Abstract
Trace elements (Zn, Cu, Pb, and Cd) in root and leaf tissues of the gray mangrove (Avicennia marina) and in corresponding sediment samples were studied. Samples were taken from the inflow/outflow points in two distinct habitats, i.e., the Hara Protected Area and the Azini Bay, of Hormozgan Province in south Iran. Heavy metal concentrations (μg g−1 of dry weight) in the sediments of the Hara Protected Area ranged from 16.0 to 68.0 for Pb, 15.0 to 52.0 for Zn, 9.0 to 27.0 for Cu, and 1.0 to 3.3 for Cd. In the Azini Bay, these concentrations ranged from 7.1 to 27.5 for Pb, 17.1 to 55.9 for Zn, 12.1 to 37.9 for Cu, and 0.2 to 2.3 for Cd. The accumulation trend of heavy metal concentrations in the roots of A. marina was in the order Pb (16.1) > Zn (15.8) > Cu (9.3) > Cd (1.3) μg g−1 of dry weight in the Hara Protected Area and in the order Zn (13.7) > Cu (9.4) > Pb (5.5) > Cd (0.6) μg g−1 of dry weight in the Azini Bay. The value of translocation factor (TLF) was smaller than 1 in both regions. It was estimated from 0.44 to 0.62 in the Hara Protected Area and from 0.51 to 1.01 in the Azini Bay. The enrichment coefficient for root (ECR) varied from 0.32 to 0.93 in the Hara Protected Area and from 0.32 to 0.51 in the Azini Bay. The ratio of heavy metals in leaves/sediments (ECL) also varied from 0.01 to 0.67 in the Hara Protected Area and from 0.01 to 0.47 in the Azini Bay. The enrichment coefficient for leaf (ECL) was always lower than ECR in both regions. Based on the above findings, A. marina can be regarded as an excluder for the heavy metals examined in this study, given its low efficiency in translocating and accumulating the heavy metals in the shoots. Apart from serving as a baseline for the study area, findings could be useful for mitigating heavy metal contamination in these sensitive ecosystems through possible phytomanagement using gray mangrove.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals have posed numerous environmental problems in most coastal and aquatic ecosystems for a long time (Islam and Tanaka 2004; Rai 2008). Despite the fact that they are components of the earth’s crust (Alloway 2013) and occur naturally in all ecosystems (Harborne 2014), their concentrations are considerably increased by human activities (Sharma et al. 2016). The main challenge with heavy metals is that, unlike organic pollutants, these inorganic pollutants are not decomposable (Achary et al. 2017). Therefore, they are considered major environmental pollutants (Sahu 2016) and have been in the center of attention in the recent years due to the adverse impacts on different ecosystems (Naji and Ismail 2012; Nath et al. 2014a; Willemsen et al. 2016). These elements find their way into the aquatic environments by natural factors, industrial and agricultural activities, as well as urban sewage and can impair the bioactivities and the quality of aquatic systems (Alloway 2013). They can accumulate in the bodies of marine organisms through the food chain (Cabrita et al. 2017), and the growing rate of their accumulation expose people who feed on these organisms to risks (Nachev and Sures 2016).
In Iran, mangrove ecosystems in the coastal areas of Hormozgan Province, widely known as the Hara forests, create a notorious coastal environment of major value (Masnavi et al. 2016). During the recent decades, these coastal areas have been subject to pollution by trace elements due to progressive industrialization and urbanization near these areas, which largely affect trace element accumulation and distribution, along with natural processes. Therefore, remedial measures are required to mitigate potential impacts of these hazardous trace elements (Mondal et al. 2018). Mangroves are known to play an effective role in alleviating metal pollutants through accumulating them in their own tissues (Zhou et al. 2010; Usman et al. 2013; Nath et al. 2014b) and have been reported to exhibit significant phytoremediation potential for certain heavy metals (Einollahipeer et al. 2013; Kaewtubtim et al. 2016; Kaewtubtim et al. 2018).
