Abstract
The present study compared accumulation of heavy metals in a mangrove swamp dominated by Kandelia obovata with that by Sonneratia apetala in Pearl River Estuary, China. The results showed that the concentrations of heavy metals at all sediment depths in the S. apetala site were significantly higher than that in K. obovata. The geo-accumulation index and potential ecological risk index also showed that S. apetala sediment had a higher contamination of heavy metals, especially Cd. S. apetala significantly altered the biogeochemical cycles of Cd, lead (Pb), nickel (Ni) and chromium (Cr). In S. apetala sediment, TOC played an important role in sequestering heavy metals as reflected by its positive correlations with Zn and Pb. This study demonstrated the importance of plant species in altering soil quality and heavy metal accumulation, and S. apetala is more efficiently working as a pollution barrier than K. obovata.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mangroves are important intertidal estuarine wetlands that provide a unique ecological environment for diverse fauna and flora (Smee et al. 2017). However, mangroves are also one of the most threatened tropical environments, which have been damaged by reclamation for urbanization, agriculture, mariculture and tourism, inadequate protection from insect infestations and anthropogenic pollution such as heavy metals (Friess et al. 2012). From 2000 to 2012, the degradation and annual losses of mangroves, particularly because of rapid coastal development, varied from 0.16 to 0.39% (Hamilton and Casey 2016). In China, the area of mangroves between the mid-1950s and 1990s also decreased from over 40,000 to 15,122 ha (Peng et al. 2008). In the early 1990s, several mangrove reforestation projects have been initiated by local governments in China to plant indigenous and exotic mangrove species, which increased the mangrove forest area to 22,000 ha (Lin and Liu 2003). Sonneratia apetala, because of its fast growth and high adaptability, has been introduced to mainland China from Bangladesh since 1985 for mangrove reforestation (Chen and Ma 2015). Since then, most of the studies on S. apetala focus on its physiology, growth, reproduction, carbon storage and ecological adaptation (Liu et al. 2014; Hossain et al. 2016), and only a few has reported its effect on biogeochemical cycling (Li et al. 2017a, b).
The sediment in mangrove wetlands has been considered a sink of contamination and a record of anthropogenic inputs of pollution (El-Said and Youssef 2013). Heavy metal pollution has been reported to disturb the establishment and growth of mangrove seedlings, affect mangrove health and pose toxicity to mangrove ecosystems (Sruthi et al. 2017). The toxicity and ecological risk of heavy metals to plants and ecosystems are related to the behavior and fate of heavy metals in sediments and are controlled by sediment properties, such as pH, organic matter, grain size, salinity, oxidation–reduction potential and benthic animals. (Sundaramanickam et al. 2016; Wu et al. 2016). On the other hand, sediment properties are affected by the presence of mangrove plants (Weng et al. 2014). Zhang et al. (2010) reported that S. apetala had a potential to remove nutrients and heavy metals in a simulated wetland. Rhizophora apiculata could remove chromium in a phytoremediation system (Richter et al. 2016). However, how different mangrove plants affect the distribution and accumulation of heavy metals in the sediment is not clear, even though such knowledge is necessary to evaluate the role of mangrove species in the remediation of heavy metal-contaminated coastal environments.
Shenzhen is a rapidly developed and urbanized modern city in the eastern coastal area of the Pearl River Estuary, China, and both native (Kandelia obovata) and exotic (S. apetala) mangrove plants are found along its coastlines. As a native mangrove species, K. obovata is widely spread along the coastline of south China and is quite adaptive to adverse environmental conditions (Lu et al. 2007). Shajing–Xixiang area of Shenzhen was adjacent to the catchment of the Dongbao River which was heavily polluted by heavy metals (Wu et al. 2016). The Dongbao River (22°46′N, 113°47′E) connected to the Pearl River Estuary is regarded as one of the most polluted urban rivers in China because sewages from more than 7000 metals and electronics factories are discharged directly to the river without any proper treatment (Song et al. 2016). So far, no systematic research on the ecological risk assessment of heavy metal contamination in this mangrove wetland has been conducted. In August of 2015, the growth of exotic S. apetala in the study area was found to have severe leaf shedding, but the adjacent K. obovata community appeared to have normal and vigorous growth. Previous studies reported that S. apetala had faster growth and more adaptive characteristics than K. obovata (Zan et al. 2003; Li et al. 2017a). However, it is not clear why these two species respond differently to similar heavy metal-polluted environment. We hypothesize that the different tolerance between S. apetala and K. obovata may be related to the different accumulations of heavy metal in sediment dominated by the two species. The present study aims to compare the effects of S. apetala and K. obovata on the accumulation of heavy metals in sediments and other physicochemical sediment properties. The potential ecological risk of heavy metals in S. apetala and K. obovata sediments was also assessed.
Materials and methods
Study area
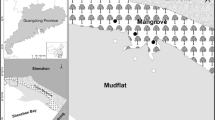
The studied mangrove forest (Shajing–Xixiang area in Shenzhen) is located in the eastern coastal area of the Pearl River Estuary, China (Fig. 1). The mangrove forest is dominated by native mangrove plants, namely K. obovata and Aegiceras corniculatum, while Acanthus ilicifolius, Bruguiera gymnorrhiza, Avicennia marina and Excoecaria agallocha are also found in this forest. Some introduced mangrove species, including Rhizophora stylosa, Laguncularia racemose and S. apetala are also found in this area. The mean annual temperature is 23.0 °C, with the highest temperature in July (36.1 °C) and the lowest in January (3.9 °C). The mean annual precipitation is 1935.8 mm, most of which occur from May to September. The tides are semi-diurnal with an average tidal range of 1.9 m (Yang et al. 2014).
Sediment collection and pretreatment
In August of 2016, the study area was uniformly divided into six sampling points along the coast with a distance of about 200 m between two consecutive sampling points (Fig. 1). Sampling points 1–3 were dominated by S. apetala, while K. obovata was dominant in sampling points 4–6. At each sampling point, three random surface sediment samples (0–10 cm) were collected using acid-washed PVC pipes (each was 40 cm long and 7.5 cm internal diameter). Core sediment samples (0–30 cm) were also collected at sample points 2 and 5 (each in triplicate) for analyzing the history of heavy metal pollution in this area. The sediment cores were immediately sliced into 10 cm increments with a plastic knife after collection, and the subsamples were placed in plastic bags, sealed and transported back to the laboratory on the same day.
