Abstract
The concentrations and distribution of selected heavy metals in epipelic and benthic sediments of Cross River Estuary mangrove swamp were studied to determine the extent of anthropogenic inputs from industrial activities and to estimate the effects of seasonal variations on geochemical processes in this tropical estuarine ecosystem. The analysis shows that the mean concentrations (mg/kg, dw) of Cu, Cr, Fe, Ni, Pb, V and Zn vary from 24.1–32.4, 19.9–27.4, 666.7–943.5, 15.2–30.3, 8.8–24.7, 2.2–6.9 and 140.1–188.9, respectively. An important observation is that, in general, lowest metal concentrations are found during the dry season, compared to wet season. Pollution load index (PLI) and index of geoaccumulation (I geo) revealed overall low values but the enrichment factors (EFs) for Cr, Zn, and V were high, and this reflects the intensity of anthropogenic inputs related to industrial discharge into the estuary. The mean concentrations of Zn, Cu and to some extent Ni exceeded the Effects Range—Low (ERL) and Threshold Effect Level (TEL) values in majority of the samples studied, indicating that there may be some ecotoxicological risk to organisms living in these sediments. The inter-element relationship revealed the identical source of elements in the sediments of the studied area. The concentration of heavy metals reported in this work will be useful as baselines for comparison in future sediment quality studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Estuaries, which are dynamic, complex and unique systems, are among the most productive marine ecosystems in the world (Chapman and Wang 2001). The pollution problems in estuaries are characterized by interconnected, complicated interactions, often making the interpretation of the disturbance effects in such ecosystems complex and confusing. In addition, these dynamic physical, chemical and geological conditions can hinder efforts to assess the impact of anthropogenic activity on estuarine biotic integrity. Thus, it is critical that sedimentary metal contamination in estuaries be properly and fully assessed.
The Cross River Basin encompasses an area of about 70,000km2 of which 50,000km2 lies within Nigeria and 20,000km2 lies within Cameroon. From observed physiological, ecological and zoogeographical discontinuity, the Nigerian section is here termed the lower Cross River Basin (4°58′ N, 8°21′ E). The Cross River enters Nigeria at about 40km upstream of Ikom. An ecologically significant floodplain is developed in the lower course of the Cross River. There is some delta formation in this region and, flowing through mangrove swamp, the main river is an ecosystem, which feeds a network of brackish water channels which all discharge into the Atlantic Ocean in the Bight of Bonny (Teugels et al. 1992).
Heavy metal concentrations in harbor or estuarine sediments usually are quite high due to significant anthropogenic metal loadings carried by upstream of tributary rivers (Paetzel et al. 2003; Muniz et al. 2004; Guerra-Garcia and Garcia-Gomez 2005). The sediments serve as a metal pool that can release metals to the overlying water via natural or anthropogenic processes, causing potential adverse health effects to the ecosystems (Dickinson et al. 1996; Poulton et al. 1996; Fatoki and Mathabatha 2001; McCready et al. 2006). Moreover, marine organisms or biota can take up metals, which in turn enhances the potential of some metals entering into food chain.
High levels of heavy metals in sediments do not necessarily reflect anthropogenic influence, but instead may be of a diagenetic origin (Zwolsman et al. 1993) or grain size effects (Loring and Rantala 1992). Since metals from both natural and anthropogenic sources accumulate in sediments, it is often difficult to determine what fraction of sedimentary metal load comes from which source. A crucial step for pollution assessment of sediment is to establish the expected natural background concentration levels (Niencheskil et al. 1994), from which various approaches can be used to quantify anthropogenic inputs.
A study of the distribution, enrichment, accumulation and seasonal variation of heavy metals in sediments of Cross River Estuary swamp is important to the assessment of the possible influence of anthropogenic activities on Cross River waters (Rubio et al. 2000; Huang and Lin 2003; Hung and Hsu 2004; Morillo et al. 2004; Adamo et al. 2005; Vald’es et al. 2005; Alagarsamy 2006). In the present study, we carried out physicochemical characterization of the epipelic and benthic sediments of Cross River Estuary mangrove ecosystem, namely pH, clay, silt, sand, total organic carbon (TOC), nutritive salts, total organic nitrogen (TON), exchangeable cations, and we examined the distribution of heavy metals such as Pb, Cr, Cu, Zn, Fe, Ni, and V in the epipelic and benthic sediments in both wet and dry seasons. Our assessment of sediment contamination was based on (1) sediment quality guidelines (SQGs) used by US EPA (2) degree of contamination using the enrichment factor, the geo-accumulation index (I geo) and the pollution load index (PLI); and (3) applications of two sets of guidelines: ERL/ERM and TEL/PEL and mean toxic units (Aloupi and Angelidis 2001; Woitke et al. 2003; Reddy et al. 2004; Selvaraj et al. 2004; MacDonald et al. 2000; Perin et al. 1997; Long and MacDonald 1998; Pedersen et al. 1998; Tomllinson et al. 1980). A statistical correlation analysis between sediment metal concentrations was also performed to determine the possible anthropogenic sources of the heavy metal concentrations in the Cross River sediments.
The measurement of trace element concentrations and distribution in marine environment leads to better understanding of their behavior in aquatic environment and is important for detecting the sources of pollution (Forstner and Wittman 1979). To date, there are scarce data pertaining to elemental composition of sediment profiles in this important estuary. Hence, the present collaborative study has been initiated with the following objectives: (1) to evaluate the geochemical factors that affect the seasonal distribution of the heavy metals including possible anthropogenic influences and their interaction in the sediment types of Cross River Estuary mangrove ecosystem, (2) to assess its ecological environmental risk, thus contributing to the knowledge and management of this region in future. Our results would provide a baseline against which future anthropogenic effects can be evaluated.