In Sydney, Australia, the root epidermis was the main barrier to the transport of Pb into Avicennia marina trees, while Cu and Zn had always higher concentrations in the cellular wall of the leaves than inside the cells (MacFarlane and Burchett 2000). Copper and Pb concentrations were higher in root and leaf tissues of A. marina than in sediments of mangrove forests in the same area, while a robust linear relationship between concentrations of those metals in sediments and root tissues was noted (MacFarlane et al. 2003). In Sirik Azini creek, Iran, the concentrations of heavy metals in leaves of A. marina were higher than those in sediments, resulting in bioconcentration factors higher than 1 for certain metals (Parvaresh et al. 2011). In Qeshm Island of Iran, the highest Pb and Cd concentrations in leaves of A. marina were 34.5 and 3.5 ppm, respectively, while the lowest concentrations in the stems were 2.0 and 0.05 ppm, respectively (Shirvani Mahdavi et al. 2012), with heavy metals accumulating more in the leaves than in the stems. The accumulation of heavy metals in the sediments and tissues of A. marina trees in Qeshm Island, Iran, followed different patterns, with Ni being the dominant metal in sediments and root tissues, whereas Cu was the dominant metal in stems and leaves (Einollahipeer et al. 2013). In Thailand, the bioconcentration factor (BCF) values for the roots of A. marina were 8.1 for Zn, 1.5 for Cu, 10.1 for Pb, and 10.6 for Cd, while for the leaves, the respective values were 4.7, 1.7, 4.1, and 12.2 (Kaewtubtim et al. 2016). Furthermore, A. marina was considered a heavy metal excluder species, since roots accumulated certain heavy metals in higher quantities than other plant parts (Kaewtubtim et al. 2018). Despite efforts undertaken to evaluate pollution levels in the Persian Gulf, continuous monitoring and additional research on accumulation of pollutants in local marine flora are essential for identifying gaps in our knowledge that could be used for better management of these sensitive ecosystems.
Because the sediments of mangrove forests in Hormozgan Province of the Persian Gulf can accumulate heavy metals, the objective of this study was to estimate the accumulation of heavy metals in the surface sediments, including the metals Zn, Cu, Pb, and Cd, and the distribution of those metals in gray mangrove, seeking an answer to the question whether local A. marina trees could be used for phytomanagement of heavy metals in this sensitive environment through exploitation for decorative purposes, timber used for building dwellings and boats, as well as fuel-wood for cooking and heating. To this end, ecological-biological indices, such as translocation factor (TLF), bioconcentration factor (BCF), and enrichment factors (EF) were also used.
Materials and methods
Study area
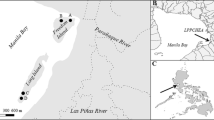
The study was conducted at two mangrove sites of Hormozgan Province, Iran (Fig. 1). The Hara Protected Area, between Qeshm Island and Bandar Khamir (26° 40′ to 26° 59′ N, 55° 21′ to 55° 52′ E), and the Azini Bay are located in the international wetland of the Gaz and Hara river deltas (26° 10′ to 26° 28′ N, 57° 01′ to 57° 14′ E). The former is a relatively virgin area that was officially registered in the Biosphere Reserve Directory of UNESCO’s Man and Biosphere Programme, and the latter is also virgin, but with a high commuting rate of fishing boats. The main factors threatening these wetlands include the cutting of mangrove trees for fuel or feed, petroleum pollutions, and the pollution caused by urban and rural sewage as well as the development of shrimp farming industry. It is estimated that about 2000 trips are daily made by boats in the Azini Bay.
Sampling and measurement of heavy metals
We used point count sampling method to randomly select five stations in each habitat, so as to cover the maximum part of the region and to meet the objectives of the study. This is a method where data are collected at points dispersed across an area where sampling needs to be done (Southwood and Henderson 2000). Samples were taken from the five inflow/outflow points in each habitat and in total, 10 points (Fig. 1). All points were sampled at full low tide on a daily basis from 10:00 to 15:00 in October to December of 2015. Plant and sediment samples were collected from the field. Taxonomic identification of A. marina was made in situ with the help of the experts of Jonoub Keifiat-Azma company and of an expert of the Environment Protection Organization of Hormozgan Province who contributed to the field phase of the research. In all individual sites, the leaves, roots, and sediment were sampled in three replications. In total, 15 samples of sediment, root, and leaf were collected from each habitat. For each sample, almost 15 leaves and parts of the roots of several trees were gathered. Then, they were washed with water right away (not allowing rinsates to reach the sediment) and packaged in plastic bags labeled with the site name and the sampling date.
The collected leaves and roots were also washed with distilled water in the laboratory. Then, the leaves were oven-dried at 60 °C and the roots at 101 °C for 24 h (Memmert Universal Oven UN55, Germany). The dried samples were ground in a mortar. Next, 1 g was digested with a hot plate for 1 h at 40 °C and for 3 h at 140 °C after adding concentrated nitric acid and hydrogen peroxide (1:5 ratio). During the leaf and root harvest, a certain amount of surrounding sediment was also collected with a plastic spatula and a steel-made shovel. Then, the samples were placed in acid-pickled polyethylene containers labeled with the station name and the sampling date and transported to laboratory in an icebox where they were cooled down to − 20 °C. The samples were kept at this temperature until analysis.
