., Stajnbaher, D., & Schenck, F. J. (2003). Fast and easy multi residue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. Journal of AOAC International, 86, 412–431.
Bagheri, A., Behbahani, M., Amini, M. M., Sadeghi, O., Taghizade, M., Baghayi, L., & Salarian, M. (2012a). Simultaneous separation and determination of trace amounts of Cd(II) and Cu(II) in environmental samples using novel diphenylcarbazide modified nanoporous silica. Talanta, 89, 455–461.
Bagheri, A., Behbahani, M., Amini, M. M., Sadeghi, O., Tootoonchi, A., & Dahaghin, Z. (2012b). Preconcentration and separation of ultra-trace palladium ion using pyridine-functionalized magnetic nanoparticles. Microchimica Acta, 178, 261–268.
Bagheri, A., Taghizadeh, M., Behbahani, M., Asgharinezhad, A. A., Salarian, M., Dehghani, A., Ebrahimzadeh, H., & Amini, M. M. (2012c). Synthesis and characterization of magnetic metalorganic framework (MOF) as a novel sorbent, and its optimization by experimental design methodology for determination of palladium in environmental samples. Talanta, 99, 132–139.
Behbahani, M., Bagheri, A., Amini, M. M., Sadeghi, O., Salarian, M., Najafi, F., & Taghizadeh, M. (2013a). Application of multiwalled carbon nanotubes modified by diphenylcarbazide for selective solid phase extraction of ultra traces Cd(II) in water samples and food products. Food Chemistry, 141, 48–53.
Behbahani, M., Najafi, F., Bagheri, S., Kalate Bojdi, M., Salarian, M., & Bagheri, A. (2013b). Application of surfactant assisted dispersive liquid–liquid microextraction as an efficient sample treatment technique for preconcentration and trace detection of zonisamide and carbamazepine in urine and plasma samples. Journal of Chromatography. A, 1308, 25–31.
Behbahani, M., Akbari Ghareh Tapeh, N., Mahyari, M., Pourali, A. R., Golrokh Amin, B., & Shaabani, A. (2014a). Monitoring of trace amounts of heavy metals in different food and water samples by flame atomic absorption spectrophotometer after preconcentration by amine-functionalized graphene nanosheet. Environmental Monitoring and Assessment, 186, 7245–7257.
Behbahani, M., Ali Akbari, A., Amini, M. M., & Bagheri, A. (2014b). Synthesis and characterization of pyridine-functionalized magnetic mesoporous silica and its application for preconcentration and trace detection of lead and copper ions in fuel products. Analytical Methods, 6, 8785–8792.
Behbahani, M., Babapour, M., Amini, M. M., Sadeghi, O., Bagheri, A., Salarian, M., & Rafiee, B. (2014c). Separation/enrichment of copper and silver using titanium dioxide nanoparticles coated with poly-thiophene and their analysis by flame atomic absorption spectrophotometry. American Journal of Analytical Chemistry, 4, 90–98.
Behbahani, M., Bide, Y., Salarian, M., Niknezhad, M., Bagheri, S., Bagheri, A., & Nabid, M. R. (2014d). The use of tetragonal star-like polyaniline nanostructures for efficient solid phase extraction and trace detection of Pb (II) and Cu (II) in agricultural products, sea foods, and water samples. Food Chemistry, 158, 14–19.
Behbahani, M., Esrafili, A., Bagheri, S., Radfar, S., Kalate Bojdi, M., & Bagheri, A. (2014e). Modified nanoporous carbon as a novel sorbent before solvent-based de-emulsification dispersive liquid–liquid microextraction for ultra-trace detection of cadmium by flame atomic absorption spectrophotometry. Measurements, 51, 174–181.
Behbahani, M., Gorji, T., Mahyari, M., Salarian, M., Bagheri, A., & Shaabani, A. (2014f). Application of polypropylene amine dendrimers (POPAM)-grafted MWCNTs hybrid materials as a new sorbent for solid-phase extraction and trace determination of gold (III) and palladium (II) in food and environmental samples. Food Analytical Methods, 7, 957–966.
Behbahani, M., Najafi, F., Amini, M. M., Sadeghi, O., Bagheri, A., & Ghareh Hassanlou, P. (2014g). Solid phase extraction using nanoporous MCM-41 modified with 3, 4-dihydroxybenzaldehyde for simultaneous preconcentration and removal of gold (III), palladium (II), copper (II) and silver (I). Journal of Industrial and Engineering Chemistry, 20, 2248–2255.
