Abstract
The present study investigates the groundwater quality for domestic and irrigation purposes in a coastal aquifer in the West Godavari delta region based on geochemical evaluation, integrated multivariate statistical analysis and entropy water quality index (EWQI). The study area is underlain by the Quaternary sediments with unconsolidated to semi consolidated sand, silt and clay formation. In the study, the significant hydrochemical facies of groundwater observed were Na-Mg-Cl-HCO3−, Na-Cl-HCO3− and Mg-Na-Cl-HCO3−. The results revealed that the area occupies high salinity groundwater controlled mainly by evaporation and also by rock weathering-solubilization to some extent. The concentrations of major cations and anions decreased in the order: Na+ > K+ > Mg2+ > Ca2+ = Cl− > HCO3− > SO42− > NO3−. The chemical constituents of the samples TA (85%), TDS (100%), TH (83%), Mg2+ (91%), Cl−(81%) and SO42 (12%) exceeded the limits, making them unfit for drinking. Based on EWQI (53.3–143.4), nearly 70% of groundwater samples were of poor to very poor quality for drinking, which required treatment, and the remaining 30% of samples were unsuitable for domestic purposes. Some samples of the irrigation suitability parameters (Na%, SAR, RSC, PI, CAI, KR and CCR) exhibit moderate to good categories, which can be used for irrigation with proper management. The multivariate statistical analysis was performed to understand the relationships among the chemical constituents present in groundwater. TDS is highly correlated with EC, TH, Ca2+, Mg2+, Na+, K+, HCO3− and Cl−. Principal component analysis (PCA) applied to the datasets showed that the first three PCs accounted for 65% of total variance cumulatively 94.5% for a total of 7 PCs. The PCA results indicate that the variation of groundwater quality is possibly attributed to various anthropogenic and geogenic factors, rock–water interactions and ion exchange processes in groundwater. The uncontrolled drawl of subsurface waters and aqua forming at an advanced rate when compared with recharge has led to this coastal aquifer being in a critical stage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater is a vital natural resource and has a significant role in the global economy. For irrigation purposes, groundwater is a reliable source of water and can be used in a flexible manner (USGS 2001). According to the World Bank (2012), the largest consumer of groundwater in the world is India, with an estimated annual groundwater use of 230 km3. Owing to the pressure created on hydrologic and hydrogeologic systems, the quality of groundwater is being degraded particularly in coastal areas across the globe. In coastal regions, seawater intrusion and salinization of groundwater because of overexploitation of freshwater aquifers will establish a negative water balance (Ferguson and Gleeson 2012). Hydrochemical studies of groundwater have vigorously been conducted by several researchers globally to identify and interpret the human-induced impact on groundwater chemistry (Sahu and Sikhdar 2008; Gibrilla et al. 2011; Aly et al. 2014; Brindha and Kavitha 2015; Sarikhani et al. 2015; Li et al. 2016; Diana et al. 2017; Wagh et al. 2018; He et al. 2019; Loh et al. 2020). The multiusage of groundwater for drinking, agricultural and industrial purposes, fisheries and energy production depends considerably on its quality (Iscen et al. 2008). The soil structure and crop yields are adversely affected by the presence of salts in irrigation waters. Arid and semi-arid climate regions are particularly vulnerable to salinity because of variations in rainfall and temperatures that lead to high evaporation (Jalali 2007; Houatmia et al. 2016). The soils of agricultural areas have created environmental problems like water resource contaminants and health risks for human beings due to the vigorous usage of fertilisers and agrochemicals (Shindo et al. 2006; Scanlon et al. 2007; Jiang et al. 2009). Zakaria et al. (2021) conducted groundwater quality studies in the Anayari catchment area, which is predominantly dependent on groundwater for agricultural purposes. They found that the water containing a low percentage of Na+ with moderate salinization can usually be used for irrigation purposes without any prior treatment. In the recent years, with an increasing number of chemical and physical variables in groundwater, a wide range of conventional tools and techniques of statistical methods have been applied for proper analysis and interpretation of data (Belkhiri et al. 2010; Machiwal and Jha 2010). Hierarchical cluster analysis, being a simple but efficient approach, was applied by researchers to distinguish the multivariate similarities in groundwater quality. Principal Component Analysis (PCA)/Factor Analysis (FA) and Cluster Analysis (CA) explains the dataset matrixes for understanding environmental systems and the quality of water influenced either by natural or anthropogenic conditions (Lee et. al. 2001; Subyani and Ahmadi 2010; Dudeja et al. 2011; Guggenmos et al. 2011; Blake et al. 2016; Ravikumar et al. 2017; Sayad et al. 2017; Khelif and Boudoukha 2018; Paul et al. 2019; Sandeep et al. 2020). Multivariate statistical techniques can be employed to analyze large datasets on water quality with the minimum loss of vital information (Simeonov et al. 2003; Jauhir et al. 2011; Gulgundi and Shetty 2018). The alluvial aquifer system is the dominant type of aquifer in the coastal area. The coastal alluvial aquifer is relatively vulnerable to contamination by seawater. It is hard to restore its fresh groundwater condition which makes groundwater unsuitable for drinking as well as agriculture use (Jeen et al. 2001; Chidambaram et al. 2009; Mohapatra et al. 2011; Swarna Latha and Nageswara Rao 2012; Guler et al. 2012; Reddy 2013; CGWB 2014; Sajjil Kumar 2016; Alfrrah et al. 2018; Sivakarun et al. 2020). The conversion of agriculture and marshy lands into aquaculture, which uses large scale saline water from creeks and urban industrialization lead to the alteration of freshwater aquifers in coastal regions. Therefore, understanding the hydrochemical characteristics of the coastal groundwater is essential to prevent saline intrusion and its associated problems (Prasanna et al. 2011; Thilagavathi et al. 2019). The residents of coastal regions in India are facing severe drinking water quality problems in comparison with other regions.