To measure the heavy metals, the sediment samples were oven-dried at 101 °C for 24 h. The dried samples were powdered in a mortar and screened through a 69-μm sieve to have an homogenous mixture. Then, 1 g of the sediments was digested by adding a mixture of nitric acid and concentrated perchloric acid (1:4) and placed at room temperature for 1 h and at 140 °C for 3 h. Next, the digested samples were infiltrated through a 42-μm Whatman filter and adjusted to a certain volume by adding twice-distilled water. The concentrations of the sample metals were measured by an atomic absorption spectrophotometer (UNICO - UV/Vis 4802, USA). The atomic absorption spectroscopy (AAS) technique is a spectro-analytical procedure for the quantitative determination of chemical elements employing the absorption of optical radiation (light) by free atoms in the gaseous state. In this study, we followed the procedure described by El Nemr et al. (2016). Percentage recovery values were all above 95%.
Data analysis
The data of laboratory measurements are presented as mean ± standard error of the mean (SEM). The potential of the plants to uptake, accumulate, and translocate the heavy metal was estimated by calculating the translocation factor (TLF), the bioconcentration factor (BCF), and the enrichment factor (EF) as explicitly described below:
Translocation factor
It specifies the rate of translocation of heavy metals from plant root to shoot. TLF values > 1 represent metal accumulator species and TLF values < 1 represent metal repellent species (Mattina et al. 2003). The TLF was calculated by Eq. (1) (Sasmaz and Sasmaz 2017):
Bioconcentration factor
It is determined as the ratio of the concentration of heavy metals in shoot to their concentration in exchangeable form in soil (Barla et al. 2017). The BCF can be calculated by Eq. (2) (Sasmaz and Sasmaz 2017):
Enrichment factor
The ratio of heavy metals in a leaf to sediment is called enrichment coefficient of leaf (ECL), and the ratio of heavy metals in a root to sediment is called enrichment coefficient of root (ECR). The EF is a good way to distinguish the natural and human-made sources of pollution (Abrahim and Parker 2008). The classification of A. marina according to values of EF is presented in Table 1.
Results
Concentration of heavy metals in sediments
Mean concentrations of Zn, Cu, Pb, and Cd were 35.7 ± 2.5, 15.1 ± 1.2, 49.8 ± 3.4, and 1.4 ± 0.1 μg g−1 dry matter (DM) in sediments of the Hara Protected Area and 31.8 ± 2.8, 23.9 ± 1.9, 17.3 ± 1.5, and 1.1 ± 0.2 μg g−1 DM in sediments of the Azini Bay, respectively (Table 2). Mean concentrations of the heavy metals were in the order of Pb > Zn > Cu > Cd in the Hara Protected Area and in the order of Zn > Cu > Pb > Cd in the Azini Bay.
Concentration of heavy metals in root and leaf tissues of gray mangrove
Total average concentrations of Zn in root tissue of A. marina in the Hara Protected Area and the Azini Bay were 15.8 ± 0.9 and 13.7 ± 1.0 μg g−1 DM, respectively (Table 3). The respective values were 9.3 ± 0.6 and 9.4 ± 0.6 μg g−1 DM for Cu, while these were 16.1 ± 1.0 and 5.5 ± 0.4 μg g−1 DM for Pb, respectively. Also, A. marina had 1.3 ± 0.2 μg g−1 DM Cd with a variation range of 0.3–2.7 in their roots in the Hara Protected Area and 0.6 ± 0.1 μg g−1 DM Cd with a variation range of 0.2–1.0 in the Azini Bay. Mean Zn concentrations in the leaves of A. marina were 7.0 ± 0.7 and 9.1 ± 0.5 μg g−1 DM in the Hara Protected Area and the Azini Bay, respectively (Table 4). The respective values were 5.7 ± 0.5 and 6.6 ± 0.4 μg g−1 DM for Cu, while these were 7.3 ± 0.8 and 2.8 ± 0.3 μg g−1 DM for Pb, respectively. Also, mean Cd concentration in the roots of A. marina was estimated at 0.6 ± 0.1 μg g−1 DM with a variation range of 0.2–1.6 in the Hara Protected Area and 0.6 ± 0.0 μg g−1 DM with a variation range of 0.3–0.8 in the Azini Bay. Mean concentrations of all metals were higher in the roots than in the leaves (Fig. 2). Moreover, mean concentrations followed a similar trend in the two sampled areas for all metals, except for Zn, which showed higher concentrations in the roots in the Hara Protected Area, but higher concentrations in the leaves in the Azini Bay (Fig. 2).
Mean comparison of the elements in the roots of A. marina between the two sampling sites by t test showed significant differences between mean concentrations of Pb and Cd (P < 0.05) (Table 5). Also, significant differences were observed between the two studied regions in Zn and Pb concentrations in the leaves (P < 0.05) (Table 6). The results of the t test for comparing mean concentrations of the elements between roots and leaves of A. marina in the Hara Protected Area and the Azini Bay showed significant higher concentrations of the four heavy metals in the former region (P < 0.05) (Table 7). The same trend was also observed in the Azini Bay, except for Cd, where the mean Cd concentrations in roots and leaves did not differ significantly (P > 0.05) (Table 8).