Behbahani, M., Najafi, F., Bagheri, S., Kalate Bojdi, M., Ghareh Hassanlou, P., & Bagheri, A. (2014h). Coupling of solvent-based de-emulsification dispersive liquid–liquid microextraction with high performance liquid chromatography for simultaneous simple and rapid trace monitoring of 2, 4-dichlorophenoxyacetic acid and 2-methyl-4-chlorophenoxyacetic acid. Environmental Monitoring and Assessment, 186, 2609–2618.
Behbahani, M., Sadeghi Abandansari, H., Salarian, M., Babapour, M., Bagheri, A., & Nabid, M. R. (2014i). Synthesis and application of a thermosensitive tri-block copolymer as an efficient sample treatment technique for preconcentration and ultra-trace detection of lead ions. Microchimica Acta, 181, 129–137.
Behbahani, M., Salarian, M., Bagheri, A., Tabani, H., Omidi, F., & Fakhari, A. (2014j). Synthesis, characterization and analytical application of Zn (II)-imprinted polymer as an efficient solid-phase extraction technique for trace determination of zinc ions in food samples. Journal of Food Composition Analysis, 34, 81–89.
Behbahani, M., Ghareh Hassanlou, P., Amini, M. M., Moazami, H. R., Sadeghi Abandansari, H., Bagheri, A., & Hassan Zadeh, S. (2015). Selective solid-phase extraction and trace monitoring of lead ions in food and water samples using new lead-imprinted polymer nanoparticles. Food Analytical Methods, 8, 558–568.
Doner, G., & Ege, A. (2005). Determination of copper, cadmium and lead in seawater and mineral water by flame atomic absorption spectrometry after coprecipitation with aluminum hydroxide. Analytica Chimica Acta, 547, 14–17.
Drozdzynski, D., & Kowalska, J. (2009). Rapid analysis of organic farming insecticides in soil and produce using ultra-performance liquid chromatography/tandem mass spectrometry. Analytical and Bioanalytical Chemistry, 394, 2241–2247.
Ebrahimzadeh, H., & Behbahani, M. (2013). A novel lead imprinted polymer as the selective solid phase for extraction and trace detection of lead ions by flame atomic absorption spectrophotometry: synthesis, characterization and analytical application. Arabian Journal of Chemistry. doi:10.1016/j.arabjc.2013.09.017.
Environmental Protection Agency (EPA), Ambient water quality criteria for silver, EPA 4405-80-071, Office of Water Regulations, Washington, DC, 1980.
Fathi, M. R., Pourreza, N., & Purweis, S. (2009). Determination of silver by flame atomic absorption spectrometry after preconcentration on naphthalene modified with dithizone. Journal of the Chinese Chemical Society, 56(4), 725–728.
Ghorbani-Kalhor, E., Behbahani, M., & Abolhasani, J. (2014). Application of Ion-imprinted polymer nanoparticles for selective trace determination of palladium ions in food and environmental samples with the aid of experimental design methodology. Food Analytical Methods. doi:10.1007/s12161-014-0057-7.
Hossien-poor-Zaryabi, M., Chamsaz, M., Heidari, T., Hossein Arbab Zavar, M., Behbahani, M., & Salarian, M. (2014). Application of dispersive liquid–liquid micro-extraction using mean centering of ratio spectra method for trace determination of mercury in food and environmental samples. Food Analytical Methods, 7, 352–359.
Kolthoff, I, M, Elving, P, J,. (Eds.), Treatise on analytical chemistry, part II, vol. Interscience, New York. 1966.
Mohammadi, S. Z., Afzali, D., Taher, M. A., & Baghelani, Y. M. (2009). Ligandless dispersive liquid–liquid microextraction for the separation of trace amounts of silver ions in water samples and flame atomic absorption spectrometry determination. Talanta, 80, 875–879.
Nabid, M. R., Sedghi, R., Bagheri, A., Behbahani, M., Taghizadeh, M., Oskooie, H. A., & Heravi, M. M. (2012). Preparation and application of poly(2-amino thiophenol)/MWCNTs nanocomposite for adsorption and separation of cadmium and lead ions via solid phase extraction. Journal of Hazardous Materials, 203–204, 93–100.
Nabid, M. R., Sedghi, R., Behbahani, M., Arvan, B., Heravi, M. M., & Abdi Oskooie, H. (2014). Application of poly 1, 8‐diaminonaphthalene/multiwalled carbon nanotubes‐COOH hybrid material as an efficient sorbent for trace determination of cadmium and lead ions in water samples. Journal of Molecular Recognition, 27, 421–428.
Naeemullah, T. M., Kazi, T. G., Citak, D., & Soylak, M. (2013). Pressure-assisted ionic liquid dispersive microextraction of vanadium coupled with electrothermal atomic absorption spectrometry. Journal of Analytical and Atomic Spectrometry, 28, 1441–1445.