Keeping this in to consideration, the present study was carried out to evaluate the hydrochemical characteristics and quality of groundwater and its suitability for domestic use and irrigation in an alluvial coastal aquifer using multivariate statistical techniques. The main aim of the present study is to assess the quality of water based on the entropy weighted water quality index (EWQI) for drinking purposes.
Study area
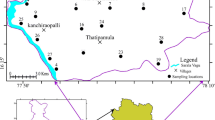
The study area is located in the West Godavari delta region of coastal Andhra Pradesh (AP) in Southern India. The district of West Godavari in AP is bounded by the districts of East Godavari in the North and Krishna in the South, Telangana State in the West and the Bay of Bengal in the East. The area under research lies between 16º 19' N to 16º 40' N latitudes and 81º 19' E to 81º 43' E longitudes (Fig. 1). It has a 23 km coastline covered with natural vegetation, cashew, casuarina and coconut plantations on its sandy tracts. The study area receives rainfall mostly from the south-west monsoon (June to September) and the average annual rainfall recorded is about 875 mm. The climate is maritime tropical humid with the maximum of 38 °C in May. The River Godavari is a major river and its tributaries, namely the Tammileru, Yarrakalva and Ramileru, flow through the West Godavari district, providing an abundant water supply for vast tracts of agriculture fields and aquaculture ponds. The river Godavari bifurcates into Gautami Godavari and Vasishta Godavari in the district region. The Gautami Godavari river marks the district boundary on the right side and drains through the present study area before ultimately debouching into the Bay of Bengal at Antarvedi.
The delta area is aided by the large canal system and numerous other drains. The oceanic saline water from creeks is also extensively used for aqua farming near the coastal tracts. The largest shallow freshwater lake in Asia is Kolleru Lake, in the southwestern part of the study area, and is designated as a wetland of international importance under the international Ramsar Convention. The study area accommodates nearly 0.5 million people, spread over several villages. Agriculture and aquaculture are the predominant activities found in the study area. The area is known for the large scale production of paddy, sugarcane, pulses, oilseeds, coconuts, etc. and it is considered to be one of the largest aqua farming regions of the country. The study area has been infested by a huge number of fish and prawn ponds during the last three decades, resulting in an ecological and environmental imbalance (Swarna Latha and Hema Malini 2018).
General geology and geomorphology
Geologically, the study area is underlain by the quaternary sediments with unconsolidated to semi consolidated sand, silt and clay formations. In general, the delta sediments consist of brown, grey, gravelly sands and silty clay. The thickness of the sediments gradually increases towards the sea and is of the order of 400 m in the Godavari delta (Raju et al. 1994; Ramesh 2008). The quaternary sediments comprise thick layers of alluvium, gravel and colluvial deposits, beach sand, kankar and soils of various types. Different geomorphic features such as flood plains, alluvial plains, levees, paleochannels, beach ridges, active tidal flats, mudflats, swamps and backwaters etc. are observed. Flood plains are built up of alluvium carried by the river during floods and is deposited in the sluggish water. The flat or nearly level sloping ground of these flood plains yields high groundwater potential zones. Beach ridges are low dunes formed as continuous mounds of beach materials (sand, gravel, shingle, etc.) parallel to the shoreline. Another important feature is tidal flats, which are characteristically extensive, nearly horizontal, marshy or barren stretches of land alternately covered and uncovered by the rise and fall of the tides. It consists of unconsolidated sediments, mostly mud and sand. Soils predominantly are deep black clay and sandy; and to some extent, gravelly, dark brown and silty soils. Groundwater extraction structures in the study region are mainly open, bore or tube wells. The average depth of the dug well recorded is 7 m below ground level (m bgl). The borewell depth varies from 10 to 65 m. The average fluctuation of the water table is recorded at 0.91 m in the study area (CGWB 2017).
Materials and methods
A total of fifty eight (58) groundwater samples were collected with proper care from the bore wells covering the entire study area during May 2017 (Fig. 1). The 1L polyethylene bottles were used for collecting groundwater and were properly rinsed with distilled water before carrying out the sampling. At the sampling location, the bottles were rinsed several times with the same bore well water to avoid any contamination before filling. These samples were cautiously sealed and labelled and taken to the laboratory to carry out the analysis within a week. The samples were preserved by adding appropriate reagents in the laboratory by adopting standard protocols (APHA 1998). The pH, electrical conductivity (EC) and total dissolved solids (TDS) were analyzed using a multi parameter digital meter. Total alkalinity (TA), total hardness (TH), calcium (Ca2+), bicarbonates (HCO3−) and chlorides (Cl−) were measured by the titration method, sodium (Na+) and potassium (K+) by flame photometer whereas sulphates (SO42−) were analyzed by spectrophotometry. Magnesium (Mg2+) was estimated by the formulae [TH-(2.5 × CaH)]/4.1 (Todd and Mays 2005). The result of ionic balance shows that the error for groundwater samples was ≤ 10%. The analytical results of chemical parameters of groundwater are presented in Table 1. The Bureau of Indian Standards (BIS 2012) was considered for comparing chemical constituents in groundwater for its utilization for both domestic and agricultural purposes.
Entropy weighted water quality index (EWQI)
The water quality index methods provide better and more valid information to ascertain quality issues more accurately. The entropy weighted water quality index (EWQI) is one such method which explains the quality of water with a numerical number by integrating all the analyzed hydrochemical parameters. This improved water quality index model is considered to be more reliable and accurate as it greatly reduces the biased weight assignment to the quality parameters. EWQI is calculated as explained below in five steps (Wu et al. 2018; Subba Rao et al. 2020; Adimalla 2021):
Step 1 The eigenvalue matrix “X” which is associated with the water quality parameters estimated by the following equation:
where, m (i = 1, 2, 3, 4, …, m) represents water samples and n (j = 1, 2, 3, 4, ……., n) represents the total number of hydrochemical parameters of each sample.