Biological indices of A. marina in the studied region
The values of ECR, ECL, and TLF of heavy metals in A. marina in the Hara Protected Area and the Azini Bay are summarized in Tables 9 and 10, respectively. Accordingly, heavy metals TLF, i.e., the translocation rate of heavy metals from root to leaf tissues, varied from 0.44 to 0.62 in the Hara Protected Area and from 0.51 to 1.01 in the Azini Bay. In the Hara Protected Area, the highest TFL value of 0.62 was related to Cu and the lowest TLF value of 0.44 to Zn, while the highest value was related to Cd and the lowest to Pb in the Azini Bay. ECR, i.e., the ratio of heavy metals in roots/sediments, varied from 0.32 to 0.93 in the Hara Protected Area and from 0.32 to 0.51 in the Azini Bay. Total average ECR was smaller than 1 for all metals. In both habitats, the highest ECR value was related to Cd and the lowest one to Pb. ECL, i.e., the ratio of heavy metals in leaves/sediments, varied from 0.01 to 0.67 in the Hara Protected Area (Table 9) and from 0.01 to 0.47 in the Azini Bay (Table 10). Mean ECL was smaller than 1 for all metals. The highest ECL value in the Hara Protected Area was obtained for Cd and the lowest one for Pb, while Cd and Zn exhibited the highest and the lowest values of ECL in the Azini Bay. Averaged over studied areas, there was a steady amount in the TLF and ECR for Zn and Cu, while this amount was reduced for Pb and raised for Cd (Fig. 3). Moreover, there was a low amount in ECL in all metals, except from Cd, which showed a significant higher value than the other metals (Fig. 3).
Discussion
In this study, Zn, Cu, Pb, and Cd concentrations were determined in the root and leaf tissues of gray mangrove (A. marina) in coastal environments of Hormozgan Province of Iran. Also, biological indices, such as the translocation factor (TLF), the bioconcentration factor (BCF), and the enrichment factor (EF) for characterizing the potential of gray mangrove to uptake, accumulate, and translocate the heavy metal were calculated. The phytoremediating role of gray mangroves, especially in contaminated areas, has been described in earlier studies (Suresh and Ravishankar 2004; Mac Farlane et al. 2007), but despite efforts undertaken to evaluate pollution levels in the Persian Gulf, data on the response of A. marina to heavy metal contamination in the coastal zone of Hormozgan, Iran, remain limited. From this point of view, the present study adds novel evidence on the response of A. marina to accumulation and translocation of metal pollutants that could be useful for potential phytomanagement of trace metal contamination in these sensitive ecosystems.
The accumulation trend of heavy metals in the sediment was in the order of Pb > Zn > Cu > Cd of the Hara Protected Area and in the order of Zn > Pb > Cu > Cd in the Azini Bay. Similarly, the accumulation trend in the roots of A. marina was in the order of Pb > Zn > Cu > Cd in the Hara Protected Area, but in the order of Zn > Cu > Pb > Cd in the Azini Bay. The leaf exhibited the same accumulation trend as the sediment. According to the accumulation trends of heavy metals, we can say that since the roots are in direct contact with the sediment, the accumulation of metals in the sediment may influence their accumulation trend in roots; whereas, in other tissues, the accumulation trend may differ from that of the sediment. The trend of heavy metal accumulation (expressed in μg g−1 DM) in different tissues of A. marina in the Hara Protected Area and the Azini Bay, as compared with other aquatic sites in the world, are shown in Table 11. It appears that the concentrations of heavy metals in the tissues of A. marina in the two studied areas are comparable or lower than those of other areas, probably reflecting the low contamination levels in both studied areas.
Translocation and bioaccumulation factors of the studied metals showed a variety of trends, indicating a different uptake capability of the heavy metals at the different tissues of A. marina. Previous studies noted the role of A. marina in purifying coastal sediments and reducing metallic pollutants by accumulation in plant tissues and immobilization in the medium (MacFarlane et al. 2003). The decreased level of translocation of the metals from the roots to the leaves of A. marina could be probably related to the use of the metals in physiological processes of plant growth, but this assumption was not tested in the current study. TLF represents the rate of translocation of heavy metals from root to shoot/leaf tissues (Sasmaz and Sasmaz 2017) and is used to estimate the potential of a plant to clean the environment from heavy metals (Sasmaz et al. 2008). The values of TLF were smaller than 1 in both Hara Protected Area and Azini Bay. Likewise, the ECR and ECL, which show the ratio of heavy metals in root and leaf to total heavy metals in sediment, respectively, were smaller than 1 for all metals in both regions. The values of ECR and ECL reflect the potential of a plant to accumulate and translocate the trace metals and the above findings mean that A. marina can be regarded as a repellent species for the heavy metals examined in this study, given its low efficiency in translocating and accumulating the heavy metals in the shoots. The roots of A. marina had higher EF than the leaves for all metals, implying higher potential of roots to accumulate the metals. This finding means that the roots of A. marina have a critical capacity to accumulate the heavy metals, as also mentioned in a previous study (MacFarlane and Burchett 2000). Moreover, the EF values were smaller than 2 in all cases, showing that A. marina lacked or had low enrichment in both study sites (Table 1). The lower BCF and TLF values indicate that gray mangrove adopted an exclusion strategy for the studied heavy metals.