2 Materials and Methods
2.1 Study Area
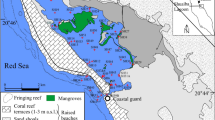
The study area (Fig. 1) is the coastal zone of Nigeria in West Africa, which lies between the River Niger Delta (7°30′ E) and Rio del Ray (8°30′ E) in the Cameroon Republic (Tahal Consultants 1979; Tuegels et al. 1992). Within this coastal stretch, mangroves occur in the estuaries of Imo River, Qua Iboe River and Cross River (Fig. 1). The three estuaries are connected to each other by means of inter-riverine creeks, constituting a homogeneous ecological unit. In this study, only the mangrove swamp ecosystem of the freshwater dominated euryhaline Cross River was investigated. The mangrove ecosystem was flooded twice daily and crude oil spillage occurred on 22nd November, 2001 in the vicinity of the Qua Iboe estuary. The sampling sites were located near the urban and industrial centres of Cross River and Akwa Ibom states of Nigeria. In addition to harbor activities, industrial facilities of oil operators are installed in these areas. Likely sources for metal contamination associated with these facilities include petroleum exploration, pipeline vandalization by saboteurs, crude oil trading along Nigeria–Cameroon border, fish cultivation and farming.
2.2 Sampling
Sampling for Cross River Estuary mangrove sediments was conducted between June, 2003 and February, 2004, representing the peak periods of wet and dry seasons in Nigeria. Three sampling sites—James Town (Station-1), Oron (Station-2) and Nwaniba (Akwa Akpa Uruan–Oron River confluence point, Station-3) were selected. Intertidal sediment samples were obtained with a 6.5cm diameter corer to a depth of 10cm. Subtidal sediment samples were collected using a Shipek Grab sampler (0.1 m2). Sediments collected were stored in clean plastic bottles in a cold container until analysis was carried out. Triplicate samples were usually obtained at each site (Popek 2003; Radojevic and Bashkin 1999). A total of 72 samples were taken from all sites investigated. Monthly samplings of intertidal and subtidal sediments were carried out during the wet (June 2003–September 2003) and dry (November 2003–February 2004) seasons. To determine the expected natural background levels, sediment samples were also collected and analyzed from areas remotely located from anthropogenic inputs.
Sediment samples from the various sampling locations in each site were pooled together and composite samples from the three sites of the ecosystem were air dried and 2mm sieved prior to analysis.
2.3 Sediment Characterization
Sediment physicochemical parameters were determined using standard procedures (Radojevic and Bashkin 1999). Fast changing parameters, such as pH, salinity, and temperature were measured in the field using portable multi-probe quality meter (Model U7, Horiba Ltd). Chloride, nitrate, sulphate, carbonate and exchangeable cations were determined using standard procedures (Radojevic and Bashkin 1999; Black et al. 1961). Total organic carbon (TOC) content was determined by the rapid wet oxidation method based on Walkey and Black procedure (Jacobsen 1992; Page et al. 1982; AOAC 1975). Total organic nitrogen (TON) was determined by classical Kjeldahl digestion followed by distillation. TON in the distillates was determined by spectrophotometry. Particle size distribution (grain size analysis) was determined by the hydrometer method (AOAC 1975; Juo 1979).
2.4 Analysis of Heavy Metals
The sediment samples were dried at 70–80°C for 48 h and thereafter gently ground with rolling pin to disaggregate the samples but not break down the grains themselves, and sieved to collect less than 63 μ grain sizes. The sediment samples were digested as described by Ho et al. (2003) and Miroslav and Vladimir (1999). Precisely, 2.0g of sediment was digested with a solution of concentrated HNO3 (0.3 ml) and HCl (6.0 ml) to near dryness and allowed to cool before 20 ml of 5.0 M HNO3 (1 M = 1 mol dm−3) solutions were added. The solutions were allowed to stay overnight and filtered. The filtrates were transferred into 100ml volumetric flask and made up to the mark with 0.5 M HNO3 (Binning and Baird 2001).
A reagent blank (without sample) was prepared using a mixture of HNO3 and HCl, and the entire sequence of steps was followed as described for the sample preparation. The sample solution and the blank were analyzed for the concentrations of extractable heavy metals (Cr, Pb, Fe, Ni, V, Zn, and Cu) using an inductively coupled plasma spectrophotometer (Optima 3000—Optima 3000—Perkin Elmer). The analysis was duplicated to verify the precision of the method of digestion. The instrumental detection limits (IDL) were: 0.02, 0.002, 0.01, 0.01, 0.002, 0.01, and 0.01 mg kg−1 for Cr, Pb, Fe, Ni, V, Zn and Cu, respectively. Duplicates and method blanks were employed to test for precision, accuracy and reagent purity used in the analytical procedures.
2.5 Preparation of Standards
In order to reduce the detrimental effects of overlapping spectral interferences on element quantitation during metal analyses, an interelement correction standard was prepared by using standardized solution of metal ions prepared from their salts. A mixture of commercially available 100mg kg−1 stock solutions (Analar Grade) of Cr3+, Pb2+, Fe2+, Ni2+, V2+, Zn2+ and Cu2+ were prepared as interelement working standard solution to verify that the overlapping lines do not cause the detection of elements at concentration above methods detection limits (MDLs) (Popek 2003).