The water content was determined by the weight loss after drying at 105 °C for 48 h. Plant roots and stones were removed from the sediment. The sediment was then dried, ground into powder and passed through a 0.5-mm sieve before the analysis of pH, electronic conductivity (EC), total organic carbon (TOC), total nitrogen (TN) and total heavy metals (Cd, Cu, Zn, Pb, Ni and Cr). Sediment pH and EC in the sediment slurry (sediment:water = 1:2.5 w/v) were determined using pH meter (sartouris PB-10, Germany) and conductivity meter (LABORATORY BENCHTOP METERS, China). The TOC and TN were measured by CHNSO elemental analyzer Vario EL Cube (Elementar, Germany). For heavy metal determination, 2-g sediment sample was digested in a mixture of 9 ml nitric acid (HNO3), 3 ml hydrofluoric acid (HF) and 1 ml hydrochloric acid (HCl) in a microwave. The heavy metals concentrations in the digest were analyzed using inductively coupled plasma-atomic emission spectrometry (ICP-AES, Leeman, USA). The internal standard method was used to test the recovery of heavy metals in sediment samples. The recoveries of heavy metals ranged from 95.35 to 106.41%.
To determine the possible biological effects of combined metals in both S. apetala and K. obovata sites, the mean PEL quotients (m-p-Q) determined by Long et al. (1998) were calculated using the following formula:
where Ci is the sediment concentration of metal (i), PELi is the PEL of metal (i) and n is the number of metal. The classification of toxicity probability for biota described by Long et al. (1998) suggested that m-P-Q < 0.1 indicated an 8% probability of being toxic, and m-P-Q values of 0.11–1.5, 1.51–2.3 and > 2.3 indicated 21, 49 and 73% probability of being toxic, respectively.
The potential ecological risk coefficient (\( E_{\text{r}}^{i} \)) and potential ecological risk index (RI) were used to evaluate the ecological risk of each heavy metal, as well as multiple heavy metals, based on the following formula: \( E_{\text{r}}^{i} = T_{\text{r}}^{i} \cdot C_{\text{f}}^{i} = T_{\text{r}}^{i} \cdot C_{\text{s}}^{i} /C_{\text{n}}^{i} \), where \( T_{\text{r}}^{i} \) is the toxic-response factor of heavy metal i, \( C_{\text{f}}^{i} \) is the contamination factor of heavy metal i, \( C_{\text{s}}^{i} \) is the measured concentration of heavy metal i in the sediment, and \( C_{\text{n}}^{i} \) is the background value of heavy metals i, adopted from Li and Zheng (1988). The toxic-response factors for Cd, Cr, Cu, Pb and Zn were 30, 2, 5, 5 and 1, respectively (Hakanson 1980). RI was calculated as the sum of all six risk factors of heavy metals using the formula: \( {\text{RI}} = \sum\nolimits_{i = 1}^{n} {E_{\text{r}}^{i} } \), where n is the number of heavy metals analyzed in the sample (n = 6 in the present study). Heavy metal pollution was also characterized by the geo-accumulation index (Igeo), defining by the following equation: Igeo = log2 (Cn/1.5Bn), where Cn is the measured content of the metal n and Bn is the background value of the metal (Li and Zheng 1988). The constant factor 1.5 was introduced to analyze natural fluctuations in the contents of a given substance in the environment with very small anthropogenic influences.
Statistical analysis
All data were expressed as the mean ± standard deviation (SD) of three replicates. A two-way analysis of variance (ANOVA) was used to test any significant differences in sediment heavy metal concentrations and other properties between S. apetala and K. obovata sites and among different depths. If the interaction is significant at p < 0.05, a Student t test would be performed to determine any difference in these properties between S. apetala and K. obovata sites at each sediment depth. Multivariate statistical analyses were conducted to determine pollution sources and relationships among heavy metals and other sediment properties. Principal component analysis (PCA) was used to investigate the potential pollution sources (natural or anthropogenic) and the relationships between heavy metals. Hierarchical cluster analysis (HCA) of the normalized data set was carried out using Ward’s method with Euclidean distances as a measure of similarity (Gotelli and Ellison 2004). The classification is based on visual observation of the dendrogram. Pearson’s coefficient was calculated to examine the relationships among TOC, TN, pH and heavy metals and confirmed the results obtained by multivariate analysis. All statistical analyses were done using Statistical Product and Service Solution (SPSS) 16.0.
Results and discussion
Physicochemical properties of sediment
The sediment in the S. apetala site had significantly higher TOC content than that in the K. obovata site at all depths, except the deepest layer (Fig. 2). A two-way ANOVA revealed that TOC was significantly affected by both site and depth factors, and the interaction of these two sources of variations was also significant (Table 1S). Li et al. (2017a) found that S. apetala produced larger amounts of root materials and leaf litter than the native K. obovata, thus providing more carbon inputs to the sediment. In both sites, TOC content in sediments decreased from surface (3.48% in average) to bottom layers (1.34%), which may be related to the higher letter supply in the upper sediment layer by mangrove plant. These data are consistent with findings of previous studies with more organic matter distributed in surface sediment (Perry and Berkeley 2009; Zhou et al. 2010). Li et al. (2017b) estimated the sedimentation rate in Shenzhen Bay was around 1.38 cm year−1, so the present 30-cm sediment core recorded the anthropogenic activities of recent 20 years. The TN content in the surface sediment (0–15 cm) in the S. apetala site was significantly higher than in the K. obovata site, but there was no significant difference between two sites at deeper sediment layers (Fig. 2, Table 1S). The root system of S. apetala is different from K. obovata, with more fine roots and pneumatophores that can enhance the adsorption and retention of nitrogen from surrounding inputs (Lugomela and Bergman 2002). Compared to site, the effect of depth on TN contents in both sites is not significant (Table 1S). Similar to TN, only site (but not depth) significantly influenced the EC, pH and water content in sediment, and their values in the S. apetala site were significantly higher than that in K. obovata, with a few exceptions such as EC and water content in both sites were comparable at the last two deepest layers (25–30 cm). The values of EC in the S. apetala site were also higher than in the K. obovata site. On the other hand, the sediment in the K. obovata site (pH 6.5–6.6) was slightly acidic than that in the S. apetala site (pH 7.0–7.6). The alkalinity in S. apetala site may improve the deposition of heavy metals in sediment. The acidity in mangrove sediment was due to the decomposition and oxidation of FeS2 and FeS leading to more production of humic acids (Tam and Wong 2000). However, the litter production and decomposition rate of S. apetala were reported to be faster than that of K. obovata (Liu et al. 2014). In fact, the field condition of mangrove forest is so complex, and the litter decomposition is highly dependent on the surrounding environment, such as pH, electronical conductivity, organic matter and particle size (Laing et al. 2009; Antoniadis et al. 2017). So why the K. obovata sediment in the present study was more acidic deserved more in-depth research.