The highest TLF from root to leaf tissues was related to Pb in the Hara Protected Area and to Cd in the Azini Bay. The higher TLF for Pb or Cd and the lower one for Zn reflect the high potential of root in accumulating Zn and the high potential of leaf in accumulating Pb or Cd, including possible atmospheric heavy metal deposition. The higher translocation rates of Pb and Cd to the leaves can be a defensive mechanism against excessive accumulation, which reduces the concentrations of these harmful elements when the leaves are shed. Unnecessary heavy metals were accumulated in the leaves of the spotted mangrove tree (Rhizophora stylosa) and then repelled as the leaves were shed (Zheng et al. 1997). High bioavailability of Pb and Cd for plant uptake was probably the responsible factor for the increased concentrations of those metals in the leaf tissues as compared to the sediment (Yim and Tam 1999). Bioavailability depends largely on the concentrations and chemical forms (species) of the metals in the pore water, which are controlled by numerous complex interacting processes (Kim et al. 2015). Higher Cd concentration in the shoots than other elements is in line with previous reports (Nirmal Kumar et al. 2011) and could be probably associated with the high root-to-shoot translocation rates of Cd, as previously reported for other plant species (Sękara et al. 2005; Lu et al. 2008; Dong et al. 2017). Cd accumulation ability was weakened in roots of A. marina and the translocation factor increased in stems and leaves with increase of stress duration (Jian et al. 2017). Nonetheless, much lower Cd concentration in such tissues as roots and leaves as compared to other elements can be related with the lower demand of these tissues for Cd and with the fact that Cd is not lost with leaf shedding. In a study on the transfer of heavy metals from roots to leaves, Zn had the lowest concentration in the leaves and its weak translocation to the leaf tissues was related to the fact that the white mangrove trees (Laguncularia racemosa) tolerated Zn in the roots and did not require to repel this metal through leaf shedding (Machado et al. 2002). Low translocation rate of Zn from the sediment to the leaves was reported by a previous study (Zheng et al. 1997), which suggested that the lower Zn bioavailability in the sediment than other metals was the main reason for its lower concentration in the leaves.
The EF is a good way to distinguish the natural source and the human-made pollution (Salem et al. 2017). The present study showed that the highest value of EF was related to Cd and the lowest to Pb. Moreover, the EF was higher for all metals in the roots of A. marina than in the leaves. Higher EF values in the roots than in the leaves reflect a higher potential of roots to accumulate metals (Sasmaz et al. 2008). This response may be due to the aerial roots of A. marina, which supply oxygen requirements for the oxidation at lower layers and substrate, resulting in high accumulation of metals in the roots. The exploration of EF revealed that all tissues were able to receive the studied heavy metals (Zn, Cu, Pb, and Cd) from the sediment. Previous research showed that the essentiality of some elements (like Zn and Cu) for plants and inessentiality of others were the main reason for their different accumulation rates during the metabolic process (Kaewtubtim et al. 2016; Willemsen et al. 2016). In an examination of the inflow rate of heavy metals from the sediment and their bioaccumulation in the leaves, Cu and Zn had lower bioaccumulation rates (MacFarlane and Burchett 2002). Similarly, the present study showed that the root of A. marina was an optimum transporter of heavy metals from the environment to the aboveground tissues. Sharifan and Davari (2010) studied Cu concentration in A. marina in Qeshm Island and estimated Cu translocation rate from root to leaf at 0.89 and BCF at 0.84. They argued that since Cu is an essential element for the plant, its concentration is constant across the different parts of the plant. Copper and Zn have important roles to play in the processes of chloroplasts, the synthesis of proteins and enzymatic activities, growth hormones, and the metabolism of the carbohydrates (Hänsch and Mendel 2009; Mousavi 2011).