3 Results and Discussion
3.1 Sediment Quality Characteristics
Table 1 shows mean values and standard deviation of pH, total organic carbon (%), total organic nitrogen (%), nutritive salts (mg/kg), exchangeable cations (mg/kg), salinity (%) and percentages of sand, silt and clay in sediment samples studied. The sediments of Cross River Estuary mangrove ecosystem have shown vary pH from slightly acidic for epipelic sediment (mean values of 5.9 and 6.5 in wet and dry seasons respectively) to neutral in benthic sediment (mean values of 7.1 and 7.4 in wet and dry seasons respectively). The acidic nature is mainly pronounced in epipelic sediment during the two seasons, and this can be attributed to the decomposition of mangrove litter and hydrolysis of tannin in mangrove plants releasing various kinds of organic acids (Liao 1990).
The sediment samples show a variable admixture of sand, silt and clay. Results show that sand (>63μm) was the main component of all sediment samples, with a range from 60.5% to 69.9%. Mean clay contents were in the range of 21.3% to 27.1%. The dominance of sand fraction might be as a result of high energy level in the estuary, giving the depositional area a sandy beach environment. A variable amount of erosion and depositions in this estuary in both seasons is reflected from variable admixture of sand and clay fractions in individual sediment. The high levels of nutritive salts (CO3 2−, SO4 2−, Cl−, NH4 + and NO3 −) in sediments analyzed for both seasons were indicative of the influences of human mediated activities. Crude oil pollution has also been associated with increase in nutritive salt and salinity levels of aquatic ecosystems (Rhykered et al. 1995 and Ward et al. 1980) and may have contributed to the high concentrations of SO4 2−, Cl−, and NH4 + salts in the epipelic sediments of Cross River Estuary mangrove swamps during both seasons.
Sediments are important sources of micronutrients. Micronutrients such as Mg, Ca, Na and K are abundant natural elements and are important in ensuring optimal primary and secondary productivity of the marine and brackish ecosystems. These elements together contribute about 37% to the normal salinity level of the sea, with Na supplying about 31% (Duxbury 2000). The concentrations of the micronutrients obtained in the present study were relatively stable (RSD < 15%) during both seasons and did not exceed the natural occurrence levels reported for cations (e.g. 1350, 410, 10,500 and 390 mg/L for Mg, Ca, Na and K respectively) in tropical seawater (Hem 1985).
The mean TOC levels in sediment analyzed ranged from 4.0% to 11.3%. The TOC levels of epipelic sediments (10.2% and 11.3%) are higher in both seasons than that of benthic sediments (4.0% and 7.0%). Previous studies have found that higher TOC (>3.0%) levels are typically associated with fine sediments and lower TOC levels with coarse sediments (Salomons and Forstner 1984; Cho et al. 1999). The relatively high concentrations of TOC in epipelic sediments studied reflect “high” organic matter flux to epipelic sediments of Cross River Estuary mangrove ecosystem which can be related to the decomposition of mangrove litter and hydrolysis of tannin in mangrove plants releasing various kinds of organic matter and acids. (Liao 1990). The total organic nitrogen (TON) ranged from 0.29% to 0.46%. The benthic sediment collected during the dry season has the least nitrogen content of 0.29%, while others fell within the same range of 0.4–0.46%. The palaeoenvironmental significance of the C/N ratio and its usefulness as an organic matter identifier has been emphasized by Meyers (1994). The C/N ratios have been used to distinguish between organic matter inputs in estuaries, since autochthonous marine organisms rich in protein material have C/N values (4–10) much lower than terrestrial plants (>20) (Kawamura and Ishiwatari 1981; Meyers 1994 and 1997). In this work, the C/N ratios of benthic sediments were 13.8 and 15.6 for both seasons, indicating an input of different mixtures of land and aquatic organic matter. These values are similar to those found in marine Sapropel, where organic matter is composed of a mixture of vascular plant debris and algal plus bacteria remains (Meyers 1994). The C/N ratio of epipelic sediments were >20, indicating terrigenous organic matter, mainly from vascular plant detritus or grassy material.
3.2 Heavy Metal Distribution
Table 2 presents the range, mean and standard deviation of heavy metals in sediments of Cross River Estuary mangrove swamp. From all metals studied, Fe showed the highest mean concentration in the sediment types at both seasons, followed by Zn. The maximum mean concentration values for Cu (32.4 mg kg−1), Zn (188.9 mg kg−1), Cr (27.4 mg kg−1), Fe (943.5 mg kg−1), Ni (30.3 mg kg−1) and V (6.9 mg kg−1) were obtained in sediments (epipelic and benthic) collected during the wet season. Intensive fishing activities, sewage drainage from the mainland and other industrial activities are possible potential sources for the enrichment of these elements during the wet season. The concentrations of Cu, Fe, Zn, V in epipelic sediments are higher than the levels observed in subtidal sediments of Cross River estuarine ecosystem. The enhanced concentrations of these metals in epipelic sediment in both seasons may be ascribed to industrial activities and terrestrial runoffs. The variation may also be attributed to increase absorption, sedimentation and flocculation dynamics that take place in the estuary (Matagi et al. 1998).