Vertical profiles of sediment physicochemical properties in K. obovata (black triangle) and S. apetala (black square) sites in eastern coastal area of the Pearl River Estuary, China (mean ± SD of three replicates; the asterisk “*” and “**” at each sediment depth indicated significant differences between two sites at p < 0.05 and p < 0.01, respectively, according to Student t test)
Heavy metal concentrations in sediments
The sediment concentrations of Cd, Cu, Zn, Ni and Cr in the S. apetala site were significantly higher than in the K. obovata site at most sediment depths (Fig. 3), indicating that S. apetala could enhance the accumulation of heavy metals in mangrove sediment. A two-way ANOVA revealed that Cd and Ni were only affected by site; Cu, Zn and Cr were affected by site, depth and their interaction, while site and depth had no significant effects on sediment Pb concentrations (Table 1S). Previous studies have showed that the vertical profile of heavy metals could provide a rapid insight into their degree of pollution over time, and the geochemistry and waste discharge affected the vertical distributions of heavy metals (Qiao et al. 2013; Dias-Ferreira et al. 2016). In the present study, the vertical profiles varied between the two sites and were heavy metal specific. In the S. apetala site, Zn and Cu levels decreased with depths, while the depth changes were less obvious in the K. obovata site. In the S. apetala site, the depth profiles of Cu and Zn concentrations were comparable to that of TOC (Fig. 2), suggesting the close relationship between organic matter and heavy metals. Marchand et al. (2006) found that the high specific surface area of organic matter enhanced the sorption with metals. The vertical variations of Cr in both sites were significant but without any obvious trend. The steady levels of Cd, Pb and Ni along sediment depths in both sites indicated the inputs of these heavy metals did not increase with time even though the areas have intensive human activities and rapid economic development in recent decades. This also suggested that the pollution control policy implemented by local government during the recent 20 years as described by Li et al. (2017b) has been effective. The awareness of public on environmental protection and conservation of natural resources has been strengthened, and many mangrove swamps in Shenzhen, China have been under effective protection and scientific management in recent years (Li et al. 2013b).
Vertical profiles of sediment heavy metals concentrations (μg g−1) in K. obovata (black triangle) and S. apetala (black square) sites in eastern coastal areas of the Pearl River Estuary, China (mean ± SD of three replicates; the asterisk “*” and “**” at each sediment depth indicated significant differences between two sites at p < 0.05 and p < 0.01, respectively, according to Student t test)
Based on the background levels of heavy metals reported by Gan et al. (2010), Cd, Cu, Zn and Pb in surface sediments (0–5 cm) in both K. obovata and S. apetala sites were above their respective background levels (Table 2, Table 2S), indicating anthropogenic activities had a direct impact on the concentrations of heavy metals in these sediments. On the contrary, the Cr concentrations in sediments in both sites were similar to the background value, demonstrating the present sediment was not contaminated by Cr and its presence in sediments was primarily lithogenic origin. Similar low levels of Cr were also detected in sediments from Shantou Bay without any source of Cr such as from mechanical engineering and steel industry (Qiao et al. 2013).
Previous studies have found that the sediment properties could significantly affect metal mobility in the intertidal sediment and floodplain (Laing et al. 2009; Frohne et al. 2014). The correlations among different heavy metals were dependent on sites and metal species (Table 1). In the K. obovata site, the correlations between TOC and heavy metals could be positive, negative or no correlations, while significant correlations were all positive in the S. apetala site. The pneumatophores of S. apetala may trap more efficiently suspended sediments to accumulate more TOC and heavy metals in the sediment (MacFarlane et al. 2007). The positive correlations between TOC and two heavy metals (Zn and Pb) in the S. apetala site and between TOC and Cu in the K. obovata site indicated that sediment organic matter played an important role in the distribution pattern of heavy metals (Samuel and Phillips 1988). In sediment, organic matter increased the sediment retention capacity and created ligands that roots are unable to absorb, which improved the retention of heavy metals by organic matter in the sediment (Laing et al. 2009; Antoniadis et al. 2017). Sundaramanickam et al. (2016) found that organic matter in sediment acted as a metal binding agent and complexed with Fe and Ni during both pre- and post-monsoon seasons along Parangipettai, India. However, the correlations between TOC and other heavy metals such as Cd and Ni in both sites were not significant, and the value between TOC and Cr in the K. obovata site was even negative. Paramasivam et al. (2015) reported that the concentrations of heavy metals were not controlled by a single factor, instead a combination of geochemical support phase and their mixed association affected the metal level. This implied that the sediment heavy metals in the present mangrove swamp may come from different sources of contamination and the roles of two mangrove species in retaining heavy metals and their fates may be different. Similar to TOC, the correlations between TN and heavy metals in the K. obovata site were also complex. On the contrary, no significant correlations were found in the S. apetala site, suggesting that TN had little effect on the accumulation of heavy metals in this site.
The present mangrove swamp is located in the downstream of the catchment of the Dongbao River (with a distance of 20 km), the largest manufacturing base of metal and electronics industries in the Pearl River Delta (Wu et al. 2016). This explained why the concentrations of heavy metals in present sediment samples such as Cu, Zn and Ni were relatively high (Table 2), when compared to the other mangroves in China which are less affected by industries and the mangrove wetlands in other parts of the world. The mean Cd and Pb concentrations were higher than other mangrove wetlands, except Galatea, Panama (Guzman and Jiménez 1992) and South Port Klang, Malaysia (Sany et al. 2013), which had comparable Cd and Pb as the present study, respectively. The concentrations of Cr in this study were also higher than other wetlands but lower than other sediments in the Pearl River, South China (104.68 μg g−1) (Bai et al. 2011). Additionally to anthropogenic inputs, the high concentrations of heavy metals in the present mangrove sediments may be attributed to the large amount of suspended and fine particles discharged from the distributaries into the Pearl River Estuary which could enhance the retention and precipitation of heavy metals due to large surface area of fine particles (Fernandes and Nayak 2015; Zhao et al. 2017).