Step 2 The standardization process “yij” is evaluated and then standard evaluation matrix “Y” is obtained as:
where xij is the initial matrix; (xij)min and (xij)max are the minimum and maximum values of the hydrochemical parameters of the samples, respectively.
Step 3 The entropy “(ej)” and entropy weight “(wj)” are computed using the equations as follows:
The entropy weight function is based on the discrete probability distribution.
where, \(n_{ij} = - \frac{{\left( {1 + y_{ij} } \right)}}{{\mathop \sum \nolimits_{i = 1}^{m} \left( {1 + y_{ij} } \right)}}\).
The degree of diversity (d) possessed by each criteria is evaluated as:
The weight for each criteria is given by
Step 4 The quality rating scale “qj” of the “j” parameter is calculated as:
where, “Vj” is the concentration value of chemical parameter “j” and “Sj” is the standard limit of BIS of parameter “j”.
Step 5 EWQI is calculated by using the following equation.
The computed EWQI for each sampling station has been grouped into five categories, namely, excellent (EWQI < 25), good (between 25 and 50), poor (50–100), very poor (100–150) and extremely poor (EWQI > 150) for human consumption. The standard values and the corresponding entropy weights are presented in Table 2.
Irrigation suitability parameters
The following selected parameters were computed for assessing the groundwater suitability for irrigation purpose.
where the concentration of ions used in the calculations are in meq/L except for KR and CR for which mg/L used.
The results of all irrigation quality parameters are given in Table 3. Multivariate statistical analysis methods, including principal component analysis, factor analysis, and correlation, were used to analyze the groundwater chemistry characteristics. XLSTAT 2018 was utilized for preparing graphs and data table analysis. The Piper, USSL, Wilcox diagrams were generated using Aquachem 2014 software.
Results and discussion
The box plot helps in summarizing the distribution of a data set by the median, the variation, the skewness, outliers and extreme values in a graphical form. From Fig. 2, it is noted that TA, EC, TDS, Mg2+, HCO3−, SO42− are approaching normality. The data for the variables Ca2+, Na+, Cl− and NO3− depart from a normal distribution only in skewness. There are outliers for pH, TH and K+ but the data departs from a normal distribution only in the skewness. The unexpected outliers may be due to the usage of fertilizers in agricultural and aqua pond regions. The abundance of chemical parameters is as follows: Na+ > K+ > Mg2+ > Ca2+ = Cl− > HCO3− > SO42− > NO3−.
Hydro chemical processes
The Piper (1944) plot explains the evolutionary trends of water quality parameters in order to classify the similarities and differences in the chemical composition of water into certain water types. The groundwaters were categorized into different hydrochemical facies based on major cations Ca2+, Mg2+, Na+, K+ and major anions HCO3−, Cl−, SO42− using Piper’s trilinear diagram (Fig. 3). The prominent types shown are Na-Mg-Cl-HCO3−, Na-Cl-HCO3− and Mg–Na–Cl–HCO3−. It can be observed from the plot that the majority of groundwater samples fall into the field of 4 suggesting that strong acids exceed weak acids. The exceeding primary salinity (field 7) and alkalies exceeding alkaline earths (field 2) are also found (Table 4). The samples in the Na–Mg–Cl facies indicate the leaching of primary/secondary salts and exchange of ions from the clay deposits. The mechanism controlling the geochemical process of groundwater with respect to atmospheric precipitation, rock–water interaction and evaporation, has been presented by Gibbs plot (1970) for the present study (Fig. 4). The ratio of dominant cations (Na+ + K+)/(Na+ + K+ + Ca2+) and anions (Cl−/(Cl− + HCO3−) against TDS was plotted. It is found that most sampling points fall towards evaporation dominance, indicating that the groundwater has high salinity controlled by evaporation and also rock weathering solubilization. Cation exchange is the influencing factor controlling hydrochemical processes. The limited interaction of rock water generally includes the chemical weathering of the rocks, the precipitation dissolution of secondary carbonates and the exchange of ions between the water and the clay minerals.
Suitability of groundwater use for drinking
The chemical constituents present in the groundwater show a wide variation in different individual parameters (Table 1). The pH of groundwater samples ranged from 7.2 to 8.7, with a mean of 7.8, indicating the slightly alkaline nature of groundwater in the study area. The concentrations of chemical constituents in groundwater and their effects on human health are presented in Table 5. The minimum and maximum values of alkalinity ranged from 124 to 466 mg/L with a standard deviation of 81.8. Above 250 mg/L, the concentration of total alkalinity in the water gives an unpleasant taste (BIS 2012). Nearly 85% of the water samples in the study area contain alkalinity values higher than the desirable limits. The high alkalinity values in the study area are raised due to the action of carbonates on the basic materials in the soil, which gives water an unpleasant taste. EC fluctuated from 1675 to 3881 µS/cm, with a mean of 2800 µS/cm while TDS ranged between 985 and 2283 mg/L, with a mean of 1647 mg/L. High EC is probably resulted from the dissolved inorganic substances largely present in the water (Hem, 1985). All groundwater samples recorded TDS levels above the desirable limits (more than 500 mg/L) and 84% of samples had TDS levels greater than 2000 mg/L, indicating that they are unfit for drinking (BIS 2012).TH as CaCO3 varied from 114 to 688 mg/L with a mean of 386. There are ten samples that fall within the desirable limits and the remaining samples (83% of total samples > 300 mg/L) fall into the very hard water category. The cations Ca2+, Mg2+, Na+ and K+ ranged between 11–69 mg/L, 10–139 mg/L, 98–414 mg/L, 29–143 mg/L, respectively. The anions HCO3−, Cl−, SO42− and NO3− from 162 to 610 mg/L, 156–602 mg/L, 28–227 mg/L, 2–41 mg/L, respectively.