The V/Ni ratios of the sediments at both seasons are given in Table 2. The values (0.09–0.41) comply with emissions from oil-related industries, and comparable to the reported values for Nigerian crude oils (Olajire and Oderinde 1993). The low V/Ni ratios indicate the oil-forming continent environment. Although large amounts of vanadium and nickel are associated to metallo-organic complexes under euxinic conditions (Lewan and Maynard 1982; Breit and Wanty 1991), a substantial presence of bacterially generated H2S limits the availability of nickelous cations because of the formation of NiS complexes, whereas vanadyl or trivalent vanadium are available (Lewan 1984). From V/(V + Ni) ratio (Table 2), it is evident that all the ratios from Cross River Estuary ecosystem fall below the range (0.54–0.80) proposed by Hatch and Leventhal (1992) as indicative of anoxic conditions. The V/(V + Ni) ratios of sediments studied (0.08–0.29) are lower than 0.54, and thus are related to oxic conditions at the seafloor.
3.3 US EPA Sediment Quality Guidelines
Sediments were classified as non-polluted, moderately polluted and heavily polluted, based on the SQG of US EPA (Perin et al. 1997). Concentrations of heavy metals in sediments of Cross River Estuary mangrove swamp are summarized in Table 3. Average data of sedimentary metals from areas remotely located from anthropogenic inputs are used to provide natural background concentrations (Essien 2007). Result in Table 3 shows that concentrations of Zn, Cr, Cu and V were for all sediments higher than natural background concentration, while concentrations of Fe (except in W E) were for other sediments lower than natural background concentrations. The concentrations of Ni (except in D E), and Pb (except in W E and D E) were also higher than the natural background concentrations.
Sediments, contaminated with Zn are considered “moderately polluted” per the SQG (Fig. 2). Similarly, Cu (except in D B), Cr (except in D E and D B), Ni (except in W E and D E), were also “moderately polluted” using the SQG.
3.4 Pollution Load Index (PLI)
In order to understand the contamination state of heavy metals, Tomllinson’s pollution load index (PLI) (Tomllinson et al. 1980) was calculated using the heavy metal data and average natural background concentrations of the metals (Essien 2007). The PLI is obtained as a contamination factor (CF) of each metal with respect to the natural background value in the sediment (Angulo 1996) by applying the following equations:
where,
- CF:
-
contamination factor, n=number of metals
- \(\overline C _{{\text{metal}}} \) :
-
mean metal concentration in polluted sediments
- \(\overline C _{{\text{background}}\;{\text{value}}} \) :
-
mean natural background value of that metal.
The PLI represents the number of times by which the metal content in the sediment exceeds the average natural background concentration, and gives a summative indication of the overall level of heavy metal toxicity in a particular sample.
The contamination factor and pollution load index are given in Table 3. Our result shows that the CF values of Ni (in D E), Pb (in W E and D E), V (in D B) and Fe (except W E) in the study area are low (<1). However, CF values for others show higher values (>1) due to influence of external discrete sources like industrial and agricultural runoff and anthropogenic inputs. The values of pollution load index (Table 3) were greater than unity (>1) in all the sediment types for both seasons. An overall assessment indicates that this estuarine mangrove ecosystem is considered to be of pollution concern in view of crude oil spillages that have been occurring in the region.
3.5 Enrichment Factor and Index of Geo-Accumulation
To assess the relative contributions of natural and anthropogenic metal inputs to sediments, it is necessary to have some idea of what natural concentrations are expected to be in sediments from a given region. Thus for the purpose of assessing anthropogenic inputs to sediments in this region, we use data from samples taken from remotely located areas from anthropogenic influences to establish metal–normalizer relationships to which the data generated from epipelic and benthic sediments are compared. Iron (Fe) was chosen as geochemical normalizer because of its conservative nature during diagenesis (Berner 1980). Regression analysis of the clay fraction on Fe also shows weak positive relationship (Fig. 3), indicating the association of iron with clay fraction.
The extent of sediment contamination was assessed using the enrichment factor (EF) and geo-accumulation index (I geo) (Aloupi and Angelidis 2001; Woitke et al. 2003; Reddy et al. 2004; Selvaraj et al. 2004). EF is a good tool to differentiate the metal source between anthropogenic and naturally occurring (Morillo et al. 2004; Selvaraj et al. 2004; Adamo et al. 2005; Vald’es et al. 2005). According to this technique, metal concentrations were normalized to textural or compositional characteristics of sediments. Normalizing elements relative to Fe or Al is widely used to compensate for both the granulometric and mineralogical variability of metal concentrations in sediment (Chapman and Wang 2001). EF has also been used as an indication of degree of pollution or environmental contamination or both (Feng et al. 2004; Zhang and Liu 2002). The EF is defined as:
where (X/Fe)sample is the ratio of heavy metal (X) to Fe in the sample of interest, and (X/Fe)background is the natural background value of the metal Fe ratio.
EF values were interpreted as suggested by Birth (2003) for metals studied with respect to natural background concentration. EF < 1 indicates no enrichment, EF < 3 is minor enrichment, EF = 3–5 is moderate enrichment, EF = 5–10 is moderately severe enrichment, EF = 10–25 is severe enrichment, EF = 25–50 is very severe enrichment and EF > 50 is extremely severe enrichment.
Table 4 presents the mean EF values of the metals studied with respect to the natural background concentration. All the metals except Cu, Ni (in W E and D E), Pb (in W E and D E) and V (in D B) showed high EF values (>1.5), indicating that they have originated from non-crustal sources, i.e. anthropogenic pollution. The benthic sediments are more enriched with Pb, Cr and Ni than epipelic sediments. Nickel and Pb had the least EF values (<1.0) in epipelic sediments analyzed for both seasons, indicating no enrichment and originated from crustal source. Going by Birth (2003) interpretation, Cr is moderately enriched in all the sediments while Cu, V, Zn, Ni (in W B and D B) and Pb (in W B and D B) are minor enrichment.