The concentrations of heavy metals in the present six sampling points showed significant fluctuations (Table 2). In theory, the concentrations of heavy metals should decrease from point 1 to point 6 with increasing distance to the estuary of Dongbao River; however, the results did not follow this or even show the opposite trend. For instance, the Cd concentration at point 1 was lower than that at points 3 and 6, and point 3 had a significantly higher Cd concentration. On the contrary, point 3 had lower Cu concentration than point 2, despite their close proximity to the river. Point 2 had the lowest Cd but the highest level was at point 3. Similar results as Cd were found in Pb, indicating the similar anthropogenic origin of these two heavy metals. On the other hand, the lowest and highest levels of Ni and Cr were detected at point 5 and point 1, respectively. The highest levels of Cu and Zn were found at point 1 but this point did not contain the highest levels of other heavy metals. It has been suggested that the heavy metal concentrations in sediments are not only affected by the inputs, the dispersal by water current and the mobility of heavy metals in sediment may also affect the accumulation (Zheng et al. 2008; Wu et al. 2016). The major causes of the variations in heavy metal accumulation in the present mangrove sediment may be related to the anthropogenic inputs (including industrial and domestic sewage), the effect of river flow and the types of vegetation. Detailed investigations are needed to obtain a comprehensive understanding of such spatial variation.
Evaluation of heavy metal risk
In the present study, mean sediment Cd levels in both sites exceeded the Class III standard for Marine Sediment Quality of China GB 18668-2002 (SEPA 2002), mean Cu levels were above that of Class II (except for mean Cu at S. apetala above Class III), while mean Pb levels were above those of Class I (Table 2, Table 2S), indicating Cd was the most problematic heavy metal, irrespective of plant species. Previous studies also reported that the Pearl River Estuary was heavily contaminated by Cd, due to the prevalence of electroplating industries (Li et al. 2007; Wu et al. 2016). Compared to Cd, mean Zn levels in the S. apetala site varied from Class II to Class III and were more polluted than that in the K. obovata site. Among all heavy metals, Cr was the least polluted one, with mean Cr levels in the S. apetala site ranging from Class I to Class II, and was lower than Class I in the K. obovata site. The lower contamination of Zn and Cr than Cd indicated that the anthropogenic source of these two metals was under control, which was related to the effectively managed discharges from mechanical engineering and steel industries in this region (Cheung et al. 2003).
In the present study, the concentrations of Cd, Cu, Zn, Pb and Cr at all sediment depths in the K. obovata site were higher than the respective threshold effect level (TEL) but below the probable effect level (PEL) (Fig. 3, Table 2S). The TEL and PEL of the sediment quality guidelines (SQGs) have been applied to assess which heavy metal might adversely affect aquatic organisms (MacDonald et al. 2000; Rumisha et al. 2016). Generally, adverse biological effects are rarely expected at levels below the TEL, whereas the PEL is defined as the level above which adverse effects are expected to occur more often (Costa-Böddeker et al. 2017). The present findings suggested that adverse biological effects caused by these metals may be observed occasionally. The Ni levels in the K. obovata site were much higher than the PEL value, indicating that adverse effects caused by Ni are expected to occur more often. In the S. apetala site, the levels of Cd, Cu and Ni at all sediment depths were higher than the respective PEL values, Zn and Pb concentrations were higher than TEL but lower than the PEL values (except Zn higher than PEL at 0–5 cm). The Cr levels reached up to the PEL benchmark, except at 5–10- and 25–30-cm-deep sediments. Based on the TEL and PEL criteria, the contamination of heavy metals in the S. apetala site was higher than that in the K. obovata site, especially for Cd, Cu and Zn. The higher mean values of Cd, Cu and Ni in the S. apetala site than the PEL values indicated more attention should be paid to this site.
In the present study, the mean PEL quotient (m-p-Q) determined by Long et al. (1998) at all sediment depths in the K. obovata site was 0.90, ranging from 0.11 to 1.5 (Fig. 4), indicating that the combination of the six metals in the K. obovata site had a 21% probability of being toxic. This value was similar to that recorded in previous studies conducted in intertidal Bohai Bay (Gao and Li 2012) and the coastal Shandong Peninsula (Li et al. 2013a). The mean PEL quotient in the S. apetala site at all sediment depths was 1.73 (from 1.51 to 2.3), suggesting a 49% probability of being toxic and further confirmed that this site was more contaminated and problematic than the K. obovata site.
Potential ecological risks of heavy metals have previously been used for a better understanding of heavy metal pollution (Rumisha et al. 2016). In this study, the pattern of \( E_{\text{r}}^{i} \) of each heavy metal was more or less the same as its concentration (Figure 1S), while the ecological risk of Cd was very high (\( E_{\text{r}}^{i} \ge 320 \)). In the K. obovata site, the mean \( E_{\text{r}}^{i} \) values of six studied heavy metals followed the sequence Cd (4196.24 ± 336.85) > Cu (36.30 ± 11.36) Ni (27.63 ± 3.71) > Pb (19.71 ± 1.81) > Zn (4.78 ± 0.27) > Cr (3.22 ± 1.16), and the same sequence was also found in the S. apetala site [Cd (8441.94 ± 811.72) > Cu (188.38 ± 33.31) > Ni (42.44 ± 5.35) > Pb (20.10 ± 2.91) > Zn (8.30 ± 1.22) > Cr (5.29 ± 1.00)]. It is clear that although Cd concentrations were significantly lower than other heavy metals (Fig. 3), the highest \( E_{\text{r}}^{i} \) values of Cd (Figure 1S) demonstrated its strongest toxicity than the other metals. For the other heavy metals, the ecological risk of Cu was low to moderate in the K. obovata site, but was high in the S. apetala site. The ecological risk of Zn, Pb, Ni and Cr was low (\( E_{\text{r}}^{i} < 40 \)) in both sites, except Ni in the S. apetala site (\( E_{\text{r}}^{i} \) about 40). According to grades of potential ecological risk, the values of RI ranged from 3896.36 to 4618.38 in the K. obovata site and the ranges in the S. apetala site were from 7984.71 to 10,099.06, both sites were classified as at very high risk (Table 3S). The percentages of Cd accounting for the total risk were 97.85 and 96.94% in the K. obovata and S. apetala sites, respectively, indicating that Cd was the main heavy metal posing a very high risk to the environment. Similar high Cd contamination was detected in many other coastal areas in China, such as geo-accumulation index of − 1.62 to 2.85 in Pearl River Estuary (Zhao et al. 2017), \( E_{\text{r}}^{i} \) of 414.36–573.82 in Bohai Bay (Chai et al. 2014), pollution load index of 0.40–0.67 in Shantou Bay (Qiao et al. 2013), revealing that the coastal area is contaminated by Cd, irrespective to its geographical location.