Calcium bicarbonate is the prime cause of the hardness in water. Concentrations of Ca2+ and Mg2+ are well below the permissible limits. In seawater, magnesium is present in large quantities. High magnesium in the groundwater causes scaling in boilers, pipes and water heaters and abdominal disorders etc. and is not desirable for domestic use. Higher values of Na+ (mean 250 mg/L) and K+ (mean 95.2 mg/L) were found in the groundwater and may be attributed to saline water intrusion, discharge of aquaculture wastewaters and domestic sewage (Thilagavathi, et. al. 2019; Sivakarun et. al. 2020). Normally, these ions are not toxic to humans, but excess intake causes hypertension and vomiting, etc. Whereas K+ is an essential element for plants and animals. Cl− directly relates to the mineral content of water and is mostly identified by the salt taste in potable water. Only 19% of groundwater samples showed less than 250 mg/L which are acceptable for drinking as per BIS. It explains that the probable cause for the abnormal concentration of chloride is the seawater intrusion and rocks in the study region. SO42− concentrations in 7 locations were recorded as slightly high and all the samples of NO3− fell under the permissible limits of BIS. Overall, the majority of water quality parameters of the groundwater samples analyzed in the study area were recorded above desirable levels.
Groundwater quality based on EWQI
The results of the entropy weighted water quality index (EWQI) to quantify the quality of groundwater for the purpose of drinking in each location of the study area are given in Table 6. The spatial distribution of EWQI is presented in Fig. 5. The computed EWQI values for groundwater samples in the study ranged between 53.3 and 143.3 with an average of 99. Overall, the results indicate that the quality of groundwater is poor to extremely poor category. Fifty percent of total groundwater samples have shown more than 100 EWQI value, indicating that they are unfit for drinking or domestic use (Wu et al. 2018). The remaining 34% of samples were classified as very poor quality water, which is also unsuitable for drinking. Only 16% of samples show poor quality, which indicates that these sampling location’s groundwater may be marginally utilized for domestic use. None of the samples were found in the excellent to good quality of groundwater category.
Suitability of groundwater use for irrigation
In the study area, the groundwater samples were analyzed for monitoring the suitability of quality for irrigation purposes. It can be observed from Table 7, the groundwater recorded as high to very high salinity condition (Richards 1954). About 85% of total samples have recorded electrical conductivity that is very high (> 2250 μS/cm). High EC in the water proportionate to the salt content explains that the groundwater can severely affect the plants and soils, thus reducing productivity. The Na% ranged between 41 and 87.9 meq/L with a mean of 63 meq/L. Nearly 69% of water samples were found to have high levels of sodium (> 60%), thus not suitable even for irrigation (Swarna Latha and Nageswara Rao 2012). The ratio of Cl−/HCO3− of groundwaters can provide the level of salinization effect in a region (Weiner 2000). The Cl−/HCO3−ratio is shown above 2 in twenty-four samples out of 58, indicating the possible signatures of seawater intrusion into the land as the area is adjacent to the coast and the aqua ponds are continuously pumped by saline water (Desai et al. 1979).
High sodium content may destroy the soil structure and affect plant growth (Wilcox 1948). Only two samples (Nos. 54 and 55) fall under the permissible to doubtful category and the remaining samples are in the doubtful to unsuitable category (Fig. 6 and Table 8). The SAR values in the study area vary from 2.0 to 13.2 meq/L and nearly 45% of samples exhibit increased problems as SAR > 6 meq/l (Herman Bouwer 1978).
Wilcox (1948) diagram represents the presence of sodium content in the groundwaters
The influence of evaporation can be examined with the help of Na+ vs Cl− plots. The graph (Fig. 7a) provides a strong evidence of halite dissolution which results from aquifer salts and seawater intrusion. It was found that the dominant anion and cation present in water are Cl– and Na + , respectively. The enrichment of Na+ and K+ concentrations is accompanied by the increase of Cl− ions that probably occurs due to dissolution of soil salts (Manusree et al. 2009; Srinivasamoorthy et al. 2011; Rao et al. 2017; Senthilkumar et al. 2018). The majority of samples fall in injuriously contaminated by saline water intrusion indicating the mixing of fresh water aquifers with the saline water (Fig. 7b). The enrichment of Na + groundwater samples may due to cation exchange process. The progress in the salinization can occur with the groundwater recharge through Ca2+ exchange with Na+ as the clay soils and marine salts dominate the region. The Durov diagram (Fig. 8) explains that the Na+ + K+ enrichment with HCO3− dominance in the groundwater of study region may be influenced by the dissolution of clays and marine salts and the subsequent replacement of alkaline earths with the alkalis. The high TDS in groundwater also indicated the dissolution of soil salts and anthropogenic sources in the study region (Manjusree et al. 2009; Chidambaram et al. 2018).
According to the U.S. Salinity Laboratory Diagram (USDA 1955), more than 80% of the water samples come under the fields of C4S2, C4S1, C4S3 and C3S2, indicating high-very high salinity and low–high alkali water (Fig. 9 and Table 9). The groundwater is not suitable for irrigation in the drainage restriction as it leads to low permeability and poor cultivability. RSC varied from − 6.1 to 3.8 meq/L with a mean of − 1.8 meq/L in the study area. More than 82% of samples show negative values and are safe for irrigation purposes. The best irrigation practises must be adopted to use the marginal RSC water for irrigation. The high concentration of Na+, Ca2+, Mg2+ and HCO3− in irrigation water can affect the soil’s permeability condition. More than 80% of the groundwater samples are not suitable for irrigation purposes. (Donen 1964). The range of KR values is 0.7–8.5 mg/L and most groundwater samples (91%) are recorded above 1, hence the groundwater is fit for irrigation (Kelley 1951). The CR values (0.7–5.3 mg/L) recorded in the study area indicate the corrosive nature of water, thus it cannot be transported through the metal pipes.