In this study, BCF was found to be 8.1 for Zn, 1.5 for Cu, 10.1 for Pb, and 10.6 for Cd in the root tissues of A. marina and 4.7 for Zn, 1.7 for Cu, 4.1 for Pb, and 12.2 for Cd in the leaf tissues. These findings indicate high accumulation of Zn and Pb in the roots compared with the shoots and almost equal accumulation of Cu in both tissues. Moreover, the ratio of the concentrations of heavy metals in leaf to root was 1:2.3 (Zn), 1:1.6 (Cu), 1:2.2 (Pb), and 1:2.2 (Cd) in the Hara Protected Area and 1:1.5 (Zn), 1:1.4 (Cu), 1:1.9 (Pb), and 1:1 (Cd) in the Azini Bay. Likewise, the ratio of heavy metal concentrations in leaf to sediment was found to be 1:5.11 (Zn), 1:2.6 (Cu), 1:6.8 (Pb), and 1:2.4 (Cd) in the Hara Protected Area and 1:3.5 (Zn), 1:3.6 (Cu), 1:6.1 (Pb), and 1:1.9 (Cd) in the Azini Bay. In total, it can be said that higher concentrations of metals in the sediment and roots as opposed to leaves can be associated with a lower demand of those metals by the leaves than by the roots. This requirement is remarkable especially for Cu. Mangrove plants are known to accumulate trace elements in their aboveground tissues at different concentrations because some metals are considered necessary for growth and survival (MacFarlane and Burchett 1999). Therefore, metal accumulation in these plants is driven by basic metabolic requirements of the plants. For example, Cu and Zn are essential growth elements and are more mobile than non-essential elements like Pb, a fact that leads in variable accumulation in plant tissues (MacFarlane et al. 2003, 2007). Moreover, gray mangrove may act as a heavy metal phytostabilizer close to contaminant sources (Singh 2012; Birch et al. 2013) by ‘fixing’ heavy metals in the rhizosphere at concentrations greater than underlying sediments. This reaction can be regarded as a physiological mechanism of translocation prevention of the metals to aboveground tissues, which limits toxicity to plants and exclusion of metals via leaf litter. Mangrove ecosystems along coastal areas play a crucial role in filtering sediments and material deposits in marine ecosystems. Thus, the ability of Avicennia spp. to fix heavy metals in their tissues is very important in phytoremediation, which focuses on ecological processes and functions for gentle remediation options of ecosystem services in contaminated areas, including production of usable biomass for the production of renewable energy or provide feedstock for the circular bioeconomy. In light of the above, local A. marina trees could be an option for phytomanagement of heavy metals in this sensitive environment, keeping contaminants of this group at low availability levels to the soil.
Conclusions
The role of gray mangrove as a biofilter of heavy metals in coastal environments of Hormozgan Province, Iran, was explored. The study provided actual heavy metal accumulations in the sediment-plant ecosystem of mangrove forest, being important in designing the long-term management and conservation policies for the managers of mangrove forest. Overall, accumulation of heavy metals in the sediment may determine their accumulation in the root, while the variation of heavy metal concentrations in other tissues may differ from that of the sediment. Despite heavy metal contamination levels in the coastal environments of Hormozgan Province, Iran, A. marina has successfully adapted itself to this stress condition with a mechanism for selective uptake of only necessary minerals. In light of the above, local A. marina trees could be used for potential phytomanagement of heavy metals in this sensitive environment through exploitation for decorative purposes, timber used for building dwellings and boats, as well as fuel wood for cooking and heating, as it could bear and accumulate (immobilize) common trace metals.
References
Abrahim G, Parker R (2008) Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Tamaki Estuary, Auckland, New Zealand. Environ Monit Assess 136:227–238
Achary MS, Satpathy K, Panigrahi S, Mohanty A, Padhi R, Biswas S, Prabhu R, Vijayalakshmi S, Panigrahy R (2017) Concentration of heavy metals in the food chain components of the nearshore coastal waters of Kalpakkam, southeast coast of India. Food Control 72:232–243
Alloway BJ (2013) Sources of heavy metals and metalloids in soils. In: Alloway BJ (ed) Heavy metals in soils. Springer, Dordrecht, The Netherlands, pp 11–50
Barla A, Shrivastava A, Majumdar A, Upadhyay MK, Bose S (2017) Heavy metal dispersion in water saturated and water unsaturated soil of Bengal delta region, India. Chemosphere 168:807–816