The distribution pattern of the geo-accumulation index (Igeo), a normalization technique that has been widely applied to the assessment of heavy metal contamination in soils and sediments (Costa-Böddeker et al. 2017), of each heavy metal was more or less the same as its concentration and \( E_{\text{r}}^{i} \) value, with lower Igeo values in the K. obovata site than in the S. apetala site, except Pb (Figure 2S). In the K. obovata site, the mean Igeo values of the six metals followed an increasing order of Cd (7.78 ± 0.13) > Cu (2.21 ± 0.43) > Ni (1.86 ± 0.18), Zn (1.67 ± 0.08), Pb (1.37 ± 0.13) > Cr (0.49 ± 0.01), but the order in the S. apetala site was slightly different, with Cd (8.79 ± 0.12) > Cu (4.65 ± 0.24) > Zn (2.52 ± 0.28), Ni (2.48 ± 0.15) > Pb (1.41 ± 0.21) > Cr (0.79 ± 0.26). According to Müller (1969), heavy metals in the K. obovata site could be classified into four groups based on the pollution potential: (1) unpolluted to moderately polluted (0 < Igeo < 1), which was the case of Cr; (2) moderately contaminated (1 < Igeo < 2), the case of Ni, Zn and Pb; (3) moderately to heavily contaminated, the case of Cu; and (4) extremely contaminated (Igeo > 5), the case of Cd. Similar grouping was also found in the S. apetala site, except the case of Cu (4 < Igeo < 5) that was heavily to extremely contaminated, and Zn and Ni (2 < Igeo < 3) which were moderately to heavily contaminated. These results demonstrated that the sediment in the present mangrove swamp was extremely contaminated, and the risk of Cd to environments must not be ignored as it is a widespread concern.
Multivariate statistical analysis
Multivariate analysis (PCA and HCA) is effective at providing suggestions on the sources and pathways of heavy metal contamination (Sundaramanickam et al. 2016). Table 3 shows the results of PCA obtained by applying varimax rotation for heavy metals in S. apetala and K. obovata sites. Factor loading is excellent with the values higher than 0.71 and is poor with values lower than 0.32 (Garcia et al. 2004). In both sites, Cd, Cu and Zn had strong positive loadings on PC1. In the K obovata site, Pb and Ni had positive loadings on PC1 and 2, with only Cr had a positive loading on PC2; while in the S. apetala site, Pb and Ni had positive loading on PC1, with Cr had a positive loading on both PC1 and 2. Generally, the pollution caused Cu and Zn is mainly related to sewage discharges (Gu et al. 2014), the main source of Pb is atmospheric deposition (Huang et al. 2014), and Ni mainly comes from alloy, manufacture of coins, magnets and household utensils (Gu et al. 2012). The PC1 in the S. apetala site accounted for 60.5% of variation while the value was only 39.6% in the K. obovata site, implying that the former site was more affected by anthropogenic contamination. In both sites, Cr was not a serious concern (Fig. 3, Table 2), indicating that there was no anthropogenic contamination of Cr and its presence was due to natural sources.
In the dendrogram obtained from HCA, all heavy metals in the K. obovata site were grouped into three clusters, with cluster 1 consisting of Cd, Zn and Cu, cluster 2 of Pb and cluster 3 of Ni and Cr (Fig. 5). The three metals in cluster 1 also had significant correlation with each other (Table 1), demonstrating that they originated from the same source, such as discharges of industrial and domestic sewage. The grouping of Cu and Zn in the same cluster in both sites implied their similar anthropogenic origin, which could be agrochemical sources, manufactured goods (paints, cosmetics and batteries) and agricultural fertilizers (D’Adamo et al. 2014). However, other clusters in the S. apetala site were different, its cluster 1 was made up of Cd, Pb and Ni; cluster 2 was Cu and Zn and cluster 3 was Cr. The analysis also revealed two distinct clusters, one was the cluster 3 of K. obovata site including Ni and Cr and the other was the cluster 3 of S. apetala site that includes only Cr. These results are consistent with the output from the PCA analysis. The present study demonstrated that the effects of the exotic S. apetala on the accumulation of heavy metals in sediment differed from that of the native K. obovata, with the former species exerted stronger effects on the physicochemical properties of sediments and heavy metal accumulation under the similar pollution source. The different hydrological characteristics and sources of pollution may be the main factors affecting the sources and pathways of heavy metals which deserve further investigations. The more accumulation of heavy metals in the S. apetala sediment may cause the severe shedding of leaves in this study area, but the actual link between the accumulation of heavy metals in sediment, their transfer to mangrove plants, their effects on leaf shedding and even plant death need more detailed research.