Principal component and factor analysis
The dataset of analyzed parameters was verified for variable reduction by PCA and FA using Kaiser–Meyer–Olkin and Bartlett’s sphericity tests. The results of the KMO and ρ were 0.58 and less than 0.001, respectively, hence the dataset was used for analysis (Wang et al. 2013). The results of the principal factors, eigenvalues, explained variance and varimax–rotated loads are summarized in Table 7. EC (0.92), TDS (0.92), HCO3− (0.71), TA (0.69), TH (0.69), Mg2+ (0.6) in factor 1 while in factor 2, Na+ (0.88) and Cl− (0.88) were recorded. The first three PCs accounted for 65% of total variance cumulatively 94.5% for a total of 7 PCs. The scree plot showing the positive component loadings of all PCs is presented in Fig. 10. The first factor explained 33.3% of the total variance with strong positive loadings on EC, TDS, TH, HCO3−, TA and limited loading on NO3−. This could be due to the influence of carbonate weathering as the main source of these minerals. Factor 2 contributed 20.3% of the total variance with high positive loadings on Na+ and Cl− which probably due to seawater intrusion. Factor 3 accounts for 10.8% of the total variance. The closely related parameters were SO42− and K+; this was probably due to the application of organic and inorganic fertilizers, manure and sewage. With the loading of Mg2+, factor 4 contributed 9.74% to the total variance; this indicates the impact of clay minerals and rock weathering. All the hydrochemical parameters applied by Pearson’s correlation indicating that TDS was significantly correlated with EC, TH, Ca2+, Mg2+, Na+, K+, HCO3− and Cl− (Table 10). The Na+ and Cl−, TA and HCO3− are correlated highly significant and are the main source of TDS.
Conclusions
The evolution of groundwater chemistry was explained through geochemical plots, ionic ratios, bivariate scatter plots, principal component and factor analysis for the coastal aquifer of Southern India. The chemical constituents in the groundwater were determined through EWQI for their suitability for drinking purposes. The average ionic concentration found in the study area is Na+ > K+ > Mg2+ > Ca2+ = Cl− > HCO3− > SO42− > NO3−. The following observations made during the study:
-
The high concentrations of Na+, Cl− and SO42− found in the groundwater may be attributed to the dissolution of mineral phases in the aquifer systems.
-
The result of PCA and FA analysis revealed that the factors responsible for the variation in the groundwater chemistry are weathering, leaching of secondary salts, reverse ion exchange, seawater intrusion and agricultural return flow.
-
All the groundwater samples when compared with BIS for potability indicating the groundwater in the study area is unfit for drinking in the majority of the areas.
-
The entropy water quality index values shown that nearly 85% of groundwater samples indicating very poor quality of water. Hence, remedial measures must be taken for utilizing groundwater for drinking purpose.
-
The quality indices for irrigation reveal that the groundwater studied in the locations ranges between the good and moderate categories, hence the water can be used for irrigational purposes with proper management.
Various anthropogenic activities such as intense agricultural and aquaculture practices, aquaculture waste discharge without treatment etc. are also the probable causes of deterioration of the quality of water. This research database provides baseline information that may be used for detecting significant trends more precisely with the help of modern tools like the Geographic Information System. Further research need to be conducted to identify both geogenic and anthropogenic sources of the contamination of groundwater, so that it will help the authorities in implementing an appropriate water management programmes at local level.
Data availability
All data generated or analyzed during this study are included in this article.
References
Adimalla N (2021) Application of the entropy weighted water quality index (EWQI) and the pollution index of groundwater (PIG) to assess groundwater quality for drinking purposes: a case study in a rural area of Telangana State India. Arch Environ Contam Toxicol 80:31–40. https://doi.org/10.1007/s00244-020-00800-4
Alfarrah N, Walraevens K (2018) Groundwater overexploitation and seawater intrusion in coastal areas of arid and semi-arid regions. Water 10:143. https://doi.org/10.3390/w10020143
Aly AA, Al-Omran AM, Alharby MM (2014) The water quality index and hydrochemical characterization of groundwater resources in Hafar Albatin Saudi Arabia. Arab J Geosci 8:4177–4190. https://doi.org/10.1007/s12517-014-1463-2
APHA (1998) Standard methods for the examination of water and wastewater. American Public Health Association, Washington
Belkhiri L, Boudoukha A, Mouni L, Baouz T (2010) Application of multivariate statistical methods and inverse geochemical modeling for characterization of groundwater—a case study: Ain Azel plain (Algeria). Geoderma 159(3–4):390–398. https://doi.org/10.1016/j.geoderma.2010.08.016
BIS. (2012). Drinking water-specification. Bureau of Indian standards, New Delhi IS: 10500: Vol. Second rev.
Blake S, Henry T, Murray J, Flood R, Muller MR, Jones AG, Rath V (2016) Compositional multivariate statistical analysis of thermal groundwater provenance: a hydrogeochemical case study from Ireland. Appl Geochem 75:171–188. https://doi.org/10.1016/j.apgeochem.2016.05.008
Brindha K, Kavitha R (2015) Hydrochemical assessment of surface water and groundwater quality along Uyyakondan channel. South India Environ Earth Sci 73(9):5383–5393. https://doi.org/10.1007/s12665-014-3793-5
CGWB. (2014). Central Ground Water Board Report on status of ground water quality in coastal aquifers of India. Ministry of Water Resources. India, Faridabad, Govt. of. India, New Delhi.
CGWB. (2017). Groundwater year book of Andhra Pradesh. Central Ground Water Board, Ministry of Water Resources, Government of India, New Delhi.