Birch GF, Chang CH, Lee JH, Churchill LJ (2013) The use of vintage surficial sediment data and sedimentary cores to determine past and future trends in estuarine metal contamination (Sydney Estuary, Australia). Sci Total Environ 454–455:542–561
Cabrita MT, Padeiro A, Amaro E, dos Santos MC, Leppe M, Verkulich S, Hughes KA, Peter H-U, Canário J (2017) Evaluating trace element bioavailability and potential transfer into marine food chains using immobilised diatom model species Phaeodactylum tricornutum, on King George Island, Antarctica. Mar Pollut Bull 121:192–200
Dong Q, Xu PX, Wang ZL (2017) Differential cadmium distribution and translocation in roots and shoots related to hyper-tolerance between tall fescue and Kentucky bluegrass. Front Plant Sci 8:113
Einollahipeer F, Khammar S, Sabaghzadeh A (2013) A study on heavy metal concentration in sediment and mangrove (Avicenia marina) tissues in Qeshm Island, Persian Gulf. J Novel Appl Sci 2:498–504
El Nemr A, El-Said GF, Ragab S, Khaled A, El-Sikaily A (2016) The distribution, contamination and risk assessment of heavy metals in sediment and shellfish from the Red Sea coast, Egypt. Chemosphere 165:369–380
Hänsch R, Mendel RR (2009) Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr Opin Plant Boil 12:259–266
Harborne JB (2014) Introduction to ecological biochemistry. Academic Press, London, UK
Islam MS, Tanaka M (2004) Impacts of pollution on coastal and marine ecosystems including coastal and marine fisheries and approach for management: a review and synthesis. Mar Pollut Bull 48:624–649
Jian L, Chongling Y, Daolin D, Haoliang L, Jingchun L (2017) Accumulation and speciation of Cd in Avicennia marina tissues. Int J Phytoremediat 2:1000–1006
Kaewtubtim P, Meeinkuirt W, Seepom S, Pichtel J (2016) Heavy metal phytoremediation potential of plant species in a mangrove ecosystem in Pattani Bay, Thailand. Appl Ecol Environ Res 14:367–382
Kaewtubtim P, Meeinkuirt W, Seepom S, Pichtel J (2018) Phytomanagement of radionuclides and heavy metals in mangrove sediments of Pattani Bay, Thailand, using Avicennia marina and Pluchea indica. Mar Pollut Bull 127:320–333
Kim R-Y, Yoon J-K, Kim T-S, Yang JE, Owens G, Kim K-R (2015) Bioavailability of heavy metals in soils: definitions and practical implementation—a critical review. Environ Geochem Health 37:1041–1061
Lu L, Tian S, Yang X, Wang X, Brown P, Li T, He Z (2008) Enhanced root-to-shoot translocation of cadmium in the hyperaccumulating ecotype of Sedum alfredii. J Exp Bot 59:3203–3213
MacFarlane GR, Burchett MD (1999) Zinc distribution and excretion in the leaves of grey mangrove, Avicennia marina (Forsk.) Vierh. Environ Exp Bot 41:167–175
MacFarlane G, Burchett M (2000) Cellular distribution of copper, lead and zinc in the grey mangrove, Avicennia marina (Forsk.) Vierh. Aquat Bot 68:45–59
MacFarlane G, Burchett M (2002) Toxicity, growth and accumulation relationships of copper, lead and zinc in the grey mangrove Avicennia marina (Forsk.) Vierh. Mar Environ Res 54:65–84
MacFarlane G, Pulkownik A, Burchett M (2003) Accumulation and distribution of heavy metals in the grey mangrove, Avicennia marina (Forsk.) Vierh.: biological indication potential. Environ Pollut 123:139–151
MacFarlane GR, Koller CE, Blomberg SP (2007) Accumulation and partitioning of heavy metals in mangroves: a synthesis of field-based studies. Chemosphere 69:1454–1464
Machado W, Silva-Filho E, Oliveira R, Lacerda L (2002) Trace metal retention in mangrove ecosystems in Guanabara Bay, SE Brazil. Mar Pollut Bull 44:1277–1280
Masnavi MR, Amani N, Ahmadzadeh A (2016) Ecological landscape planning and design strategies for mangrove communities (Hara forests) in South-Pars Special Economic Energy Zone, Asalouyeh-Iran. Environ Nat Resour Res 6:44–57
Mattina MI, Lannucci-Berger W, Musante C, White JC (2003) Concurrent plant uptake of heavy metals and persistent organic pollutants from soil. Environ Pollut 124:375–378
Mondal P, Reichelt-Brushett AJ, Jonathan MP, Sujitha SB, Sarkar SK (2018) Pollution evaluation of total and acid-leachable trace elements in surface sediments of Hooghly River Estuary and Sundarban Mangrove Wetland (India). Environ Sci Pollut Res 25:5681–5699
Mousavi SR (2011) Zinc in crop production and interaction with phosphorus. Aust J Basic Appl Sci 5:1503–1509
Nachev M, Sures B (2016) Environmental parasitology: parasites as accumulation bioindicators in the marine environment. J Sea Res 113:45–50
Naji A, Ismail A (2012) Sediment quality assessment of Klang Estuary, Malaysia. Aquat Ecosyst Health Manag 15:287–293
Nath B, Birch G, Chaudhuri P (2014a) Assessment of sediment quality in Avicennia marina-dominated embayments of Sydney Estuary: the potential use of pneumatophores (aerial roots) as a bio-indicator of trace metal contamination. Sci Total Environ 472:1010–1022