Conclusions
The present study reveals the pollution of heavy metals in a mangrove swamp in the eastern coastal areas of the Pearl River Estuary in China, and the influence of mangrove plant species on the accumulation of heavy metals in the sediment. Compared to Kandelia obovata (native species), Sonneratia apetala (exotic species) significantly changed the sediment physicochemical properties, with significantly higher values of TOC, TN, water content, electronic conductivity and pH, which may affect the accumulation of heavy metals in the sediment. The sediment in the S. apetala site retained and accumulated more Cd, Cu, Zn, Pb, Ni and Cr than that in the K. obovata site, and its organic matter played an important role in retaining Zn and Pb in sediment. The S. apetala site not only accumulated more heavy metals, its potential ecological risk index and geo-accumulation index were also higher than the K. obovata site. According to the mean PEL quotient, heavy metals in the S. apetala site had a 49% probability of toxicity, higher than that of 21% in the K. obovata site. The PCA and HCA results further demonstrate that exotic S. apetala affected the biogeochemical cycles of Cd, Cu, Ni and Cr more than the native K. obovata. Among the six heavy metals investigated, Cd levels in both sites were very high, exceeding the background value, Class III standard for marine sediment quality and the PEL, and was the most problematic heavy metals. More attention on Cd contamination and ways to control its inputs, especially in the site dominated by the exotic S. apetala, are urgently needed.
References
Antoniadis, V., Levizou, E., Shaheen, S. M., Ok, Y. S., Sebastian, A., Baum, C., et al. (2017). Trace elements in the soil-plant interface: Phytoavailability, translocation, and phytoremediation: A review. Earth Science Reviews, 171, 621–645.
Bai, J., Xiao, R., Cui, B., Zhang, K., Wang, Q., Liu, X., et al. (2011). Assessment of heavy metal pollution in wetland soils from the young and old reclaimed regions in the Pearl River Estuary, South China. Environmental Pollution, 159, 817–824.
Bodin, N., N’Gom-Kâ, R., Kâ, S., Thiaw, O. T., Tito de Morais, L., Le Loc’h, F., et al. (2013). Assessment of trace metal contamination in mangrove ecosystems from Senegal, West Africa. Chemosphere, 90, 150–157.
Chai, M. W., Shi, F. C., Li, R. L., & Shen, X. X. (2014). Heavy metal contamination and ecological risk in Spartina alterniflora marsh in intertidal sediments of Bohai Bay, China. Marine Pollution Bulletin, 84, 115–124.
Chen, Q., & Ma, K. M. (2015). Research overview and trend on biological invasion in mangrove forests. Chinese Journal of Plant Ecology, 39, 283–299.
Cheung, K. C., Poon, B. H. T., Lan, C. Y., & Wong, M. H. (2003). Assessment of metal and nutrient concentrations in river water and sediment collected from the cities in the Pearl River Delta, South China. Chemosphere, 52, 1431–1440.
Costa-Böddeker, S., Hoelzmann, P., Thuyên, L. X., Hug, H. D., Nguyen, H. A., Richter, O., et al. (2017). Ecological risk assessment of a coastal zone in Southern Vietnam: Spatial distribution and content of heavy metals in water and surface sediments of the Thi Vai Estuary and Can Gio Mangrove Forest. Marine Pollution Bulletin, 114, 1141–1151.
Cuong, D. T., & Obbard, J. P. (2006). Metal speciation in coastal marine sediments from Singapore using a modified BCR-sequential extraction procedure. Applied Geochemistry, 21, 1335–1346.
D’Adamo, R., Specchiulli, A., Cassin, D., Botter, M., Zonta, R., & Fabbrocini, A. (2014). The effect of floods on sediment contamination in a microtidal coastal lagoon: The lagoon of Lesina, Italy. Archives of Environmental Contamination and Toxicology, 67, 297–309.
Dias-Ferreira, C., Pato, R. L., Varejão, J. B., Tavares, A. O., & Ferreira, A. J. D. (2016). Heavy metal and PCB spatial distribution pattern in sediments within an urban catchment—contribution of historical pollution sources. Journal of Soils and Sediments, 16, 2594–2605.
El-Said, G. F., & Youssef, D. H. (2013). Ecotoxicological impact assessment of some heavy metals and their distribution in some fractions of mangrove sediments from Red Sea, Egypt. Environmental Monitoring and Assessment, 185, 393–404.
Fernandes, M. C., & Nayak, G. N. (2015). Speciation of metals and their distribution in tropical estuarine mudflat sediments, southwest coast of India. Ecotoxicology and Environmental Safety, 122, 68–75.
Friess, D. A., Phelps, J., Leong, C. R., Lee, W. K., Wee, A. K. S., Sivasothi, N., et al. (2012). Mandai mangrove, Singapore: Lessons for the conservation of Southeast Asia’s mangroves. Raffles Bulletin of Zoology, 25, 55–65.
Frohne, T., Rinklebe, J., & Diaz-Bone, R. A. (2014). Contamination of floodplain soils along the Wupper River, Germany, with As Co, Cu, Ni, Sb, and Zn and the impact of pre-definite redox variations on the mobility of these elements. Soil and Sediment Contamination, 23, 779–799.
Gan, H. Y., Liang, K., & Zheng, Z. C. (2010). Background values, contamination assessment and zoning of heavy metals in sediments of the Pearl River Estuary. Earth and Environment, 38, 344–350.
Gao, X. L., & Li, P. M. (2012). Concentration and fractionation of trace metals in surface sediments of intertidal Bohai Bay, China. Marine Pollution Bulletin, 64, 1529–1536.
Garcia, J. H., Li, W., Arimoto, R., Okrasinski, R., Greenlee, J., Walton, J., et al. (2004). Characterization and implication of potential fugitive dust sources in the Paso del Norte region. Science of the Total Environment, 325, 95–112.
Gotelli, N. J., & Ellison, A. M. (2004). A primer of ecological statistics (1st ed.). Sunderland, MA: Sinauer Associates.
Gu, Y. G., Lin, Q., Jiang, S. J., & Wang, Z. H. (2014). Metal pollution in Zhelin Bay surface sediments inferred from a sequential extraction technique, South China Sea. Marine Pollution Bulletin, 81, 256–261.
Gu, Y. G., Wang, Z. H., Lu, S. H., Jiang, S. J., Mu, D. H., & Shu, Y. H. (2012). Multivariate statistical and GIS-based approach to identify source of anthropogenic impacts on metallic elements in sediments from the mid Guangdong coasts China. Environmental Pollution, 163, 248–255.
Guzman, H. M., & Jiménez, C. E. (1992). Contamination of coral reefs by heavy metals along the Caribbean coast of Central America (Costa Rica and Panama). Marine Pollution Bulletin, 24, 554–561.
Hakanson, L. (1980). An ecological risk index for aquatic pollution control: A sediment logical approach. Water Research, 14, 975–1001.