Chidambaram S, Prasanna MV, Ramanathan AL, Vasu K, Hameed S, Warrier UK, Srinivasamoorthy K, Manivannan T, Tirmalesh K, Anandhan P, Johnsonbabu G (2009) A Study on the factors affecting the stable isotopic composition in precipitation of Tamil Nadu. Inida Hydro Process 23:1792–1800. https://doi.org/10.1002/hyp.7300
Chidambaram S, Sarathidasan J, Srinivasamoorthy K et al (2018) Assessment of hydrogeochemical status of groundwater in a coastal region of southeast coast of India. Appl Water Sci 8:27. https://doi.org/10.1007/s13201-018-0649-2
Desai BI, Gupta SK, Shah MV, Sharma SC (1979) Hydrochemical evidence of sea water intrusion along the Mangrol-Chorwad coast of Saurashtra Gujarat. Hydrol Sci J 24(1):71–82. https://doi.org/10.1080/02626667909491835
Diana AS, Madhuri SR, Tirumalesh K (2017) Evaluation of groundwater quality and suitability for irrigation and drinking purposes in southwest Punjab, India using hydrochemical approach. Appl Water Sci 7:3137–3150. https://doi.org/10.1007/s13201-016-0456-6
Doneen LD (1964) Notes on water quality in agriculture. Published as a water science and engineering, paper 4001. Department of water sciences and engineering. University of California, Davis
Dudeja D, Kumar Bartarya S, Biyani AK (2011) Hydrochemical and water quality assessment of groundwater in Doon valley of outer Himalaya Uttarakhand India. Environ Monit Assess 181:183–204. https://doi.org/10.1007/s10661-010-1823-7
Ferguson G, Gleeson T (2012) Vulnerability of coastal aquifers to groundwater use and climate change. Nat Clim Change 2:342–345. https://doi.org/10.1038/nclimate1413
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170(3962):1088–1090. https://doi.org/10.1126/science.170.3962.1088
Gibrilla EA, Bam KP, Adomako D, Ganyaglo S, Osae S, Akiti TT, Kebede S, Achoribo E, Ahialey E, Ayanu G, Agyeman EK (2011) Application of water quality index (WQI) and multivariate analysis for groundwater quality assessment of the Birimian and Cape coast Granitoid complex: Densu river basin of Ghana. Water Qual Expo Health 3:63. https://doi.org/10.1007/s12403-011-0044-9
Guggenmos MR, Daughney CJ, Jackson BM, Morgenstern U (2011) Regional-scale identification of groundwater-surface water interaction using hydrochemistry and multivariate statistical methods, Wairarapa valley, New Zealand. Hydrol Earth Syst Sci 15:3383–3398. https://doi.org/10.5194/hess-15-3383-2011
Güler C, Kurt MA, Alpaslan M, Akbulut C (2012) Assessment of the impact of anthropogenic activities on the groundwater hydrology and chemistry in Tarsus coastal plain (Mersin, SE Turkey) using fuzzy clustering, multivariate statistics and GIS techniques. J Hydrol 414:435–451. https://doi.org/10.1016/j.jhydrol.2011.11.021
Gulgundi MS, Shetty A (2018) Groundwater quality assessment of urban Bengaluru using multivariate statistical techniques. Appl Water Sci 8:43. https://doi.org/10.1007/s13201-018-0684-z
He X, Wu J, He S (2019) Hydrochemical characteristics and quality evaluation of groundwater in terms of health risks in Luohe aquifer in Wuqi county of the Chinese Loess Plateau, northwest China. Human Ecol Risk Assess Int J 25(1–2):32–51. https://doi.org/10.1080/10807039.2018.1531693
Herman B (1978) Groundwater hydrology. McGraw-Hill, New York
Houatmia F, Azouzi R, Charef A, Bedir M (2016) Assessment of groundwater quality for irrigation and drinking purposes and identification of hydrogeochemical mechanisms evolution in Northeastern Tunisia. Environ Earth Sci 75:746. https://doi.org/10.1007/s12665-016-5441-8
Iscen CF, Emiroglu Ö, Ilhan S, Arslan N, Yilmaz V, Ahiska S (2008) Application of multivariate statistical techniques in the assessment of surface water quality in Uluabat lake, Turkey. Environ Monit Assess 144:269–276. https://doi.org/10.1007/s10661-007-9989-3
Jalali M (2007) Salinization of groundwater in arid and semi-arid zones: an example from Tajarak, western Iran. Environ Geol 52:1133–1149. https://doi.org/10.1007/s00254-006-0551-3
Jeen SW, Kim JM, Ko KS, Yum B, Chang HW (2001) Hydrogeochemical characteristics of groundwater in a mid-western coastal aquifer system Korea. Geosci J 5(4):339–348. https://doi.org/10.1007/s12303-018-0065-5
Jiang Y, Yuexia Wu, Groves C, Yuan D, Kambesis P (2009) Natural and anthropogenic factors affecting the groundwater quality in the Nandong karst underground river system in Yunan China. J Contam Hydrol 109(1–4):49–61. https://doi.org/10.1016/j.jconhyd.2009.08.0
Juahir H, Zain SM, Yusoff MK, Hanidza TT, Armi AM, Toriman ME, Mokhtar M (2011) Spatial water quality assessment of Langat river basin (Malaysia) using environmetric techniques. Environ Monit Assess 173(1–4):625–641. https://doi.org/10.1007/s10661-010-1411-x
Kelley WP (1951) Alkali soils, their formation, properties, and reclamation. Reinhold Publishing Corporation. A. C. S. Monograph series, New York (No. 111)
Khelif S, Boudoukha A (2018) Multivariate statistical characterization of groundwater quality in Fesdis, East of Algeria. J Water Land Dev 37(1):65–74. https://doi.org/10.2478/jwld-2018-0026
Knobeloch L, Salna B, Hogan A, Postle J, Anderson H (2000) Blue babies and nitrate-contaminated well water. Environ Health Perspect 108(7):675–678. https://doi.org/10.1289/ehp.00108675
Lalitha S, Kalaivani D, Selvameena R, Barani AV (2004) Assay on quality of water samples from medical college area in Thanjavur India. Indian J Environ Protect 24(12):925–930