Nath B, Chaudhuri P, Birch G (2014b) Assessment of biotic response to heavy metal contamination in Avicennia marina mangrove ecosystems in Sydney Estuary, Australia. Ecotoxicol Environ Saf 107:284–290
Nazli MF, Hashim NR (2010) Heavy metal concentrations in an important mangrove species, Sonneratia caseolaris, in Peninsular Malaysia. Environ Asia 3:50–55
Nirmal Kumar IJ, Sajish PR, Nirmal Kumar R, Basil G, Shailendra V (2011) An assessment of the accumulation potential of Pb, Zn and Cd by Avicennia marina (Forssk.) Vierh. in Vamleshwar mangroves, Gujarat, India. Not Sci Biol 3:36–40
Parvaresh H, Abedi Z, Farshchi P, Karami M, Khorasani N, Karbassi A (2011) Bioavailability and concentration of heavy metals in the sediments and leaves of grey mangrove, Avicennia marina (Forsk.) Vierh, in Sirik Azini Creek, Iran. Biol Trace Elem Res 143:1121–1130
Rai PK (2008) Heavy metal pollution in aquatic ecosystems and its phytoremediation using wetland plants: an ecosustainable approach. Int J Phytoremediat 10:133–160
Ramos e Silva CA, Da Silva A, De Oliveira S (2006) Concentration, stock and transport rate of heavy metals in a tropical red mangrove, Natal, Brazil. Mar Chem 99:2–11
Sahu K (2016) Heavy metal pollution of air, water and soil—a review. SGAT Bull 16
Salem ZB, Laffray X, Al-Ashoor A, Ayadi H, Aleya L (2017) Metals and metalloid bioconcentrations in the tissues of Typha latifolia grown in the four interconnected ponds of a domestic landfill site. J Environ Sci 54:56–68
Sasmaz A, Obek E, Hasar H (2008) The accumulation of heavy metals in Typha latifolia L. grown in a stream carrying secondary effluent. Ecol Engin 33:278–284
Sasmaz M, Sasmaz A (2017) The accumulation of strontium by native plants grown on Gumuskoy mining soils. J Geochem Explor 181:236–242
Sękara A, Poniedzialek M, Ciura J, Jędrszczyk E (2005) Cadmium and lead accumulation and distribution in the organs of nine crops: implications for phytoremediation. Pol J Environ Stud 14:509–516
Sharifan HR, Davari A (2010) Bioaccumulation and distribution of heavy metals in gray mangrove (Avicennia marina): case study of the tropical areas of Persian Gulf. World Food System. Proceedings of ‘World Food System: a contribution from Europe’, Tropentag 2010, September 14–16, Zurich, Switzerland, p. 211
Sharma S, Rana S, Thakkar A, Baldi A, Murthy R, Sharma R (2016) Physical, chemical and phytoremediation technique for removal of heavy metals. J Heavy Met Tox Dis 1:2
Shirvani Mahdavi E, Khajeh Rahimi A, Vakili Amini H (2012) Pb and Cd accumulation in Avicennia marina from Qeshm Island. Iran J Fish Sci 11:867–875
Singh S (2012) Phytoremediation: a sustainable alternative for environmental challenges. Int J Green Herb Chem 1:133–139
Suresh B, Ravishankar GA (2004) Phytoremediation—a novel and promising approach for environmental clean-up. Crit Rev Biotechnol 24:97–124
Southwood TRE, Henderson PA (2000) Ecological methods, 3rd edn. Wiley-Blackwell, Oxford, UK
Subramanian A, Vannucci M (2004) Status of Indian mangroves: pollution status of the Pichavaram mangrove area, south-east coast of India. Mangrove management and conservation. United Nations University Press, Tokyo, Japan, pp 59–75
Usman ARA, Alkredaa RS, Al-Wabel MI (2013) Heavy metal contamination in sediments and mangroves from the coast of Red Sea: Avicennia marina as potential metal bioaccumulator. Ecotoxicol Environ Saf 97:263–270
Vardanyan LG, Ingole BS (2006) Studies on heavy metal accumulation in aquatic macrophytes from Sevan (Armenia) and Carambolim (India) lake systems. Environ Int 32:208–218
Willemsen PWJM, Horstman E, Borsje BW, Friess D, Dohmen-Janssen CM (2016) Sensitivity of the sediment trapping capacity of an estuarine mangrove forest. Geomorphology 273:189–201
Yim M, Tam N (1999) Effects of wastewater-borne heavy metals on mangrove plants and soil microbial activities. Mar Pollut Bull 39:179–186
Zheng WZ, Chen X, Lin P (1997) Accumulation and biological cycling of heavy metal elements in Rhizophora stylosa mangroves in Yingluo Bay, China. Mar Ecol Prog Ser 159:293–301
Zhou Y-W, Zhao B, Peng Y-S, Chen G-Z (2010) Influence of mangrove reforestation on heavy metal accumulation and speciation in intertidal sediments. Mar Pollut Bull 60:1319–1324
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Ghasemi, S., Siavash Moghaddam, S., Rahimi, A. et al. Phytomanagement of trace metals in mangrove sediments of Hormozgan, Iran, using gray mangrove (Avicennia marina). Environ Sci Pollut Res 25, 28195–28205 (2018). https://doi.org/10.1007/s11356-018-2684-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2684-9