The I geo values for the metals studied were calculated using the Muller’s (1979) expression:
where C X is the measured content of the metal X and B X is the natural background concentration of metal X.
Based on the I geo data and Muller’s geo-accumulation indexes, the contamination level with respect to each metal studied is ranked in Table 4 (I geo class). The Geoaccumulation index values (I geo) showed very low values (<0) in some of the cases except Cr (>1.0 in all the sediments), Ni (0.22 for W B), Pb (0.19 for W B), V (0.75, 0.77 and 0.61 for W E, W B and D E respectively) and Zn (0.28 and 0.24 for W E and D E respectively), indicating sediments of Cross River Estuary mangrove ecosystem are uncontaminated to moderately contaminated as a result of anthropogenic activities.
3.6 Ecotoxicological Assessment of Heavy Metals Concentrations in Sediments
To evaluate the sediment contamination and potential ecotoxicological effects associated with the observed concentration of contaminants, two sets of SQGs developed for marine and estuarine ecosystems (MacDonald et al. 2000; Long and MacDonald 1998) were applied in this study to assess the ecotoxicological potential of heavy metal concentrations in sediments (a) the effect range—low (ERL)/ effect range—median (ERM); and (b) the threshold effect level (TEL)/probable effect level (PEL) values (Table 5). Low range values (ERLs/TELs) are concentrations below which adverse effects upon sediment dwelling fauna would infrequently be expected. In contrast, the ERMs and PELs represent chemical concentrations above which adverse effects are likely to occur (Long and MacDonald 1998).
The two different ways of comparison that have been included in this study are: the number of single-species limits values exceeded and the mean quotient calculable from the two empirically derived sets of SQGs using PEL and ERM values. Table 5 gives the number of all samples in three ranges of chemical concentrations where adverse biological effects are expected rarely (<TEL/ERL), occasionally (≥TEL/ERL and <PEL/ERM) and frequently (≥PEL/ERM). For Pb and Cr, all the sediment samples were in the minimal effect—range (<TEL/ERL); while for Cu, Ni, and Zn, a large proportion (50–100%) of samples were in the minimal effect—range (<TELs/ERLs). For all the metals studied, none of the sediments were in the probable effect—range and effect range median (≥PELs/ERMs).
In order to determine the possible biological effect of combined toxicant groups, one can calculate the mean quotient for a large range of contaminants. This mean ERM quotient (m − ERM − Q) has been calculated according to Long et al. (1998) as follows:
where C i is the sediment concentration of compound i, ERM i is the ERM for compound i and n is the number of compound i.
Similarly, the mean PEL quotient (m − PEL − Q) can be calculated according to the equation:
where, PEL i is the PEL for compound i.
Mean ERM quotients have been related to probability of toxicity (Long and MacDonald 1998 and Long et al. 2000) based on the analysis of matching chemical and toxicity data from 1068 samples from the USA estuaries. The mean ERM quotient of <0.1 has a 12% probability of being toxic; a mean ERM quotient of 0.11–0.5 has a 30% probability of toxicity; a mean ERM quotient of 0.51–1.5 has a 40% of being toxic and a mean ERM quotient of >1.50 has a 74% of toxicity. According to this classification, all the sediment samples studied can be classified as “medium–low priority” sites with 30% probability of toxicity. Similarly, the mean PEL quotient ranged from 0.30 for D E to 0.38 for W B. Although, the mean PEL quotients have not been related to probability of toxicity, but the values of mean PEL quotients obtained for these sediments are low, and as such will be expected to have low percentage probability of being toxic.
3.7 Inter-Element Correlations
The correlation coefficient between element pairs is calculated in order to deduce the possible sources of the metals in the sediment samples. As seen in Table 6, the concentrations of several metals were strongly correlated with each other. The best correlations in sediments collected in the wet season were between Cu–Cr, Cu–Fe, Cu–V, Cr–Pb, Fe–Pb, Cr–Zn, Pb–Zn, Pb–V (r ≥ 0.95; P < 0.05; df = 10); and in sediments collected in dry season, between Zn–V, Cu–Fe (r ≥ 0.95, P < 0.05; df = 10). Historically, rivers have been identified as the main source of Cu, Ni and Zn, atmospheric deposition as an important component of Pb and Zn contamination, oil spillage as the main source of Pb, V and Ni and direct effluent discharge as the main source of Cd and Cr (Apte et al. 1989, Owens 1984, Olajire and Oderinde 1993). The correlation coefficients between the concentrations of the different metals indicate strong links between Cu, Cr, Pb, Zn and V, which probably reflects their related origin (Table 6).
4 Conclusion
The combined use of different approaches for evaluating sediment metal contamination facilitates a comprehensive interpretation of the sedimentary characteristics in terms of the background influences and ecotoxicological risks. It is observed that, in general, lowest metal concentrations are found during the dry season, compared to the wet season. The seasonal variation of heavy metals in sediments in the estuarine environment are influenced by so many factors including geochemical and biogeochemical processes like sedimentation, precipitation and flocculation of particulate substances (Che et al. 2003), and hence it is difficult to find the major one. Our results suggest that for heavy metal contaminants, there are important historical signatures among the epipelic and subtidal sediments. The estuarine environment is getting polluted with Zn, Cu, Cr and Ni and the affected sites may be classified as “moderately polluted” by these heavy metals. The enrichment of Cr, V and Zn reflects the intensity of anthropogenic pollution.