Lee JY, Cheon JY, Lee KK, Lee SY, Lee MH (2001) Statistical evaluation of geochemical parameter distribution in a ground water system contaminated with petroleum hydrocarbons. J Environ Qual 30(5):1548–1562. https://doi.org/10.2134/jeq2001.3051548x
Li P, Wu J, Hui Q (2016) Hydrochemical appraisal of groundwater quality for drinking and irrigation purposes and the major influencing factors: a case study in and around Hua county China. Arab J Geosci 9:15. https://doi.org/10.1007/s12517-015-2059-1
Loh YSA, Akurugu BA, Manu E, Aliou A (2020) Assessment of groundwater quality and the main controls on its hydrochemistry in some voltaian and basement aquifers, northern Ghana. Groundw Sustain Dev. https://doi.org/10.1016/j.gsd.2019.100296
Machiwal D, Jha MK (2010) Tools and Techniques for Water Quality Interpretation. In: Krantzberg A, Tanik G, AntunesdoCarmo A, Indarto JS, Ekdal A (eds) Advances i. Scientific Research Publishing Inc, California, pp 211–252
Manjusree TM, Sabu J, Thomas J (2009) Hydrogeochemistry and groundwater quality in the coastal sandy clay aquifers of Alappuzha district, Kerala. J Geol Soc India 74:459–468
Meride Y, Ayenew B (2016) Drinking water quality assessment and its effects on residents health in Wondo genet campus Ethiopia. Environ Syst Res 5:1. https://doi.org/10.1186/s40068-016-0053-6
Mohapatra PK, Vijay R, Pujari PR, Sundaray SK, Mohanty BP (2011) Determination of processes affecting groundwater quality in the coastal aquifer beneath Puri city. India: a multivariate statistical approach. Water Sci Technol 64:809–817. https://doi.org/10.2166/wst.2011.605
Paul R, Brindha K, Gowrisankar G, Tan ML, Singh MK (2019) Identification of hydrogeochemical processes controlling groundwater quality in Tripura, northeast India using evaluation indices, GIS, and multivariate statistical methods. Environ Earth Sci 78(15):470. https://doi.org/10.1007/s12665-019-8479-6
Piper AM (1944) A graphic procedure in the geochemical interpretation of water-analyses. Trans Am Geophys Union 25(6):914–928. https://doi.org/10.1029/TR025i006p00914
Prasanna MV, Chidambaram S, Hameed SA, Krishnara J, Srinivasamoorthy K (2011) Hydrogeochemical analysis and evaluation of groundwater quality in the Gadilam river basin Tamil Nadu, India. J Earth Sys Sci 120:85–98. https://doi.org/10.1007/s12040-011-0004-6
Raju DSN, Ravindran CN, Mishra PK, Chidambaram L, Saxena RK (1994) Stratigraphy and Paleoenvironments of the Godavari clay in the Krishna-Godavari basin India. Indian J Pet Geol 3(2):33–43
Ramesh NR (2008) Quaternary geology of the deltas of Andhra Pradesh. J Geol Soc India 72:438–439
Rao NS, Vidyasagar G, SuryaRao P et al (2017) Chemistry and quality of groundwater in a coastal region of Andhra Pradesh, India. Appl Water Sci 7:285–294. https://doi.org/10.1007/s13201-014-0244-0
Ravikumar P, Somashekar RK (2017) Principal component analysis and hydrochemical facies characterization to evaluate groundwater quality in Varahi river basin, Karnataka state India. Appl Water Sci 7(2):745–755. https://doi.org/10.1007/s13201-015-0287-x
Reddy AGS (2013) Evaluation of hydrogeochemical characteristics of phreatic alluvial aquifers in southeastern coastal belt of Prakasam district, South India. Environ Earth Sci 68:471–485. https://doi.org/10.1007/s12665-012-1752-6
Richards LA (1954) Diagnosis and improvement of saline and alkali soils United States Department of Agriculture Agriculture Handbook No. 60. Soil Sci 78:154
Sahu P, Sikdar PK (2008) Hydrochemical framework of the aquifer in and around east Kolkata wetlands, west Bengal India. Environ Geol 55(4):823–835. https://doi.org/10.1007/s00254-007-1034-x
Sajil Kumar PJ (2016) Deciphering the groundwater–saline water interaction in a complex coastal aquifer in South India using statistical and hydrochemical mixing models. Model Earth Syst Environ 2(194):1–11. https://doi.org/10.1007/s40808-016-0251-2
Sandeep R, Baldev S, Deswal S (2020) Groundwater quality analysis of northeastern Haryana using multivariate statistical techniques. J Geol Soc India 95:407–416. https://doi.org/10.1007/s12594-020-1450-z
Sarikhani R, Dehnavi AG, Ahmadnejad Z, Kalantari N (2015) Hydrochemical characteristics and groundwater quality assessment in Bushehr Province, SW Iran. Environ Earth Sci 74:6265–6281. https://doi.org/10.1007/s12665-015-4651-9
Sayad L, Djabri L, Bouhsina S, Bertrand C, Hani A, Chaffai H (2017) Hydrochemical study of Drean-Annaba aquifer system (NE Algeria). J Water Land Dev 34:259–263. https://doi.org/10.1515/jwld-2017-0061
Scanlon BR, Jolly I, Sophocleous M, Zhang L (2007) Global impacts of conversions from natural to agricultural ecosystems on water resources: quantity versus quality. Water Resour Res 43(W03437):1–20. https://doi.org/10.1029/2006WR005486
Senthilkumar S, Gowtham B, Sundararajan M, Chidambaram S, Francis LJ, Prasanna MV (2018) Impact of landuse on the groundwater quality along coastal aquifer of Thiruvallur district, South India. Sustain Water Resour Manag 4:849–873. https://doi.org/10.1007/s40899-017-0180-x
Shindo J, Okamoto K, Hiroyuki K (2006) Prediction of the environmental effects of excess nitrogen caused by increasing food demand with rapid economic growth in eastern Asian countries, 1961–2020. Ecol Model 193(3–4):703–720. https://doi.org/10.1016/j.ecolmodel.2005.0
Simeonov V, Stratis JA, Samara C, Zachariadis G, Voutsa D, Anthemidis A, Sofoniou M, Kouimtzis T (2003) Assessment of the surface water quality in northern Greece. Water Res 37(17):4119–4124. https://doi.org/10.1016/S0043-1354(03)00