Hamilton, S. E., & Casey, D. (2016). Creation of a high spatio-temporal resolution global data-base of continuous mangrove forest cover for the 21st century (CGMFC-21). Global Ecology and Biogeography, 25, 729–738.
Hossain, S. J., Iftekharuzzaman, M., Haque, M. A., Saha, B., Moniruzzaman, M., Rahman, M. M., et al. (2016). Nutrient compositions, antioxidant activity, and common phenolics of Sonneratia apetala (Buch.-Ham.) fruit. International Journal of Food Properties, 19, 1080–1092.
Huang, P., Li, T. G., Li, A. C., Yu, X. K., & Hu, N. J. (2014). Distribution, enrichment and sources of heavy metals in surface sediments of the North Yellow Sea. Continental Shelf Research, 73, 1–13.
Laing, G. D., Rinkklebe, J., Vandecasteele, B., Meers, E., & Tack, F. M. G. (2009). Trace metal behavior in estuarine and riverine floodplain soil and sediments: A review. Science of the Total Environment, 407, 3972–3985.
Li, F. L., Yang, L., Zan, Q. J., Shin, P. K. S., Cheung, S. G., Wong, Y. S., et al. (2017a). Does energetic cost for leaf construction in Sonneratia change after introduce to another mangrove wetland and differ from native mangrove plants in South China? Marine Pollution Bulletin. https://doi.org/10.1016/j.marpolbul.2017.02.056.
Li, G. G., Hu, B. Q., Bi, J. Q., Leng, Q. N., Xiao, C. Q., & Yang, Z. C. (2013a). Heavy metals distribution and contamination in surface sediments of the coastal Shandong Peninsula (Yellow Sea). Marine Pollution Bulletin, 76, 420–426.
Li, J., & Zheng, C. J. (1988). Environmental background values data sheet. Beijing: China Environmental Science Press.
Li, L. Q. (2008). Heavy metals in mangrove wetland of China. Master thesis. Xiamen University.
Li, Q. S., Wu, Z. F., Chu, B., Zhang, N., Cai, S. S., & Fang, J. H. (2007). Heavy metal in coastal wetland sediments of the Pearl River Estuary, China. Environmental Pollution, 149, 158–164.
Li, R. L., Chai, M. W., Li, R. Y., Xu, H. L., He, B., & Qiu, G. Y. (2017b). Influence of introduced Sonneratia apetala on nutrients and heavy metals in intertidal sediments, South China. Environmental Science and Pollution Research, 24, 2914–2927.
Li, Y., Ru, Z. Z., Cheng, H. R., Wang, Z. L., & Zan, X. (2013b). Study on management modes of mangrove wetlands in Shenzhen, China. Guangdong Forestry Science and Technology, 29, 31–37. (in Chinese with English abstract).
Lin, Z. D., & Liu, H. M. (2003). Protection and management countermeasures for mangrove resource in Guangdong Province. Central South Forest Inventory and Planning, 22, 35–38.
Liu, L. N., Li, F. L., Yang, Q., Tam, N. F. Y., Liao, W. B., & Zan, Q. J. (2014). Long-term differences in annual litter production between alien (Sonneratia apetala) and native (Kandelia obovata) mangrove species in Futian, Shenzhen, China. Marine Pollution Bulletin, 85, 747–753.
Long, E. R., Field, L. J., & MacDonald, D. D. (1998). Predicting toxicity in marine sediments with numerical sediment quality guidelines. Environmental Toxicology and Chemistry, 17, 714–727.
Lu, H. L., Yan, C. L., & Liu, J. C. (2007). Low-molecular-weight organic acids exuded by mangrove (Kandelia candel (L.) Druce) roots and their effect on cadmium species change in the rhizosphere. Environmental and Experimental Botany, 61, 159–166.
Lugomela, C., & Bergman, B. (2002). Biological N2-fixation on mangrove pneumatophores: Preliminary observations and perspectives. Ambio, 31, 612–613.
MacDonald, D. D., Ingersoll, C. G., & Berger, T. A. (2000). Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Archives of Environmental Contamination and Toxicology, 39, 20–31.
MacFarlane, G. R., Koller, C. E., & Blomberg, S. P. (2007). Accumulation and partitioning of heavy metals in mangroves: A synthesis of field-based studies. Chemosphere, 69, 1454–1464.
Marchand, C., Lallier-Verges, E., Baltzer, F., Alberic, P., Cossa, D., & Baillif, P. (2006). Heavy metals distribution in mangrove sediments along the mobile coastline of French Guiana. Marine Chemistry, 98, 1–17.
Müller, G. (1969). Index of geoaccumulation in the sediments of the Rhine River. GeoJournal, 2, 108–118.
Paramasivam, K., Ramasamy, V., & Suresh, G. (2015). Impact of sediment characteristics on the heavy metal concentration and their ecological risk of surface sediments of Vaigai river, Tamilnadu, India. Spectrochimica Acta Part A Molecular and Biomolelcular Spectroscopy, 137, 397–407.
Peng, Y. S., Zhou, Y. W., & Chen, G. Z. (2008). The restoration of mangrove wetland: A review. Acta Ecologica Sinica, 28, 786–797. (in Chinese with English abstract).
Perdomo, L., Ensminger, I., Espinos, L. F., Elsters, C., Wallner-Kersanach, M., & Schnetters, M. L. (1998). The mangrove ecosystem of the Ciénaga Grande de Santa Marta (Colombia): Observations on regeneration and trace metals in sediment. Marine Pollution Bulletin, 37, 393–403.
Perry, C. T., & Berkeley, A. (2009). Intertidal substrate modification as a result of mangrove planting: Impacts of introduced mangrove species on sediment microfacies characteristics. Estuarine, Coastal and Shelf Science, 81, 225–237.
Qiao, Y. M., Yang, Y., Zhao, J. G., Tao, R., & Xu, R. H. (2013). Influence of urbanization and industrialization on metal enrichment of sediment cores from Shantou Bay, South China. Environmental Pollution, 182, 28–36.
Richter, O., Nguyen, H. A., Nguyen, K. L., Nguyen, V. P., Biester, H., & Schmidt, P. (2016). Phytoremediation by mangrove trees: Experimental studies and model development. Chemical Engineering Journal, 294, 389–399.