It is proposed that continuous monitoring and further studies on the level of these heavy metals should be carried out in the near future to ascertain long-term effects of anthropogenic impact and to assess the effectiveness of reducing the impact of human activity to upgrade the brackish environment in the Cross River Estuary on the Cameroon–Nigerian boarder as the exploration and crude oil trading activities influence the geochemical processes in the estuarine systems.
References
Adamo, P., Arienzo, M., Imperato, M., Naimo, D., Nardi, G., & Stanzione, D. (2005). Distribution and partition of heavy metals in surface and sub-surface sediments of Naples city port. Chemosphere, 61, 800–809. doi:10.1016/j.chemosphere.2005.04.001.
Alagarsamy, R. (2006). Distribution and seasonal variation of trace metals in surface sediments of the Mandovi estuary west coast of India. Estuarine, Coastal and Shelf Science, 67, 333–339. doi:10.1016/j.ecss.2005.11.023.
Aloupi, M., & Angelidis, M. O. (2001). Geochemistry of natural and anthropogenic metals in the coastal sediments of the island of Lesvos, Aegean Sea. Environmental Pollution, 113, 211–219. doi:10.1016/S0269-7491(00)00173-1.
Angulo, E. (1996). The Tomllinson pollution load index applied to heavy metals ‘Mussel-Watch’ data: a useful index to assess coastal pollution. The Science of the Total Environment, 187, 19–56. doi:10.1016/0048-9697(96)05128-5.
AOAC (1975). Methods for soil analysis (12th ed.). Washington, DC: Association of Official Analytical Chemist.
Apte, S. C., Gardner, M. J., Gunn, A. M., Ravenscroft, J. E., & Vale, J. (1989). Trace metals in the Severn estuary—a reappraisal pp. 1–9. Marlow: Water Research Centre.
Berner, R. A. (1980). Early diagenesis. A theoretical approach. Princeton, NJ: Princeton University Press.
Binning, K., & Baird, D. (2001). Survey of heavy metals in the sediments of the Swatkop River Estuary, Port Elizabeth South Africa. Water S.A, 24(4), 461–466.
Birth, G. A. (2003). A scheme for assessing human impacts on coastal aquatic environments using sediments. In: Woodcoffe, C.D; Furness, R.A (eds.), Coastal GIS 2003. Wollongong University Papers in Centre for Maritime Policy, 14, Australia.
Black, C. A., Evans, D. D., White, J. L., Esminger, L. B., & Clark, F. E. (1961). Methods of soil analysis 2. Chemical and microbiological properties. Madison: American Society of Agronomy.
Breit, G. N., & Wanty, R. B. (1991). Vanadium accumulation in carbonaceous rocks: a review of geochemical controls during deposition and diagenesis. Chemical Geology, 91, 83–97. doi:10.1016/0009-2541(91)90083-4.
Chapman, P. M., & Wang, F. (2001). Assessing sediment contamination in estuaries. Environmental Toxicology and Chemistry, 20, 3–22. doi:10.1897/1551-5028(2001)020<0003:ASCIE>2.0.CO;2.
Che, Y., He, Q., & Lin, W. Q. (2003). The distribution of particulate heavy metals and its indications to the transfer of sediments in the Changjiang estuary and Hangzhons bay, China. Marine Pollution Bulletin, 46, 123–131. doi:10.1016/S0025-326X(02)00355-7.
Cho, Y.-G., Lee, C. B., & Choi, M.-S. (1999). Geochemistry of surface sediments off the southern and western coast of Korea. Marine Geology, 159, 111–129. doi:10.1016/S0025-3227(98)00194-7.
Dickinson, W. W., Dunbar, G. B., & Mclead, H. (1996). Heavy metal history from cores in Wellington Harbour, New Zealand. Environmental Geology, 27, 59–69. doi:10.1007/BF00770603.
Duxbury, D. S. (2000). Introduction to the world’s ocean (6th ed.). UK: McGraw Hill.
Essien, J. P. (2007). Ecology and Physiological diversity of heterotrophic and hydrocarbon utilizing microorganisms in sediments of the Niger Delta Mangrove Swamp ecosystem. Ph.D. Thesis, University of Calabar, Calabar, Nigeria, p 500.
Fatoki, O. S., & Mathabatha, S. (2001). An assessment of heavy metal pollution in the East London and Port Elizabeth harbours. Water S.A., 27, 233–240.
Feng, H., Han, X., Zhang, W., & Yu, L. (2004). A preliminary study of heavy metal contamination in Yangtze River intertidal zone due to urbanization. Marine Pollution Bulletin, 49, 910–915. doi:10.1016/j.marpolbul.2004.06.014.
Forstner, U., & Wittman, G. T. W. (1979). Metal pollution in the aquatic environment p. 486. Berlin: Springer.
Guerra-Garcia, J. M., & Garcia-Gomez, J. C. (2005). Assessing pollution levels in sediments of a harbour with two opposing entrances: environmental implications. Journal of Environmental Management, 77, 1–11. doi:10.1016/j.jenvman.2005.01.023.
Hatch, J. R., & Leventhal, J. S. (1992). Relationship between inferred redox potential of the depositional environment and geochemistry of the Upper Pennsylvanian (Missourian) Stark Shale Member of the Dennis Limestone, Wabaunsee County, KS, USA. Chemical Geology, 99, 65–82. doi:10.1016/0009-2541(92)90031-Y.
Hem, J. D. (1985). Study and interpretation of the chemical characteristics of natural waters (3rd ed., pp. 2254–2263). US Geological Surveys Water Supply Paper.