Naeemullah, K. T. G., Tuzen, M., Shah, F., Afridi, H. I., & Citak, D. (2014). Development of a new green non-dispersive ionic liquid microextraction method in a narrow glass column for determination of cadmium prior to couple with graphite furnace atomic absorption spectrometry. Analytica Chimica Acta, 812, 59–64.
Omidi, F., Behbahani, M., Sadeghi Abandansari, H., Sedighi, A., & Shahtaheri, S. J. (2014a). Application of molecular imprinted polymer nanoparticles as a selective solid phase extraction for preconcentration and trace determination of 2, 4-dichlorophenoxyacetic acid in the human urine and different water samples. Journal Environmental Health Science Engineering, 12, 137–146.
Omidi, F., Behbahani, M., Samadi, S., Sedighi, A., & Shahtaheri, S. J. (2014b). Coupling of molecular imprinted polymer nanoparticles by high performance liquid chromatography as an efficient technique for sensitive and selective trace determination of 4-chloro-2-methylphenoxy acetic acid in complex matrices. Iran J Public Health, 43, 645–657.
Rezaei, F., Yamini, Y., Moradi, M., & Ebrahimpour, B. (2013). Solid phase extraction as a cleanup step before microextraction of diclofenac and mefenamic acid using nanostructured solvent. Talanta, 105, 173–178.
Salarian, M., Ghanbarpour, A., Behbahani, M., Bagheri, S., & Bagheri, A. (2014). A metal-organic framework sustained by a nanosized Ag12 cuboctahedral node for solid-phase extraction of ultra traces of lead (II) ions. Microchimica Acta, 181, 999–1007.
Saracoglu, S., Soylak, M., & Elci, L. (2003). Separation/preconcentration of trace heavy metals in urine, sediment and dialysis concentrates by coprecipitation with samarium hydroxide for atomic absorption spectrometry. Talanta, 59, 287–293.
Shamsipur, M., Javanbakht, M., Ghasemi, Z., Ganjali, M. R., Lippolis, V., & Garau, A. (2002). Separation, preconcentration and determination of trace amounts of silver ion in aqueous samples using octadecyl silica membrane disks modified with some recently synthesized mixed aza-thioether crowns containing 1, 10-phenanthroline sub-unit and atomic absorption spectrometry. Separation and Purification Technology, 28(2), 141–147.
Silvestre, C. I. C., Santos, J. L. M., Lima, J. L. F. C., & Zagatto, E. A. G. (2009). Liquid–liquid extraction in flow analysis: a critical review. Analytica Chimica Acta, 652, 54–65.
Soager, R, Metallic raw materials dictionary, Bank Tobel, Zurich, 1984.
Sobhi, H. R., Yamini, Y., Esrafili, A., & Adib, M. (2008). Extraction and determination of 2-pyrazoline derivatives using liquid phase microextraction based on solidification of floating organic drop. Journal of Pharmaceutical and Biomedical Analysis, 48, 1059–1063.
Soylak, M., & Cay, R. S. (2007). Separation/preconcentration of silver(I) and lead(II) in environmental samples on cellulose nitrate membrane filter prior to their flame atomic absorption spectrometric determinations. Journal of Hazardous Materials, 146, 142–147.
Tuzen, M., Unsal, Y. E., Soylak, M., Akkirman, Z., & Hazer, B. (2013). Solid-phase extraction of lead and copper on a polyhydroxybutyrate-b-polydimethyl siloxane (PHB-b-PDMS) block copolymer disc and flame atomic absorption spectrometric determination of them in water and food samples. International Journal of Food Science and Technology, 48, 2384–2390.
Unsal, Y. E., Tuzen, M., & Soylak, M. (2014). Speciation of chromium by the combination of dispersive liquid microextraction and microsample injection flame atomic absorption spectrometry. Turkish Journal of Chemistry, 38, 173–181.
Wua, X., & Yang, Y. (2011). Heavy metal (Pb, Co, Cd, Cr, Cu, Fe, Mn and Zn) concentrations in harvest-size white shrimp Litopenaeus vannamei tissues from aquaculture and wild source. Journal Food Composition Analysis, 24, 62.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Omidi, F., Behbahani, M., Shahtaheri, S.J. et al. Trace monitoring of silver ions in food and water samples by flame atomic absorption spectrophotometry after preconcentration with solvent-assisted dispersive solid phase extraction. Environ Monit Assess 187, 361 (2015). https://doi.org/10.1007/s10661-015-4568-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-4568-5