Rumisha, C., Mdegela, R. H., Kochzius, M., Leermakers, M., & Elskens, M. (2016). Trace metals in the giant tiger prawn Penaeus monodon and mangrove sediments of the Tanzania coast: Is there a risk to marine fauna and public health? Ecotoxicology and Environmental Safety, 132, 77–86.
Samuel, N. L., & Phillips, D. J. H. (1988). Distribution, variation and impacts of trace elements in San Francisco Bay. Marine Pollution Bulletin, 19, 413–425.
Sany, T. S. B., Salleh, A., Rezayi, M., Saadati, N., Narimany, L., & Tehrani, G. M. (2013). Distribution and contamination of heavy metal in the coastal sediments of Port Klang, Selangor, Malaysia. Water, Air, and Soil pollution, 224, 1–18.
SEPA (State Environmental Protection Administration of China). (2002). Marine Sediment Quality (GB 18668-2002). Beijing: Standards Press of China.
Smee, D. L., Sanchez, J. A., Diskin, M., & Trettin, C. (2017). Mangrove expansion into salt marshes alters associated faunal communities. Estuarine, Coastal and Shelf Science, 187, 306–313.
Song, M., Yin, P., Zhao, L., Jiao, Z., Duan, S., & Lu, G. (2016). Distribution and risk assessment of heavy metals in sediments of the Pearl River Estuary, southern China. Soil and Sediment Contamination, 25, 101–116.
Sruthi, P., Shackira, A. M., & Puthur, J. T. (2017). Heavy metal detoxification mechanisms in halophytes: An overview. Wetlands Ecology and Management, 25, 129–148.
Sundaramanickam, A., Shanmugam, N., Cholan, S., Kumaresan, S., Madeswaran, P., & Balasubramanian, T. (2016). Spatial variability of heavy metals in estuarine, mangrove and coastal ecosystems along Parangipettai, Southeast coast of India. Environmental Pollution, 218, 186–195.
Tam, N. F. Y., & Wong, Y. S. (2000). Spatial variation of heavy metals in surface sediments of Hong Kong mangrove swamps. Environmental Pollution, 110, 195–205.
Usman, A. R. A., Alkredaa, R. S., & Al-Wabel, M. I. (2013). Heavy metal contamination in sediments and mangroves from the coast of Red Sea: Avicennia marina as potential metal bioaccumulator. Ecotoxicology and Environmental Safety, 97, 263–270.
Weng, B. S., Huang, Y., Liu, J. C., Lu, H. L., & Yan, C. L. (2014). Alleviated toxicity of cadmium by the rhizosphere of Kandelia obovata (S.L.) Yong. Bulletin of Environmental Contamination and Toxicology, 93, 603–610.
Wu, Q. H., Zhou, H. C., Tam, N. F. Y., Tian, Y., Tan, Y., Zhou, S., et al. (2016). Contamination, toxicity and speciation of heavy metals in an industrialized urban river: Implications for the dispersal of heavy metals. Marine Pollution Bulletin, 104, 153–161.
Yang, Q., Lei, A. P., Li, F. L., Liu, L. N., Zan, Q. J., Shin, P. K. S., et al. (2014). Structure and function of soil microbial community in artificially planted Sonneratia apetala and S. caseolaris forests at different stand ages in Shenzhen Bay, China. Marine Pollution Bulletin, 85, 754–763.
Zan, Q. J., Wang, B. S., Wang, Y. J., & Zhang, W. Y. (2003). Ecological assessment on the introduced Sonneratia caseolaris and S. apetala at the mangrove forest of Shenzhen Bay, China. Acta Botanica Sinica, 45, 544–551.
Zhang, J. E., Liu, J. L., Ouyang, Y., Liao, B. W., & Zhao, B. L. (2010). Removal of nutrients and heavy metals from wastewater with mangrove Sonneratia apetala Buch-Ham. Ecological Engineering, 36, 807–812.
Zhao, G. M., Ye, S. Y., Yuan, H. M., Ding, X. G., & Wang, J. (2017). Surface sediment properties and heavy metal pollution assessment in the Pearl River Estuary, China. Environmental Science and Pollution Research, 24, 2966–2979.
Zheng, N., Wang, Q. C., Liang, Z. Z., & Zheng, D. M. (2008). Characterization of heavy metal concentrations in the sediments of three freshwater rivers in Huludao City, Northeast China. Environmental Pollution, 154, 135–142.
Zhou, Y. W., Zhao, B., Peng, Y. S., & Chen, G. Z. (2010). Influence of mangrove reforestation on heavy metal accumulation and speciation in intertidal sediments. Marine Pollution Bulletin, 60, 1319–1324.
Acknowledgements
This work was financially supported by the Program of Science and Technology of Guangdong Province (Study on ecological investigation and protection patterns of typical coastal Mangroves in Guangdong Province), and the Program of Science and Technology of Shenzhen (JCYJ20160330095549229, KQJSCX20160226110414), and the Program of Assessment and Restoration of Mangrove Geiwei of Shenzhen Bay. Minwei Chai is responsible for the operation of this research and writing this article. Ruili Li is the principal investigator of this research and provides useful suggestions. Nora Fung Yee Tam and Qijie Zan make a great contribution in the revision of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure
1S The potential ecological risk coefficients (\( E_{\text{r}}^{i} \)) of sediment heavy metals (Cd, Cu, Zn, Pb, Ni and Cr) and the potential ecological risk index (RI) in K. obovata (black triangle) and S. apetala (black square) sites in eastern coastal areas of the Pearl River Estuary, China (mean ± standard deviation of three replicates). (PPTX 93 kb)
Figure
2S The geo-accumulation index (Igeo) of sediment heavy metals in K. obovata (black triangle) and S. apetala (black square) sites in the eastern coastal areas of the Pearl River Estuary, China (mean ± standard deviation of three replicates). (PPTX 90 kb)
Rights and permissions
About this article
Cite this article
Chai, M., Li, R., Tam, N.F.Y. et al. Effects of mangrove plant species on accumulation of heavy metals in sediment in a heavily polluted mangrove swamp in Pearl River Estuary, China. Environ Geochem Health 41, 175–189 (2019). https://doi.org/10.1007/s10653-018-0107-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-018-0107-y