Ho, S. T., Tsai, I. J., & Yu, K. C. (2003). Correlation among aqua-regia extractable heavy metals in vertical river sediments. Diffuse Pollution Conference, Dublin, 14, 12–18.
Huang, K. M., & Lin, S. (2003). Consequences and implication of heavy metal spatial variations in sediments of the Keelung River drainage basin, Taiwan. Chemosphere, 53, 1113–1121. doi:10.1016/S0045-6535(03)00592-7.
Hung, J. J., & Hsu, C. L. (2004). Present state and historical changes of trace metal pollution in Kaoping coastal sediments, southwestern Taiwan. Marine Pollution Bulletin, 49, 986–998. doi:10.1016/j.marpolbul.2004.06.028.
Jacobsen, S. T. (1992). Chemical reaction and air change during the decomposition of organic matter. Resources, Conservation and Recycling, 6, 529–539.
Juo, A. S. R. (1979). Selected methods for soil and plant analysis: manual series p. 70. Ibadan: International Institute of Tropical Agriculture (IITA).
Kawamura, K., & Ishiwatari, R. (1981). Polyunsaturated fatty acids in a lacustrine sediment as a possible indicator of paleoclimate. Geochimica et Cosmochimica Acta, 45, 149–155. doi:10.1016/0016-7037(81)90158-7.
Lewan, M. D. (1984). Factor controlling the proportionality of vanadium to nickel in crude oils. Geochimica et Cosmochimica Acta, 48, 2231–2238. doi:10.1016/0016-7037(84)90219-9.
Lewan, M. D., & Maynard, J. B. (1982). Factors controlling enrichment of vanadium and nickel in the bitumen of organic sedimentary rocks. Geochimica et Cosmochimica Acta, 46, 2547–2560. doi:10.1016/0016-7037(82)90377-5.
Liao, J. F. (1990). The chemical properties of the mangrove Solonchak in the northeast part of Hainan Island. Acta Scientiarum Naturalium Universitatis Sunyatseni, 9(4), 67–72 Supp.
Long, E. R., Field, L. J., & MacDonald, D. D. (1998). Predicting toxicity in marine sediments with numerical sediment quality guidelines. Environmental Toxicology and Chemistry, 17, 714–727. doi:10.1897/1551-5028(1998)017<0714:PTIMSW>2.3.CO;2.
Long, E. R., & MacDonald, D. D. (1998). Recommended uses of empirically derived, sediment quality guidelines for marine and estuarine ecosystems. Human and Ecological Risk Assessment, 4(5), 1019–1039. doi:10.1080/10807039891284956.
Long, E. R., MacDonald, D. D., Smith, S. L., & Calder, F. O. (1995). Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environmental Management, 19, 81–97. doi:10.1007/BF02472006.
Long, E. R., MacDonald, D. D., Severn, C. G., & Hong, C. B. (2000). Classifying probabilities of acute toxicity in marine sediments with empirically derived sediment quality guidelines. Environmental Toxicology and Chemistry, 19, 2598–2601. doi:10.1897/1551-5028(2000)019<2598:CPOATI>2.3.CO;2.
Loring, H. D., & Rantala, R. (1992). Manual for the geochemical analyses of marine sediments and suspended particulate matter. Earth-Science Reviews, 32, 235–283. doi:10.1016/0012-8252(92)90001-A.
MacDonald, D. D., Ingerosoll, C. G., & Berger, T. A. (2000). Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Archives of Environmental Contamination and Toxicology, 39, 20–31. doi:10.1007/s002440010075.
Matagi, S. V., swai, D., & mugabe, R. (1998). heavy metal removal mechanisms in wetlands. African J. Tropical Hydrobiol. Fisher., 8, 23–35.
McCready, S., Birch, G. F., & Long, E. R. (2006). Metallic and organic contaminants in sediments of Sydney harbour, Australia and vicinity—a chemical dataset for evaluating sediment quality guidelines. Environment International, 32, 455–465. doi:10.1016/j.envint.2005.10.006.
Meyers, P. A. (1994). Preservation of elemental and isotopic source identification of sedimentary organic matter. Chemical Geology, 144, 289–302. doi:10.1016/0009-2541(94)90059-0.
Meyers, P. A. (1997). Organic geochemical proxies of paleoceanographic, paleolimnologic and paleoclimatic processes. Organic Geochemistry, 27, 213–250. doi:10.1016/S0146-6380(97)00049-1.
Miroslav, R., & Vladimir, N. B. (1999). Practical environmental analysis p. 466. Cambridge, UK: The Royal Society of Chemistry.
Morillo, J., Usero, J., & Gracia, I. (2004). Heavy metal distribution in marine sediments from the southwest coast of Spain. Chemosphere, 55, 431–442. doi:10.1016/j.chemosphere.2003.10.047.
Muller, G. (1979). Schwermetalle in den sediments des Rheins-Veran-derngren seitt. 1971. Umschan, 79, 778–783.
Muniz, P., Danula, E., Yannicelli, B., Garcia-Alonso, J., Medina, G., & Bicego, M. C. (2004). Assessment of contamination by heavy metals and petroleum hydrocarbons in sediments of Monterideo harbour (Uruguay). Environment International, 29, 1019–1028. doi:10.1016/S0160-4120(03)00096-5.
Niencheskil, L. F. H., Windom, L. H., & Smith, R. (1994). Distribution of particulate trace metal in Patos lagoon estuary (Brazil). Marine Pollution Bulletin, 28(2), 96–102. doi:10.1016/0025-326X(94)90545-2.