Sivakarun N, Udayaganesan P, Chidambaram S, Venkatramanan S, Prasanna MV, Pradeep K, Banajarani P (2020) Factors determining the hydrogeochemical processes occurring in shallow groundwater of coastal alluvial aquifer India. Geochemistry 80(125623):1–16. https://doi.org/10.1016/j.chemer.2020.125623
Srinivasamoorthy M, Vasanthavigar S, Chidambaram S, Anandan P, Sharma VS (2011) Characterization of groundwater chemistry in an eastern coastal area of Cuddalore district Tamil Nadu. J Geol Soc India 78:549–558
Subba Rao N, Sunitha B, Adimalla N, Chaudhary M (2020) Quality criteria for groundwater use from a rural part of Wanaparthy district, Telangana state, India, through ionic spatial distribution (ISD), entropy water quality index (EWQI) and principal component analysis (PCA). Environ Geochem Health 42(2):579–599. https://doi.org/10.1007/s10653-019-00393-5
Subyani AM, Al Ahmadi ME (2010) Multivariate statistical analysis of groundwater quality in Wadi Ranyah Saudi Arabia JAKU. Earth Sci 21(2):29–46. https://doi.org/10.4197/Ear.21-2.2
Swarna Latha P, Hema Malini B (2018) Land cover change detection analysis using remote sensing and GIS techniques: a study on part of West Godavari Delta Region, Andhra Pradesh India. Trans Inst Indian Geogr 40(2):241–247
Swarna Latha P, Nageswara Rao K (2012) An integrated approach to assess the quality of groundwater in a coastal aquifer of Andhra Pradesh India. Environ Earth Sci 66(8):2143–2169. https://doi.org/10.1007/s12665-011-1438-5
Thilagavathi R, Chidambaram S, Thivya C, Tirumalesh K, Venkatramanan S, Pethaperumal S, Prasanna MV, Ganesh N (2019) Influence of variations in rainfall pattern on the hydrogeochemistry of coastal groundwater—an outcome of periodic observation. Environ Sci Pollut Res Int 26(28):29173–29190. https://doi.org/10.1007/s11356-019-05962-w
Todd DK, Mays LW (2005) Groundwater Hydrology, 3rd edn. Wiley, New York
USDA (1955) Water: The Yearbook of Agriculture. The United States Department of Agriculture.
USGS (2001) What is Groundwater. Clark DW, Briar DW. Open-file report, reprinted April 2001.
Wagh V, Panaskar D, Aamalawar ML, Lolage YP, Mukate S, Narshimma A (2018) Hydrochemical characterisation and groundwater suitability for drinking and irrigation uses in semiarid region of Nashik, Maharashtra India. Hydrosp Anal 2(1):43–60. https://doi.org/10.21523/gcj3.18020104
Wang Y, Wang P, Bai Y, Tian Z, Li J, Shao X, Mustavich LF, Li B (2013) Assessment of surface water quality via multivariate statistical techniques: A case study of the Songhua River Harbin region, China. Jour Hydro Env Res 7(1):30–40. https://doi.org/10.1016/j.jher.2012.10.003
Wei Li, Ruben O, Natalia C, Haizhou L (2017) Mechanisms on the impacts of alkalinity, pH, and chloride on persulfate-based groundwater remediation. Environ Sci Technol 51(7):3948–3959. https://doi.org/10.1021/acs.est.6b04849
Weiner ER (2000) Applications of environmental chemistry-a practical guide for environmental professionals. Lewis Publishers, New York
WHO (2003a) Chloride in Drinking-water. Background document for development WHO Guidelines for Drinking-water Quality. World Health Organization. WHO/SDE/WSH/03.04/03.
WHO (2003b) pH in Drinking-water Background document for development of WHO Guidelines for Drinking-water Quality. World Health Organization .WHO/SDE/WHO/03.04/12.
WHO (2004) Sulfate in Drinking-water Background document for development of WHO Guidelines for Drinking-water Quality. World Health Organization. WHO/SDE/WSH/03.04/114.\
WHO (2009) Calcium and Magnesium in Drinking-water: Public health significance. Cotruvo J, Bartram J, eds. World Health Organization, Geneva
Wilcox LV (1948) The quality of water for irrigation use. US Department of Agricultural Technical Bulletin 1962, Washington
World Bank (2012) India groundwater: a valuable but diminishing resource.
Wu C, Wu X, Qian C, Zhu G (2018) Hydrogeochemistry and groundwater quality assessment of high fluoride levels in the Yanchi endorheic region, northwest China. Appl Geochem 98:404–417. https://doi.org/10.1016/j.apgeochem.2018.10.016
Zakaria N, Anornu G, Adomako D, Owusu-Nimo F, Abass G (2021) Evolution of groundwater hydrogeochemistry and assessment of groundwater quality in the Anayari catchment. Groundw Sustain Dev. https://doi.org/10.1016/j.gsd.2020.100489
Acknowledgements
The author also thanks Dr. K. Nageswara Rao, Assistant Professor of Geography, IGNOU, New Delhi, for his help carrying out the analysis and the Editor-in-Chief and anonymous reviewers for providing valuable suggestions to improve the manuscript.
Funding
The author is grateful to the University Grants Commission, New Delhi for funding provided towards the present work in the form of Post-Doctoral Fellowship for women (F.No.15-1/2013-14/PDFWM-2013-14-GE-AND-19638 (SAII) Dated 18-Apr-2014), Swarna Latha P.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that there is no conflict of Interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Latha, P.S. Evaluation of groundwater quality for domestic and irrigation purposes in a coastal alluvial aquifer using multivariate statistics and entropy water quality index approach: a case study from West Godavari Delta, Andhra Pradesh (India). Environ Earth Sci 81, 275 (2022). https://doi.org/10.1007/s12665-022-10387-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-022-10387-9