Olajire, A. A., & Oderinde, R. A. (1993). Trace metals in Nigerian crude oils and their heavy-end distillates. Bulletin of the Chemical Society of Japan, 66(2), 630–632. doi:10.1246/bcsj.66.630.
Owens, M. (1984). Severn estuary—an appraisal of water quality. Marine Pollution Bulletin, 15, 41–47. doi:10.1016/0025-326X(84)90460-0.
Paetzel, M., Nes, G., Leifsen, L. O., & Schrader, H. (2003). Sediment pollution in the Vagen, Bergen harbour, Norway. Environmental Geology, 43, 476–483.
Page, A. L., Miller, R. H., & Keeney, D. R. (1982). Methods of soil analysis. Part 2. Chemical and microbiological properties (2nd ed., p. 1159). American Society of Agronomy, Madison, WI, pp. 538–580.
Pedersen, F., Bjornestad, E., Andersen, H. V., Kjolholt, J., & Poll, C. (1998). Characterization of sediments from Copenhagen harbour by use of biotests. Water Science and Technology, 37(6–7), 233–240. doi:10.1016/S0273-1223(98)00203-0.
Perin, G., Bonardi, M., Fabris, R., Simoncini, B., Manente, S., Tosi, L., et al. (1997). Heavy metal pollution in central Venice Lagoon bottom sediments: evaluation of the metal bioavailability by geochemical speciation procedure. Environmental Technology, 18, 593–604.
Popek, E. P. (2003). Sampling and analysis of environmental pollutants: a complete guide p. 356. USA: Academic.
Poulton, D. J., Morris, W. A., & Coakley, J. P. (1996). Zonation of contaminated bottom sediments in Hamilton harbour as defined by statistical classification techniques. Water Quality Research Journal of Canada, 31, 505–528.
Radojevic, M., & Bashkin, V. N. (1999). Practical environmental analysis (p. 465). Royal Society of Chemistry, Thomas Graham House, Cambridge CB4 OWF, UK.
Reddy, M. S., Basha, S., Sravan Kumar, V. G., Joshi, H. V., & Ramachandraiah, G. (2004). Distribution, enrichment and accumulation of heavy metals in coastal sediments of Alang–Sosiya ship scrapping yard, India. Marine Pollution Bulletin, 48, 1055–1059. doi:10.1016/j.marpolbul.2003.12.011.
Rhykered, R. I., Weaver, R. W., & Mclnnes, K. J. (1995). Influence of salinity on bioremediation of oil in soil. Environmental Pollution, 90, 127–130. doi:10.1016/0269-7491(94)00087-T.
Rubio, B., Nombela, M. A., & Vilas, F. (2000). Geochemistry of major and trace elements in sediments of the Ria de Vigo (N W Spain): an assessment of metal pollution. Marine Pollution Bulletin, 40, 968–980. doi:10.1016/S0025-326X(00)00039-4.
Salomons, W., & Forstner, U. (1984). Metal in the hydrocycle p. 349. Berlin: Springer.
Selvaraj, K., Ram Mohan, V., & Szefer, P. (2004). Evaluation of metal contamination in coastal sediments of the Bay of Bengal, India: geochemical and statistical approaches. Marine Pollution Bulletin, 49, 174–185.
Tahal Consultants (1979). (Nigeria) Ltd. Qua Iboe River Basin Pre-feasibility study. Cross River Basin Development Authority, Nigeria, vol. 2, Annex II: A 1–14.
Teugels, G. C., Reid, F. M., & King, R. P. (1992). Fishes of the Cross River Basin (Cameroon–Nigeria): taxonomy, zoogeography, ecology and conservation. Annals Science Zoologiques, 1, 216–248.
Tomllinson, D. C., Wilson, J. G., Harris, C. R., & Jeffrey, D. W. (1980). Problems in the assessment of heavy metals levels in estuaries and the formation of pollution index. Helgoländer Wissenschaftliche Meeresuntersuchungen, 33, 566–569.
Vald’es, J., Vargas, G., Sifeddine, A., Ortlieb, L., & Guinez, M. (2005). Distribution and enrichment of heavy metals in Mejillones Bay (23°S), Northern Chile: geochemical and statistical approach. Marine Pollution Bulletin, 50, 1558–1568.
Ward, D. M., Atlas, R. M., Boehm, P. D., & Calder, J. A. (1980). Microbial biodegradation and the chemical evolution of Amoco Cadiz oil pollutants. Ambio, 9, 277–283.
Woitke, P., Wellmitz, J., Helm, D., Kube, P., Lepom, P., & Litheraty, P. (2003). Analysis and assessment of heavy metal pollution in suspended solids and sediments of the river Danube. Chemosphere, 51, 633–642.
Zhang, J., & Liu, C. L. (2002). Riverine composition and estuarine geochemistry of particulate metals in China weathering features, anthropogenic impact and chemical fluxes. Estuarine, Coastal and Shelf Science, 54, 1051–1070.
Zwolsman, I. G. J., Berger, W. G., & Vaneck, M. T. G. (1993). Sediment accumulation rates, historical input, post depositional mobility and retention of major elements and trace metals in salt marsh sediments of the Scheldt estuary, SW Netherlands. Marine Chemistry, 44, 73–94.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Essien, J.P., Antai, S.P. & Olajire, A.A. Distribution, Seasonal Variations and Ecotoxicological Significance of Heavy Metals in Sediments of Cross River Estuary Mangrove Swamp. Water Air Soil Pollut 197, 91–105 (2009). https://doi.org/10.1007/s11270-008-9793-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-008-9793-x